Abstract
AIM: To evaluate the demographic characteristics and clinical phenotypes of inflammatory bowel disease (IBD) in a geographic area in Northeastern Brazil.
METHODS: This retrospective study was conducted at the Hospital of the Federal University of Piauí in Northeastern Brazil. Demographic characteristics and clinical phenotypes of IBD were analyzed in relation to the time of diagnostic confirmation, which was defined as the date of disease onset. Data were collected between January 2011 and December 2012 and included all census patients 18 years of age or older during that period for whom there was diagnostic confirmation of Crohn’s disease (CD), ulcerative colitis (UC), or unclassified colitis according to the Montreal criteria. We also analyzed the period of time between the onset of clinical manifestations and the diagnosis of IBD (delay in the diagnosis). Statistical analyses included means and standard deviations for numeric variables and the Pearson χ2 adherence test for nominal variables. The annual index occurrence and overall prevalence of IBD at our institution were also calculated, with P values < 0.05 indicating statistical significance. This study was approved by the Institutional Ethics and Research Committee.
RESULTS: A total of 252 patients with IBD were included, including 152 (60.3%) UC patients and 100 (39.7%) CD patients. The clinical and demographic characteristics of all patients with IBD showed a female to male ratio of 1.3:1.0 and a mean age of 35.2 (SD = 14.5) years. In addition, the majority of patients were miscegenated (171, 67.9%), had received higher education (157, 62.4%), lived in urban areas (217, 86.1%), and were under the age of 40 years (97, 62.5%). For patients with CD, according to the Montreal classification, the predominant features present from the onset of disease were an age between 17 and 40 years (A2); colonic disease location (L2); and nonstricturing, nonfistulizing disease behavior (B1). However, approximately one-quarter of all CD patients demonstrated perineal involvement. We also observed considerable delay in the diagnosis of IBD throughout the entire study period (mean = 35.5 mo). In addition, the annual index occurrence rose from 0.08 to 1.53 cases/105 inhabitants/year during the study period, and the prevalence rate was 12.8 cases/105 inhabitants in 2012. Over the last two decades, there was a noted increase in the frequency of IBD in the study area.
CONCLUSION: In this study, there was a predominance of patients with UC, young people under 40 years of age, individuals with racial miscegenation, and low annual incomes.
Keywords: Inflammatory bowel diseases, Crohn’s disease, Ulcerative colitis, Epidemiology, Human Development Index
Core tip: This study addressed the demographic characteristics and clinical phenotypes of inflammatory bowel disease (IBD) patients in Northeastern Brazilian, where living conditions are poor and there is a lack of data on this subject. Over the last two decades, there was a noted increase in the frequency of IBD in the study area, although there was considerable delay in disease diagnosis throughout the study period. There was a predominance of patients with ulcerative colitis, but there was no difference between males and females in terms of disease frequency. Most individuals were aged below 40 years, had miscegenated ethnic characteristics, and received low annual incomes.
INTRODUCTION
Inflammatory bowel disease (IBD) encompasses a group of chronic and idiopathic inflammatory diseases preferentially affecting the gastrointestinal tract (GIT). Two subcategories, Crohn’s disease (CD) and ulcerative colitis (UC), have been highlighted[1]. CD is characterized by discontinuous and transmural inflammation that can involve any segment of the GIT, sometimes presenting stenotic or penetrating behavior with the formation of abscesses and fistulas[2]. UC is an inflammatory process confined to the mucosa and submucosa of the large intestine, with a characteristic gradient of greater to minor severity in the distal to proximal direction[3,4]. Unclassified colitis is defined when the disease involves only the large intestine and presents superimposed clinical and endoscopic characteristics of both CD and UC[5,6].
The incidence and prevalence of IBD are higher in countries with greater economic development, especially in the northern countries of Western Europe, Canada, the United States of America, Australia, and New Zealand[7,8]. In recent decades, there has also been an increase in these rates in countries of Southern and Eastern Europe and, to a lesser extent, the Middle East, North Africa, and some Asian countries[9-11]. In Latin America, there are few epidemiological studies of IBD, although some studies have reported growth in the frequencies of both CD and UC in this region, despite the low incidence of these diseases[12,13].
In Brazil, epidemiological studies of IBD are also very scarce, although increased frequencies of outpatient visits and hospitalizations in the major urban centers of Brazil have been observed[14-16]. However, no studies have been conducted with large Brazilian territorial coverage regarding the demographic and clinical aspects of IBD.
The purpose of this study was to address the lack of data on IBD in the state of Piauí, an area in the Brazilian Northeast, where living conditions are considered the worst (Figure 1). In recent decades, Brazil has experienced a continuous increase in the Human Development Index (HDI) from 0.590 in 1990 to 0.718 in 2010, although the country still stands at 84th in United Nations rankings. In Piauí, the HDI was reportedly lower (0.646 in 2010) than the HDI of southern and southeastern Brazilian states (HDIs between 0.731 and 0.783) and the federal capital (an HDI of 0.824)[17].
Figure 1.
Study area (Piauí State), located in the tropical zone in the northeastern region of Brazil.
The main objective of this study was to identify the demographic characteristics and clinical phenotypes of IBD in a geographic area in Northeastern Brazil with a low HDI. In addition, we sought to calculate the annual index rate for the occurrence and prevalence of IBD at our institution.
MATERIALS AND METHODS
Study location
The study was conducted at the Hospital of the Federal University of Piauí (HU-UFPI), which is considered a reference center for the treatment of patients with IBD. The strategic location of this hospital in the capital of Piauí, Teresina, and its inclusion in a computerized public health network result in the referral of patients from all other hospitals and public health centers throughout the state. In the state of Piauí, approximately 85% of the population receives health care solely through the public system, and our institution has been the only public hospital in the state to care for patients above 15 years of age with IBD.
