Abstract
AIM: To determine the best cut-off value between the early and late recurrence periods after the initial recurrence of hepatocellular carcinoma (HCC).
METHODS: The clinical records of 404 patients who underwent macroscopic curative hepatectomy for HCC between 1980 and 2010 were retrospectively examined. We divided the 252 patients experienced a recurrence of HCC into two groups, the early and late recurrence groups using the “minimum P-value” approach. Factors for early recurrence were investigated using all 404 patients, and factors related to late recurrence were investigated in the patients who were confirmed to be recurrence free at the end of the early recurrence period.
RESULTS: For the 252 patients who experienced a recurrence, the optimal cut-off value for differentiating early and late recurrence based on the overall survival after initial recurrence was 17 mo (5-year overall survival after initial recurrence: 15.4% vs 36.3%, P = 0.000018). Cox proportional hazard analysis identified early recurrence (P = 0.003) as one of the independent prognostic factors associated with overall survival after initial recurrence. A logistic regression model showed that an alpha-fetoprotein level > 100 ng/mL (P < 0.001), multiple HCC (P < 0.001), serosal invasion (P = 0.031), and microvascular invasion (P = 0.012) were independent factors associated with early recurrence, whereas the only independent factor related to late recurrence was liver cirrhosis (P = 0.002).
CONCLUSION: Seventeen months after hepatectomy is a useful cut-off value between early and late recurrence of HCC based on the prognosis and different etiologies.
Keywords: Early recurrence, Late recurrence, Hepatocellular carcinoma, Hepatectomy, Minimum P-value approach
Core tip: The optimal cut-off value for differentiating early and late recurrence after hepatectomy for the hepatocellular carcinoma based on the overall survival after initial recurrence was 17 mo, and this cut-off may distinguish recurrences with different etiologies.
INTRODUCTION
Hepatocellular carcinoma (HCC) is one of the most common malignant tumors worldwide, especially in East Asian countries[1]. Several studies have demonstrated that patients who underwent resection for HCC more recently had better survival results in the past decade[2,3]. Whereas, the time interval between resection for HCC and recurrence has been reported to influence the survival time after recurrence[4], and the early recurrence of HCC is now recognized as an important condition with a poor prognosis[4-8].
Recurrent HCC can originate from either metastases from the primary tumor or a multicentric occurrence. Several authors have stated that early recurrence might primarily represent metastasis from the primary tumor, whereas late recurrence is most likely due to a multicentric occurrence[9-11]. However, the term “early recurrence” has never been clearly defined. The period from 6 mo to 2 years after hepatectomy has been used as the early recurrence period in previous reports[6,10,12]. There is no consensus regarding the meaning of the terms “early recurrence” and “late recurrence” using an evidence-based cut-off value to provide the greatest difference in prognosis between the two groups.
The aim of this study was to determine the best cut-off value between the early and late recurrence periods based on the difference in the prognosis of the two groups after the initial recurrence of HCC. In addition, we investigated factors that may contribute individually to early and late recurrence and evaluated the differences in the prognostic factors for survival between the early and late recurrence groups.
MATERIALS AND METHODS
Patients
A total of 404 patients underwent macroscopic curative hepatectomy for HCC between 1980 and 2010 at the Department of Surgery, Division of Digestive Surgery, Kyoto Prefectural University of Medicine, and all of these patients were analyzed in this study. There were 316 men and 88 women included in this study. The mean ± SD age was 62.0 ± 9.7 years. Underlying liver diseases included cirrhosis in 210 patients (52.0%) and non-cirrhotic liver diseases in 194 patients (48.0%). A total of 90 patients were seropositive for hepatitis B surface antigen, and 186 were seropositive for antibodies to hepatitis C. One patient died within 30 d of the surgery as a result of acute renal failure. According to Child’s classification system modified by Pugh et al[13], 393 patients (97.2%) were grouped in class A and 11 (2.8%) in class B. The mean ± SD tumor diameter was 4.1 ± 3.0 cm. Hepatectomies and the tumor location were defined according to Couinaud’s definition of liver segmentation[14].
Treatment
The indications for hepatectomy and the type of surgical procedure used were usually determined based on the patients’ liver function, which was primarily assessed using the Makuuchi criteria, which comprise preoperative measurements of ascites, the serum bilirubin level and the indocyanine green retention rate at 15 min (ICGR15)[15]. A total of 350 patients underwent anatomical resection, and 54 underwent non-anatomical resection. Anatomical hepatectomy was defined here as the removal of a Couinaud’s hepatic segment (subsegment) or segments confined by tumor-bearing portal tributaries. Limited resection or enucleation smaller than Couinaud’s segment (subsegment) was defined as non-anatomical resection. Adjuvant therapy was not administrated during the current study.
