Abstract
AIM: To study at what temperature the oxygen carried by the perfusate meets liver requirements in a model of organ perfusion.
METHODS: In this study, we correlated hypoxia inducible factor (HIF)-1α expression to the perfusion temperature and the hepatic oxygen uptake in a model of isolated perfused rat liver. Livers from Wistar rats were perfused for 6 h with an oxygenated medium at 10, 20, 30 and 37 °C. Oxygen uptake was measured by an oxygen probe; lactate dehydrogenase activity, lactate release and glycogen were measured spectrophotometrically; bile flow was gravitationally determined; pH of the perfusate was also evaluated; HIF-1α mRNA and protein expression were analyzed by real time-polymerase chain reaction and ELISA, respectively.
RESULTS: Livers perfused at 10 and 20 °C showed no difference in lactate dehydrogenase release after 6 h of perfusion (0.96 ± 0.23 vs 0.93 ± 0.09 mU/min per g) and had lower hepatic damage as compared to 30 and 37 °C (5.63 ± 0.76 vs 527.69 ± 45.27 mU/min per g, respectively, Ps < 0.01). After 6 h, tissue ATP was significantly higher in livers perfused at 10 and 20 °C than in livers perfused at 30 and 37 °C (0.89 ± 0.06 and 1.16 ± 0.05 vs 0.57 ± 0.09 and 0.33 ± 0.08 nmol/mg, respectively, Ps < 0.01). No sign of hypoxia was observed at 10 and 20 °C, as highlighted by low lactate release respect to livers perfused at 30 and 37 °C (121.4 ± 12.6 and 146.3 ± 7.3 vs 281.8 ± 45.3 and 1094.5 ± 71.7 nmol/mL, respectively, Ps < 0.02), and low relative HIF-1α mRNA (0.40 ± 0.08 and 0.20 ± 0.03 vs 0.60 ± 0.20 and 1.47 ± 0.30, respectively, Ps < 0.05) and protein (3.72 ± 0.16 and 3.65 ± 0.06 vs 4.43 ± 0.41 and 6.44 ± 0.82, respectively, Ps < 0.05) expression.
CONCLUSION: Livers perfused at 10 and 20 °C show no sign of liver injury or anaerobiosis, in contrast to livers perfused at 30 and 37 °C.
Keywords: Anaerobiosis, Hypoxia inducible factor-1α, Ischemia, Liver transplantation, Machine perfusion
Core tip: Among the techniques developed to improve the preservation of marginal organs for transplantation, hypothermic perfusion is the preferred choice. We show that it is possible to perfuse a rat liver at 20 °C without incurring ischemia. We evaluated liver injury, energetic status, lactate release, and hypoxia inducible factor-1α expression. Results show that symptoms of ischemia appear at temperatures > 20 °C, whereas there is no detectable advantage below 20 °C. These findings have interesting implications in liver preservation; maintaining the liver in a mild metabolic state could be useful for pharmacologic treatment and regeneration of the energetic status in ATP-depleted organs.
INTRODUCTION
Orthotopic liver transplantation is the treatment of choice for end-stage liver disease. The employment of this technique is limited by the shortage of viable donor organs. Recently, the donor acceptance criteria for organ retrieval have been expanded, including livers with low degrees of steatosis[1] and grafts from non-heart-beating donors[2].
The use of marginal livers for organ transplantation emphasizes a fundamental flaw of conventional cold storage: despite all improvements, marginal organs are at greater risk of preservation-associated primary nonfunction because of increased sensitivity to preservation-induced ischemia/reperfusion injury[3]. Increasing grades of donor liver steatosis were associated with worse initial poor function[4,5] due to impaired metabolism of the steatotic hepatocytes[6,7], to the crystallization of lipids during cold ischemia[8], and to an increased sensitivity to oxygen radicals during reperfusion[9]. Livers from non-heart-beating donors exhibited postoperative biliary complications[10,11] due to the superimposing effects of cold and warm ischemia[12].
In order to overcome the limits of cold storage, animal studies concerning preservation by machine perfusion are flourishing; this technique reducing ischemic injury is usually associated with organ preservation. Different settings have been tested. St Peter and colleagues showed that oxygenated, normothermic (sanguineous) machine perfusion recovers ischemic livers to a viable level[13]. In a clinical trial, Guarrera et al[14] demonstrated improved clinical parameters and shorter duration of hospital stay in patients who received grafts stored by hypothermic machine perfusion in comparison to patients who received grafts preserved by cold storage. Tolboom et al[15] showed that, in a rat liver transplantation model, the survival rate after 4 wk was 100% for animals receiving livers preserved by subnormothermic machine perfusion; on the contrary, no cold stored graft survived after transplantation.
The mechanism by which machine perfusion better preserves marginal livers is not yet fully understood, nor a rationale was given for applying particular perfusion conditions. Nonetheless, cold storage compromises the ability to reoxidize NAD(P)H through mitochondrial respiration[16], whereas machine perfusion is always associated with ATP and glycogen recovery[17,18], suggesting a decisive role of oxygenation in the control of ischemic damage during preservation. For these reasons, it is of primary importance to ensure adequate oxygenation during perfusion. The issue of oxygenation is strictly related to perfusion temperature; both the oxygen carried by the perfusate and liver oxygen requirement are strongly related to temperature, with the first decreasing and the second exponentially increasing at increasing temperatures[19].
