Abstract
AIM: To investigate the correlation among tumor markers, curative resection, and recurrence in gastric cancer.
METHODS: The patients with preoperative tumor makers [Carcinoembryonic antigen, Carbohydrate antigen (CA) 19-9, and CA 125] and elective gastrectomy between January 2000 and December 2009 at Chungbuk National University Hospital were enrolled in this study. We analyzed the relationship among the tumor makers, curative resection and recurrence, retrospectively.
RESULTS: Among the 679 patients with gastric cancer, curative resection was 93.6% (n = 636) and non-curative resection was 6.4% (n = 43). The independent risk factors for the non-curative resection were tumor location and the positivity of preoperative serum CA 19-9 and CA 125 levels. After curative resection, the independent prognostic risk factors for recurrence in curative resection were gender, stage, and preoperative increased serum CA 125 level (HR = 2.431, P =0.020), in a multivariate analysis.
CONCLUSION: Preoperative CA 125 is a useful predictive biomarker for curative resection and prognostic biomarker for recurrence in gastric cancer patients.
Keywords: Gastric Cancer, Tumor Marker, Carcinoembryonic antigen, Carbohydrate antigen 19-9, Carbohydrate antigen 125
Core tip: Tumor marker such as carcinoembryonic antigen, Carbohydrate antigen (CA) 19-9, and CA 125 in gastric cancer are usual tools for predicting prognosis or monitoring. The aim of this study was to investigate the correlation among tumor markers, curative resection, and recurrence in gastric cancer. Our data showed that preoperative CA 19-9 and CA 125 are independent risk factor of non-curative operation. And preoperative CA 125 is independent risk factor for recurrence after curative operation. Preoperative CA 125 is considered useful marker for predicting curative operation and prediction of recurrence after curative resection in gastric cancer patients.
INTRODUCTION
Serum tumor marker is a simple and convenient study for predicting prognosis or monitoring, and it is widely being used in a gastrointestinal malignancy[1]. Carcinoembryonic antigen (CEA) is a glycoprotein that is often elevated in the serum of patients with variety malignancies such as gastric, pancreatic, colorectal, breast and lung cancer[2]. Carbohydrate antigen 19-9 (CA 19-9)is an incomplete glycolipid antigen of the Lewis blood group, and it can be increased in colorectal, liver, ovarian, bile duct and gastric cancer[3]. CEA and CA 19-9 are known prognostic risk factors in gastric cancer[4,5]. In American Joint Committee on Cancer (AJCC) 7th edition, CEA and CA 19-9 been recognized as prognostic factors, but Cancer antigen 125 (CA 125) is not[6]. CA 125 is a heterogeneous cell membrane glycoprotein and it is related with malignant conditions such as ovarian, uterine, lung, or pancreatic cancers[7]. CEA is related to liver metastasis, peritoneal metastasis, histologic type and CA 19-9 is related to T, N stage, and peritoneal dissemination[8]. CA 125 is related to peritoneal dissemination[9]. Peritoneal dissemination, after curative gastrectomy with extended lymphadectomy, is most common recurrence pattern in the east[10]. As diagnosis of peritoneal dissemination is not high as that of distant metastasis and direct invasion of adjacent organs in preoperative image studies[11], surgeons face to unforeseen non-curative operation. It has been reported that CA 125 is related with peritoneal dissemination in gastric cancer[9], but there were few or no studies concerning the correlation between CA 125 and prognosis.
This study was to clarify prognostic value of preoperative CA 125 for prognostic biomarker, and to investigate the correlation between tumor markers (CEA, CA 19-9 and CA 125) and prediction of curative resection in gastric cancer patients.