Pathological examination
All resected liver specimens were cut at a thickness of approximately 5 mm, and microscopic sections were viewed after staining with hematoxylin and eosin. The pathologic diagnosis and classification of the resected HCC tissues were performed according to The General Rules for the Clinical and Pathological Study of Primary Liver Cancer. Tumors were staged using the TNM classification scheme of the International Union Against Cancer[16]. Liver cirrhosis was defined as formation of regenerative nodules with surrounding fibrotic septa in liver parenchyma.
Follow-up
The follow-up evaluations of the patients involved hepatic ultrasonography, computed tomography, and measurement of the serum alpha-fetoprotein (AFP) level and the serum protein induced by vitamin K absence II level every 3-6 mo. Disease-free survival (DFS) was defined as the interval between surgery and the date of diagnosis of the first recurrence or the last follow-up. Overall survival (OS) was defined as the interval between surgery and the date of death caused by HCC recurrence or the last follow-up. The median follow-up duration was 44.6 mo.
Optimal cut-off value between early and late recurrence of HCC
Of the 404 patients, 252 patients (62.3%) experienced a recurrence of HCC. We divided the 252 patients experienced a recurrence of HCC into two groups, the early and late HCC recurrence after hepatectomy groups. The “minimum P-value” approach, which was performed using the log-rank test for the overall survival after the initial recurrence of HCC, was used to determine the best cut-off with which to divide up patients based on their overall survival after the initial recurrence of HCC[17,18]. The clinicopathological data were analyzed and compared between patients in the early and late HCC recurrence groups.
Treatment for the hepatic recurrence of HCC
Local treatment for the initial hepatic recurrence of HCC consisted of percutaneous ethanol injection therapy, radiofrequency ablation and repeat hepatectomy. Repeat hepatectomy was performed in 32 patients, and local ablation methods were used in 30 patients. Transarterial chemoembolization (TACE) was performed in 148 patients using the Seldinger technique[19] with iodized oil (Lipiodol) or gelatin sponge cubes as an embolus material and adriamycin (10-30 mg) and mitomycin C (10-20 mg) as anti-cancer drugs.
Statistical analysis
We performed univariate analyses of the clinical and pathologic factors that were potentially associated with overall survival. Survival was calculated using the Kaplan-Meier method and was compared between groups using the log-rank test. For the purpose to compare the prognostic value of the early recurrence to that of the late recurrence, Cox proportional hazard model was used in analysis of categorical variables influencing overall survival after initial recurrence using 252 patients who developed recurrence of HCC. Factors for early recurrence after the initial hepatectomy for HCC were investigated using all 404 patients who underwent hepatectomy for HCC. Factors related to late recurrence were investigated in the patients who were confirmed to be recurrence free at the end of the early recurrence period. All significant factors identified in the univariate analysis were entered into a multivariate regression analysis to identify independent factors. A P value < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS for Windows 11.5 (SPSS, Chicago, IL).
RESULTS
The cumulative 5-year overall survival (5-year OS) and disease-free survival (5-year DFS) rates for all 404 patients together were 58.8% and 30.9%, respectively. In the 252 patients who experienced a recurrence of HCC, the optimal cut-off value between early and late recurrence for dividing patients into two groups based on the greatest difference in overall survival after initial recurrence was 17 mo (P = 0.000018) by using the minimum P value approach (Figure 1). The 107 patients who experienced an initial recurrence of HCC within 17 mo were defined as the early recurrence group, and the 145 patients who had an initial recurrence after more than 17 mo were defined as the late recurrence group. Figure 2 shows the comparison of the overall survival curves after the initial hepatic recurrence between the early and late recurrence groups. The OS after initial recurrence in the late recurrence group was significantly better than that of the early recurrence group (5-year OS after initial recurrence: 36.3% vs 15.4%, P = 0.000018).
Figure 1.
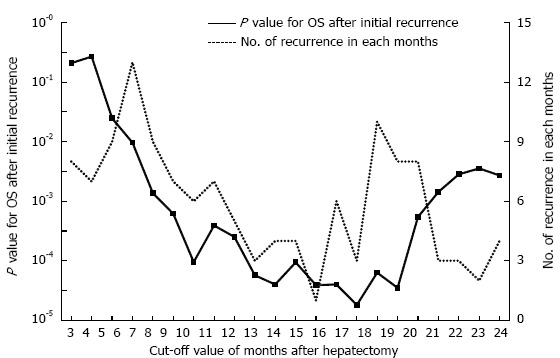
Kaplan-Meier analysis of the interval between the initial hepatectomy and recurrence. The minimum P value approach indicated that the most significant cut-off value for the interval prior to recurrence time was 17 mo. OS: Overall survival.
Figure 2.
