Abstract
AIM: To investigate the clinicopathological significance and prognostic value of caveolin-1 (CAV-1) in both tumor and stromal cells in colorectal cancer (CRC).
METHODS: A total of 178 patients with CRC were included in this study. The correlation between CAV-1 expression and clinicopathologic features and survival was studied.
RESULTS: CAV-1 expression was detected in tumor and stromal cells. The expression of stromal CAV-1 was closely associated with histological type (P = 0.022), pathologic tumor-node-metastasis stage (P = 0.047), pathologic N stage (P = 0.035) and recurrence (P = 0.000). However, tumor cell CAV-1 did not show any correlation with clinical parameters. Additionally, the loss of stromal CAV-1 expression was associated with shorter disease-free survival (P = 0.000) and overall survival (P = 0.000). Multivariate analysis revealed that the loss of stromal CAV-1 expression was an independent prognostic factor for both overall survival (P = 0.014) and disease-free survival (P = 0.006).
CONCLUSION: The loss of stromal CAV-1 expression in CRC was associated with poor prognosis and could be a prognostic factor for CRC patients.
Keywords: CAV-1, Stroma, Colorectal cancer, Prognosis
Core tip: Caveolin-1 (CAV-1), an essential structural protein of the endocytic caveolae plasma membrane, plays a major role in modulating tumorigenic processes. Recent studies have revealed that the loss of stromal CAV-1 results in an activated tumor microenvironment and is significantly related to tumor recurrence and a poor prognosis for many tumors. However, the association between CAV-1 and colorectal cancer remains unknown. In our study, we observed CAV-1 expression in both tumor and stromal cells. Our results demonstrate that the loss of stromal CAV-1 is an independent predictor of poor overall survival and disease-free survival, whereas CAV-1 expression in tumor cells has no prognostic value.
INTRODUCTION
Colorectal cancer (CRC) is the third most common cancer and the third leading cause of cancer mortality in the United States, with an estimated 142820 new cases and 50830 deaths per year[1]. Over the past ten years, the rate of death from CRC has declined by 3%, but 30% to 40% of CRC patients will develop distant metastases, and 50% will die of CRC recurrence[2].
The tumor microenvironment has recently been recognized to play an important role in determining tumor initiation and progression[3-6]. The tumor microenvironment is composed of immune cells, stromal cells (including cancer-associated fibroblasts (CAFs), adipocytes, and bone marrow-derived progenitors), and the vasculature[5,7]. Under normal physiological conditions, the stroma serves as a critical barrier to prevent malignant transformation. However, during neoplastic tumorigenesis, the stroma facilitates tumor progression and metastasis in response to molecular signals derived from carcinoma cells and other host cell types.
Caveolin-1 (CAV-1), an essential structural protein of endocytic caveolae plasma membrane invaginations, is present in most mammalian cells, such as adipocytes, endothelial cells, pneumocytes, fibroblasts, and smooth muscle cells[8,9]. CAV-1 plays a major role in modulating tumorigenic processes through its various functions, such as gene regulation, membrane trafficking, and signal transduction[10-12]. However, CAV-1 shows a compartment-dependent role in tumors. Furthermore, the role of CAV-1 is controversial in epithelial tumor cells. Several authors have proposed that high CAV-1 expression in tumor cells predicts poor survival[13,14]. In contrast, El-Gendi et al[15] reported that CAV-1 expression in tumor cells has no prognostic value. Recent studies have revealed that the loss of CAV-1 in the tumor stroma results in an activated tumor microenvironment and is significantly related to early tumor recurrence, metastasis, and poor clinical outcome in breast cancer, prostate cancer, and other solid tumors[16-19].
However, the association between CAV-1 and colorectal cancer remains unknown. Thus, to explore the relationship between CAV-1 expression and colorectal cancer, we analyzed CAV-1 expression in CRC stromal cells and tumor cells in a series of human CRC tissue sections.
MATERIALS AND METHODS
Patients and specimens
Colorectal tissue samples were obtained from the pathology department at the First Affiliated Hospital of Sun Yat-sen University between May 2004 and November 2009. The patients included in this study fulfilled the following criteria: (1) pathologically confirmed diagnosis of colorectal adenocarcinoma; (2) no treatment prior to curative excision of the primary tumor; (3) no previous malignancy or second primary tumor; (4) no severe coincident diseases; and (5) the availability of clinical information and follow-up data. The tumors were classified according to the American Joint Committee on Cancer staging manual (7th edition).
All patients were followed every 3 mo for the first year, every 6 mo from the second to fifth years and every 12 mo after 5 years. The study protocol was approved by the Ethics and Scientific Committee of the First Affiliated Hospital of Sun Yat-sen University and conforms to the Declaration of Helsinki. All patients and their families provided written informed consent prior to surgery for their information to be stored in the hospital database and used for research.