Diagnosis of IBD
The diagnosis of IBD was established according to previously developed criteria for CD and UC[1], including clinical, ileocolonoscopic, laboratory, and histopathological aspects as well as computed tomography (CT) or magnetic resonance imaging enterography studies of the small intestine. When necessary, we performed endoscopic examinations of the upper GIT to evaluate the esophagus, stomach, and duodenum. All patients underwent investigation for gastroenteritis (coproculture) and intestinal parasites (stool test). In view of the high prevalence of enteroparasitoses in the study region, all patients received antiparasitic treatment with albendazole, secnidazole, and ivermectin regardless of the outcome of the stool examinations. The differential diagnosis of intestinal tuberculosis was based on clinical data, chest radiography, Mantoux intradermal testing, and the histological results of biopsy specimens. Mansonic schistosomiasis is endemic in many areas of Northeastern Brazilian, although there were no outbreaks in the region covered by this study.
Study design
The study was designed to describe the demographic and clinical characteristics of patients with IBD at the time of diagnostic confirmation, which was defined as the date of disease onset. This retrospective study involved a cohort of patients who were in clinical follow-up at HU-UFPI. The subjects’ demographic and clinical data were collected directly from the medical records of the Digestive System Unit of HU-UFPI and were supplemented with patient interviews during periodic outpatient clinical reviews.
The data were collected between January 2011 and December 2012 and included all census patients 18 years of age or over for whom there was diagnostic confirmation of CD, UC, or unclassified colitis. Individuals who received disease diagnoses during childhood or adolescence but who were at least 18 years of age at the time of data collection were also included.
The dependent variables included diagnosis (CD or UC), classification of CD and UC according to the Montreal criteria[6], and the period of time between the onset of clinical manifestations and the diagnosis of IBD. The independent demographic variables included age, gender, race, education, family income, and residence in an urban or rural area. The independent clinical variable was a family history of IBD. Patients who were diagnosed with unclassified colitis at the onset of the disease were included in the CD or UC groups, considering the subsequent diagnostic definition established during clinical follow-up of these individuals.
Statistical analysis
To perform statistical analyses, we first created a database using Microsoft Excel, the results of which are presented in tables and graphs. The following analyses were used: means and standard deviations for numeric variables and the Pearson χ2 adherence test for nominal variables (gender, race, education, and income). These variables were compared with the respective census data for the population of the state of Piauí. The significance level used for all tests was 5%.
We also calculated the annual index occurrence and the prevalence rate of IBD in our hospital based on the annual frequency of IBD and annual population data from the state of Piaui (85% of people over 15 years of age, as explained in the “study location” section) for the period from 1988 to 2012, according to census data from the Brazilian government (Instituto Brasileiro de Geografia e Estatística)[18].
Ethical considerations
This study was approved by the Research Ethics Committee of our institution (CAAE: 0140.0.045.000-11), and ethical principles for medical research involving human subjects were observed during all stages, including ensuring the anonymity of patients. All participants were adequately informed about the study and signed an informed consent form authorizing their inclusion in the study.
RESULTS
The confirmation of IBD diagnosis for all patients included in this study occurred between 1988 and 2012. Two hundred fifty-two consecutive patients treated in the IBD outpatient unit were included, of which 152 (60.3%) had UC and 100 (39.7%) had CD. The age at disease onset ranged from 12 years to 82 years, with a mean of 35.2 (SD = 14.5) years. The mean ages for the onset of CD and UC were 32.9 (SD = 13.6) years and 36.8 (SD = 14.8) years, respectively. Figure 2 shows the frequency distribution of the ages of patients with CD and UC at the time of diagnosis.
Figure 2.
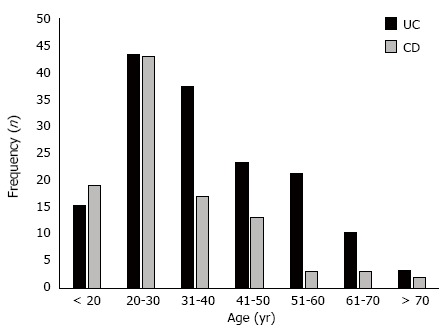
Distribution of patients with ulcerative colitis and Crohn’s disease according to age group in Piauí State (Brazil), 1988-2012. UC: Ulcerative colitis; CD: Crohn’s disease.
Regarding gender, there was a male to female ratio of 1.2 to 1.0 in the group of patients with CD, but there was no significant association with gender upon statistical analysis (P = 0.32). Patients with UC were predominantly female, with a female to male ratio of 1.8 to 1.0; this association was statistically significant (P = 0.005). Table 1 shows the demographic aspects of the study subjects and the population characteristics of the state of Piauí for comparison and statistical analysis. The patients' clinical features according to the Montreal[6] classification are shown in Table 2 for CD and Table 3 for UC.
Table 1.