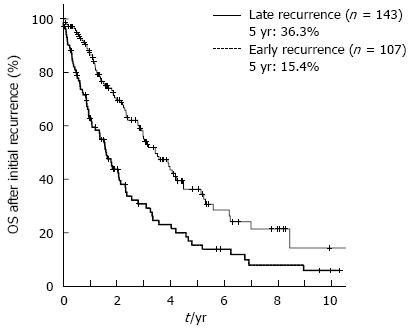
Comparison of the overall survival curves after initial hepatic recurrence for the early and late recurrence groups. The overall survival (OS) after initial recurrence in the late recurrence group was significantly better than that of the early recurrence group (5-year OS after initial recurrence: 36.3% vs 15.4%, P = 0.000018).
Table 1 shows the results of the univariate and multivariate analyses of prognostic factors associated with overall survival after initial recurrence. Cox proportional hazard analysis identified an ICGR15 ≥ 10% (P = 0.021), liver cirrhosis (P = 0.048), multiple HCC (P = 0.021), infiltrating growth (P = 0.008), and early recurrence (P = 0.001) as independent prognostic factors associated with overall survival after initial recurrence.
Table 1.
Results of the univariate and multivariate analyses of prognostic factors
| Total (n) | 5-yr OS after initial recurrence | MST (mo) | Univariate analysis P |
Multivariate analysis |
||
| Hazard ratio (95%CI) | P | |||||
| Age (yr) | 0.726 | |||||
| < 60 | 115 | 29.9% | 30.6 | |||
| ≥ 60 | 137 | 24.1% | 32.3 | |||
| Sex | 0.464 | |||||
| Male | 204 | 26.3% | 30.6 | |||
| Female | 48 | 32.7% | 38.1 | |||
| Indocyanine green retention rate at 15 min | 0.023 | 0.021 | ||||
| < 10% | 58 | 47.4% | 41.1 | 1 | ||
| ≥ 10% | 194 | 21.3% | 28.4 | 1.680 (1.081, 2.613) | ||
| Underlying liver disease | 0.017 | 0.048 | ||||
| Other | 110 | 37.3% | 39.7 | 1 | ||
| Cirrhosis | 142 | 20.0% | 27.4 | 1.422 (1.003, 2.017) | ||
| Alpha-fetoprotein (ng/mL) | 0.010 | |||||
| < 100 | 163 | 29.4% | 35.8 | |||
| ≥ 100 | 89 | 23.1% | 21.3 | |||
| Type of resection | 0.561 | |||||
| Non-anatomical resection | 52 | 17.5% | 33.4 | |||
| Anatomical resection | 200 | 28.5% | 28.5 | |||
| Number of tumors | 0.009 | 0.021 | ||||
| Single | 177 | 31.7% | 37.6 | 1 | ||
| Multiple | 75 | 17.0% | 21.6 | 1.530 (1.066, 2.196) | ||
| Growth pattern | 0.001 | 0.008 | ||||
| Expanding | 229 | 28.8% | 33.9 | 1 | ||
| Infiltrating | 23 | 12.4% | 14.3 | 1.963 (1.191, 3.234) | ||
| Histological differentiation | 0.001 | |||||
| Poorly differentiated | 27 | 10.6% | 11.1 | |||
| Others | 225 | 28.7% | 35.7 | |||
| Capsule | 0.242 | |||||
| Absent | 54 | 13.1% | 25.4 | |||
| Present | 198 | 31.5% | 33.4 | |||
| Serosal invasion | 0.905 | |||||
| Negative | 218 | 26.2% | 33.3 | |||
| Positive | 34 | 31.7% | 21.6 | |||
| Microvascular invasion | 0.012 | |||||
| Absent | 185 | 27.0% | 35.7 | |||
| Present | 67 | 26.8% | 14.4 | |||
| Surgical margin | 0.839 | |||||
| Negative | 232 | 26.7% | 28.7 | |||
| Positive | 20 | 34.3% | 48.7 | |||
| Tumor size (mm) | 0.182 | |||||
| < 50 | 187 | 25.0% | 26.9 | |||
| ≥ 50 | 65 | 20.3% | 16.6 | |||
| Recurrence period | < 0.001 | 0.001 | ||||
| Early group (< 17 mo) | 107 | 15.4% | 19.2 | 1.735 (1.237, 2.432) | ||
| Late group (≥ 17 mo) | 145 | 36.3% | 41.1 | 1 | ||
The results of the univariate and multivariate analyses of prognostic factors associated with overall survival after initial recurrence in the 252 patients who developed recurrence of hepatocellular carcinoma (HCC) after hepatectomy for HCC. MST: Median survival time.