In our previous works, we evaluated the machine perfusion at subnormothermic temperature for the preservation of ischemic[20] and steatotic[6] rat livers, in a model of ex vivo reperfusion. In this work, we studied how the liver responds to different perfusion conditions, with the goal of determining at what temperature the oxygen carried by the perfusate and the liver oxygen requirement meet or, from a different point of view, at what temperature the liver switches from aerobiosis to anaerobiosis, taking the road to ischemia.
We perfused rat livers at various temperatures saturating the perfusion solution with O2:CO2 (95%:5%). The considered temperature range allows for maintenance of homogeneous perfusion conditions, as long-term liver perfusion at 4 °C is usually performed at lower flow rate, and may require additives to prevent cell swelling. For these reasons, we did not include livers perfused at 4 °C in the experimental design. Liver injury, function, and energetic status were evaluated. The switch to anaerobic metabolism was evaluated using lactate release and mRNA/protein expression of hypoxia inducible factor (HIF)-1α, a transcription factor that precociously responds to decreases in available oxygen in the cellular environment[21].
MATERIALS AND METHODS
Animals and surgery and liver perfusion
Male Wistar rats (Harlan Laboratories Inc., Indianapolis, IN, United States) weighing 250-300 g were allowed free access to water and food until the beginning of all experiments. The use and care of animals in this experimental study were approved by the Italian Ministry of Health and by the University Commission for Animal Care. All surgeries were performed under anesthesia, and all efforts were made to minimize suffering. Rats were anesthetized with sodium pentobarbital (40 mg/kg, ip) and livers were isolated as already described[20,22]. Briefly, after median laparotomy followed by bilateral subcostal incisions, the animals received 200 U of heparin per 100 g of body weight via the inferior vena cava (5000 IU/mL; Marvecs Services, Agrate Brianza, MI, Italy). The bile duct was cannulated with 50 G polyethylene tubing (Intramed; Becton, Dickinson, and Co., Franklin Lakes, NJ, United States), and the portal vein was cannulated with a 16 G catheter (Johnson and Johnson, New Brunswick, NJ, United States). The liver was washed out with 50 mL of modified Krebs-Henseleit buffer via the portal vein cannula, then was freed from ligaments, removed and placed in a jacketed chamber for perfusion at different temperatures. At the end of liver perfusion, liver samples were immediately snap frozen in liquid nitrogen and stored at -80 °C.
Livers were divided into four experimental groups depending on perfusion temperature: 10, 20, 30 or 37 °C (n = 6/group). Livers were placed in an organ chamber, connected to a recirculating perfusion system, and perfused for 6 h. The perfusion medium was a modified Krebs-Henseleit buffer[18] continuously gassed with O2:CO2 (95%:5%). Perfusion flow was kept constant at 2.6 mL/min per gram[20].
Assays
Liver parenchyma viability was assessed through release of lactate dehydrogenase (LDH) into the effluent perfusate, as described by Bergmeyer et al[23]. The perfusion temperature was continuously monitored with a probe placed inside the isolated organ chamber. The portal venous pressure was continuously measured throughout the perfusion by means of a water column connected to the portal vein inflow catheter; pre-calibration was performed each time just before connecting the liver to the circuit. The basal perfusion pressure was 12-14 mmHg. Dissolved oxygen in the perfusion solution was measured with a probe (OXY 340i; WTW GmbH, Weilheim, Germany) at intervals of 1 h, both in the inlet and outlet perfusion solution; oxygen delivery rate and liver oxygen uptake rate (OUR) were calculated. The pH was continuously evaluated both in the perfusion solution reservoir and in a reference solution, consisting of the perfusion buffer kept at the same temperature and pO2 conditions but not circulated through the liver.
Tissue ATP was measured with the luciferin-luciferase method using the ATPlite luminescence assay kit (Perkin Elmer Inc., Waltham, MA, United States) according to the manufacturer’s instructions with minor changes. Briefly, frozen tissue was homogenized in ice-cold 100 mmol/L phosphate buffer with 3 mmol/L EDTA; the homogenate was immediately precipitated in 30% trichloroacetic acid and centrifuged at 3000 × g for 15 min at 4 °C. The supernatant was diluted 50× in 100 mmol/L phosphate buffer and assayed[20].
The glycogen assay was performed as described by Bennett et al[24]. Frozen samples were homogenized in a solution of 10% HClO4 and centrifuged at 280 × g for 15 min. The pellets were resuspended in 2 mL of deionized H2O. Samples (0.1 mL) were mixed with 0.2 mL of 5% phenol and 1 mL of H2SO4. After 30 min, absorbance at 490 nm was measured[24].
HIF-1α mRNA was analyzed using real-time-PCR using total RNA isolated from frozen liver samples with TRI reagent (Sigma-Aldrich, St. Louis, MO, United States)[25]. The cDNA was generated using iScript Supermix (Bio-Rad Laboratories Inc., Hercules, CA, United States). The RNA was assayed by measuring the absorbance at 260/280 nm. HIF-1α, ubiquitin C and GAPDH gene amplification efficiencies were established by means of calibration curves (108.8, 98.6 and 97.4%, respectively). The expression of the housekeeping gene remained constant in the considered experimental group. Sequences for the primers used are: HIF-1α: 5′-ACA AGA AAC CGC CTA TGA CG-3′ (forward) 3′-TAA ATT GAA CGG CCC AAA AG-5′ (reverse); ubiquitin C: 5′-CAC CAA GAA CGT CAA ACA GGA A-3′(forward), 3′-AAG ACA CCT CCC CAT CAA ACC-5′ (reverse); GAPDH: 5′-AAC CTG CCA AGT ATG ATG AC-3′ (forward), 5′-GGA GTT GCT GTT GAA GTC GTC A-3′ (reverse). Gene expression was analyzed using Platinum Sybr Green qPCR mix UDG (Life Technologies of Thermo Fisher Scientific Inc., Waltham, MA, United States). Ubiquitin c and GAPDH were used as reference genes. The amplification was performed through two-step cycling (95-60 °C) for 45 cycles, in an ABI prism 7000 sequence detection system (Applied Biosystems of Thermo Fisher Scientific), following the instructions of the supplier. All samples were assayed in duplicate. The results were normalized to the endogenous controls, and fold change of the gene expression was calculated using threshold cycle (Ct) values.