Demographic characteristics of the population of the state of Piauí (Brazil) in 2010 and of patients with inflammatory bowel disease (total), Crohn’s disease, and ulcerative colitis according to gender, race, education, family income, and residence (urban or rural) in Piauí (Brazil), 1988-2012
| Demographic variables | General population of Piauí1: (n = 3118360) |
IBD phenotype |
|||
| CD (n = 100) | UC (n = 152) | Overall IBD (n = 252) | |||
| n (%) | n (%) | n (%) | |||
| Gender | Male | 49.0% | 54 (54.0) | 55 (36.2) | 109 (43.3) |
| Female | 51.0% | 46 (46.0) | 97 (63.8) | 143 (56.7) | |
| χ2 test | - | P = 0.32 | P = 0.00 | P = 0.07 | |
| Race | Miscegenated | 64.0% | 64 (64.0) | 107 (70.4) | 171 (67.9) |
| White | 24.4% | 26 (26.0) | 34 (22.4) | 60 (23.8) | |
| Black | 9.4% | 10 (10.0) | 10 (6.6) | 20 (7.9) | |
| Yellow | 2.2% | 0 (0) | 1 (0.6) | 1 (0.4) | |
| χ2 test | - | P = 0.50 | P = 0.24 | P = 0.18 | |
| Education (yr of schooling) | Uneducated and < 9 yr | 58.2%2 | 25 (25.0) | 70 (46.0) | 95 (37.8) |
| ≥ 9 yr | 41.8% | 75 (75.0) | 82 (53.9) | 157 (62.4) | |
| χ2 test | - | P = 0.00 | P = 0.00 | P = 0.00 | |
| Residence | Urban area | 65.8% | 93 (93.0) | 124 (81.6) | 217 (86.1) |
| Rural area | 34.2% | 7 (7.0) | 28 (18.4) | 35 (13.9) | |
| χ2 test | - | P = 0.00 | P = 0.00 | P = 0.00 | |
| Average income | Monthly3 | 247.00 USD | 643.50 USD | 555.40 USD | 590.40 USD |
Source: Brazilian Institute of Geography and Statistics (Instituto Brasileiro de Geografia e Estatística - IBGE [database online], 2010);
Individuals aged > 18 yr;
State of Piauí = income per capita, study population: family income. IBD: Inflammatory bowel disease; CD: Crohn’s disease; UC: Ulcerative colitis.
Table 2.
Clinical features of patients with Crohn’s disease at diagnosis according to the Montreal classification6 in Piauí (Brazil), 1988-2012
| Phenotypic elements | n (%) | Female, n (%) | Male, n (%) | |
| Age at diagnosis (A) | A1: ≤ 16 yr old | 8 (8.0) | 2 (25.0) | 6 (75.0) |
| A2: 17-40 yr old | 71 (71.0) | 30 (42.3) | 41 (57.7) | |
| A3: > 40 yr old | 21 (21.0) | 14 (66.7) | 7 (33.3) | |
| Disease location (L) | L1: Terminal ileum | 15 (15.0) | 7 (46.7) | 8 (53.3) |
| L2: Colonic | 36 (36.0) | 17 (47.2) | 19 (52.8) | |
| L3: Ileocolonic | 17 (17.0) | 7 (41.2) | 10 (58.8) | |
| L4: Isolated upper disease | 7 (7.0) | 1 (14.3) | 6 (85.7) | |
| L1, L2 or L3: Concomitant with L4 | 25 (25.0) | 14 (56.0) | 11 (44.0) | |
| Disease behavior (B) | B1: Nonstricturing, nonfistulizingB1 + p (perianal disease modifier) | 69 (69.0)19 (27.0) | 33 (47.8) 7 (36.8) | 36 (52.2)12 (63.2) |
| B2: StricturingB2 + p (perianal disease modifier) | 18 (18.0) 3 (16.7) | 6 (33.3) 2 (66.7) | 12 (66.7) 1 (33.3) | |
| B3: Penetrating1B3 + p (perianal disease modifier) | 13 (13.0) 5 (38.5) | 7 (53.8) 2 (40.0) | 6 (46.2) 3 (60.0) | |
Rectovaginal fistula = 4; Entero-cutaneous fistula = 6; Entero-enteric fistula = 2; Acute perforated abdomen = 1.
Table 3.
Clinical aspects of patients with ulcerative colitis (n = 152) at the time of diagnosis according to the modified Montreal classification in Piauí (Brazil), 1988-2012
| Phenotypic elements | n (%) | Female, n (%) | Male, n (%) | |
| Age at diagnosis (A)1 | A1: ≤ 16 yr old | 7 (4.6) | 3 (42.9) | 4 (57.1) |
| A2: 17-40 yr old | 88 (57.9) | 56 (63.6) | 32 (36.4) | |
| A3: > 40 yr old | 57 (37.5) | 38 (66.7) | 19 (33.3) | |
| Disease extent (E) | E1: Ulcerative proctitis | 14 (9.2) | 9 (64.3) | 5 (35.7) |
| E2: Keft-sided ulcerative colitis | 93 (61.2) | 63 (67.7) | 30 (32.3) | |
| E3: Extensive ulcerative colitis (pancolitis) | 45 (29.6) | 25 (55.6) | 20 (44.4) | |
| Disease severity (S) | S1: Mild | 41 (27.0) | 28 (68.3) | 13 (31.7) |
| S2: Moderate | 60 (39.5) | 40 (66.7) | 20 (33.3) | |
| S3: Severe | 51 (33.5) | 29 (56.9) | 22 (43.1) | |
Age at diagnosis is not a phenotypic element of the Montreal classification for ulcerative colitis.
The annual rate of new IBD cases increased slowly between 1988 and 1998, corresponding to a rate of one to five new patients per year in that decade. In the last years of the twentieth century and the first decade of this century, significant increases in the gross annual frequencies of these diseases were observed, reaching a rate of 25 new cases of IBD (CD = 12, UC = 13) in 2012. In this context, given both the natural population growth of the state of Piauí and the gross annual rate of new cases identified in this study, the annual index occurrence of IBD at our institution was 0.08 cases/105 inhabitants/year in 1988, and this rate reached its peak in 2007 with 1.53 cases/105 inhabitants/year (Figure 3). In 2012, the prevalence of IBD at our institution was 12.8 cases/105 inhabitants.