Recurrence was observed in 107 patients within 17 mo after initial hepatectomy. Seven patients developed only distant metastatic disease (lung metastasis in two, bone metastasis in two, lymph node metastasis in two, and brain metastasis in one), 4 patients developed both intrahepatically and extrahepatically (lung metastasis in two and adrenal grand metastasis in two), and the other 96 patients developed intrahepatic recurrences. In addition, we examined the risk factors for early recurrence within 17 mo after the initial hepatectomy for HCC using all 404 patients who underwent hepatectomy for HCC. Table 2 shows the results of the χ2 test and the logistic regression model used to analyze the 12 clinicopathological factors related to early recurrence. An AFP level ≥ 100 ng/mL (P < 0.001), multiple HCC (P < 0.001), serosal invasion (P = 0.031), and Microvascular invasion (P = 0.012) were identified as independent factors associated with early recurrence. Nine patients underwent repeat hepatectomy for the recurrence of HCC within 17 mo, and pathological data of recurrent HCC of 5 patients were available. All of them were same differentiation with primary tumor.
Table 2.
Results of the univariate and multivariate analyses of the clinicopathologic factors
| Total (n) | Early group (n = 107) | Others (n = 297) | Univariate analysis P |
Multivariate analysis |
||
| RR (95%CI) | P | |||||
| Age (yr) | 0.290 | |||||
| < 60 | 175 | 51 | 124 | |||
| ≥ 60 | 229 | 56 | 173 | |||
| Sex | 0.933 | |||||
| Male | 316 | 84 | 232 | |||
| Female | 88 | 23 | 65 | |||
| Indocyanine green retention rate at 15 min | 0.193 | |||||
| < 10% | 110 | 24 | 86 | |||
| ≥ 10% | 294 | 83 | 211 | |||
| Underlying liver disease | 0.225 | |||||
| Other | 194 | 46 | 148 | |||
| Cirrhosis | 210 | 61 | 149 | |||
| Alpha-fetoprotein (ng/mL) | < 0.001 | < 0.001 | ||||
| < 100 | 280 | 52 | 228 | 1 | ||
| ≥ 100 | 124 | 55 | 69 | 2.939 (1.788, 4.830) | ||
| Number of tumors | < 0.001 | < 0.001 | ||||
| Single | 306 | 63 | 243 | 1 | ||
| Multiple | 98 | 44 | 54 | 2.633 (1.548, 4.445) | ||
| Growth pattern | 0.002 | |||||
| Expanding | 372 | 91 | 281 | |||
| Infiltrating | 32 | 16 | 16 | |||
| Capsule | 0.230 | |||||
| Absent | 82 | 26 | 56 | |||
| Present | 322 | 81 | 241 | |||
| Serosal invasion | 0.006 | 0.031 | ||||
| Negative | 358 | 87 | 271 | 1 | ||
| Positive | 46 | 20 | 26 | 2.110 (1.069, 4.164) | ||
| Microvascular invasion | < 0.001 | 0.012 | ||||
| Absent | 312 | 64 | 248 | 1 | ||
| Present | 92 | 43 | 49 | 2.001 (1.163, 3.441) | ||
| Surgical margin | 0.081 | |||||
| Negative | 374 | 95 | 279 | |||
| Positive | 30 | 12 | 18 | |||
| Tumor size (mm) | 0.010 | |||||
| < 50 | 312 | 73 | 239 | |||
| ≥ 50 | 92 | 34 | 58 | |||
The results of the univariate and multivariate analyses of the clinicopathologic factors contributing to early recurrence after the initial hepatectomy for hepatocellular carcinoma.
Recurrence was observed in 145 patients after more than 17 mo. Two patients developed distant metastatic disease (lung metastasis in one and brain metastasis in one), one patient developed both intrahepatically and extrahepatically (bone metastasis), and the other 142 patients developed intrahepatic recurrences. Factors related to late recurrence (≥ 17 mo) were investigated in the 236 patients who were confirmed to be recurrence free at 17 mo after the initial hepatectomy for HCC. Table 3 shows the results of the univariate and multivariate analyses of the clinicopathologic factors contributing to late recurrence, independent factor related to late recurrence was only liver cirrhosis (P = 0.002). Twenty-three patients underwent repeat hepatectomy for the recurrence of HCC after more than 17 mo, and pathological data of recurrent HCC of 12 patients were available. Nine of them were same differentiation with primary tumor. On the other hand, 2 of them were well differentiated HCC, despite primary tumors were moderately differentiated HCC.
Table 3.