At the end of the preservation, the nuclear fraction was immediately isolated from fresh tissue with the Nuclear Extraction Kit (Cayman Chemical Co., Ann Arbor, MI, United States). The HIF-1α protein expression was analyzed on the nuclear fraction with an ELISA kit (HIF-1α Transcription Factor Assay Kit; Cayman). The lactate was assayed using the Lactate Colorimetric Assay Kit (BioVision Inc., Milpitas, CA, United States).
Statistical analysis
Data are presented as the mean ± SE. Statistical analyses for multiple comparisons were performed using one-way analyses of variance tests with Bonferroni’s corrections.
RESULTS
Liver OUR and oxygen delivery rate
In our experiments, OUR was constant during 6 h of perfusion at 10, 20, and 30 °C. At 37 °C, perfusion OUR dropped after 3 h, probably due to massive necrosis of the liver (Figure 1A). We observed a strong linear correlation between the basal OUR and the perfusion temperature (R2 = 0.9979). A dependence of oxygen delivery rate on temperature was also observed; the available oxygen in the perfusion solution decreased with perfusion temperature (data not shown).
Figure 1.
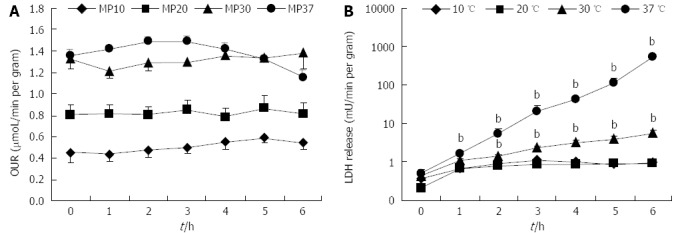
Liver oxygen uptake rate and lactate dehydrogenase release rate in rat livers perfused for 6. A: OUR; and B: Lactate-dehydrogenase (LDH) release rate over 6 h of perfusion at 10, 20, 30 or 37 °C (n = 6/group; bP < 0.01 vs 20 °C). OUR: Oxygen uptake rate.
Release of LDH, portal pressure and bile production
Livers perfused at 10 and 20 °C showed a very low level of LDH release; livers perfused at 30 and 37 °C released significantly more LDH at the end of perfusion in comparison to livers perfused at 20 °C (Ps < 0.01). Furthermore, LDH release rate was near zero in 10 and 20 °C perfusion groups, suggesting a stationary condition, whereas it increased exponentially in livers perfused at 30 and 37 °C (Figure 1B).
At the starting time-point, portal pressure was associated with perfusion temperature. Basal pressure was higher in livers perfused at 10 °C (5.8 ± 0.2 mmHg), intermediate in livers perfused at 20 and 30 °C (4.9 ± 0.1 mmHg and 4.9 ± 0.2 mmHg, respectively), and lower at 37 °C (4.2 ± 0.1 mmHg). Livers perfused at 20 and 30 °C had identical portal pressure and differed significantly from the other groups (Ps < 0.01). During perfusion, pressure did not significantly change within each group, with the exception of the 37 °C group, where portal pressure significantly increased from the 3rd hour of perfusion, rising rapidly to out-of-scale values after the 4th hour (data not shown).
The increase in basal bile flow was logarithmically proportional to perfusion temperature. Basal bile flow in livers perfused at 10 and 20 °C was very similar, and was significantly different in comparison to livers perfused at both 30 and 37 °C (Ps < 0.05) (Figure 2A). Bile flow remained constant in livers perfused at 10 and 20 °C during the whole perfusion. On the contrary, bile flow fell rapidly after 2 h of perfusion in livers perfused at 30 °C, and especially at 37 °C (Figure 2B).
Figure 2.
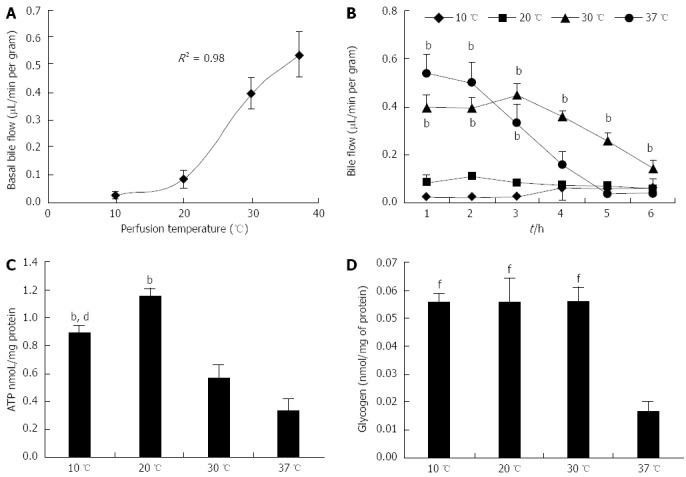
Bile flow, ATP and glycogen levels in rat livers perfused for 6 h. A: Basal bile flow; B: Bile flow during perfusion; C: ATP content; and D: Glycogen content in livers perfused for 6 h at 10, 20, 30 or 37 °C (n = 6/group; B: bP < 0.01 vs 10 and 20 °C; C: bP < 0.01 vs 30 and 37 °C, dP < 0.01 vs 20 °C; D: fP < 0.01 vs 37 °C).