Figure 3.
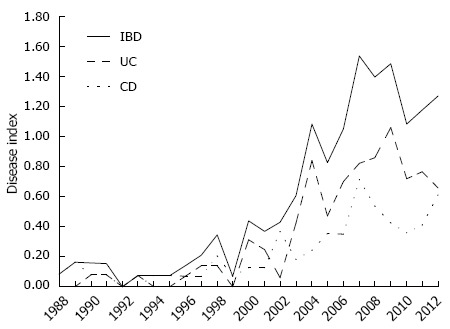
Annual index occurrence of inflammatory bowel disease (total), ulcerative colitis, and Crohn’s disease in Piauí State (Brazil) in the period from 1988-2012. IBD: Inflammatory bowel disease; UC: Ulcerative colitis; CD: Crohn’s disease.
Throughout the study period, there was considerable delay in the diagnosis of IBD. In particular, the mean time (in mo) between the onset of clinical manifestations and the diagnosis of IBD throughout the entire study period was 35.5 mo.
Regarding the etiopathogenesis of IBD, we analyzed two aspects: a family history of UC or CD and tobacco use. There was a history of IBD among first- and second-degree relatives in 29/252 (11.5%) cases, including 16/100 (16.0%) CD patients and 13/152 (8.6%) UC patients. A personal history of previous or current smoking was noted in 53/252 (21.0%) of all patients with IBD, including 21/100 (21.0%) with CD and 32/152 (21.1%) with UC.
DISCUSSION
Historical data for overall IBD geographic distribution worldwide have consistently shown higher rates of incidence and prevalence in more developed countries, the populations of which are predominantly Caucasian[12]. More recently, IBD has been detected with increasing frequency across all continents, including less developed countries, affecting people with different ethnic characteristics[9,10,19].
This study was conducted in a Brazilian region with the lowest socioeconomic human development indicators. In line with the low HDI, the average income per capita of the population of the state of Piauí (2965.00 USD per year) is well below the average per capita income of Brazil (4602.12 USD per year), while the average family income of participating patients in this research was higher [7084.80 (SD = 531.50) USD][18]. In this Brazilian region with poor living conditions, IBD is still a rare clinical condition compared to countries with high HDIs, where incidence rates are historically much higher, usually from 10.0 to 20.0 cases/105 inhabitants/year as well as higher than 20.0 cases/105 inhabitants/year[8]. However, in the 25 years of this study, we found that there was a gradual increase in the annual index occurrence at our hospital, reaching 1.53 cases/105 inhabitants/year and culminating in an intermediate prevalence rate corresponding to 12.8 cases/105 inhabitants in 2012. Our results were still much lower compared to those reported by Victoria et al[15] for the period of 1986 to 2005 in a more industrialized area of Southeastern Brazil, where the incidence rates rose from 1.0 to 8.0 cases/105 inhabitants/year and the prevalence increased from 1.2 to 20.5 cases/105 inhabitants in the same period. However, our results are consistent with the findings of researchers from other Brazilian regions[20,21] and South American countries[12,13], who have observed higher frequencies of CD and UC in hospitals based in South America. This finding suggests that IBD is also increasing in Latin America, even in regions with specific geographical, climatic, and socioeconomic characteristics that differ from those where IBD was commonly reported a few decades ago.
We assumed that the above data pertaining to the annual index occurrence and prevalence rates were not the true incidence and prevalence rates for IBD in the entire population of the state of Piauí but instead represent only an estimated statistical calculation, as this was not the main focus of the study design. In addition, other relevant factors in this regard should be emphasized, including the possibility that patients were diagnosed and treated without being referred to our institution and the lack of a state-wide registry of billing codes to verify that all IBD patients were identified and registered. Despite these potential biases, and considering that there are no epidemiological studies of IBD in this Brazilian region, we believe that the annual index occurrence and prevalence rates found in this study are representative of the true rates in the state of Piauí, which have yet to be properly calculated in future studies.
The racial phenotype of Brazil’s population is extremely heterogeneous. In particular, the population of Brazil has historically been influenced by individuals with European, African, Asian, and Amerindian ancestries, and there is significant miscegenation variability across geographical regions of the country. The general population of the state of Piauí, where the subjects of this study resided, predominately consists of individuals with miscegenated ethnic characteristics, with only a small portion of people with unique characteristics of white or black race and with little representation of Asian or purely indigenous individuals[18]. The subjects participating in our study also exhibited an ethnic profile similar to the general population where they reside; that is, the study showed no correlation between racial phenotype and the occurrence of IBD. Therefore, it appears that the ethnic characteristics of the study population differ from the pattern established in countries where there are higher traditional IBD incidence and prevalence rates, i.e., countries with a predominance of Caucasian individuals[22]. In fact, this miscegenated aspect of IBD patients in this region of Brazil is in keeping with the results of other studies that characterized these diseases as clinical entities emerging in the most diverse latitudes and longitudes of the planet, which are gradually and increasingly affecting other races and ethnicities in addition to Caucasians[11,23-26].
The literature data show that there are usually similar prevalence rates of IBD in men and women, although some studies have reported a slight predominance in males[27,28]. Our study showed that for UC patients, there was a significantly greater prevalence of women with this disease. These results are consistent with those reported by Kleinumbing-Júnior et al[14] in Southern Brazil.