Results of the univariate and multivariate analyses of the clinicopathologic factors
| Total (n) | Late group (n = 145) | Without recurrence (n = 91) | Univariate analysis P |
Multivariate analysis |
||
| RR (95%CI) | P | |||||
| Age (yr) | 0.125 | |||||
| < 60 | 95 | 64 | 31 | |||
| ≥ 60 | 141 | 81 | 60 | |||
| Sex | 0.194 | |||||
| Male | 189 | 120 | 69 | |||
| Female | 47 | 25 | 22 | |||
| Indocyanine green retention rate at 15 min | 0.008 | |||||
| < 10% | 70 | 34 | 36 | |||
| ≥ 10% | 166 | 111 | 55 | |||
| Underlying liver disease | 0.002 | 0.002 | ||||
| Other | 123 | 64 | 59 | 1 | ||
| Cirrhosis | 113 | 81 | 32 | 2.333 (1.359, 4.008) | ||
| Alpha-fetoprotein (ng/mL) | 0.199 | |||||
| < 100 | 187 | 111 | 76 | |||
| ≥ 100 | 49 | 34 | 15 | |||
| Number of tumors | 0.069 | |||||
| Single | 194 | 114 | 80 | |||
| Multiple | 42 | 31 | 11 | |||
| Growth pattern | 0.878 | |||||
| Expanding | 225 | 138 | 87 | |||
| Infiltrating | 11 | 7 | 4 | |||
| Capsule | 0.905 | |||||
| Absent | 45 | 28 | 17 | |||
| Present | 191 | 117 | 74 | |||
| Serosal invasion | 0.411 | |||||
| Negative | 216 | 131 | 85 | |||
| Positive | 20 | 14 | 6 | |||
| Microvascular invasion | 0.641 | |||||
| Absent | 199 | 121 | 78 | |||
| Present | 37 | 24 | 13 | |||
| Surgical margin | 0.703 | |||||
| Negative | 224 | 137 | 87 | |||
| Positive | 12 | 8 | 4 | |||
| Tumor size (mm) | 0.113 | |||||
| < 50 | 193 | 114 | 79 | |||
| ≥ 50 | 43 | 31 | 12 | |||
The results of the univariate and multivariate analyses of the clinicopathologic factors contributing to late recurrence in the 236 patients who were confirmed to be recurrence-free at 17 mo after the initial hepatectomy for hepatocellular carcinoma.
DISCUSSION
The early recurrence of HCC after the initial hepatectomy has been reported to be a prognostic factor for survival after recurrence[4-8]. However, in previous reports, the term “early recurrence” has not been defined based on the best cut-off value for prognosis between the early and late recurrence groups. Hayashi et al[5] and Shah et al[6] used 1 year as the period of early recurrence without any sufficient reasons. Park et al[7] and Lu et al[8] used 6 mo as the period of early recurrence because multinodular recurrence most often recurred within 6 mo after surgery. Imamura et al[11] used 2 years as the period of early recurrence after the initial hepatectomy because after the early peak for recurrence at approximately 1 year postoperatively, the recurrence rate decreased but persisted over a long period, resulting in a second peak at 4 years after surgery. We could not found any previous studies that classified patients into early and late recurrence groups based on the assessment of the best cut-off value to provide the largest difference in prognosis between the two groups. In this study, we analyzed the cut-off value between early and late recurrence based on minimum P-value approach, and we found that the best cut-off value was 17 mo after the initial hepatectomy. This is the first study to calculate the most significant cut-off value between early and late recurrence based on the prognosis after the initial recurrence of HCC. The minimum P-value approach has been proposed as a means of reducing the risk of missing a significant association[17,18]. However, this approach may give false positive associations. The potential validity and use of such an approach is currently further tested using the clinical material of the present and other studies.
There are two possible causes of HCC recurrence, metastasis from primary tumor and metachronously multicentric occurrence. Both types of disease may have been present before hepatectomy in the early postoperative recurrent cases. Some authors have noted that early recurrence might represent primarily metastasis from primary tumor, whereas late recurrence might most likely be due to multicentric occurrence[8,9,20]. However, it is usually difficult to distinguish intrahepatic recurrences of different etiologies because histopathological analysis is not performed in clinical practice. Previously reported risk factors for early recurrence include PIVKAII, AFP, Milan criteria status, nonanatomic resection, microscopic vascular invasion, intrahepatic metastasis, and positive surgical margins[5-8,11]. In this study, serosal invasion, multiple tumors, microvascular invasion, and an AFP ≥ 100 ng/mL, which were only primary tumor factors, were associated with early recurrence after the initial hepatectomy. In contrast, the factors associated with late recurrence included only liver cirrhosis, which are the host-related factor reflecting the increased carcinogenicity of the liver. Consistent with our results, Imamura et al[11] and Poon et al[10] reported that the grade of hepatitis activity and cirrhosis, respectively, are risk factors for late recurrence. These findings support the hypothesis that early recurrence is mainly influenced by factors related to the status of the primary tumor and late recurrence is chiefly caused by a second primary lesion and not by the initial tumor stage. Moreover, 17 mo after the initial hepatectomy may be a good cut-off value to distinguish recurrences of different etiologies, which strongly influence the prognosis after recurrence.