Immunohistochemistry
Tissue section immunohistochemistry was performed according to the instructions provided by the manufacturer. Briefly, 4 μm formalin-fixed, paraffin-embedded tissue sections were deparaffinized and rehydrated through a graded alcohol series. Antigen retrieval was performed in 10 mmol/L sodium citrate (pH 6.0) for 5 min using a pressure cooker and the sections were allowed to cool for 45 min at room temperature. After blocking with 3% hydrogen peroxide for 10 min to inactivate endogenous peroxidase, the slides were washed three times with phosphate-buffered saline and incubated with 10% goat serum for 30 min at room temperature. The sections were then incubated with an anti-CAV-1 antibody (rabbit monoclonal, dilution 1:100, Cell Signaling Technology, CST) overnight at 4 °C, and the slides were then incubated with an HRP-conjugated sheep anti-rabbit secondary antibody (GTVision; Shanghai, China) for 30 min at room temperature. Immunoreactivity was revealed using 3,3’-diaminobenzidine and counterstained with Mayer’s hematoxylin. A known positive tissue sample (breast cancer slide) was used as a positive control.
Evaluation of immunostaining
The staining of stromal CAV-1 was scored semi-quantitatively as negative (0, no staining), weak (1, either diffuse weak staining or strong staining in less than 30% of the stromal cells) or strong (2, strong staining of 30% or more of the stromal cells)[20]. We also evaluated CAV-1 expression in tumor cells. Any expression in tumor cells was considered to be positive CAV-1 staining[20]. The immunostaining results were evaluated by two independent pathologists.
Statistical analysis
All statistical calculations were carried out using SPSS 17.0 statistical software. The associations between CAV-1 expression and various clinical parameters were evaluated using the Mann-Whitney test, Spearman’s test or χ2 test. The Kaplan-Meier test was used to evaluate disease-free survival (DFS) and overall survival (OS), and the survival curve was compared using the Log rank test. Multivariate Cox regression models were applied to evaluate CAV-1 expression and other prognostic factors with respect to DFS and OS. All P-values are two-sided and were considered statistically significant at the level of < 0.05.
RESULTS
The present study included 178 matched colorectal cancer tissues and 30 paraneoplastic normal tissues, which were more than 5 cm from the primary tumor sites. Of the 178 CRC patients, 103 were male (57.9%) and 75 (42.1%) were female. The median age was 54 years (range: 24-85 years). The median follow-up period was 50 (range: 9-127) months for all patients.
CAV-1 expression was observed in both the tumor and stromal cells. Additionally, adipocytes, endothelial cells, and perineurial cells showed strong CAV-1 expression and served as internal positive controls. Analyses for CAV-1 expression in the stroma revealed positive expression in 129 patients (72.5%) and negative expression in 49 patients (27.5%). Of the 129 positive cases, 50 (28.1%) had a score of 1, and 79 (44.4%) had a score of 2. Representative examples are shown in Figure 1. The correlations between stromal CAV-1 and clinical variables are listed in Table 1. The expression of stromal CAV-1 was closely associated with histological type (P = 0.022), pathologic tumor-node-metastasis (TNM) stage (P = 0.047), pathologic N stage (P = 0.035) and recurrence (P < 0.001). However, there was no significant correlation between the expression level of CAV-1 and age, gender, tumor size, tumor location, pathologic T stage, or tumor cell CAV-1.
Figure 1.
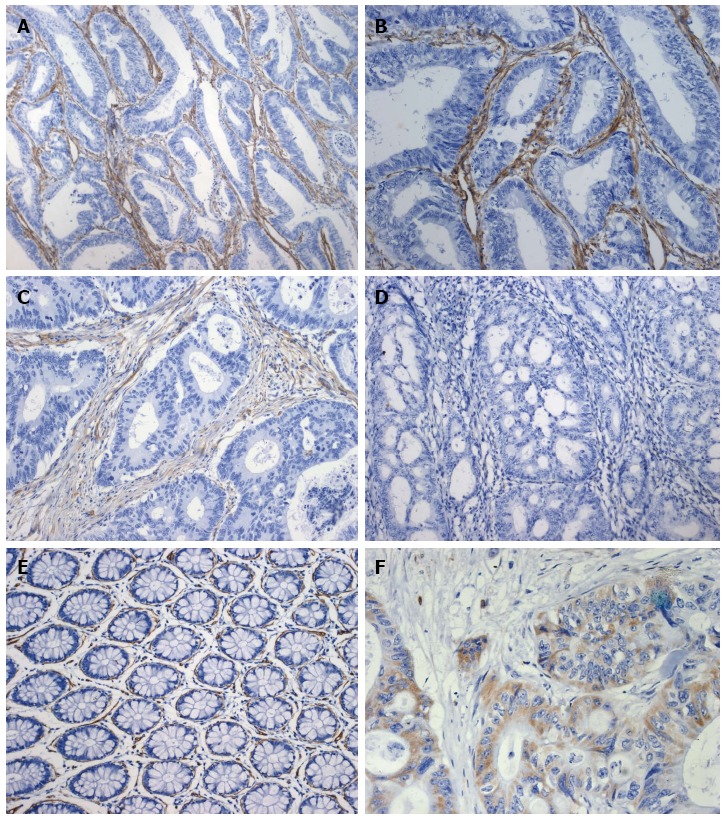
Immunohistochemical staining for caveolin-1. High caveolin-1 (CAV-1) expression in colorectal cancer stroma (A: × 200; B: × 400). Panel C (× 200) shows moderate stromal CAV-1 expression, and panel D (× 200) shows negative stromal CAV-1 expression. Panel E (× 200) depicts normal colorectal tissue. Panel F (× 400) demonstrates CAV-1 expression in colorectal tumor cells.