In relation to the patient’s age at CD diagnosis, we observed disease occurrence in all age groups, although there was a predominant initial involvement in young individuals, with a well-pronounced peak incidence between 21 and 30 years of age. Our data are in agreement with the results reported by Thia et al[29] in a large population-based study, in which most patients were aged between 17 and 40 years (A2 in the Montreal classification). When segments of the GIT were considered individually, the topographic region most affected by CD in our study was the large intestine. However, the overall involvement of the small intestine above the distal ileum (L4 alone or associated with L1-L3) was well above that reported by other studies in the literature. Considering the behavior of CD, our results are similar to those reported by other researchers[14,27-30]. Approximately one-quarter of all CD patients demonstrated perineal involvement from the time of disease onset, which was more predominant in patients with involvement of the terminal ileum.
As observed in the group of patients with CD, the onset of UC occurred in groups of younger individuals. In UC, the highest peak incidence occurred in the age groups between 21 and 40 years, although this disease also achieved significant frequency in middle-aged individuals, with a second peak in the age groups between 41 and 60 years. As a result, IBD has strong social, educational, economic, and family impacts on affected individuals, as the phases of disease activity coincide with the period of life when they are in full educational activity, starting their professional career, and forming their family bases. Our results are very similar to those reported by Manninen et al[31] in Finland and the multicenter study conducted by Tozun et al[32] in Turkey. However, studies of other populations, such as the multicenter study conducted by Ng et al[19] in Asia-Pacific countries, the review by Rocchi et al[33] on IBD in Canada, and the population-based study conducted by Vind et al[34] in Denmark, have indicated that UC can start at all ages, commencing with a peak incidence in the first decades of life but maintaining a plateau of new cases in all subsequent age groups, even after 60 years of age.
Regarding the extent of UC, there are variations in the results presented in various studies, but in general, there is a higher incidence of distal UC (proctitis) in the initial presentation of the disease, followed by left UC and, to a lesser degree, pancolitis[3]. Our series disagreed on this point, as we observed a higher frequency of involvement of left UC and a low frequency of involvement of the rectum (E2 > E3 > E1, according to the Montreal classification for UC). These results are similar to those of studies by Zeng et al[27] in China and Lakatos et al[28] in Hungary. Regarding the profile of clinical severity, there was a slight predominance of the moderate form of UC, followed by the mild and severe forms (S2 > S3 > S1).
Given the low frequency of IBD in Brazil until a few decades ago, it has been difficult for physicians to readily recognize these diseases as a result of various factors, including the lack of IBD-related knowledge among health professionals and the lack of adequate diagnostic resources. For these reasons, there has been a delay in making correct IBD diagnoses in Brazil. We observed that there was a noted reduction in the time interval between the onset of clinical manifestations and the diagnosis of IBD in the last three 5-yr periods; the mean delay in diagnosis was initially 67.5 mo, although this time decreased to 40.7 mo and more recently to 25.1 mo. We can likely ascribe this fact to the opening of specialized services for the treatment of IBD in HU-UFPI, with improvements in physical facilities and complementary examinations, in addition to better training of health care staff responsible for patient service. Currently, the time required for IBD diagnosis in the state of Piaui is still well above the few months of delay observed by Gower-Rousseau et al[30] in France and Vind et al[34] in Denmark, although similar to results obtained by Zeng et al[27] in China and Manninen et al[31] in Finland.
Our analysis of educational data indicated that patients with IBD had a higher level of education than the general population of the state where they reside, which signifies that they had fully completed primary education, had at least 9 years of schooling, and had attended or completed high school or higher education. The results of the demographic profile of the subjects of our research, including educational level and age at disease onset, were similar to the results reported in other epidemiological studies of IBD; this profile consisted of disease onset at any age, but affecting mainly young people and tending to occur in individuals with higher educational levels[28,30,35-37].
Family history is considered the main risk factor for the onset of IBD because of several studies that demonstrated the existence of familial aggregation, concordance between monozygotic twins, and a greater prevalence in Ashkenazi Jews. The involvement of CD or UC in a family member indicates a significant increase for the risk of a first-degree relative also having the same disease[2,3,38,39]. In this sense, our results revealed that 4.0% of patients with IBD also had first-degree relatives with one of these diseases, and this rate was increased to 11.5% when second-degree relatives were also considered. In this regard, there was a greater association with family history in the CD group (16.0%) than in the UC group (8.6%).
In addition to familial aggregation, other correlations between IBD and environmental factors have been considered. We analyzed some of the factors that may influence the pathogenesis of IBD in this group of patients, including the recent population migration to urban centers. In the last 50 years, there has been an acceleration of population migration from rural to urban areas in Brazil, although this phenomenon has become more significant only in the last 30 years in Northeastern Brazil. In the period from 1980 to 2010, the urban Brazilian population increased sharply from 67.6% to 84.4% in all regions of Brazil, from 50.5% to 73.2% in Northeastern Brazil, and from 42.0% to 65.8% in the state of Piaui[18]. The progressive increase in the annual index occurrence of IBD in this study (Figure 2) coincides precisely with the period in which the migratory wave of rural populations to urban areas was observed. In fact, most patients with IBD in our study resided in urban areas, while only 14% lived in the countryside when the disease was diagnosed. It is possible that the level of higher education among patients with IBD may be related to increased access to education in urban areas, in contrast to the lower education of the general population of the state of Piauí, rather than to an etiopathogenic association or an increased risk to develop these diseases. However, further studies need to be conducted in this developing and newly urbanized population group to assess the impact of social changes, including lifestyle, eating habits, types of occupation and other environmental factors, on the risk of IBD emergence. Such a study would make it possible to demonstrate whether there is in fact a positive association between the environment in urban areas, possibly related to lifestyle and eating habits, and the onset of both CD and UC, as previously suggested in several studies[40-44].