ATP and glycogen in tissue
ATP and glycogen were measured in tissue samples frozen at the end of the 6-h perfusions. ATP in livers perfused at 20 °C was significantly higher in comparison to both 30 and 37 °C (Ps < 0.01). Interestingly, the ATP content in the 20 °C perfusion group was also higher when compared to livers perfused at 10 °C (P < 0.01) (Figure 2C). The 37 °C group had significantly lower glycogen content in comparison to the other three groups (P < 0.01); no difference was observed between the 10, 20 and 30 °C groups (Figure 2D).
pH and lactic acid release
We observed that there was no significant acidification during perfusion at 10 and 20 °C. On the contrary, at 30 °C, the pH of the perfusion solution was significantly lower in comparison to the basal values (P < 0.01); the pH also significantly dropped at 37 °C starting at the 3rd hour of perfusion, in comparison to the pH values at 10 and 20 °C for the same time points (Ps < 0.01) (Figure 3A).
Figure 3.
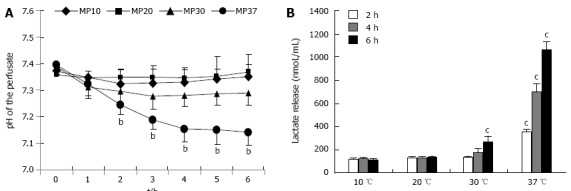
pH levels and lactate release in rat livers perfused for 6 h. A: Normalized pH; B: Lactic acid release in livers perfused for 6 h at 10, 20, 30 or 37 °C (n = 6/group; bP < 0.01 vs 10 and 20 °C; cP < 0.05 vs 10 and 20 °C).
To justify the pH fall at higher perfusion temperatures, we evaluated lactic acid release in the perfusion buffer as an index of anaerobiotic metabolism. Livers perfused at 10 and 20 °C did not release lactic acid during perfusion. In livers perfused at 30 °C, lactic acid concentrations at the 2nd hour of perfusion were identical to the respective time points at 10 and 20 °C, but increased significantly in the subsequent time points (Ps < 0.05) (Figure 2B). In livers perfused at 37 °C, lactic acid was significantly higher at the 2nd hour of perfusion, and increased dramatically during perfusion (Ps < 0.05).
HIF-1α mRNA and protein expression
HIF-1α mRNA and protein expression were assayed to identify which livers were perfused in hypoxic conditions. We observed a slight increase of HIF-1α mRNA expression in livers perfused at 30 °C (P < 0.05 vs 10 and 20 °C) and a more accentuated rise of HIF-1α mRNA expression in livers perfused at 37 °C (P < 0.01 vs 10 and 20 °C) (Figure 4A). Livers perfused at 10 and 20 °C did not show an increase in HIF-1α mRNA expression in comparison to control livers. We used an ELISA kit to evaluate HIF-1α protein expression in liver tissues at the end of perfusion, obtaining results similar to those observed for HIF-1α mRNA expression (Figure 4B).
Figure 4.
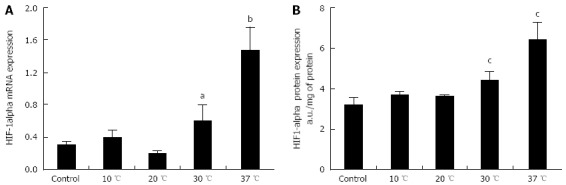
Hepatic hypoxia inducible factor-1α expression in rat livers perfused for 6 h. A: mRNA; and B: Protein expression of hypoxia inducible factor (HIF)-1α in livers perfused for 6 h at 10, 20, 30 or 37 °C (n = 6/group; aP < 0.05, bP < 0.01 vs 10 and 20 °C; cP < 0.05 vs 10 and 20 °C).
DISCUSSION
The difficulty in perfusing the liver with acellular solutions at normothermic temperatures results from two different causes: (1) as stated by Henry’s Law, oxygen solubility decreases at higher temperatures; and (2) at increasing temperature, liver metabolism increases proportionally. These two aspects act synergistically so that oxygen carried by the perfusate and liver oxygen requirement inevitably diverge at increasing temperatures. Because of this, one of the major drawbacks of acellular perfusion at normothermic temperatures is the inadequate oxygenation of the liver parenchyma, which leads to anaerobic glycolysis and acidosis[26]. In the isolated perfused rat liver model, this problem is partially solved by raising the perfusion flow at higher-than-physiologic levels; by speeding up the flow, the oxygen carried to the parenchyma will increase; on the other hand, according to Poiseuille’s Law, a higher flow is not associated with an abnormal increase in physiologic portal pressure, due to the lower viscosity of acellular solutions compared to blood[26,27]. In our model, we increased the flow through the portal vein from the physiologic value of 1.7 to 2.6 mL/min per gram, obtaining a basal pressure similar to the physiologic portal pressure. Unfortunately, this procedure is not sufficient to fulfill liver oxygen requirement at 37 °C. In our previous works, we showed that machine perfusion at subnormothermic temperature in a model of ex vivo reperfusion better preserves ischemic[20] and steatotic[6] rat livers with respect to conventional preservation.
