Abstract
AIM: To study the “hospital type-outcome” and “volume-outcome” relationships in patients with esophageal cancer who receive non-surgical treatments.
METHODS: A total of 6106 patients with esophageal cancer diagnosed between 2008 and 2011 were identified from a national population-based cancer registry in Taiwan. The hospital types were defined as medical center and non-medical center. The threshold for high-volume hospitals was based on a median volume of 225 cases between 2008 and 2011 (annual volume, > 56 cases) or an upper quartile (> 75%) volume of 377 cases (annual volume > 94 cases). Cox regression analyses were used to determine the effects of hospital type and volume outcome on patient survival.
RESULTS: A total of 3955 non-surgically treated patients were included in the survival analysis. In the unadjusted analysis, the significant prognostic factors included cT, cN, cM stage, hospital type and hospital volume (annual volume, > 94 vs ≤ 94). The 1- and 3-year overall survival rates in the non-medical centers (36.2% and 13.2%, respectively) were significantly higher than those in the medical centers (33.5% and 11.3%, respectively; P = 0.027). The 1- and 3-year overall survival rates in hospitals with an annual volume of ≤ 94 (35.3% and 12.6%, respectively) were significantly higher than those with an annual volume of > 94 (31.1% and 9.4%, respectively; P = 0.001). However, in the multivariate analysis, the hospital type was not statistically significant. Only cT, cN, and cM stages and hospital volume (annual volume > 94 vs ≤ 94) were independent prognostic factors.
CONCLUSION: Whether the treatment occurs in medical centers is not a significant prognostic factor. High-volume hospitals were not associated with better survival rates compared with low-volume hospitals.
Keywords: Cancer registry, Esophageal cancer, Hospital volume, Hospital type, Survival
Core tip: The hospital type-outcome and volume-outcome relationships in patients with esophageal cancer who receive surgical resection are well established. However, little is known concerning the hospital type- and volume-outcome relationships in patients without surgical resection. Our population-based study, including 3955 non-surgically treated patients, showed that the medical center is not a significant prognostic factor. Moreover, the high-volume hospitals were not associated with better survival rates compared with the low-volume hospitals.
INTRODUCTION
Even though multidisciplinary approaches and several combinations of therapies, such as surgery, chemotherapy, and radiotherapy, have been applied to treat esophageal cancer, the prognosis of patients with esophageal cancer is poor. Moreover, a large number of patients develop either locoregional recurrence or distant metastasis shortly after curative treatments; the prognosis for these patients is dismal[1]. To improve outcome, centralized care for esophageal cancer patients has been proposed. Several authors have suggested that referring patients to specialized units that have healthcare professionals with adequate experience may improve the quality of care as well as patient survival[2-4]. Indeed, hospital type-outcome analyses have demonstrated better outcome in university hospitals. For example, Dikken et al[5] reported that the 3-mo mortality rate after esophagectomy was 2.5% in university hospitals and 4.4% in non-university teaching hospitals, which was a significant difference (P < 0.05). Moreover, Verhoef et al[6] reported that the 5-year survival rate for surgical patients was 49.2% for the university hospitals versus 32.6% for the teaching non-university hospitals and 27.3% for the non-teaching hospitals (P < 0.05). The results of the hospital volume-outcome analysis supported the impact of volume on patient survival. For example, Birkmeyer et al[7] analyzed the Surveillance, Epidemiology, and End Results database and reported an absolute difference in 5-year likelihood of survival rates after esophagectomy for cancer between the low-volume hospitals (17%) and the high-volume hospitals (34%). A recent meta-analysis also demonstrated a long-term survival benefit after esophageal cancer resection for the high-volume hospitals (HR = 0.82; 95%CI: 0.75-0.90) compared with their low-volume counterparts[8].
However, the majority of the reports focused on the effect of hospital type and volume among patients who had undergone esophagectomy. There are few studies concerning how hospital type and volume influence the survival rate in patients without surgical resection. Therefore, we aimed to study the differences in patient and tumor characteristics according to hospital type and volume categories in this population-based study. We emphasized whether hospital type or volume affected the prognosis in patients with esophageal cancer who received non-surgical treatments.