Table 1.
Association of stromal caveolin-1 expression with clinicopathologic parameters n (%)
| Variable |
Cav-1 |
P-value | ||
| 0 | 1 | 2 | ||
| Age (yr) | 0.0951 | |||
| ≤ 50 | 22 (33.8) | 19 (29.2) | 24 (36.9) | |
| > 50 | 27 (23.9) | 31 (27.4) | 55 (48.7) | |
| Gender | 0.4041 | |||
| Male | 26 (25.2) | 29 (28.2) | 48 (46.6) | |
| Female | 23 (30.7) | 21 (28.0) | 31 (41.3) | |
| Tumor size (cm) | 0.6881 | |||
| < 5 | 28 (27.7) | 30 (29.7) | 43 (42.6) | |
| ≥ 5 | 21 (27.3) | 20 (26.0) | 36 (46.8) | |
| Tumor location | 0.3781 | |||
| Colon | 32 (30.8) | 28 (26.9) | 44 (42.3) | |
| Rectum | 17 (23.3) | 22 (30.1) | 34 (46.6) | |
| Histological type | 0.0222a | |||
| Well | 1 (12.5) | 4 (50.0) | 3 (37.5) | |
| Moderate | 31 (23.8) | 35 (26.9) | 64 (49.2) | |
| Poor | 17 (42.5) | 11 (27.5) | 12 (30.0) | |
| Pathologic T stage | 0.1182 | |||
| T1 | 1 (50.0) | 0 (0) | 1 (50.0) | |
| T2 | 0 (0) | 5 (35.7) | 9 (64.3) | |
| T3 | 45 (29.2) | 44 (28.6) | 65 (42.2) | |
| T4 | 3 (37.5) | 1 (12.5) | 4 (50.0) | |
| Pathologic N stage | 0.0352a | |||
| N0 | 10 (16.4) | 20 (32.8) | 31 (50.8) | |
| N1 | 23 (29.1) | 23 (29.1) | 33 (41.8) | |
| N2 | 16 (42.1) | 7 (18.4) | 15 (39.5) | |
| Pathologic TNM stage | 0.0472a | |||
| I | 0 (0) | 4 (50.0) | 4 (50.0) | |
| II | 10 (18.9) | 16 (30.2) | 27 (50.9) | |
| III | 39 (33.3) | 30 (25.6) | 48 (41.0) | |
| Tumor cell Cav-1 | 0.6971 | |||
| Negative | 26.7 (61.8) | 29.1 (63.8) | 76 (44.2) | |
| Positive | 3 (50.0) | 0 (0) | 3 (50.0) | |
| Recurrence | < 0.0011a | |||
| No | 28 (24.1) | 68 (58.6) | ||
| Yes | 21 (36.2) | 10 (17.2) | ||
Mann-Whitney test;
Spearman’s test;
P < 0.05, stromal CAV-1 vs clinical variables. CAV-1: Caveolin-1; TNM: Tumor-node-metastasis.
Interestingly, with regard to CAV-1 expression in tumor cells, we only observed 6 tissues that were positive for expression in our study. However, as presented in Table 2, tumor cell CAV-1 did not showed any correlation with clinical parameters.
Table 2.
Association between tumor cell caveolin-1 expression and clinicopathologic parameters n (%)
| Variable |
Cav-1 |
P-value | |
| Negative | Positive | ||
| Age (yr) | 1.000 | ||
| ≤ 50 | 63 (96.9) | 2 (3.1) | |
| > 50 | 109 (96.5) | 4 (3.5) | |
| Gender | 0.698 | ||
| Male | 100 (97.1) | 3 (2.9) | |
| Female | 72 (96.0) | 3 (4.0) | |
| Tumor size (cm) | 0.237 | ||
| < 5 | 96 (95.0) | 5 (5.0) | |
| ≥ 5 | 76 (98.7) | 1 (1.3) | |
| Tumor location | 0.694 | ||
| Colon | 101 (97.1) | 3 (2.9) | |
| Rectum | 71 (95.9) | 3 (4.1) | |
| Histological type | 0.498 | ||
| Well | 8 (100) | 0 (0) | |
| Moderate | 124 (95.4) | 6 (4.6) | |
| Poor | 40 (100.0) | 0 (0) | |
| Pathologic T stage | 0.073 | ||
| T1 | 2 (100.0) | 0 (0) | |
| T2 | 14 (100) | 0 (0) | |
| T3 | 150 (97.4) | 4 (2.6) | |
| T4 | 6 (75.0) | 2 (25.0) | |
| Pathologic N stage | 0.304 | ||
| N0 | 57 (93.4) | 4 (6.6) | |
| N1 | 77 (97.5) | 2 (2.5) | |
| N2 | 38 (100) | 0 (0) | |
| Pathologic TNM stage | 0.158 | ||
| I | 8 (100) | 0 (0) | |
| II | 49 (92.5) | 4 (7.5) | |
| III | 115 (98.3) | 2 (1.7) | |
| Stromal Cav-1 | 0.208 | ||
| 0 | 46 (93.9) | 3 (6.1) | |
| 1 | 50 (100) | 0 (0) | |
| 2 | 76 (96.2) | 3 (3.8) | |
| Recurrence | 0.180 | ||
| No | 110 (94.8) | 6 (5.2) | |
| Yes | 58 (100) | 0 (0) | |
CAV-1: Caveolin-1; TNM: Tumor-node-metastasis.