MATERIALS AND METHODS
A total of 679 gastric cancer patients admitted through the outpatient department of surgery from 2000 to 2009 were enrolled. All enrolled patients checked tumor marker before gastric operation and follow-up performed biannually after gastric operation. Preoperative measurement of CEA, CA19-9, and CA 125 were performed by radioimmunoassay. The normal ranges for CEA, CA 19-9, and CA 125 were: < 5 ng/mL, 25 U/mL, and 37 U/mL, respectively. We excluded remnant gastric cancer, synchronous primary malignancy, or gastric cancer with neo-adjuvant therapy. Recurrence pattern was classified into four categories: loco-regional recurrence, peritoneal dissemination, hematogenous and distant lymph node. We performed gastric cancer operation according to Japanese gastric cancer treatment guidelines[12]. Pathologic staging was conducted after the operation according to the AJCC sixth edition[13]. Non-curative operations were defined as microscopically or macroscopically residual tumor after gastric operation.
We retrospectively analyzed the relationship among a non-curative operation, recurrence and tumor marker. We employed SPSS 20.0 for Windows for statistical analyses (SPSS, Inc., Chicago, IL, United States). The χ2 and Fisher’s exact tests were used to assess clinical and pathological characteristics for univariated analysis and logistic regression test for multivariate analsysis. The disease free survival was analyzed using the Kaplan-Meier method, and significance testing was performed with the log-rank test. The Cox proportional hazards model was used for multivariate analysis. Differences with P values less than 0.05 (P < 0.05) were considered statistically significant.
RESULTS
Of the 679 patients, 447 patients were male and 232 patients were female with a mean age of 60.7 ± 11.4 years, and the median follow-up period was 32.4 mo. Forty-three patients (6.3%) received non-curative operations. The main causes of a non-curative operations were peritoneal dissemination (n = 21) followed by direct invasion (n = 16) (Table 1). Risk factors for non-curative operation were tumor location, CEA, CA 19-9, and CA 125 in univariate analysis. In a multivariate analysis, location of tumor (HR = 21.303; P < 0.001), CA 19-9 positivity (HR = 5.883; P < 0.001), and CA 125 positivity (HR = 15.549; P < 0.001) were independent risk factors for a non-curative operation. (Table 2) Recurrences after curative operation were 124 cases among 636 patients, and 64 patients had two or more recurrence site; hematogenous (n = 72), peritoneal (n = 50), loco-regional (n = 46) and distant lymph node metastases (n = 41). The 5-year disease-free survival rate after curative operation was 77.9%, and the 5-year disease-free survival rate of CEA, CA 19-9 and CA 125 are 51.3%, 51.7% and 30.8%, respectively (Figure 1). Risk factors of recurrence were tumor location, differentiation, lymphovascular invasion, perineural invasion, stage, and CEA, CA 19-9, and CA 125. In a multivariate analysis, gender, stage, and positivity of CA 125 (HR = 2.431; P = 0.020) were independent risk factors for recurrence (Table 3).
Table 1.
Causes for a non-curative operations
| Peritoneal dissemination | Direct invasion | Distant metastasis | Incomplete resection | Total | |
| Peritoneal dissemination | 15 | 3 | 3 | 21 | |
| Direct invasion | 3 | 13 | 16 | ||
| Distant metastasis | 3 | 3 | 6 | ||
| Incomplete resection | 6 | 6 | |||
| Total | 21 | 16 | 6 | 6 | 49 |
Table 2.