Analysis of the association between prior or current tobacco use and the onset of IBD in our patients indicated that the frequency was similar in both groups with CD and UC. Therefore, no protective effects or increased susceptibility to CD were observed in relation to tobacco use, as previously suggested by some epidemiological[39,42,43] and experimental studies[45]. However, this issue was not analyzed in greater depth, and there may be other factors that could adequately explain the associations between onset, phenotypes, or IBD behavior and tobacco smoking.
In conclusion, the results of our study showed that there was a predominance of IBD patients with UC. In total, there was no difference between males and females in terms of disease frequency, although there was a significantly greater UC frequency in women. Most individuals were aged below 40 years, had miscegenated ethnic characteristics, and received low annual incomes. There was also a significant increase in the annual index occurrence of IBD at our institution. The IBD prevalence rate was found to be intermediate, lying between the high rates measured in more developed countries and the low prevalence rates in other areas. There was also considerable delay in the diagnosis of IBD, which, on average, was approximately two and a half years.
ACKNOWLEDGMENTS
We are very grateful to Mr. Marcos Antônio Araújo for his assistance in formatting the database and performing the statistical analysis for this study. We are also grateful to the resident doctors Paulo Vinicius Gomes de Oliveira, Conceição de Maria de Sousa Coelho, Daniel de Alencar Macêdo Dutra, Arlene dos Santos Pinto, and Daniela Calado Lima Costa for their cooperation in collecting data.
COMMENTS
Background
Crohn’s disease (CD) and ulcerative colitis (UC) are chronic inflammatory bowel diseases (IBD) of unknown etiology and multifactorial pathophysiology, which affect the gastrointestinal tract. Historically, the incidence and prevalence of IBD have been highest in economically developed nations with a predominantly Caucasian population, such as the northern countries of Western Europe, Canada, United States, Australia and New Zealand. Recent epidemiological studies have shown a gradual increase in the prevalence of CD and UC in different continents.
Research frontiers
In Brazil, there are still few epidemiological studies regarding the demographic and phenotypic characteristics of IBD. This study was carried out at a specialized university center for the care of IBD in Northeast Brazil, a region that is much less economically developed and has a lower Human Development Index (HDI) than the South and Southeast regions of the country.
Innovations and breakthroughs
The results of the study showed some similarities with other studies: most subjects lived in urban areas, were aged under 40 years, had a higher level of education and higher family income than the population of that region. On the other hand, this population presented some other characteristics: they were predominantly female, especially patients with UC and they had ethnic characteristics similar to those of the population of the studied region: predominantly with characteristics of racial miscegenation and less interaction between white and black people. Of considerable interest was the observation that there was a marked increase in the incidence of IBD in the studied region during recent decades.
Applications
The study results suggest that IBD have become more frequent in recent decades in this region of Brazil, and they affect populations with different racial and socioeconomic characteristics than those with a historically high prevalence and incidence of CD and UC.
Terminology
The HDI, the index adopted by the United Nations, classifies countries into: developed (HDI from 0.800 to 1.000, i.e., very high development), developing (HDI from 0.700 to 0.799 and HDI from 0.600 to 0.699, i.e., high and medium human development, respectively) and underdeveloped countries (HDI from 0.500 to 0.599 and HDI from 0.000 to 0.499, i.e., low and very low human development, respectively). According to this classification, Brazil has a high human development (HDI = 0.730). However, the region studied has a medium human development (HDI = 0.646).
Peer review
This is an excellent descriptive study in which the authors analyzed the increased frequency of IBD in a region of Brazil that has a medium human development index. The study results showed that IBD are expanding to other parts of the world, and they also occur in populations other than those that have a higher prevalence of individuals with Caucasian ethnic characteristics.
Footnotes
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: April 4, 2014
First decision: May 13, 2014
Article in press: August 28, 2014
P- Reviewer: Huang VW S- Editor: Ding Y L- Editor: A E- Editor: Zhang DN
References
- 1.Bernstein CN, Fried M, Krabshuis JH, Cohen H, Eliakim R, Fedail S, Gearry R, Goh KL, Hamid S, Khan AG, et al. World Gastroenterology Organization Practice Guidelines for the diagnosis and management of IBD in 2010. Inflamm Bowel Dis. 2010;16:112–124. doi: 10.1002/ibd.21048. [DOI] [PubMed] [Google Scholar]
- 2.Baumgart DC, Sandborn WJ. Crohn’s disease. Lancet. 2012;380:1590–1605. doi: 10.1016/S0140-6736(12)60026-9. [DOI] [PubMed] [Google Scholar]