A multivariate analysis showed that liver cirrhosis, an ICGR15 ≥ 10%, an infiltrating growth pattern, multiple HCC and early recurrence were independent prognostic factor associated with overall survival after initial recurrence. The four former parameters were related to liver function and HCC stage, which have been previously reported to be important prognostic factors[21-23]. It is not surprising that early recurrence was also an independent prognostic factor, because early recurrence represents mainly metastatic recurrence, and it may be associated with the presence of minimal metastasis at the time of the initial hepatectomy. In contrast, late recurrence represents mainly multicentric recurrence. To prevent early and late recurrence, different adjuvant therapeutic methods may be required.
In patients with a high risk of early recurrence after the initial hepatectomy for HCC, early detection through close postoperative surveillance is necessary. To date, various systemic adjuvant treatments, such as chemotherapy, immunotherapy, and TACE, have been tried[24,25]. There are several systematic reviews on the role of neoadjuvant/adjuvant therapy for HCC treated with hepatectomy[26-30]. A clinical trial to examine the recurrence-preventing effect of sorafenib when administered after curative treatments such as resection or ablation (STORM trial) is in progress[29]. Whereas, Lau et al[27] reported that adjuvant intra-arterial 131I-lipiodol after curative liver resection provided a survival benefit in a randomized control trial. If these risk factors for early recurrence are found after resection for HCC, clinical trials involving adjuvant therapy should be performed.
Considering that the late recurrence was not related to primary tumor factors, but related only liver cirrhosis, late recurrence may be consider secondary primary tumors. The management of late recurrence should be similar to that of an initial diagnosis of HCC. Muto et al[31] mentioned that polyprenoic acid can prevent second primary tumors in patients who are clinically free of disease after their primary hepatomas are treated in a randomized controlled study, although the mechanism of action of polyprenoic acid is not fully revealed. On the other hand, Singal et al[32] reported that interferon treatment after curative resection or ablation of HCC in HCV-related cirrhotics prevents HCC recurrence and improves survival. To prevent late recurrence, the suppression of multicentric occurrence using polyprenoic acid or interferon treatment will be indicated in patients with cirrhotic livers.
In conclusion, this study demonstrates that 17 mo after hepatectomy is a good cut-off value between early and late recurrence based on the prognosis after the initial recurrence of HCC, and this cut-off may distinguish recurrences of different etiologies. The early recurrence was influenced mainly by the primary tumor stage, and late recurrence was influenced only by the liver function status. It is important to prevent early and late recurrences using different adjuvant therapeutic approaches.
COMMENTS
Background
The time interval between resection for hepatocellular carcinoma (HCC) and recurrence has been reported to influence the survival time after recurrence, and the early recurrence of HCC is now recognized as an important condition with a poor prognosis. However, there is no consensus regarding the meaning of the terms “early recurrence” and “late recurrence” using an evidence-based cut-off value to provide the greatest difference in prognosis between the two groups.
Research frontiers
The period from 6 mo to 2 years after hepatectomy has been used as the early recurrence period in previous reports without any sufficient reasons. Authors could not found any previous studies that classified patients into early and late recurrence groups based on the assessment of the best cut-off value to provide the largest difference in prognosis between the two groups.
Innovations and breakthroughs
Authors firstly examined the best cut-off value between the early and late recurrence periods based on the difference in the prognosis of the two groups after the initial recurrence of HCC. In addition, they investigated factors that may contribute individually to early and late recurrence and evaluated the differences in the prognostic factors for survival between the early and late recurrence groups.
Applications
Authors divided the patients experienced a recurrence of HCC into two groups, the early and late HCC recurrence after hepatectomy groups. The “minimum P-value” approach, which was performed using the log-rank test for the overall survival after the initial recurrence of HCC, was used to determine the best cut-off with which to divide up patients based on their overall survival after the initial recurrence of HCC.
Terminology
HCC is one of the most common malignant tumors worldwide, especially in East Asian countries. Several studies have demonstrated that patients who underwent resection for HCC more recently had better survival results in the past decade. Recurrent HCC can originate from either metastases from the primary tumor or a multicentric occurrence. Several authors have stated that early recurrence might primarily represent metastasis from the primary tumor, whereas late recurrence is most likely due to a multicentric occurrence.
Peer review
The paper shows that 17 mo is the optimal cut-off value for differentiating early and late recurrence after hepatectomy for hepatocellular carcinoma based on the overall survival after initial recurrence. The approach to the problem that is the object of the study is quite original and the paper is well written; however, some questions should be answered.