The aim of the present work was to determine at what temperature the oxygen carried by the perfusate and the liver oxygen requirement meet, allowing long-term perfusion or, conversely, at what temperature liver metabolism shifts from aerobiosis to anaerobiosis. In isolated mitochondria, respiration rate increases exponentially with temperature[28]. Considering the whole organ, Fujita et al[29] studied the oxygen requirement at different temperatures in perfused livers, and worked out an equation showing that liver oxygen requirement increases exponentially as a function of temperature. This observation has a key implication: below a certain temperature threshold, liver oxygen requirement slightly increases with temperature, whereas above such threshold it increases dramatically. The data obtained herein suggest that this threshold temperature may lie between 20 and 30 °C. In our perfusion model, the OUR of livers perfused at 10 and 20 °C was identical to the theoretical oxygen requirement according to Fujita et al[29], but uptake rate of livers perfused at 30 and 37 °C was lower than theoretical oxygen requirement, suggesting that oxygen requirements are not completely fulfilled at higher temperatures. The data obtained on liver injury and function support this hypothesis: LDH release rate was near zero in livers perfused at 10 and 20 °C, whereas LDH increased significantly in livers perfused at 30 and 37 °C.
The literature clearly shows that bile formation depends on the activity of various ATP-driven pumps[30,31], and consequently is strictly related to mitochondrial respiration rate. Due to dependence on temperature of both respiration rate[28,32] and enzyme activity[33], a similar bile flow dependence is expected. In our model, the basal bile flow followed this trend. Importantly, bile flow remained constant during perfusion at 10 and 20 °C, but dramatically dropped after 2 and 3 h of perfusion in both 30 and 37 °C perfused livers, suggesting that liver is unable to maintain baseline bile production rate, due to oxygen deficiency. Accordingly, livers perfused at 30 and 37 °C contained significantly less ATP compared to livers perfused at 20 °C. ATP content of livers perfused at 10 °C was significantly higher when compared to 30 and 37 °C groups as well, but, interestingly, was significantly lower than ATP measured in the 20 °C perfusion group. This difference may be explained by a lower coupling efficiency of oxidative phosphorylation, usually occurring at low temperatures[28]. Furthermore, while glycogen stores are not affected at 30 °C, we observed a significant reduction of liver glycogen at 37 °C.
These data suggest that livers perfused at 30 and 37 °C are in anaerobic conditions. Livers perfused at 20 and 10 °C show aerobic conditions during the whole perfusion. Accordingly, we observed that, contrary to livers perfused at 30 and 37 °C, livers perfused at 10 and 20 °C did not acidify the perfusion medium. Lactic acid release is a suitable and sensitive marker of occurring anaerobic metabolism[34]. Livers perfused at 10 and 20 °C showed identically low lactate release with release rates near zero, demonstrating that, at these temperatures, the liver can support aerobic metabolism during the perfusion interval used in our experiments. Livers perfused at 30 °C showed a significantly higher lactate release rate compared to those perfused at 10 °C and 20 °C. Livers perfused at 37 °C had dramatically higher lactate release rates, suggesting a huge difference between oxygen uptake and oxygen requirement at this temperature. Indeed, in our model of acellular perfusion, the liver cannot support aerobic metabolism at 37 °C and his metabolism is mainly anaerobiotic, wasting ATP and glycogen stores, and releasing great amounts of unprocessed lactic acid in the perfusate.
HIF-1α is a transcriptional activator of genes whose products, including glycolytic enzymes, are involved in systemic, local, and cellular responses to hypoxia, such as inducing alternative metabolic pathways that do not require O2[35]. In hypoxic conditions, HIF-1α mRNA expression can increase[36,37]. To the best of our knowledge, HIF-1α mRNA and protein expression have not been assayed in a model of rat liver perfusion. We observed an increase in both HIF-1α mRNA and protein in livers perfused at 30 °C, and a larger increase in livers perfused at 37 °C. Interestingly, expression levels did not differ in livers perfused at 10 °C and 20 °C, which were similar to controls.
These data clearly demonstrate that long term liver perfusion with simple acellular solutions is not possible above 30 °C. Livers perfused at 37 °C are evidently in anaerobic conditions; livers perfused at 30 °C seem to be in an intermediate state, showing the first signs of distress, but not as much as livers perfused at 37 °C, suggesting that the optimal temperature should certainly lie below 30 °C. Should this optimal temperature necessarily be lower than 20 °C? Our data, both those registered as a time course along perfusion period, and those assayed in the tissue, demonstrate that livers perfused at 10 and 20 °C exhibit quite similar and stable conditions, suggesting that the temperature where oxygen liver requirement and oxygen delivery meet is certainly below 30 °C, but not necessarily below 20 °C.
One of the most important applications of long-term liver perfusion is machine perfusion for organ transplantation. Recently, liver machine perfusion has been evaluated as a suitable alternative to simple cold storage, particularly for marginal organ preservation[38,39]. Furthermore, liver hypothermic machine perfusion (4-8 °C) has recently been tested in a clinical trial with encouraging results[14,40,41]. In this trial, a starch-added solution was used to perfuse livers at 0.667 mL/min per liver g, without oxygenation. Subnormothermic perfusion has not yet been used in clinical trial, although some authors referred to the subnormothermic machine perfusion as “the way in-between” that could potentially bypass the flaws of both hypothermic and normothermic machine perfusion[42,43]. The subnormothermic temperature should allow the use of low-viscosity solutions and consequently higher flow rates, sustaining a mild liver metabolism useful for restoring energy stocks before transplantation, particularly in livers from donation after cardiac death. Moreover, the avoidance of hypothermia could be useful in fatty livers, and maintaining the liver in a mild metabolic state could be useful for genetic and immunologic manipulations before transplantation[41,42].