Univariate and multivariate analysis of the risk factors for a non-curative operations n (%)
| Variables |
Univariate analysis |
Multivariate analysis |
|||
| Curative | Non-curative | P value | HR (95%CI) | P value | |
| Age, yr | 0.189 | 0.238 | |||
| < 60 | 272 (95.1) | 14 (4.9) | |||
| ≥ 60 | 364 (92.6) | 29 (7.4) | 1.599 (0.733-3.487) | ||
| Sex | 0.574 | 0.642 | |||
| Male | 417 (93.3) | 30 (6.7) | |||
| Female | 219 (94.4) | 13 (5.6) | 0.832 (0.384-1.803) | ||
| Differentiation | 0.080 | 0.082 | |||
| Differentiated | 382 (95.0) | 20 (5.0) | |||
| Undifferentiated | 254 (91.7) | 23 (8.3) | 1.937 (0.919-4.085) | ||
| Location | < 0.001 | < 0.001 | |||
| Lower 1/3 | 359 (93.2) | 26 (6.8) | |||
| Middle 1/3 | 212 (97.2) | 6 (2.8) | 0.334 (0.120-0.928) | 0.036 | |
| Upper 1/3 | 60 (90.9) | 6 (9.1) | 1.223 (0.421-3.555) | 0.711 | |
| Whole | 5 (50.0) | 5 (50.0) | 21.303 (4.985-91.036) | < 0.001 | |
| CEA | 0.038 | ||||
| Negative | 579 (94.3) | 35 (5.7) | 0.792 | ||
| Positive | 57 (87.7) | 8 (12.3) | 1.142 (0.427-3.056) | ||
| CA 19-9 | < 0.001 | < 0.001 | |||
| Negative | 586 (95.6) | 27 (4.4) | |||
| Positive | 50 (75.8) | 16 (24.2) | 5.883 (2.569-13.474) | ||
| CA 125 | < 0.001 | < 0.001 | |||
| Negative | 616 (95.7) | 28 (4.3) | |||
| Positive | 20 (57.1) | 15 (42.9) | 15.549 (6.473-37.352) | ||
CEA: Carcinoembryonic antigen; CA: Carbohydrate antigen.
Figure 1.
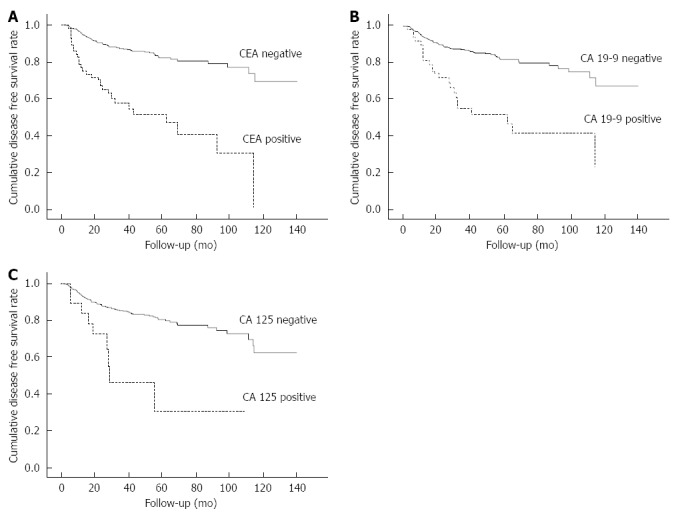
Disease free survival curve according to positivity of Carcinoembryonic antigen (A), Carbohydrate antigen 19-9 (B) and Carbohydrate antigen 125 (C).
Table 3.
Univariateand multivariate analysis of prognostic risk factors for disease-free survival after curative operations
| Variables |
Univariate analysis |
Multivariate analysis |
||
| 5-year DFS | P value | HR (95%CI) | P value | |
| Age, yr | 0.865 | 0.282 | ||
| < 60 (n =272) | 78.3% | |||
| ≥ 60 (n = 364) | 80.1% | 1.250 (0.833-1.877) | ||
| Sex | 0.194 | 0.037 | ||
| Male (n = 417) | 78.3% | |||
| Female (n = 219) | 81.7% | 0.616 (0.391-0.971) | ||
| Differentiation | 0.020 | 0.292 | ||
| Differentiated (n = 382) | 81.6% | |||
| Undifferentiated (n = 254) | 75.6% | 0.797 ( 0.524-1.215) | ||
| Location | 0.004 | 0.454 | ||
| Lower 1/3 (n = 359) | 76.0% | |||
| Middle 1/3 (n = 212) | 86.8% | 0.797 (0.489-1.297) | ||
| Upper 1/3 (n = 60) | 70.8% | 1.172 (0.643-2.135) | ||
| Whole (n = 5) | 40.0% | 0.487 (0.138-1.720) | ||
| Lymphovascular invasion | < 0.001 | 0.709 | ||
| Negative (n = 513) | 81.8% | |||
| Positive (n = 123) | 69.4% | 1.085 (0.708-1.662) | ||
| Perineural invasion | < 0.001 | 0.072 | ||
| Negative (n = 582) | 81.4% | |||
| Positive (n = 54) | 54.4% | 1.629 (0.958-2.770) | ||
| Stage | < 0.001 | < 0.001 | ||
| IA (n = 292) | 98.7% | |||
| IB (n = 102) | 89.4% | 6.613 (1.703-25.674) | 0.006 | |
| II (n = 75) | 79.8% | 15.415 (4.297-55.300) | < 0.001 | |
| IIIA (n = 69) | 49.5% | 43.857 (12.999-147.966) | < 0.001 | |
| IIIB (n = 39) | 46.2% | 66.090 (18.643-234.289) | < 0.001 | |
| IV (n = 59) | 32.7% | 93.720 (27.252-319.103) | < 0.001 | |