MATERIALS AND METHODS
The patient data were obtained from the Taiwan Cancer Registry, which is a national population-based cancer registration database organized and funded by the Health Promotion Administration, Ministry of Health and Welfare, the executive branch of the central government. The hospitals with greater than 50-bed capacity, which provide outpatient care and hospitalized cancer care, are recruited to participate in reporting all newly diagnosed malignant neoplasms to the registry. The data were collected and verified by cancer registrars at each hospital. The clinical details including sex, date of birth, date of hospitalization, care facilities, date of diagnosis, clinical stage, surgical method, surgical margin, pathological stage, treatment modality, radiation dose, and survival status were recorded. Using the International Classification of Diseases for Oncology (ICD-O-3) site codes (C15.0, C15.1, C15.2, C15.3, C15.4, C15.5, C15.8, and C15.9) and morphology codes (8052, 8070, 8071, 8072, 8073, 8074, 8076, 8077, 8083 and 8084), 6106 patients who were diagnosed with esophageal squamous cell carcinoma (ESCC) between January 1, 2008 and December 31, 2011 were identified. The treatment modalities included the following: (1) neoadjuvant chemoradiation followed by surgery (n = 850); (2) surgery alone (n = 679); (3) surgery followed by chemotherapy or/and radiotherapy (n = 622); (4) definitive chemoradiation (n = 3020); (5) radiotherapy alone (n = 442); (6) chemotherapy alone (n = 333); and (7) unknown (n = 160).
To study the hospital type-outcome relationship, the hospital types were defined as medical center and non-medical center according to Taiwan Joint Commission on Hospital Accreditation (http://www.tjcha.org.tw) based on the quality of process and outcome in healthcare performance. There are a total of 19 medical centers in Taiwan. To study the volume-outcome relationship, the hospitals were divided into quartiles (Q1-Q4) of total hospital volume between 2008 and 2011. The threshold for high-volume hospitals was based on the median (Q3-4, > 50%) volume of 225 cases between 2008 and 2011 (annual volume, > 56 cases) or upper quartile (Q4, > 75%) volume of 377 cases (annual volume, > 94 cases). A subset of 3955 patients who were treated without surgical resection was included in the outcome analysis. The outcome measures were 1- and 3-year overall survival. The survival time was defined as the number of days between the date of diagnosis and the date of death or the end of the study on December 31, 2012, whichever occurred first.
Statistical analysis
The categorical and continuous variables were compared using the χ2 test and Student’s t-test, respectively. The survival curves were plotted using the Kaplan-Meier method and were compared using the log-rank test. The differences in survival estimates were calculated using the Cox proportional hazards regression model, stratified for hospital type or volume, and adjusted for known prognostic factors. All of the statistical calculations were performed using SAS version 9.3 (SAS Institute, Inc, Cary, NC) and SPSS version 17.0 (SPSS Inc, Chicago, IL). P less than 0.05 was considered to be statistically significant.
RESULTS
The characteristics of patients according to hospital type are presented in Table 1. A total of 6106 patients received treatments for ESCC in 62 hospitals, whereas 4180 (68.5%) of 6106 patients were treated in 19 medical centers and 1926 patients (31.5%) were treated in non-medical centers. The patients who were treated in medical centers were more likely to be older than those treated in non-medical centers (57.45 ± 11.4 years vs 56.89 ± 11.6 years). As for tumor characteristics, a higher percentage of patients with advanced stage tumors was found in medical centers. The patients who were treated in medical centers had tumors of larger size (5.4 ± 3.0 cm vs 5.1 ± 3.2 cm, P = 0.006), and a higher frequency was noted to be cT3/4 (71.5% vs 69.8%, P = 0.009) and clinical node-positive tumors (75.1% vs 69.9%, P < 0.001). Furthermore, a higher proportion of the patients in medical centers than in non-medical centers received surgical resection (36.5% vs 32.6%, P = 0.009).
Table 1.