Survival analysis
Next, we evaluated the prognostic value of CAV-1 expression in colorectal cancer. Figure 2 shows that the patients who were positive for stromal CAV-1 expression had significantly longer overall survival and disease-free survival (P < 0.001 for both) than the patients who were negative. Of note, the patients with high levels of stromal CAV-1 (score = 2) had a good prognosis, with 89.8% of the patients surviving the follow-up period. Similarly, 77.4% of the patients with moderate stromal CAV-1 staining (score = 1) survived. In contrast, 46.9% of the patients who were negative for stromal CAV-1 expression (score = 0) survived. The 5-year survival rates showed similar patterns. CRC patients with high stromal CAV-1 had a good 5-year survival rate (92%), whereas CRC patients with moderate or absent stromal CAV-1 expression had progressively worse 5-year survival rates (61% and 46%, respectively). We also analyzed the prognostic significance of CAV-1 expression in tumor cells, but no prognostic significance for either OS or DFS was found (P = 0.216 and 0.189, respectively).
Figure 2.

Kaplan-Meier curves of overall survival and disease-free survival. Patients with high stromal caveolin-1 expression have good overall survival (A) and disease-free survival (B).
A univariate analysis of possible prognostic indicators identified stromal CAV-1, pathologic N stage, pathologic TNM stage and recurrence as indicators of OS, and stromal CAV-1, pathologic N stage and recurrence as indicators of DFS (Table 3). In a multivariable analysis, stromal CAV-1 expression was a significant independent prognostic factor for OS and DFS (P = 0.006, HR = 0.546, 95%CI: 0.353-0.844; P = 0.014, HR = 0.578, 95%CI: 0.373-0.895, respectively). Moreover, the results also showed that recurrence was an independent prognostic factor for OS and that TNM and recurrence were independent prognostic factors for DFS (Table 3).
Table 3.
Univariate and multivariate analyses of prognostic factors in colorectal cancer with respect to overall survival and disease-free survival
| Parameter | Univariate analysisP-value | Multivariate analysisHR (95%CI) | P-value |
| OS | |||
| Age (> 50) | 0.185 | - | 1.632 |
| Gender | 0.794 | - | 1.431 |
| Tumor location | 0.384 | - | 1.414 |
| Tumor cell Cav-1 | 0.216 | - | 0.742 |
| Stromal Cav-1 | < 0.001a | 0.578 (0.373-0.895) | 0.014a |
| Tumor size | 0.443 | - | 0.382 |
| Histological type | 0.533 | - | 0.666 |
| Pathological T stage | 0.067 | - | 0.347 |
| Pathological N stage | < 0.001a | - | 0.868 |
| TNM | 0.025a | - | 0.378 |
| Recurrence | < 0.001a | 2.065 (1.121-3.803) | < 0.001a |
| DFS | |||
| Age (> 50) | 0.165 | - | 0.218 |
| Gender | 0.664 | - | 0.833 |
| Tumor location | 0.061 | - | 0.325 |
| Tumor cell Cav-1 | 0.189 | - | 0.705 |
| Stromal Cav-1 | < 0.001a | 0.546 (0.353-0.844) | 0.006a |
| Tumor size | 0.603 | - | 0.511 |
| Histological type | 0.478 | - | 0.553 |
| Pathological T stage | 0.171 | - | 0.888 |
| Pathological N stage | 0.002a | - | 0.510 |
| TNM | 0.184 | 0.451 (0.233-0.874) | 0.018a |
| Recurrence | < 0.001a | 1.863 (1.106-2.434) | < 0.001a |
P < 0.05, univariate analysis vs multivariate analysis. CAV-1: Caveolin-1; TNM: Tumor-node-metastasis; OS: Overall survival; DFS: Disease-free survival.