| CEA | < 0.001 | 0.073 | ||
| Negative (n = 579) | 82.1% | 1.559 (0.959-2.535) | ||
| Positive (n = 57) | 51.3% | |||
| CA 19-9 | < 0.001 | 0.694 | ||
| Negative (n = 586) | 81.7% | |||
| Positive (n = 50) | 51.7% | 0.901 (0.535-1.516) | ||
| CA 125 | < 0.001 | 0.020 | ||
| Negative (n = 616) | 80.6% | |||
| Positive (n = 20) | 30.8% | 2.431 (1.153-5.123) | ||
DISCUSSION
Depth of invasion and lymph node metastasis were most important independent prognostic risk factors in gastric cancer, and currently used TNM stage is useful to predicting survival rate in each stage. However, it was difficult to estimate N stage, to predict survival rate in each stage and to decide treatment of modality in preoperative clinical TNM stage. Many prognostic risk factors of gastric cancer were investigated, and tumor marker is one of the prognostic factors of gastric cancer. CEA and CA 19-9 are the most common tumor markers for predicting prognosis in gastric cancer[14]. The sensitivities of CEA and CA 19-9 in gastric cancer are 16%-58.4% and 34.1%-64.9%[15-17]. Preoperative positivity for CEA and CA 19-9 is associated with a poor prognosis[4,5,18], but there is a few report for preoperative CA 125 in gastric cancer. The preoperative CEA and CA 19-9 were not prognostic factor for palliative gastric surgery, but it was independent prognostic factors in curative surgery[4]. The postoperative CEA positivity in early gastric cancer and postoperative CEA and CA 72-4 positivity in advanced gastric cancer were independent prognostic factors[18]. In our study, the preoperative CEA and CA 19-9 were not independent prognostic risk factors for recurrence as other study[19], interestingly preoperative CA 125 was an independent risk factor for recurrence with a HR of 2.431. In early stage (data was not shown), no recurrence observed in the patients with positivity of CA 125 in stage IA (n = 2), but there were two cases of recurrences among 4 patients with positivity of CA 125 in stage IB, one is peritoneal carcinomatosis, and the other is loco-regional recurrence, respectively. We think that this finding may be related with recurrence patterns of gastric cancer. The sensitivity of CA 125 is 6%-31.6%[20,21], and it is related with peritoneal metastasis[22]. In the Japan Clinical Oncology Group (JCOG9501) trial, recurrence patterns were peritoneal recurrence, regional lymph node recurrence, hepatic, and others were: 38.1%, 21.9%, 20.9%, and 19.7%, respectively[23]. In Korean study, recurrent patterns were observed according to time period. Hematogenous recurrence was the most common recurrent pattern in the 1990s, whereas peritoneal recurrence was the most common recurrent pattern in the 2000s. Furthermore, peritoneal dissemination was the most frequent recurrence pattern (32.1%) during the entire period[10]. In our study, peritoneal dissemination and hematogenous metastasis were the main recurrence patterns. The positivity of CA 125 may be reflected recurrent patterns of peritoneal dissemination.