These data clearly show that livers perfused at 20 °C have no sign of anaerobiosis; therefore, reducing the perfusion temperature below 20 °C is unlikely to further improve this technique. On the contrary, livers perfused at 30 °C start to show the symptoms of lack of oxygenation. The adequate oxygenation of livers preserved by perfusion at 20 °C highlights this technique’s concrete potential to avoid ischemic insult, the real culprit of the preservation injury observed in cold storage-transplanted organs. The subnormothermic temperature, allowing a less complicated setting, might also favor the successful translation of this technique from experimental studies into clinical practice.
ACKNOWLEDGMENTS
We thank Mr. Massimo Costa for his skillful technical assistance, and Mrs. Nicoletta Breda for the editing assistance. We thank Dr. Chiara Prezzavento for editing the manuscript for journal submission.
COMMENTS
Background
Cold (0-4 °C) storage is considered the gold standard in organ preservation for transplantation. Currently, as new perfusion techniques are developed to improve the preservation of marginal organs, hypothermic machine perfusion is the preferred choice, though it presents drawbacks of cold injury, a slowed down metabolism, and a more complex setting.
Research frontiers
Clinical trials show improved clinical parameters and shorter duration of hospital stay in patients who receive grafts stored by hypothermic machine perfusion in comparison to patients who receive grafts preserved by cold storage.
Innovations and breakthroughs
Currently, new perfusion techniques have being developed for the preservation of marginal livers. The preferred technique is hypothermic machine perfusion (0-4 °C). The authors demonstrated that livers perfused at 20 °C show no sign of ischemia. Therefore, reducing the perfusion temperature below 20 °C is unlikely to further improve this technique. On the contrary, livers perfused at 30 °C start to show the symptoms of lack of oxygenation.
Applications
The adequate oxygenation of livers perfused at 20 °C highlights this technique’s concrete potential to avoid cold injury and ischemic insult during liver preservation for transplantation. Furthermore, the subnormothermic temperature, allowing a less complicated setting, might also favor the successful translation of this technique from experimental studies into clinical practice. Finally, maintaining the liver in a mild metabolic state during preservation could be useful for pharmacologic, genetic and immunologic manipulations, and for regeneration of the energy status in ATP-depleted organs, avoiding both ischemic and cold-induced insult. This possibility could be extremely suitable for the preservation of livers rejected as non heart beating donor organs and steatotic organs.
Terminology
Oxygenated machine perfusion is a technique currently under development for the preservation of organs for transplantation. This technique is aimed at reducing the ischemic injury and better preserving marginal organs, which suffer cold and ischemic injury from static cold storage, the gold standard for organ preservation.
Peer review
This manuscript showed that perfusion of livers at higher temperatures (> 30 °C) leads to hypoxia and increased hypoxia inducible factor-1α expression.
Footnotes
Supported by Grant from Fondazione Cariplo, No. 2011-0439.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: June 20, 2014
First decision: July 21, 2014
Article in press: September 30, 2014
P- Reviewer: Jiang ZY S- Editor: Ma YJ L- Editor: AmEditor E- Editor: Wang CH
References
- 1.Monbaliu D, Van Gelder F, Troisi R, de Hemptinne B, Lerut J, Reding R, de Ville de Goyet J, Detry O, De Roover A, Honore P, et al. Liver transplantation using non-heart-beating donors: Belgian experience. Transplant Proc. 2007;39:1481–1484. doi: 10.1016/j.transproceed.2007.02.077. [DOI] [PubMed] [Google Scholar]
- 2.Urena MA, Moreno Gonzalez E, Romero CJ, Ruiz-Delgado FC, Moreno Sanz C. An approach to the rational use of steatotic donor livers in liver transplantation. Hepatogastroenterology. 1999;46:1164–1173. [PubMed] [Google Scholar]
- 3.Ploeg RJ, D’Alessandro AM, Knechtle SJ, Stegall MD, Pirsch JD, Hoffmann RM, Sasaki T, Sollinger HW, Belzer FO, Kalayoglu M. Risk factors for primary dysfunction after liver transplantation--a multivariate analysis. Transplantation. 1993;55:807–813. doi: 10.1097/00007890-199304000-00024. [DOI] [PubMed] [Google Scholar]
- 4.Burke A, Lucey MR. Non-alcoholic fatty liver disease, non-alcoholic steatohepatitis and orthotopic liver transplantation. Am J Transplant. 2004;4:686–693. doi: 10.1111/j.1600-6143.2004.00432.x. [DOI] [PubMed] [Google Scholar]
