Abstract
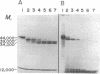
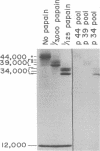
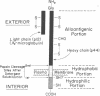
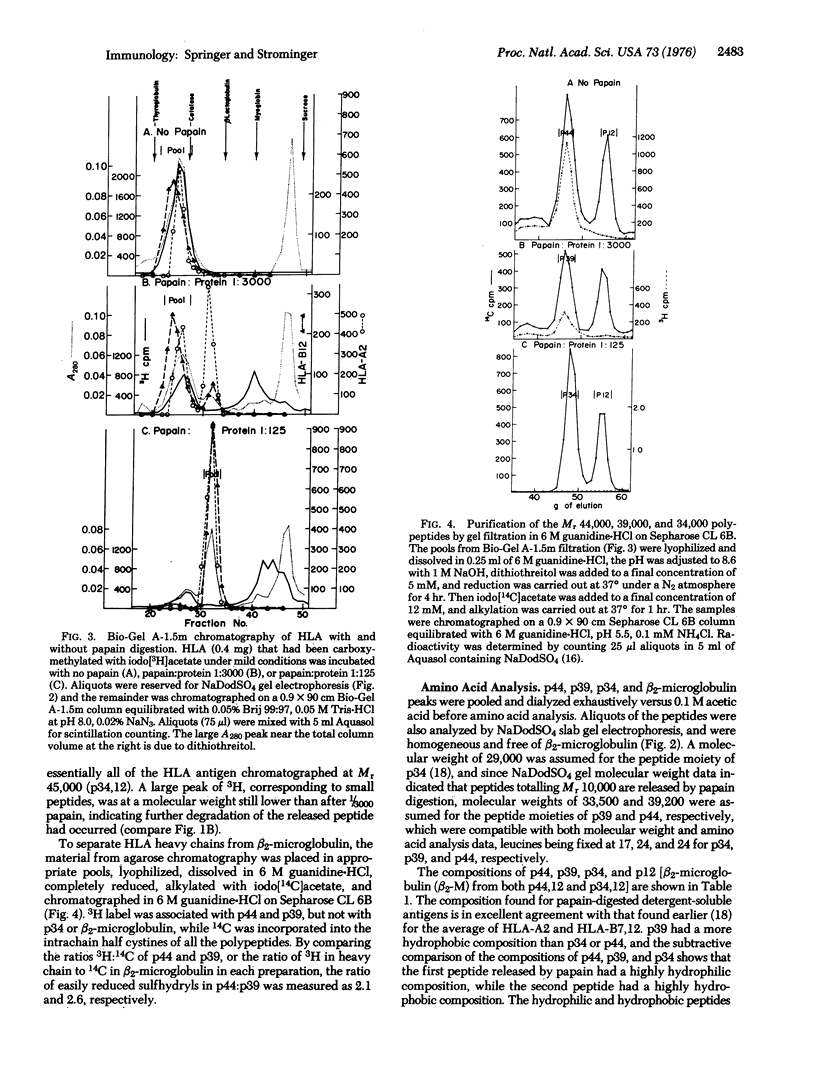
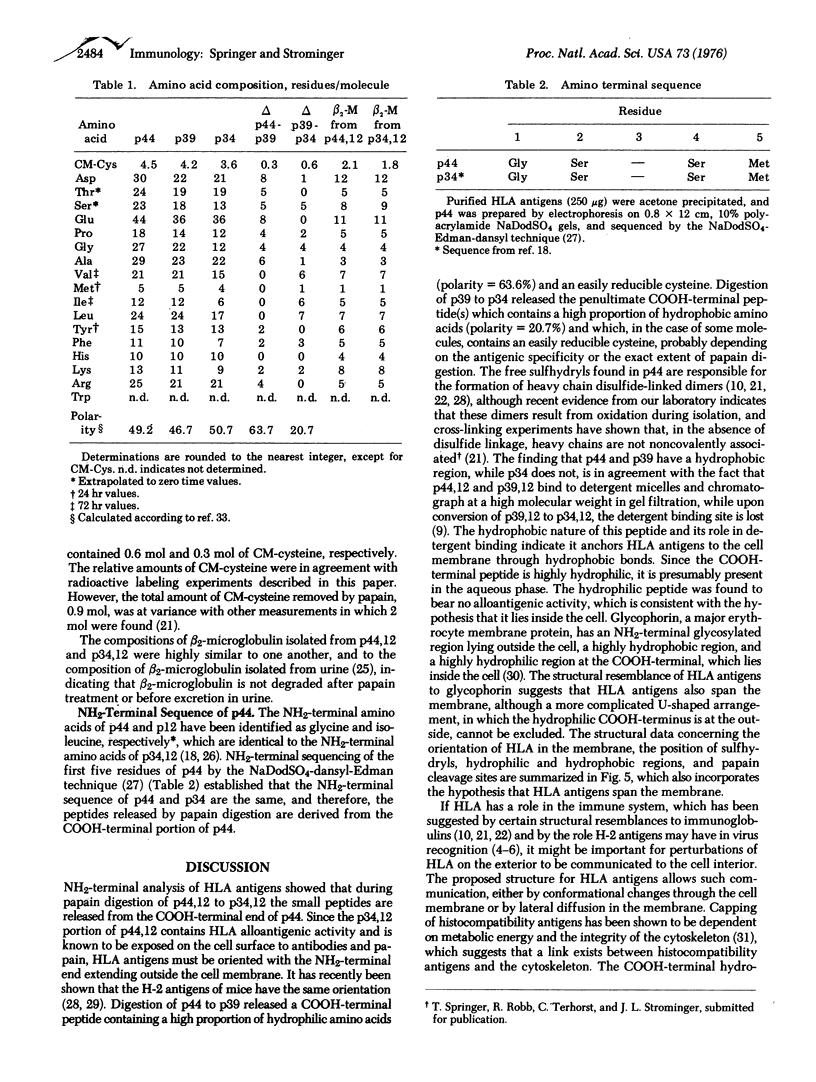
Purified, detergent-soluble HLA antigens (p44,12) are composed of a glycoprotein of molecular weight 44,000 (p44) and a peptide of molecular weight 12,000 (p12), beta2-microglobulin. Upon digestion with papain, p44,12 is converted to p39,12, then to p34,12, which retains antigenic activity. The NH2-terminal amino acid sequences of p34 and p44 are identical. p44, p39, and p34 were purified, and comparison of their amino acid compositions showed that the COOH-terminal peptide removed by the first papain cleavage is hydrophilic and contains cysteine that can be alkylated after mild reduction. The penultimate COOH-terminal peptide removed by the second papain cleavage is hydrophobic, and presumably anchors HLA antigens to the membrane. This correlates with the observation that p44,12 and p39,12 bind detergent, while p34,12 does not. The orientation and integration of HLA antigens in the lymphocyte membrane were thus defined, and the structure suggests that HLA antigens span the plasma membrane.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bach F. H., Bach M. L., Sondel P. M. Differential function of major histocompatibility complex antigens in T-lymphocyte activation. Nature. 1976 Jan 29;259(5541):273–281. doi: 10.1038/259273a0. [DOI] [PubMed] [Google Scholar]
- Berggård I., Bearn A. G. Isolation and properties of a low molecular weight beta-2-globulin occurring in human biological fluids. J Biol Chem. 1968 Aug 10;243(15):4095–4103. [PubMed] [Google Scholar]
- Bevan M. J. Interaction antigens detected by cytotoxic T cells with the major histocompatibility complex as modifier. Nature. 1975 Jul 31;256(5516):419–421. doi: 10.1038/256419a0. [DOI] [PubMed] [Google Scholar]
- Bretscher M. S., Raff M. C. Mammalian plasma membranes. Nature. 1975 Nov 6;258(5530):43–49. doi: 10.1038/258043a0. [DOI] [PubMed] [Google Scholar]
- Capaldi R. A., Vanderkooi G. The low polarity of many membrane proteins. Proc Natl Acad Sci U S A. 1972 Apr;69(4):930–932. doi: 10.1073/pnas.69.4.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cresswell P., Dawson J. R. Dimeric and monomeric forms of HL-A antigens solubilized by detergent. J Immunol. 1975 Jan;114(1 Pt 2):523–525. [PubMed] [Google Scholar]
- Cresswell P., Springer T., Strominger J. L., Turner M. J., Grey H. M., Kubo R. T. Immunological identity of the small subunit of HL-A antigens and beta2-microglobulin and its turnover on the cell membrane. Proc Natl Acad Sci U S A. 1974 May;71(5):2123–2127. doi: 10.1073/pnas.71.5.2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cresswell P., Turner M. J., Strominger J. L. Papain-solubilized HL-A antigens from cultured human lymphocytes contain two peptide fragments. Proc Natl Acad Sci U S A. 1973 May;70(5):1603–1607. doi: 10.1073/pnas.70.5.