In our study, stage I and II are 61.8%, this is why non-curative operation smaller (6.4%) than other studies (17.9%-35.8%)[24,25]. In general, diagnostic accuracy of preoperative T stage in gastric cancer is 65%-92.1%[26]. and sensitivity and specificity of hematogenous metastasis especially liver are 87.5% and 99.0%[27]. Diagnostic accuracy of preoperative for peritoneal metastasis is 30%-100%[27], but sensitivity is only 28.8%[11]. Diagnostic laparoscopy maybe useful for border line peritoneal dissemination in image study. Kapiev et al[28] reported that 29.5% of patients with borderline unresectable gastric cancer were diagnosed as peritoneal metastasis by diagnostic laparoscopy. In our study, the proportion of non-curative resection with peritoneal dissemination is low (3.1%), according to our data, diagnostic laparoscopy is not necessary even in far advanced gastric cancer. Peritoneal metastasis is the main cause of non-curative operation in our study and to avoid unnecessary operation precise diagnosis of peritoneal dissemination is important. In general, tumor markers (CEA, CA 19-9 and CA 125) are related with peritoneal dissemination[8,9,22]. There is a few report of relationship between curative resection and preoperative tumor marker. Pectasides et al[16] reported that the sensitivity of CEA and CA 19-9 in inoperable or metastatic disease gastric cancer were 48.6% and 64.9%, retrospectively. As compared to our study, their study did not checked tumor marker preoperative, and there was no data of CA 125. In our study, 24.2% and 42.9% of patients with increased levels of CA 19-9 and CA 125 received a non-curative operation. The HRs of CA 19-9 and CA 125 for non-curative operations were 4.153 and 10.796, each representing statistically significant levels. We think that the positivity of CA 19-9 and CA 125 may reflect peritoneal dissemination. Thus, it would be useful biomarker to avoid unnecessary laparotomy for patients with borderline resectability on preoperative imaging test if they show increased CA 19-9 and CA 125 levels.
In far advanced gastric cancer, the positivity of CA 19-9 and CA 125 showed a higher frequency of receiving non-curative operations, a more careful approach is necessary. Additionally, a more aggressive treatment is required even if a curative operation is performed, as preoperative increased CA 125 is related to possible recurrence.
COMMENTS
Background
Preoperative image study is a useful diagnostic tool for staging in gastric cancer. But its role in resectability and prognosis in a part of gastric cancer patients is questionable. The aim of this study was to investigate the correlation among tumor markers especially carbohydrate antigen (CA) 125, curative resection, and recurrence in gastric cancer.
Research frontiers
CA 125 is related to peritoneal dissemination, and it has been reported that CA 125 is related with peritoneal dissemination in gastric cancer. However, the clinical significant of CA 125 in gastric cancer is not clarified. The current hot spot is to clarify the role of CA 125 in gastric cancer.
Innovations and breakthroughs
Previous studies showed that CA 125 is related with peritoneal carcinomatosis in gastric cancer. But, there were few or no studies concerning the relation between preoperative CA 125 and curative operation or prognosis. In this study, the preoperative CA 125 is related with non-curative operation and poor prognosis.
Applications
In far advanced gastric cancer, the positivity of CA 125 showed a higher frequency of receiving non-curative operations, a more careful approach is necessary. Additionally, a more aggressive treatment is required even if a curative operation is performed, as preoperative increases in CA 125 are related to possible recurrence.
Peer review
This is a retrospective study that analyzes clinical significance of tumor marker in gastric cancer. The results are interesting and show that the positivity of preoperative CA 125 associated with non-curative operations and poor prognosis. This finding suggest that CA 125 is a useful tumor marker predicting non-curative operation and poor prognosis.