DISCUSSION
In this study, we evaluated the expression of CAV-1 in the stroma and tumor cells of CRC. Our results demonstrated that the loss of CAV-1 expression in the stroma is a strong and independent predictor of poor OS and DFS, whereas CAV-1 expression in tumor cells had no prognostic value. Moreover, stromal CAV-1 expression was closely associated with histological type (P = 0.022), pathologic TNM stage (P = 0.047), pathologic N stage (P = 0.035) and recurrence (P < 0.001). However, CAV-1 expression in tumor cells did not show any correlation with the clinical parameters.
Tumors, which are composed of tumor cells and stromal cells, grow within a complex tumor microenvironment. CAFs were recently demonstrated to promote tumor initiation, prevent cancer cell apoptosis, induce cancer cell proliferation, and stimulate tumor angiogenesis by secreting a large amount of growth factors, extracellular matrix components, and matrix metalloproteinases[4,6]. Although the underlying mechanisms are not fully elucidated, the loss of CAV-1 in the stroma plays a major role.
Witkiewicz et al[16] reported that the absence of stromal CAV-1 expression predicts early tumor recurrence and poor clinical outcome in human breast cancer. Di Vizio et al[21] found that the loss of stromal CAV-1 expression was closely related to disease progression and metastasis. Jia et al[18] indicated that the down-regulation CAV-1 expression in the stroma predicts lymph node metastases, early tumor recurrence, and poor prognosis in esophageal squamous cell carcinoma. Consistent with previous studies, we found that the loss of CAV-1 expression in the stroma is a strong and independent predictor of a poor prognosis in CRC. In our cohort, the patients with high levels of stromal CAV-1 had a better prognosis, with 89.8% surviving the follow-up period. Similarly, 77.4% of the patients with moderate stromal CAV-1 staining survived. In contrast, 46.9% of the patients who were negative for stromal CAV-1 expression survived. We found a similar pattern for the 5-year survival rates. CRC patients with high stromal CAV-1 had a good (92%) 5-year survival rate. In contrast, CRC patients with moderate levels or absent CAV-1 expression in the stroma had progressively worse 5-year survival rates (61% and 46%, respectively). These results suggest that stromal CAV-1 expression could be used as an important prognostic factor for CRC patients.
In accordance with previous studies, we also found that the absence of CAV-1 expression in the stroma was significantly associated with early tumor recurrence[17,20]. Patients who had lower stromal CAV-1 expression were more likely to experience tumor recurrence. This finding highlights the role of the tumor stroma in determining disease recurrence in CRC patients. Therefore, new drugs that target the tumor stroma may have unexpected effects in CRC therapies.
We also found that the loss of stromal CAV-1 expression was significantly associated with lymph node invasion. Thus, the stromal CAV-1 status may serve as a predictor of lymph node invasion. CAV-1 expression in the stroma of a biopsy sample may also be useful in deciding whether endoscopic surgery should be performed for patients with early CRC. Moreover, considering that the loss of CAV-1 expression in the stroma may predict a high risk of lymph node invasion, it would also be informative when choosing a postoperative therapy for patients with no lymph node invasion.
The role of CAV-1 in tumor cells is controversial. Steffens et al[14] reported that low CAV-1 expression in renal cell carcinoma predicts a good clinical outcome compared with patients with high CAV-1 expression. However, El-Gendi et al[15] reported that CAV-1 expression in tumor cells has no prognostic value. Additionally, Friedrich et al[22] reported that CAV-1 deficiency in Apc (min/+) mice facilitates colorectal initiation. Luo et al[23] suggested that CAV-1 could be a biomarker of early-onset CRC. In our study, we also examined CAV-1 expression in epithelial tumor cells in the same patients. However, we only found 6 patients with positive tumor cell expression, and our results revealed no significant correlation between tumor cell CAV-1 and clinical characteristics or prognosis. The discrepancy could be explained by the tumor specificity of CAV-1 expression. Additionally, variation in scoring methods could explain some of the observed discrepancies.
Although the role of CAV-1 in the tumor stroma is not fully understood, recent findings support the hypothesis that CAV-1 plays a crucial role in the stroma. Koleske et al[24] were the first to find that CAV-1 functions as a tumor suppressor in fibroblasts. The reduction of CAV-1 levels by the constitutive activation of oncogenes, such as c-Myc, v-Src, H-Ras (G12V), and Neu/ErB2, can significantly promote tumor growth[25-27]. The tumor suppressor p53 can also transcriptionally regulate stromal CAV-1 expression and p53 inactivation induces CAV-1 down-regulation and promotes tumor progression[28]. In a xenograft model, CAV-1-deficient cancer-associated fibroblasts were found to promote both tumor growth and angiogenesis[29]. Several studies have found that the loss of CAV-1 in fibroblasts is sufficient to induce the conversion of benign stromal fibroblasts to tumor-associated fibroblasts via the TGF-β pathway[30,31]. In turn, CAFs promote tumor initiation, progression, and prevent cancer cell apoptosis. Recently, Pavlides et al[32] proposed a new tumor metabolism model, the Reverse Warburg Effect. In this model, the loss of CAV-1 causes stromal CAFs to undergo autophagy and aerobic glycolysis. As a consequence, the CAFs secrete energy-rich metabolites and chemical building blocks to adjacent tumor cells to prompt growth. The results of the present study support this hypothesis and indicate that the loss of CAV-1 expression in CAFs plays an important role in supporting tumor growth.