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewenstein B. M., Freed J. H., Mole L. E., Nathenson S. G. Localization of the papain cleavage site of H-2 glycoproteins. Proc Natl Acad Sci U S A. 1976 Mar;73(3):915–918. doi: 10.1073/pnas.73.3.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodfellow P. N., Jones E. A., Van Heyningen V., Solomon E., Bobrow M., Miggiano V., Bodmer W. F. The beta2-microglobulin gene is on chromosome 15 and not in the HL-A region. Nature. 1975 Mar 20;254(5497):267–269. doi: 10.1038/254267a0. [DOI] [PubMed] [Google Scholar]
- Grey H. M., Kubo R. T., Colon S. M., Poulik M. D., Cresswell P., Springer T., Turner M., Strominger J. L. The small subunit of HL-A antigens is beta 2-microglobulin. J Exp Med. 1973 Dec 1;138(6):1608–1612. doi: 10.1084/jem.138.6.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henning R., Milner R. J., Reske K., Cunningham B. A., Edelman G. M. Subunit structure, cell surface orientation, and partial amino-acid sequences of murine histocompatibility antigens. Proc Natl Acad Sci U S A. 1976 Jan;73(1):118–122. doi: 10.1073/pnas.73.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koszinowski U., Ertl H. Lysis mediated by T cells and restricted by H-2 antigen of target cells infected with vaccinia virus. Nature. 1975 Jun 12;255(5509):552–554. doi: 10.1038/255552a0. [DOI] [PubMed] [Google Scholar]
- Nakamuro K., Tanigaki N., Pressman D. Multiple common properties of human beta2-microglobulin and the common portion fragment derived from HL-A antigen molecules. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2863–2865. doi: 10.1073/pnas.70.10.2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parham P., Terhorst C., Herrmann H., Humphreys R. E., Waterfield M. D., Strominger J. L. Immunological and chemical purity of papain-solubilized HL-A antigens. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1594–1598. doi: 10.1073/pnas.72.4.1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson P. A., Rask L., Lindblom J. B. Highly purified papain-solubilized HL-A antigens contain beta2-microglobulin. Proc Natl Acad Sci U S A. 1974 Jan;71(1):35–39. doi: 10.1073/pnas.71.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson P. A., Rask L., Sege K., Klareskog L., Anundi H., Ostberg L. Evolutionary relationship between immunoglobulins and transplantation antigens. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1612–1616. doi: 10.1073/pnas.72.4.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader J. W., Cunningham B. A., Edelman G. M. Functional interactions of viral and histocompatibility antigens at tumor cell surfaces. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5066–5070. doi: 10.1073/pnas.72.12.5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearer G. M., Rehn T. G., Garbarino C. A. Cell-mediated lympholysis of trinitrophenyl-modified autologous lymphocytes. Effector cell specificity to modified cell surface components controlled by H-2K and H-2D serological regions of the murine major histocompatibility complex. J Exp Med. 1975 Jun 1;141(6):1348–1364. doi: 10.1084/jem.141.6.