Footnotes
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: March 17, 2014
First decision: April 5, 2014
Article in press: September 16, 2014
P- Reviewer: Pompili M S- Editor: Gou SX L- Editor: A E- Editor: Zhang DN
References
- 1.Bosch FX, Ribes J, Díaz M, Cléries R. Primary liver cancer: worldwide incidence and trends. Gastroenterology. 2004;127:S5–S16. doi: 10.1053/j.gastro.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 2.Wu CC, Cheng SB, Ho WM, Chen JT, Liu TJ, P’eng FK. Liver resection for hepatocellular carcinoma in patients with cirrhosis. Br J Surg. 2005;92:348–355. doi: 10.1002/bjs.4838. [DOI] [PubMed] [Google Scholar]
- 3.Yamamoto Y, Ikoma H, Morimura R, Konishi H, Murayama Y, Komatsu S, Shiozaki A, Kuriu Y, Kubota T, Nakanishi M, et al. Changing trends in long-term outcomes after hepatic resection for hepatocellular carcinoma: A 30-year, single-center experience. Anticancer Res. 2013;33:5097–5105. [PubMed] [Google Scholar]
- 4.Shimada M, Takenaka K, Gion T, Fujiwara Y, Kajiyama K, Maeda T, Shirabe K, Nishizaki T, Yanaga K, Sugimachi K. Prognosis of recurrent hepatocellular carcinoma: a 10-year surgical experience in Japan. Gastroenterology. 1996;111:720–726. doi: 10.1053/gast.1996.v111.pm8780578. [DOI] [PubMed] [Google Scholar]
- 5.Hayashi M, Shimizu T, Hirokawa F, Inoue Y, Komeda K, Asakuma M, Miyamoto Y, Takeshita A, Shibayama Y, Tanigawa N. Clinicopathological risk factors for recurrence within one year after initial hepatectomy for hepatocellular carcinoma. Am Surg. 2011;77:572–578. [PubMed] [Google Scholar]
- 6.Shah SA, Greig PD, Gallinger S, Cattral MS, Dixon E, Kim RD, Taylor BR, Grant DR, Vollmer CM. Factors associated with early recurrence after resection for hepatocellular carcinoma and outcomes. J Am Coll Surg. 2006;202:275–283. doi: 10.1016/j.jamcollsurg.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 7.Park JH, Koh KC, Choi MS, Lee JH, Yoo BC, Paik SW, Rhee JC, Joh JW. Analysis of risk factors associated with early multinodular recurrences after hepatic resection for hepatocellular carcinoma. Am J Surg. 2006;192:29–33. doi: 10.1016/j.amjsurg.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 8.Lu X, Zhao H, Yang H, Mao Y, Sang X, Miao R, Xu Y, Du S, Xu H, Chi T, et al. A prospective clinical study on early recurrence of hepatocellular carcinoma after hepatectomy. J Surg Oncol. 2009;100:488–493. doi: 10.1002/jso.21354. [DOI] [PubMed] [Google Scholar]
- 9.Yamamoto J, Kosuge T, Takayama T, Shimada K, Yamasaki S, Ozaki H, Yamaguchi N, Makuuchi M. Recurrence of hepatocellular carcinoma after surgery. Br J Surg. 1996;83:1219–1222. [PubMed] [Google Scholar]
- 10.Poon RT, Fan ST, Ng IO, Lo CM, Liu CL, Wong J. Different risk factors and prognosis for early and late intrahepatic recurrence after resection of hepatocellular carcinoma. Cancer. 2000;89:500–507. [PubMed] [Google Scholar]
- 11.Imamura H, Matsuyama Y, Tanaka E, Ohkubo T, Hasegawa K, Miyagawa S, Sugawara Y, Minagawa M, Takayama T, Kawasaki S, et al. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol. 2003;38:200–207. doi: 10.1016/s0168-8278(02)00360-4. [DOI] [PubMed] [Google Scholar]
- 12.Ibrahim S, Roychowdhury A, Hean TK. Risk factors for intrahepatic recurrence after hepatectomy for hepatocellular carcinoma. Am J Surg. 2007;194:17–22. doi: 10.1016/j.amjsurg.2006.06.051. [DOI] [PubMed] [Google Scholar]
- 13.Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646–649. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 14.Couinaud C. [Liver lobes and segments: notes on the anatomical architecture and surgery of the liver ] Presse Med. 1954;62:709–712. [PubMed] [Google Scholar]
- 15.Makuuchi M, Kosuge T, Takayama T, Yamazaki S, Kakazu T, Miyagawa S, Kawasaki S. Surgery for small liver cancers. Semin Surg Oncol. 1993;9:298–304. doi: 10.1002/ssu.2980090404. [DOI] [PubMed] [Google Scholar]
- 16.Liver Cancer study Group of Japan. General rules for the clinical and pathological study of primary liver cancer. 2nd English ed. Tokyo: Kanehara; 2003. pp. 13–28. [Google Scholar]