- 5.Verran D, Kusyk T, Painter D, Fisher J, Koorey D, Strasser S, Stewart G, McCaughan G. Clinical experience gained from the use of 120 steatotic donor livers for orthotopic liver transplantation. Liver Transpl. 2003;9:500–505. doi: 10.1053/jlts.2003.50099. [DOI] [PubMed] [Google Scholar]
- 6.Vairetti M, Ferrigno A, Carlucci F, Tabucchi A, Rizzo V, Boncompagni E, Neri D, Gringeri E, Freitas I, Cillo U. Subnormothermic machine perfusion protects steatotic livers against preservation injury: a potential for donor pool increase? Liver Transpl. 2009;15:20–29. doi: 10.1002/lt.21581. [DOI] [PubMed] [Google Scholar]
- 7.Chavin KD, Yang S, Lin HZ, Chatham J, Chacko VP, Hoek JB, Walajtys-Rode E, Rashid A, Chen CH, Huang CC, et al. Obesity induces expression of uncoupling protein-2 in hepatocytes and promotes liver ATP depletion. J Biol Chem. 1999;274:5692–5700. doi: 10.1074/jbc.274.9.5692. [DOI] [PubMed] [Google Scholar]
- 8.Fukumori T, Ohkohchi N, Tsukamoto S, Satomi S. The mechanism of injury in a steatotic liver graft during cold preservation. Transplantation. 1999;67:195–200. doi: 10.1097/00007890-199901270-00002. [DOI] [PubMed] [Google Scholar]
- 9.Soltys K, Dikdan G, Koneru B. Oxidative stress in fatty livers of obese Zucker rats: rapid amelioration and improved tolerance to warm ischemia with tocopherol. Hepatology. 2001;34:13–18. doi: 10.1053/jhep.2001.25452. [DOI] [PubMed] [Google Scholar]
- 10.Fung JJ, Eghtesad B, Patel-Tom K. Using livers from donation after cardiac death donors--a proposal to protect the true Achilles heel. Liver Transpl. 2007;13:1633–1636. doi: 10.1002/lt.21388. [DOI] [PubMed] [Google Scholar]
- 11.Suárez F, Otero A, Solla M, Arnal F, Lorenzo MJ, Marini M, Vázquez-Iglesias JL, Gómez M. Biliary complications after liver transplantation from maastricht category-2 non-heart-beating donors. Transplantation. 2008;85:9–14. doi: 10.1097/01.tp.0000297945.83430.ce. [DOI] [PubMed] [Google Scholar]
- 12.Reddy SP, Bhattacharjya S, Maniakin N, Greenwood J, Guerreiro D, Hughes D, Imber CJ, Pigott DW, Fuggle S, Taylor R, et al. Preservation of porcine non-heart-beating donor livers by sequential cold storage and warm perfusion. Transplantation. 2004;77:1328–1332. doi: 10.1097/01.tp.0000119206.63326.56. [DOI] [PubMed] [Google Scholar]
- 13.St Peter SD, Imber CJ, Lopez I, Hughes D, Friend PJ. Extended preservation of non-heart-beating donor livers with normothermic machine perfusion. Br J Surg. 2002;89:609–616. doi: 10.1046/j.1365-2168.2002.02052.x. [DOI] [PubMed] [Google Scholar]
- 14.Guarrera JV, Henry SD, Samstein B, Odeh-Ramadan R, Kinkhabwala M, Goldstein MJ, Ratner LE, Renz JF, Lee HT, Brown RS, et al. Hypothermic machine preservation in human liver transplantation: the first clinical series. Am J Transplant. 2010;10:372–381. doi: 10.1111/j.1600-6143.2009.02932.x. [DOI] [PubMed] [Google Scholar]
- 15.Tolboom H, Izamis ML, Sharma N, Milwid JM, Uygun B, Berthiaume F, Uygun K, Yarmush ML. Subnormothermic machine perfusion at both 20°C and 30°C recovers ischemic rat livers for successful transplantation. J Surg Res. 2012;175:149–156. doi: 10.1016/j.jss.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cleta Croce A, Ferrigno A, Vairetti M, Bertone R, Freitas I, Bottiroli G. Autofluorescence spectroscopy of rat liver during experimental transplantation procedure. An approach for hepatic metabolism assessment. Photochem Photobiol Sci. 2005;4:583–590. doi: 10.1039/b503586d. [DOI] [PubMed] [Google Scholar]
- 17.Dutkowski P, Furrer K, Tian Y, Graf R, Clavien PA. Novel short-term hypothermic oxygenated perfusion (HOPE) system prevents injury in rat liver graft from non-heart beating donor. Ann Surg. 2006;244:968–976; discussion 976-977. doi: 10.1097/01.sla.0000247056.85590.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vairetti M, Ferrigno A, Rizzo V, Boncompagni E, Carraro A, Gringeri E, Milanesi G, Barni S, Freitas I, Cillo U. Correlation between the liver temperature employed during machine perfusion and reperfusion damage: role of Ca2+ Liver Transpl. 2008;14:494–503. doi: 10.1002/lt.21421. [DOI] [PubMed] [Google Scholar]
- 19.van der Plaats A, ‘t Hart NA, Verkerke GJ, Leuvenink HG, Ploeg RJ, Rakhorst G. Hypothermic machine preservation in liver transplantation revisited: concepts and criteria in the new millennium. Ann Biomed Eng. 2004;32:623–631. doi: 10.1023/b:abme.0000019181.18194.51. [DOI] [PubMed] [Google Scholar]
- 20.Ferrigno A, Rizzo V, Boncompagni E, Bianchi A, Gringeri E, Neri D, Richelmi P, Freitas I, Cillo U, Vairetti M. Machine perfusion at 20°C reduces preservation damage to livers from non-heart beating donors. Cryobiology. 2011;62:152–158. doi: 10.1016/j.cryobiol.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 21.Smith TG, Robbins PA, Ratcliffe PJ. The human side of hypoxia-inducible factor. Br J Haematol. 2008;141:325–334. doi: 10.1111/j.1365-2141.2008.07029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferrigno A, Richelmi P, Vairetti M. Troubleshooting and improving the mouse and rat isolated perfused liver preparation. J Pharmacol Toxicol Methods. 2013;67:107–114. doi: 10.1016/j.vascn.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 23.Bergmeyer HU, Bernt E, Hess B. Lactate-dehydrogenase, UV-assay with pyruvate and nadh. Methods of enzymatic analysis. New York: Academic; 1965. pp. 736–743. [Google Scholar]