Patient characteristics according to hospital type n (%)
| Variables | Total |
Medical center or not |
||
| Yes | No | P value | ||
| Patient number | 6106 | 4180 | 1926 | |
| Hospital number | 62 | 19 | 43 | |
| Sex | 0.963 | |||
| Male | 5768 (94.5) | 3949 (94.5) | 1819 (94.4) | |
| Female | 338 (5.5) | 231 (5.5) | 107 (5.6) | |
| Age (yr) | < 0.001 | |||
| < 40 | 189 (3.1) | 133 (3.2) | 56 (2.9) | |
| 40-49 | 1413 (23.1) | 908 (21.7) | 505 (26.2) | |
| 50-59 | 2272 (37.1) | 1577 (37.7) | 695 (36.1) | |
| 60-69 | 1278 (20.9) | 915 (21.9) | 363 (18.9) | |
| 70-79 | 658 (10.8) | 433 (10.4) | 225 (11.7) | |
| ≥ 80 | 296 (4.9) | 214 (5.1) | 82 (4.3) | |
| Tumor length (cm) | 0.006 | |||
| mean ± SD | 5.3 ± 3.1 | 5.4 ± 3.0 | 5.1 ± 3.2 | |
| cT stage | 0.009 | |||
| 1 | 531 (8.7) | 340 (8.1) | 191 (9.9) | |
| 2 | 838 (13.7) | 544 (13.0) | 294 (15.3) | |
| 3 | 2994 (49.0) | 2086 (49.9) | 908 (47.1) | |
| 4 | 1339 (21.9) | 901 (21.6) | 438 (22.7) | |
| Unknown | 404 (6.6) | 309 (7.4) | 95 (4.9) | |
| cN stage | < 0.001 | |||
| 0 | 1385 (22.7) | 862 (20.6) | 523 (27.2) | |
| 1/2/3 | 4488 (73.5) | 3141 (75.1) | 1347 (69.9) | |
| Unknown | 233 (3.8) | 177 (4.2) | 56 (2.9) | |
| cM stage | 0.424 | |||
| 0 | 4261 (69.8) | 2931 (70.1) | 1330 (69.1) | |
| 1 | 1731 (28.4) | 1167 (27.9) | 564 (29.3) | |
| Unknown | 114 (1.9) | 82 (2.0) | 32 (1.7) | |
| Tumor location | < 0.001 | |||
| Upper third | 1445 (23.7) | 936 (22.4) | 509 (26.4) | |
| Middle third | 2152 (35.2) | 1390 (33.3) | 762 (39.6) | |
| Lower third | 1134 (186) | 758 (18.1) | 376 (19.5) | |
| Unknown | 1375 (22.5) | 1096 (26.2) | 279 (14.5) | |
| Tumor differentiation | < 0.001 | |||
| Well | 150 (2.5) | 108 (2.6) | 42 (2.2) | |
| Moderate | 2759 (45.2) | 1781 (42.6) | 978 (50.8) | |
| Poorly | 1309 (21.4) | 916 (21.9) | 393 (20.4) | |
| Unknown | 1888 (30.9) | 1375 (32.9) | 513 (26.6) | |
| Treatment modality | 0.009 | |||
| Surgery with neoadjuvant chemoradiation | 850 (13.9) | 610 (14.6) | 240 (12.5) | |
| Surgery without neoadjuvant chemoradiation | 1301 (21.3) | 914 (21.9) | 387 (20.1) | |
| No surgery | 3955 (64.8) | 2656 (63.5) | 1299 (67.5) | |
| Hospital volume | < 0.001 | |||
| Q1-Q2 | 3137 | 1504 (36.0) | 1633 (84.8) | |
| Q3-Q4 | 2969 | 2676 (64.0) | 293 (15.2) | |
| Hospital volume | < 0.001 | |||
| Q1-Q3 | 4717 | 2791 (66.8) | 1926 (100.0) | |
| Q4 | 1389 | 1389 (33.2) | - | |
Table 2 presents the characteristics of patients according to hospital volume. There were 8 hospitals, including 7 medical centers, in quartiled 3-4, whereas only 3 medical centers were in quartile 4. There was no difference in the distribution of age and sex between high- and low-volume hospitals. However, the patients who were treated in high-volume hospitals had tumors of larger size (Q4 vs Q1-3: 5.7 ± 3.0 cm vs 5.2 ± 3.0 cm, P < 0.001), a higher frequency was noted to be cT3/4 (Q4 vs Q1-3: 78.7% vs 68.7%, P < 0.001) and clinical node-positive tumors (Q4 vs Q1-3: 82.7% vs 70.8%, P < 0.001). There was also a higher proportion of patients in high-volume hospitals than in low-volume hospitals who received surgical resections (Q4 vs Q1-3: 41.1% vs 33.5%, P < 0.001).
Table 2.