Footnotes
Supported by Research grant of Chungbuk National University in 2013.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: June 28, 2014
First decision: August 6, 2014
Article in press: September 30, 2014
P- Reviewer: Koukourakis GV, Mura B S- Editor: Qi Y L- Editor: A E- Editor: Wang CH
References
- 1.Grem J. The prognostic importance of tumor markers in adenocarcinomas of the gastrointestinal tract. Curr Opin Oncol. 1997;9:380–387. doi: 10.1097/00001622-199709040-00012. [DOI] [PubMed] [Google Scholar]
- 2.Fletcher RH. Carcinoembryonic antigen. Ann Intern Med. 1986;104:66–73. doi: 10.7326/0003-4819-104-1-66. [DOI] [PubMed] [Google Scholar]
- 3.Lamerz R. Role of tumour markers, cytogenetics. Ann Oncol. 1999;10 Suppl 4:145–149. [PubMed] [Google Scholar]
- 4.Reiter W, Stieber P, Reuter C, Nagel D, Cramer C, Pahl H, Fateh-Moghadam A. Prognostic value of preoperative serum levels of CEA, CA 19-9 and CA 72-4 in gastric carcinoma. Anticancer Res. 1997;17:2903–2906. [PubMed] [Google Scholar]
- 5.Marrelli D, Roviello F, De Stefano A, Farnetani M, Garosi L, Messano A, Pinto E. Prognostic significance of CEA, CA 19-9 and CA 72-4 preoperative serum levels in gastric carcinoma. Oncology. 1999;57:55–62. doi: 10.1159/000012001. [DOI] [PubMed] [Google Scholar]
- 6.American Joint of Commutee on Cancer. AJCC cancer staging manual 7th edition. New York: Springer; 2010. pp. 117–121. [Google Scholar]
- 7.Cragun JM. Screening for ovarian cancer. Cancer Control. 2011;18:16–21. doi: 10.1177/107327481101800103. [DOI] [PubMed] [Google Scholar]
- 8.Ucar E, Semerci E, Ustun H, Yetim T, Huzmeli C, Gullu M. Prognostic value of preoperative CEA, CA 19-9, CA 72-4, and AFP levels in gastric cancer. Adv Ther. 2008;25:1075–1084. doi: 10.1007/s12325-008-0100-4. [DOI] [PubMed] [Google Scholar]
- 9.Nakata B, Hirakawa-YS Chung K, Kato Y, Yamashita Y, Maeda K, Onoda N, Sawada T, Sowa M. Serum CA 125 level as a predictor of peritoneal dissemination in patients with gastric carcinoma. Cancer. 1998;83:2488–2492. doi: 10.1002/(sici)1097-0142(19981215)83:12<2488::aid-cncr12>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 10.Kim DH, Kim SM, Hyun JK, Choi MG, Noh JH, Sohn TS, Bae JM, Kim S. Changes in postoperative recurrence and prognostic risk factors for patients with gastric cancer who underwent curative gastric resection during different time periods. Ann Surg Oncol. 2013;20:2317–2327. doi: 10.1245/s10434-012-2700-0. [DOI] [PubMed] [Google Scholar]
- 11.Leake PA, Cardoso R, Seevaratnam R, Lourenco L, Helyer L, Mahar A, Law C, Coburn NG. A systematic review of the accuracy and indications for diagnostic laparoscopy prior to curative-intent resection of gastric cancer. Gastric Cancer. 2012;15 Suppl 1:S38–S47. doi: 10.1007/s10120-011-0047-z. [DOI] [PubMed] [Google Scholar]
- 12.Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3) Gastric Cancer. 2011;14:113–123. doi: 10.1007/s10120-011-0042-4. [DOI] [PubMed] [Google Scholar]
- 13.Greene FL, Page DL, Fleming ID, Fritz AG, Balch CM, Haller DG, Morrow M. AJCC Cancer Staging Manual. 6th ed. New York: Springer-Verlag; 2002. pp. 89–99. [Google Scholar]
- 14.Hur H, Song KY, Park CH, Jeon HM. Follow-up strategy after curative resection of gastric cancer: a nationwide survey in Korea. Ann Surg Oncol. 2010;17:54–64. doi: 10.1245/s10434-009-0676-1. [DOI] [PubMed] [Google Scholar]