- 24.Bennett LW, Keirs RW, Peebles ED, Gerard PD. Methodologies of tissue preservation and analysis of the glycogen content of the broiler chick liver. Poult Sci. 2007;86:2653–2665. doi: 10.3382/ps.2007-00303. [DOI] [PubMed] [Google Scholar]
- 25.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 26.Bessems M, ‘t Hart NA, Tolba R, Doorschodt BM, Leuvenink HG, Ploeg RJ, Minor T, van Gulik TM. The isolated perfused rat liver: standardization of a time-honoured model. Lab Anim. 2006;40:236–246. doi: 10.1258/002367706777611460. [DOI] [PubMed] [Google Scholar]
- 27.Izamis M. Meeting the oxygen requirements of an isolated perfused rat liver. Cambridge: Massachusets Institute of Technology; 2006. [Google Scholar]
- 28.Quentin E, Avéret N, Guérin B, Rigoulet M. Temperature dependence of the coupling efficiency of rat liver oxidative phosphorylation: role of adenine nucleotide translocator. Biochem Biophys Res Commun. 1994;202:816–821. doi: 10.1006/bbrc.1994.2003. [DOI] [PubMed] [Google Scholar]
- 29.Fujita S, Hamamoto I, Nakamura K, Tanaka K, Ozawa K. Isolated perfusion of rat livers: effect of temperature on O2 consumption, enzyme release, energy store, and morphology. Nihon Geka Hokan. 1993;62:58–70. [PubMed] [Google Scholar]
- 30.Stieger B, Meier Y, Meier PJ. The bile salt export pump. Pflugers Arch. 2007;453:611–620. doi: 10.1007/s00424-006-0152-8. [DOI] [PubMed] [Google Scholar]
- 31.Oude Elferink RP, Paulusma CC, Groen AK. Hepatocanalicular transport defects: pathophysiologic mechanisms of rare diseases. Gastroenterology. 2006;130:908–925. doi: 10.1053/j.gastro.2005.08.052. [DOI] [PubMed] [Google Scholar]
- 32.Klingenberg M, Grebe K, Appel M. Temperature dependence of ADP/ATP translocation in mitochondria. Eur J Biochem. 1982;126:263–269. doi: 10.1111/j.1432-1033.1982.tb06774.x. [DOI] [PubMed] [Google Scholar]
- 33.Freehold NJ. Manual of clinical enzyme measurements. Freehold, NJ: Worthington Biomedical Corporation; 1972. [Google Scholar]
- 34.Vincent JL, Dufaye P, Berré J, Leeman M, Degaute JP, Kahn RJ. Serial lactate determinations during circulatory shock. Crit Care Med. 1983;11:449–451. doi: 10.1097/00003246-198306000-00012. [DOI] [PubMed] [Google Scholar]
- 35.Semenza GL. Transcriptional regulation by hypoxia-inducible factor 1 molecular mechanisms of oxygen homeostasis. Trends Cardiovasc Med. 1996;6:151–157. doi: 10.1016/1050-1738(96)00039-4. [DOI] [PubMed] [Google Scholar]
- 36.Wiener CM, Booth G, Semenza GL. In vivo expression of mRNAs encoding hypoxia-inducible factor 1. Biochem Biophys Res Commun. 1996;225:485–488. doi: 10.1006/bbrc.1996.1199. [DOI] [PubMed] [Google Scholar]
- 37.Knudsen AR, Kannerup AS, Grønbæk H, Andersen KJ, Funch-Jensen P, Frystyk J, Flyvbjerg A, Mortensen FV. Effects of ischemic pre- and postconditioning on HIF-1α, VEGF and TGF-β expression after warm ischemia and reperfusion in the rat liver. Comp Hepatol. 2011;10:3. doi: 10.1186/1476-5926-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee CY, Mangino MJ. Preservation methods for kidney and liver. Organogenesis. 2009;5:105–112. doi: 10.4161/org.5.3.9582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Olschewski P, Gass P, Ariyakhagorn V, Jasse K, Hunold G, Menzel M, Schöning W, Schmitz V, Neuhaus P, Puhl G. The influence of storage temperature during machine perfusion on preservation quality of marginal donor livers. Cryobiology. 2010;60:337–343. doi: 10.1016/j.cryobiol.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 40.Tulipan JE, Stone J, Samstein B, Kato T, Emond JC, Henry SD, Guarrera JV. Molecular expression of acute phase mediators is attenuated by machine preservation in human liver transplantation: preliminary analysis of effluent, serum, and liver biopsies. Surgery. 2011;150:352–360. doi: 10.1016/j.surg.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 41.Henry SD, Guarrera JV. Protective effects of hypothermic ex vivo perfusion on ischemia/reperfusion injury and transplant outcomes. Transplant Rev (Orlando) 2012;26:163–175. doi: 10.1016/j.trre.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 42.van Gulik TM. New concepts in liver preservation: how the pendulum sways back. Liver Transpl. 2009;15:1–3. doi: 10.1002/lt.21642. [DOI] [PubMed] [Google Scholar]
- 43.Monbaliu D, Brassil J. Machine perfusion of the liver: past, present and future. Curr Opin Organ Transplant. 2010;15:160–166. doi: 10.1097/MOT.0b013e328337342b. [DOI] [PubMed] [Google Scholar]