- 3.Ordás I, Eckmann L, Talamini M, Baumgart DC, Sandborn WJ. Ulcerative colitis. Lancet. 2012;380:1606–1619. doi: 10.1016/S0140-6736(12)60150-0. [DOI] [PubMed] [Google Scholar]
- 4.Danese S, Fiocchi C. Ulcerative colitis. N Engl J Med. 2011;365:1713–1725. doi: 10.1056/NEJMra1102942. [DOI] [PubMed] [Google Scholar]
- 5.Nikolaus S, Schreiber S. Diagnostics of inflammatory bowel disease. Gastroenterology. 2007;133:1670–1689. doi: 10.1053/j.gastro.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. 2006;55:749–753. doi: 10.1136/gut.2005.082909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burisch J, Munkholm P. Inflammatory bowel disease epidemiology. Curr Opin Gastroenterol. 2013;29:357–362. doi: 10.1097/MOG.0b013e32836229fb. [DOI] [PubMed] [Google Scholar]
- 8.Ng SC, Bernstein CN, Vatn MH, Lakatos PL, Loftus EV, Tysk C, O’Morain C, Moum B, Colombel JF. Geographical variability and environmental risk factors in inflammatory bowel disease. Gut. 2013;62:630–649. doi: 10.1136/gutjnl-2012-303661. [DOI] [PubMed] [Google Scholar]
- 9.Baumgart DC, Bernstein CN, Abbas Z, Colombel JF, Day AS, D’Haens G, Dotan I, Goh KL, Hibi T, Kozarek RA, et al. IBD Around the world: comparing the epidemiology, diagnosis, and treatment: proceedings of the World Digestive Health Day 2010--Inflammatory Bowel Disease Task Force meeting. Inflamm Bowel Dis. 2011;17:639–644. doi: 10.1002/ibd.21409. [DOI] [PubMed] [Google Scholar]
- 10.Cosnes J, Gower-Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011;140:1785–1794. doi: 10.1053/j.gastro.2011.01.055. [DOI] [PubMed] [Google Scholar]
- 11.Ahuja V, Tandon RK. Inflammatory bowel disease in the Asia-Pacific area: a comparison with developed countries and regional differences. J Dig Dis. 2010;11:134–147. doi: 10.1111/j.1751-2980.2010.00429.x. [DOI] [PubMed] [Google Scholar]
- 12.Stanley MW, Tani EM, Skoog L. Fine-needle aspiration of fibroadenomas of the breast with atypia: a spectrum including cases that cytologically mimic carcinoma. Diagn Cytopathol. 1990;6:375–382. doi: 10.1002/dc.2840060602. [DOI] [PubMed] [Google Scholar]
- 13.Figueroa C C, Quera P R, Valenzuela E J, Jensen B C. [Inflammatory bowel disease: experience of two Chilean centers] Rev Med Chil. 2005;133:1295–1304. [PubMed] [Google Scholar]
- 14.Kleinubing-Júnior H, Pinho MSL, Ferreira LC, Bachtold GA, Merki A. The profile of outpatients with inflammatory bowel disease. ABCD Arq Bras Cir Dig (São Paulo) 2011;24:200–203. [Google Scholar]
- 15.Victoria CR, Sassak LY, Nunes HR. Incidence and prevalence rates of inflammatory bowel diseases, in midwestern of São Paulo State, Brazil. Arq Gastroenterol. 2009;46:20–25. doi: 10.1590/s0004-28032009000100009. [DOI] [PubMed] [Google Scholar]
- 16.Elia PP, Fogaça HS, Barros RG, Zaltman C, Elia CS. [Descriptive analysis of the social, clinical, laboratorial and anthropometric profiles of inflammatory bowel disease inwards patients from the “Clementino Fraga Filho” University Hospital, Rio de Janeiro, RJ, Brazil] Arq Gastroenterol. 2007;44:332–339. doi: 10.1590/s0004-28032007000400010. [DOI] [PubMed] [Google Scholar]
- 17.Programa das Nações Unidas para o Desenvolvimento (PNUD) Ranking do IDH nos estados do Brasil. Available from: http://www.pnud.org.br/arquivos/ranking-idhm-2010-uf.pdf.
- 18. Available from: http://censo2010.ibge.gov.br.
- 19.Ng SC, Tang W, Ching JY, Wong M, Chow CM, Hui AJ, Wong TC, Leung VK, Tsang SW, Yu HH, et al. Incidence and phenotype of inflammatory bowel disease based on results from the Asia-pacific Crohn’s and colitis epidemiology study. Gastroenterology. 2013;145:158–165.e2. doi: 10.1053/j.gastro.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 20.Souza MM, Belasco AGS, Aguilar-Nascimento JE. Perfil epidemiológico dos pacientes portadores de doença inflamatória intestinal do estado de Mato Grosso. [The epidemiological profile of patients with inflammatory bowel disease in the state of Mato Grosso] Rev Bras Coloproctol. 2008;28:324–328. [Google Scholar]
- 21.Salviano FN, Burgos MG, Santos EC. [Socioeconomic and nutritional profile of patients with inflammatory bowel disease at a university hospital] Arq Gastroenterol. 2007;44:99–106. doi: 10.1590/s0004-28032007000200003. [DOI] [PubMed] [Google Scholar]
- 22.Burisch J, Jess T, Martinato M, Lakatos PL. The burden of inflammatory bowel disease in Europe. J Crohns Colitis. 2013;7:322–337. doi: 10.1016/j.crohns.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 23.Ouakaa-Kchaou A, Gargouri D, Bibani N, Elloumi H, Kochlef A, Kharrat J. Epidemiological evolution of epidemiology of the inflammatory bowel diseases in a hospital of Tunis. Tunis Med. 2013;91:70–73. [PubMed] [Google Scholar]
- 24.Prideaux L, Kamm MA, De Cruz PP, Chan FK, Ng SC. Inflammatory bowel disease in Asia: a systematic review. J Gastroenterol Hepatol. 2012;27:1266–1280. doi: 10.1111/j.1440-1746.2012.07150.x. [DOI] [PubMed] [Google Scholar]
- 25.Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46–54.e42; quiz e30. doi: 10.1053/j.gastro.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 26.Sewell JL, Inadomi JM, Yee HF. Race and inflammatory bowel disease in an urban healthcare system. Dig Dis Sci. 2010;55:3479–3487. doi: 10.1007/s10620-010-1442-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zeng Z, Zhu Z, Yang Y, Ruan W, Peng X, Su Y, Peng L, Chen J, Yin Q, Zhao C, et al. Incidence and clinical characteristics of inflammatory bowel disease in a developed region of Guangdong Province, China: a prospective population-based study. J Gastroenterol Hepatol. 2013;28:1148–1153. doi: 10.1111/jgh.12164. [DOI] [PubMed] [Google Scholar]