- 15.Heptner G, Domschke S, Domschke W. Comparison of CA 72-4 with CA 19-9 and carcinoembryonic antigen in the serodiagnostics of gastrointestinal malignancies. Scand J Gastroenterol. 1989;24:745–750. doi: 10.3109/00365528909093116. [DOI] [PubMed] [Google Scholar]
- 16.Pectasides D, Mylonakis A, Kostopoulou M, Papadopoulou M, Triantafillis D, Varthalitis J, Dimitriades M, Athanassiou A. CEA, CA 19-9, and CA-50 in monitoring gastric carcinoma. Am J Clin Oncol. 1997;20:348–353. doi: 10.1097/00000421-199708000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Filella X, Fuster J, Molina R, Grau JJ, García-Valdecasas JC, Grande L, Estapé J, Ballesta AM. TAG-72, CA 19.9 and CEA as tumor markers in gastric cancer. Acta Oncol. 1994;33:747–751. doi: 10.3109/02841869409083943. [DOI] [PubMed] [Google Scholar]
- 18.Kim DH, Oh SJ, Oh CA, Choi MG, Noh JH, Sohn TS, Bae JM, Kim S. The relationships between perioperative CEA, CA 19-9, and CA 72-4 and recurrence in gastric cancer patients after curative radical gastrectomy. J Surg Oncol. 2011;104:585–591. doi: 10.1002/jso.21919. [DOI] [PubMed] [Google Scholar]
- 19.Gaspar MJ, Arribas I, Coca MC, Díez-Alonso M. Prognostic value of carcinoembryonic antigen, CA 19-9 and CA 72-4 in gastric carcinoma. Tumour Biol. 2001;22:318–322. doi: 10.1159/000050633. [DOI] [PubMed] [Google Scholar]
- 20.He CZ, Zhang KH, Li Q, Liu XH, Hong Y, Lv NH. Combined use of AFP, CEA, CA125 and CAl9-9 improves the sensitivity for the diagnosis of gastric cancer. BMC Gastroenterol. 2013;13:87. doi: 10.1186/1471-230X-13-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lai IR, Lee WJ, Huang MT, Lin HH. Comparison of serum CA72-4, CEA, TPA, CA19-9 and CA125 levels in gastric cancer patients and correlation with recurrence. Hepatogastroenterology. 2002;49:1157–1160. [PubMed] [Google Scholar]
- 22.Hwang GI, Yoo CH, Sohn BH, Shin JH, Park YL, Kim HD, Kim YS, Han WK, Pae WK. Predictive value of preoperative serum CEA, CA19-9 and CA125 levels for peritoneal metastasis in patients with gastric carcinoma. Cancer Res Treat. 2004;36:178–181. doi: 10.4143/crt.2004.36.3.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sasako M, Sano T, Yamamoto S, Kurokawa Y, Nashimoto A, Kurita A, Hiratsuka M, Tsujinaka T, Kinoshita T, Arai K, et al. D2 lymphadenectomy alone or with para-aortic nodal dissection for gastric cancer. N Engl J Med. 2008;359:453–462. doi: 10.1056/NEJMoa0707035. [DOI] [PubMed] [Google Scholar]
- 24.Huang KH, Wu CW, Fang WL, Chen JH, Lo SS, Wang RF, Li AF. Palliative resection in noncurative gastric cancer patients. World J Surg. 2010;34:1015–1021. doi: 10.1007/s00268-010-0467-7. [DOI] [PubMed] [Google Scholar]
- 25.Wang CS, Chao TC, Jan YY, Jeng LB, Hwang TL, Chen MF. Benefits of palliative surgery for far-advanced gastric cancer. Chang Gung Med J. 2002;25:792–802. [PubMed] [Google Scholar]
- 26.Kwee RM, Kwee TC. Imaging in local staging of gastric cancer: a systematic review. J Clin Oncol. 2007;25:2107–2116. doi: 10.1200/JCO.2006.09.5224. [DOI] [PubMed] [Google Scholar]
- 27.D’Elia F, Zingarelli A, Palli D, Grani M. Hydro-dynamic CT preoperative staging of gastric cancer: correlation with pathological findings. A prospective study of 107 cases. Eur Radiol. 2000;10:1877–1885. doi: 10.1007/s003300000537. [DOI] [PubMed] [Google Scholar]
- 28.Kapiev A, Rabin I, Lavy R, Chikman B, Shapira Z, Kais H, Poluksht N, Amsalam Y, Halpern Z, Markon I, et al. The role of diagnostic laparoscopy in the management of patients with gastric cancer. Isr Med Assoc J. 2010;12:726–728. [PubMed] [Google Scholar]