There are several limitations to this study. This research is a retrospective study, and there are limitations to any retrospective data collection, which is prone to bias. The relatively small sample size and the reliability of immunohistochemical techniques are other limitations.
In conclusion, our results suggest that the loss of CAV-1 expression in the stroma could be a predictor of poor clinical outcomes. As such, stromal CAV-1 levels could be used as a valuable biomarker for stratifying CRC patients into high-risk and low-risk groups at diagnosis; this information could be used to provide a more personalized approach to postoperative therapy. Further studies are needed to elucidate the mechanisms of stromal CAV-1 reduction and the tumor-stroma cross-talk that is crucial for tumor growth and metastasis.
COMMENTS
Background
Caveolin-1 (CAV-1), an essential structural protein of the endocytic caveolae plasma membrane, plays a major role in modulating tumorigenic processes. Recent studies have revealed that the loss of stromal CAV-1 results in an activated tumor microenvironment and is significantly related to early tumor recurrence, metastasis, and poor prognosis in breast cancer, prostate cancer, and other solid tumors. However, the association between CAV-1 and colorectal cancer (CRC) remains unknown.
Research frontiers
Tumors are composed of tumor cells and stromal cells. Stromal cells have recently been recognized to play an important role in determining tumor initiation and progression. Under normal physiological conditions, the stroma serves as a critical barrier to prevent malignant transformation. However, during neoplastic tumorigenesis, the stroma facilitates tumor progression and metastasis in response to molecular signals derived from carcinoma cells and other host cell types. CAV-1, an essential structural protein of endocytic caveolae plasma membrane invaginations, is present in most mammalian cells, such as adipocytes, endothelial cells, fibroblasts, and smooth muscle cells. CAV-1 plays a major role in modulating tumorigenic processes. Recent studies have revealed that the loss of CAV-1 in the tumor stroma results in an activated tumor microenvironment and is significantly related to early tumor recurrence, metastasis, and poor clinical outcome in breast cancer, prostate cancer and other solid tumors.
Innovations and breakthroughs
This study is the first to report CAV-1 expression in both tumor and stromal cells of colorectal cancer. Moreover, our results demonstrate that the loss of CAV-1 expression in the stroma is a strong and independent predictor of poor overall survival and disease-free survival, whereas CAV-1 expression in tumor cells has no prognostic value. Additionally, CAV-1 expression in the stroma was closely associated with histological type, pTNM stage, pN stage and recurrence. Conversely, CAV-1 expression in tumor cells did not show any correlation with clinical parameters.
Applications
The results suggest that stromal CAV-1 levels could be used as a valuable biomarker for stratifying CRC patients into high-risk and low-risk groups at diagnosis; this information could be used to provide a more personalized approach to postoperative therapy.
Terminology
The stroma is a component of tumors. During neoplastic tumorigenesis, the stroma facilitates tumor progression and metastasis. Caveolin-1, an essential structural protein of endocytic caveolar plasma membrane invaginations, is present in most stromal cells.
Peer review
In the manuscript “Loss of stromal caveolin-1 expression in colorectal cancer predicts poor survival”, the authors found loss of stromal CAV-1 was closely associated with histological type, pathologic TNM stage, pathologic N stage and recurrence in colorectal cancer. The higher stromal CAV-1 expression, the better patients’ survival. It indicates that CAV-1 could be a prognostic factor for patients with CRC.