- 28.Lakatos L, Kiss LS, David G, Pandur T, Erdelyi Z, Mester G, Balogh M, Szipocs I, Molnar C, Komaromi E, et al. Incidence, disease phenotype at diagnosis, and early disease course in inflammatory bowel diseases in Western Hungary, 2002-2006. Inflamm Bowel Dis. 2011;17:2558–2565. doi: 10.1002/ibd.21607. [DOI] [PubMed] [Google Scholar]
- 29.Thia KT, Sandborn WJ, Harmsen WS, Zinsmeister AR, Loftus EV. Risk factors associated with progression to intestinal complications of Crohn’s disease in a population-based cohort. Gastroenterology. 2010;139:1147–1155. doi: 10.1053/j.gastro.2010.06.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gower-Rousseau C, Vasseur F, Fumery M, Savoye G, Salleron J, Dauchet L, Turck D, Cortot A, Peyrin-Biroulet L, Colombel JF. Epidemiology of inflammatory bowel diseases: new insights from a French population-based registry (EPIMAD) Dig Liver Dis. 2013;45:89–94. doi: 10.1016/j.dld.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 31.Manninen P, Karvonen AL, Huhtala H, Rasmussen M, Collin P. The epidemiology of inflammatory bowel diseases in Finland. Scand J Gastroenterol. 2010;45:1063–1067. doi: 10.3109/00365521.2010.485323. [DOI] [PubMed] [Google Scholar]
- 32.Tozun N, Atug O, Imeryuz N, Hamzaoglu HO, Tiftikci A, Parlak E, Dagli U, Ulker A, Hulagu S, Akpinar H, et al. Clinical characteristics of inflammatory bowel disease in Turkey: a multicenter epidemiologic survey. J Clin Gastroenterol. 2009;43:51–57. doi: 10.1097/MCG.0b013e3181574636. [DOI] [PubMed] [Google Scholar]
- 33.Rocchi A, Benchimol EI, Bernstein CN, Bitton A, Feagan B, Panaccione R, Glasgow KW, Fernandes A, Ghosh S. Inflammatory bowel disease: a Canadian burden of illness review. Can J Gastroenterol. 2012;26:811–817. doi: 10.1155/2012/984575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vind I, Riis L, Jess T, Knudsen E, Pedersen N, Elkjaer M, Bak Andersen I, Wewer V, Nørregaard P, Moesgaard F, et al. Increasing incidences of inflammatory bowel disease and decreasing surgery rates in Copenhagen City and County, 2003-2005: a population-based study from the Danish Crohn colitis database. Am J Gastroenterol. 2006;101:1274–1282. doi: 10.1111/j.1572-0241.2006.00552.x. [DOI] [PubMed] [Google Scholar]
- 35.Jussila A, Virta LJ, Salomaa V, Mäki J, Jula A, Färkkilä MA. High and increasing prevalence of inflammatory bowel disease in Finland with a clear North-South difference. J Crohns Colitis. 2013;7:e256–e262. doi: 10.1016/j.crohns.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 36.Kappelman MD, Moore KR, Allen JK, Cook SF. Recent trends in the prevalence of Crohn’s disease and ulcerative colitis in a commercially insured US population. Dig Dis Sci. 2013;58:519–525. doi: 10.1007/s10620-012-2371-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Binder V. Epidemiology of IBD during the twentieth century: an integrated view. Best Pract Res Clin Gastroenterol. 2004;18:463–479. doi: 10.1016/j.bpg.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 38.Nunes T, Fiorino G, Danese S, Sans M. Familial aggregation in inflammatory bowel disease: is it genes or environment? World J Gastroenterol. 2011;17:2715–2722. doi: 10.3748/wjg.v17.i22.2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Danese S, Sans M, Fiocchi C. Inflammatory bowel disease: the role of environmental factors. Autoimmun Rev. 2004;3:394–400. doi: 10.1016/j.autrev.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 40.Qin X. Etiology of inflammatory bowel disease: a unified hypothesis. World J Gastroenterol. 2012;18:1708–1722. doi: 10.3748/wjg.v18.i15.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Soon IS, Molodecky NA, Rabi DM, Ghali WA, Barkema HW, Kaplan GG. The relationship between urban environment and the inflammatory bowel diseases: a systematic review and meta-analysis. BMC Gastroenterol. 2012;12:51. doi: 10.1186/1471-230X-12-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cabré E, Domènech E. Impact of environmental and dietary factors on the course of inflammatory bowel disease. World J Gastroenterol. 2012;18:3814–3822. doi: 10.3748/wjg.v18.i29.3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kohda K, Sawada N, Kawazoe Y. Formation of O6,7-dimethylguanine residues in calf thymus deoxyribonucleic acid treated with carcinogenic N-methyl-N-nitrosourea in vitro. Chem Pharm Bull (Tokyo) 1991;39:801–802. doi: 10.1248/cpb.39.801. [DOI] [PubMed] [Google Scholar]
- 44.Castiglione F, Diaferia M, Morace F, Labianca O, Meucci C, Cuomo A, Panarese A, Romano M, Sorrentini I, D’Onofrio C, et al. Risk factors for inflammatory bowel diseases according to the “hygiene hypothesis”: a case-control, multi-centre, prospective study in Southern Italy. J Crohns Colitis. 2012;6:324–329. doi: 10.1016/j.crohns.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 45.Verschuere S, De Smet R, Allais L, Cuvelier CA. The effect of smoking on intestinal inflammation: what can be learned from animal models? J Crohns Colitis. 2012;6:1–12. doi: 10.1016/j.crohns.2011.09.006. [DOI] [PubMed] [Google Scholar]