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer T. A., Strominger J. L., Mann D. Partial purification of detergent-soluble HL-A antigen and its cleavage by papain. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1539–1543. doi: 10.1073/pnas.71.4.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strominger J. L., Cresswell P., Grey H., Humphreys R. E., Mann D., McCuneJ, Parham P., Robb R., Sanderson A. R., Springer T. A. The immunoglobulin-like structure of human histocompatibility antigens. Transplant Rev. 1974;21(0):126–143. doi: 10.1111/j.1600-065x.1974.tb01549.x. [DOI] [PubMed] [Google Scholar]
- Strominger J. L., Humphreys R. E., McCune J. M., Parham P., Robb R., Springer T., Terhorst C. The immunoglobulin-like structure of human histocompatibility antigens. Fed Proc. 1976 Apr;35(5):1177–1182. [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Tanigaki N., Pressman D. The basic structure and the antigenic characteristics of HL-A antigens. Transplant Rev. 1974;21(0):15–34. doi: 10.1111/j.1600-065x.1974.tb01544.x. [DOI] [PubMed] [Google Scholar]
- Terhorst C., Parham P., Mann D. L., Strominger J. L. Structure of HLA antigens: amino-acid and carbohydrate compositions and NH2-terminal sequences of four antigen preparations. Proc Natl Acad Sci U S A. 1976 Mar;73(3):910–914. doi: 10.1073/pnas.73.3.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita M., Marchesi V. T. Amino-acid sequence and oligosaccharide attachment sites of human erythrocyte glycophorin. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2964–2968. doi: 10.1073/pnas.72.8.2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner M. J., Cresswell P., Parham P., Strominger J. L., Mann D. L., Sanderson A. R. Purification of papain-solubilized histocompatibility antigens from a cultured human lymphoblastoid line, RPMI 4265. J Biol Chem. 1975 Jun 25;250(12):4512–4519. [PubMed] [Google Scholar]
- Weiner A. M., Platt T., Weber K. Amino-terminal sequence analysis of proteins purified on a nanomole scale by gel electrophoresis. J Biol Chem. 1972 May 25;247(10):3242–3251. [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. Immunological surveillance against altered self components by sensitised T lymphocytes in lymphocytic choriomeningitis. Nature. 1974 Oct 11;251(5475):547–548. doi: 10.1038/251547a0. [DOI] [PubMed] [Google Scholar]
- van Someren H., Westerveld A., Hagemeijer A., Mees J. R., Meera Khan P., Zaalberg O. B. Human antigen and enzyme markers in man-Chinese hamster somatic cell hybrids: evidence for synteny between the HL-A, PGM3, ME1, and IPO-B loci. Proc Natl Acad Sci U S A. 1974 Mar;71(3):962–965. doi: 10.1073/pnas.71.3.962. [DOI] [PMC free article] [PubMed] [Google Scholar]