Footnotes
Supported by National Natural Science Foundation of China, No. 81072049.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: June 22, 2014
First decision: July 21, 2014
Article in press: September 30, 2014
P- Reviewer: Chae SC, Lu F, Sporea I S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Wang CH
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Sinclair P, Singh A, Riaz AA, Amin A. An unsolved conundrum: the ideal follow-up strategy after curative surgery for colorectal cancer. Gastrointest Endosc. 2012;75:1072–1079. doi: 10.1016/j.gie.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 3.Togo S, Polanska UM, Horimoto Y, Orimo A. Carcinoma-associated fibroblasts are a promising therapeutic target. Cancers (Basel) 2013;5:149–169. doi: 10.3390/cancers5010149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Polanska UM, Orimo A. Carcinoma-associated fibroblasts: non-neoplastic tumour-promoting mesenchymal cells. J Cell Physiol. 2013;228:1651–1657. doi: 10.1002/jcp.24347. [DOI] [PubMed] [Google Scholar]
- 5.Shimoda M, Mellody KT, Orimo A. Carcinoma-associated fibroblasts are a rate-limiting determinant for tumour progression. Semin Cell Dev Biol. 2010;21:19–25. doi: 10.1016/j.semcdb.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martinez-Outschoorn UE, Lisanti MP, Sotgia F. Catabolic cancer-associated fibroblasts transfer energy and biomass to anabolic cancer cells, fueling tumor growth. Semin Cancer Biol. 2014;25:47–60. doi: 10.1016/j.semcancer.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 7.Pavlides S, Vera I, Gandara R, Sneddon S, Pestell RG, Mercier I, Martinez-Outschoorn UE, Whitaker-Menezes D, Howell A, Sotgia F, et al. Warburg meets autophagy: cancer-associated fibroblasts accelerate tumor growth and metastasis via oxidative stress, mitophagy, and aerobic glycolysis. Antioxid Redox Signal. 2012;16:1264–1284. doi: 10.1089/ars.2011.4243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parton RG, Hanzal-Bayer M, Hancock JF. Biogenesis of caveolae: a structural model for caveolin-induced domain formation. J Cell Sci. 2006;119:787–796. doi: 10.1242/jcs.02853. [DOI] [PubMed] [Google Scholar]
- 9.Parton RG. Cell biology. Life without caveolae. Science. 2001;293:2404–2405. doi: 10.1126/science.1065677. [DOI] [PubMed] [Google Scholar]
- 10.Mercier I, Jasmin JF, Pavlides S, Minetti C, Flomenberg N, Pestell RG, Frank PG, Sotgia F, Lisanti MP. Clinical and translational implications of the caveolin gene family: lessons from mouse models and human genetic disorders. Lab Invest. 2009;89:614–623. doi: 10.1038/labinvest.2009.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Couet J, Li S, Okamoto T, Ikezu T, Lisanti MP. Identification of peptide and protein ligands for the caveolin-scaffolding domain. Implications for the interaction of caveolin with caveolae-associated proteins. J Biol Chem. 1997;272:6525–6533. doi: 10.1074/jbc.272.10.6525. [DOI] [PubMed] [Google Scholar]
- 12.Williams TM, Medina F, Badano I, Hazan RB, Hutchinson J, Muller WJ, Chopra NG, Scherer PE, Pestell RG, Lisanti MP. Caveolin-1 gene disruption promotes mammary tumorigenesis and dramatically enhances lung metastasis in vivo. Role of Cav-1 in cell invasiveness and matrix metalloproteinase (MMP-2/9) secretion. J Biol Chem. 2004;279:51630–51646. doi: 10.1074/jbc.M409214200. [DOI] [PubMed] [Google Scholar]
- 13.Qian N, Ueno T, Kawaguchi-Sakita N, Kawashima M, Yoshida N, Mikami Y, Wakasa T, Shintaku M, Tsuyuki S, Inamoto T, et al. Prognostic significance of tumor/stromal caveolin-1 expression in breast cancer patients. Cancer Sci. 2011;102:1590–1596. doi: 10.1111/j.1349-7006.2011.01985.x. [DOI] [PubMed] [Google Scholar]
- 14.Steffens S, Schrader AJ, Blasig H, Vetter G, Eggers H, Tränkenschuh W, Kuczyk MA, Serth J. Caveolin 1 protein expression in renal cell carcinoma predicts survival. BMC Urol. 2011;11:25. doi: 10.1186/1471-2490-11-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.El-Gendi SM, Mostafa MF, El-Gendi AM. Stromal caveolin-1 expression in breast carcinoma. Correlation with early tumor recurrence and clinical outcome. Pathol Oncol Res. 2012;18:459–469. doi: 10.1007/s12253-011-9469-5. [DOI] [PubMed] [Google Scholar]
- 16.Witkiewicz AK, Dasgupta A, Sotgia F, Mercier I, Pestell RG, Sabel M, Kleer CG, Brody JR, Lisanti MP. An absence of stromal caveolin-1 expression predicts early tumor recurrence and poor clinical outcome in human breast cancers. Am J Pathol. 2009;174:2023–2034. doi: 10.2353/ajpath.2009.080873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ayala G, Morello M, Frolov A, You S, Li R, Rosati F, Bartolucci G, Danza G, Adam RM, Thompson TC, et al. Loss of caveolin-1 in prostate cancer stroma correlates with reduced relapse-free survival and is functionally relevant to tumour progression. J Pathol. 2013;231:77–87. doi: 10.1002/path.4217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jia Y, Wang N, Wang J, Tian H, Ma W, Wang K, Tan B, Zhang G, Yang S, Bai B, et al. Down-regulation of stromal caveolin-1 expression in esophageal squamous cell carcinoma: a potent predictor of lymph node metastases, early tumor recurrence, and poor prognosis. Ann Surg Oncol. 2014;21:329–336. doi: 10.1245/s10434-013-3225-x. [DOI] [PubMed] [Google Scholar]