- 17.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M, Berger A, Wind P, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 18.Altman DG, Lausen B, Sauerbrei W, Schumacher M. Dangers of using “optimal” cutpoints in the evaluation of prognostic factors. J Natl Cancer Inst. 1994;86:829–835. doi: 10.1093/jnci/86.11.829. [DOI] [PubMed] [Google Scholar]
- 19.Seldinger SI. Catheter replacement of the needle in percutaneous arteriography; a new technique. Acta radiol. 1953;39:368–376. doi: 10.3109/00016925309136722. [DOI] [PubMed] [Google Scholar]
- 20.Jwo SC, Chiu JH, Chau GY, Loong CC, Lui WY. Risk factors linked to tumor recurrence of human hepatocellular carcinoma after hepatic resection. Hepatology. 1992;16:1367–1371. doi: 10.1002/hep.1840160611. [DOI] [PubMed] [Google Scholar]
- 21.Poon RT, Ng IO, Fan ST, Lai EC, Lo CM, Liu CL, Wong J. Clinicopathologic features of long-term survivors and disease-free survivors after resection of hepatocellular carcinoma: a study of a prospective cohort. J Clin Oncol. 2001;19:3037–3044. doi: 10.1200/JCO.2001.19.12.3037. [DOI] [PubMed] [Google Scholar]
- 22.Andreou A, Vauthey JN, Cherqui D, Zimmitti G, Ribero D, Truty MJ, Wei SH, Curley SA, Laurent A, Poon RT, et al. Improved long-term survival after major resection for hepatocellular carcinoma: a multicenter analysis based on a new definition of major hepatectomy. J Gastrointest Surg. 2013;17:66–77; discussion p.77. doi: 10.1007/s11605-012-2005-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shimada K, Sakamoto Y, Esaki M, Kosuge T, Morizane C, Ikeda M, Ueno H, Okusaka T, Arai Y, Takayasu K. Analysis of prognostic factors affecting survival after initial recurrence and treatment efficacy for recurrence in patients undergoing potentially curative hepatectomy for hepatocellular carcinoma. Ann Surg Oncol. 2007;14:2337–2347. doi: 10.1245/s10434-007-9415-7. [DOI] [PubMed] [Google Scholar]
- 24.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–1917. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 25.Takayama T, Sekine T, Makuuchi M, Yamasaki S, Kosuge T, Yamamoto J, Shimada K, Sakamoto M, Hirohashi S, Ohashi Y, et al. Adoptive immunotherapy to lower postsurgical recurrence rates of hepatocellular carcinoma: a randomised trial. Lancet. 2000;356:802–807. doi: 10.1016/S0140-6736(00)02654-4. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz JD, Schwartz M, Mandeli J, Sung M. Neoadjuvant and adjuvant therapy for resectable hepatocellular carcinoma: review of the randomised clinical trials. Lancet Oncol. 2002;3:593–603. doi: 10.1016/s1470-2045(02)00873-2. [DOI] [PubMed] [Google Scholar]
- 27.Lau WY, Lai EC, Leung TW, Yu SC. Adjuvant intra-arterial iodine-131-labeled lipiodol for resectable hepatocellular carcinoma: a prospective randomized trial-update on 5-year and 10-year survival. Ann Surg. 2008;247:43–48. doi: 10.1097/SLA.0b013e3181571047. [DOI] [PubMed] [Google Scholar]
- 28.Majno PE, Adam R, Bismuth H, Castaing D, Ariche A, Krissat J, Perrin H, Azoulay D. Influence of preoperative transarterial lipiodol chemoembolization on resection and transplantation for hepatocellular carcinoma in patients with cirrhosis. Ann Surg. 1997;226:688–701; discussion 701-703. doi: 10.1097/00000658-199712000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Printz C. Clinical trials of note. Sorafenib as adjuvant treatment in the prevention of disease recurrence in patients with hepatocellular carcinoma (HCC) (STORM) Cancer. 2009;115:4646. doi: 10.1002/cncr.24673. [DOI] [PubMed] [Google Scholar]
- 30.Kudo M. Adjuvant therapy after curative treatment for hepatocellular carcinoma. Oncology. 2011;81 Suppl 1:50–55. doi: 10.1159/000333259. [DOI] [PubMed] [Google Scholar]
- 31.Muto Y, Moriwaki H, Ninomiya M, Adachi S, Saito A, Takasaki KT, Tanaka T, Tsurumi K, Okuno M, Tomita E, et al. Prevention of second primary tumors by an acyclic retinoid, polyprenoic acid, in patients with hepatocellular carcinoma. Hepatoma Prevention Study Group. N Engl J Med. 1996;334:1561–1567. doi: 10.1056/NEJM199606133342402. [DOI] [PubMed] [Google Scholar]
- 32.Singal AK, Freeman DH, Anand BS. Meta-analysis: interferon improves outcomes following ablation or resection of hepatocellular carcinoma. Aliment Pharmacol Ther. 2010;32:851–858. doi: 10.1111/j.1365-2036.2010.04414.x. [DOI] [PubMed] [Google Scholar]