- 19.Zhao X, He Y, Gao J, Fan L, Li Z, Yang G, Chen H. Caveolin-1 expression level in cancer associated fibroblasts predicts outcome in gastric cancer. PLoS One. 2013;8:e59102. doi: 10.1371/journal.pone.0059102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Witkiewicz AK, Dasgupta A, Sammons S, Er O, Potoczek MB, Guiles F, Sotgia F, Brody JR, Mitchell EP, Lisanti MP. Loss of stromal caveolin-1 expression predicts poor clinical outcome in triple negative and basal-like breast cancers. Cancer Biol Ther. 2010;10:135–143. doi: 10.4161/cbt.10.2.11983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Di Vizio D, Morello M, Sotgia F, Pestell RG, Freeman MR, Lisanti MP. An absence of stromal caveolin-1 is associated with advanced prostate cancer, metastatic disease and epithelial Akt activation. Cell Cycle. 2009;8:2420–2424. doi: 10.4161/cc.8.15.9116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Friedrich T, Richter B, Gaiser T, Weiss C, Janssen KP, Einwächter H, Schmid RM, Ebert MP, Burgermeister E. Deficiency of caveolin-1 in Apc(min/+) mice promotes colorectal tumorigenesis. Carcinogenesis. 2013;34:2109–2118. doi: 10.1093/carcin/bgt142. [DOI] [PubMed] [Google Scholar]
- 23.Luo T, Wu S, Shen X, Li L. Network cluster analysis of protein-protein interaction network identified biomarker for early onset colorectal cancer. Mol Biol Rep. 2013;40:6561–6568. doi: 10.1007/s11033-013-2694-0. [DOI] [PubMed] [Google Scholar]
- 24.Koleske AJ, Baltimore D, Lisanti MP. Reduction of caveolin and caveolae in oncogenically transformed cells. Proc Natl Acad Sci USA. 1995;92:1381–1385. doi: 10.1073/pnas.92.5.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Timme TL, Goltsov A, Tahir S, Li L, Wang J, Ren C, Johnston RN, Thompson TC. Caveolin-1 is regulated by c-myc and suppresses c-myc-induced apoptosis. Oncogene. 2000;19:3256–3265. doi: 10.1038/sj.onc.1203654. [DOI] [PubMed] [Google Scholar]
- 26.Park DS, Razani B, Lasorella A, Schreiber-Agus N, Pestell RG, Iavarone A, Lisanti MP. Evidence that Myc isoforms transcriptionally repress caveolin-1 gene expression via an INR-dependent mechanism. Biochemistry. 2001;40:3354–3362. doi: 10.1021/bi002787b. [DOI] [PubMed] [Google Scholar]
- 27.Sotgia F, Martinez-Outschoorn UE, Howell A, Pestell RG, Pavlides S, Lisanti MP. Caveolin-1 and cancer metabolism in the tumor microenvironment: markers, models, and mechanisms. Annu Rev Pathol. 2012;7:423–467. doi: 10.1146/annurev-pathol-011811-120856. [DOI] [PubMed] [Google Scholar]
- 28.Razani B, Altschuler Y, Zhu L, Pestell RG, Mostov KE, Lisanti MP. Caveolin-1 expression is down-regulated in cells transformed by the human papilloma virus in a p53-dependent manner. Replacement of caveolin-1 expression suppresses HPV-mediated cell transformation. Biochemistry. 2000;39:13916–13924. doi: 10.1021/bi001489b. [DOI] [PubMed] [Google Scholar]
- 29.Bonuccelli G, Whitaker-Menezes D, Castello-Cros R, Pavlides S, Pestell RG, Fatatis A, Witkiewicz AK, Vander Heiden MG, Migneco G, Chiavarina B, et al. The reverse Warburg effect: glycolysis inhibitors prevent the tumor promoting effects of caveolin-1 deficient cancer associated fibroblasts. Cell Cycle. 2010;9:1960–1971. doi: 10.4161/cc.9.10.11601. [DOI] [PubMed] [Google Scholar]
- 30.Sotgia F, Del Galdo F, Casimiro MC, Bonuccelli G, Mercier I, Whitaker-Menezes D, Daumer KM, Zhou J, Wang C, Katiyar S, et al. Caveolin-1-/- null mammary stromal fibroblasts share characteristics with human breast cancer-associated fibroblasts. Am J Pathol. 2009;174:746–761. doi: 10.2353/ajpath.2009.080658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martinez-Outschoorn UE, Pavlides S, Whitaker-Menezes D, Daumer KM, Milliman JN, Chiavarina B, Migneco G, Witkiewicz AK, Martinez-Cantarin MP, Flomenberg N, et al. Tumor cells induce the cancer associated fibroblast phenotype via caveolin-1 degradation: implications for breast cancer and DCIS therapy with autophagy inhibitors. Cell Cycle. 2010;9:2423–2433. doi: 10.4161/cc.9.12.12048. [DOI] [PubMed] [Google Scholar]
- 32.Pavlides S, Whitaker-Menezes D, Castello-Cros R, Flomenberg N, Witkiewicz AK, Frank PG, Casimiro MC, Wang C, Fortina P, Addya S, et al. The reverse Warburg effect: aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell Cycle. 2009;8:3984–4001. doi: 10.4161/cc.8.23.10238. [DOI] [PubMed] [Google Scholar]