Patient characteristics according to hospital volume n (%)
| Variables |
Hospital volume |
Hospital volume |
||||
| Q1-Q2 | Q3-Q4 | P value | Q1-Q3 | Q4 | P value | |
| No. of annual cases | ≤ 225 | > 225 | ≤ 377 | > 377 | ||
| No. of patients | 3137 | 2969 | 4717 | 1389 | ||
| No. of hospitals | 54 | 8 | 59 | 3 | ||
| Sex | 0.295 | 0.295 | ||||
| Male | 2954 (94.2) | 2814 (94.8) | 4465 (94.7) | 1303 (93.8) | ||
| Female | 183 (5.8) | 155 (5.2) | 252 (5.3) | 86 (6.2) | ||
| Age (yr) | 0.182 | 0.56 | ||||
| < 40 | 88 (2.8) | 101 (3.4) | 142 (3.0) | 47 (3.3) | ||
| 40-49 | 715 (22.8) | 698 (23.5) | 1108 (23.3) | 305 (22.0) | ||
| 50-59 | 1194 (36.9) | 1078 (36.3) | 1762 (37.1) | 510 (36.7) | ||
| 60-69 | 629 (20.1) | 649 (21.9) | 976 (20.6) | 302 (21.8) | ||
| 70-79 | 352 (11.3) | 306 (10.3) | 496 (10.5) | 162 (11.7) | ||
| ≥ 80 | 159 (5.1) | 137 (4.6) | 233 (4.9) | 63 (4.5) | ||
| Tumor length (cm) | 0.845 | < 0.001 | ||||
| mean ± SD | 5.3 ± 3.2 | 5.3 ± 2.9 | 5.2 ± 3.0 | 5.7 ± 3.0 | ||
| cT stage | 0.005 | < 0.001 | ||||
| 1 | 245 (7.8) | 286 (9.6) | 412 (8.7) | 119 (8.6) | ||
| 2 | 466 (14.9) | 372 (12.5) | 711 (15.1) | 127 (9.1) | ||
| 3 | 1531 (48.8) | 1463 (49.3) | 2167 (45.9) | 827 (59.5) | ||
| 4 | 710 (22.6) | 629 (21.2) | 1073 (22.8) | 266 (19.2) | ||
| Unknown | 185 (5.9) | 219 (7.4) | 354 (7.5) | 50 (3.6) | ||
| cN stage | < 0.001 | < 0.001 | ||||
| 0 | 820 (26.1) | 565 (19.0) | 1183 (25.1) | 202 (14.5) | ||
| 1/2/3 | 2210 (70.5) | 2278 (76.7) | 3340 (70.8) | 1148 (82.7) | ||
| Unknown | 107 (3.4) | 126 (4.2) | 194 (4.1) | 39 (4.1) | ||
| cM stage | 0.002 | 0.014 | ||||
| 0 | 2177 (69.4) | 2084 (70.2) | 3257 (69.1) | 1004 (72.3) | ||
| 1 | 919 (29.3) | 812 (27.4) | 1362 (28.9) | 369 (26.6) | ||
| Unknown | 41 (1.3) | 73 (2.5) | 98 (2.1) | 16 (1.2) | ||
| Tumor location | < 0.001 | < 0.001 | ||||
| Upper third | 801 (25.5) | 644 (21.7) | 1192 (25.3) | 253 (18.2) | ||
| Middle third | 1223 (39.0) | 929 (31.3) | 1785 (37.8) | 367 (26.4) | ||
| Lower third | 600 (19.1) | 534 (18.0) | 894 (19.0) | 240 (17.3) | ||
| Unknown | 513 (16.4) | 862 (29.0) | 846 (17.9) | 529 (38.1) | ||
| Tumor differentiation | < 0.001 | < 0.001 | ||||
| Well | 76 (2.4) | 74 (2.5) | 128 (2.7) | 22 (1.6) | ||
| Moderate | 1611 (51.4) | 1148 (38.7) | 2256 (47.8) | 503 (36.2) | ||
| Poorly | 677 (21.6) | 632 (21.3) | 892 (18.9) | 417 (30.0) | ||
| Unknown | 773 (24.6) | 1115 (37.6) | 1441 (30.6) | 447 (32.2) | ||
| Treatment modality | < 0.001 | < 0.001 | ||||
| Surgery with neoadjuvant chemoradiation | 381 (12.1) | 469 (15.8) | 524 (11.1) | 326 (23.5) | ||
| Surgery without neoadjuvant chemoradiation | 643 (20.4) | 658 (22.2) | 1056 (22.4) | 245 (17.6) | ||
| No surgery | 2113 (67.4) | 1842 (62.0) | 3137 (66.5) | 818 (58.9) | ||
| Hospital type | < 0.001 | < 0.001 | ||||
| Medical center | 1504 (47.9) | 2676 (90.1) | 2791 (59.2) | 1389 (100.0) | ||
| Not center | 1633 (52.1) | 293 (9.9) | 1926 (40.8) | 0 | ||
Table 3 summarized the characteristics of patients according to hospital volume. There was lower frequency of clinical stage II (10.2% vs 15.4%) and higher frequency of stage III (44.6% vs 39.2%) patients in medical center compared to non-medical center hospital. There was also more clinical stage II (Q1-2 vs Q3-4: 14.1% vs 9.5%; Q1-3 vs Q4: 12.9% vs 9.3%) and less stage III (Q1-2 vs Q3-4: 40.5% vs 45.0%; Q1-3 vs Q4: 42.4% vs 43.9%) patients in low volume hospitals compared to high volume hospitals. Besides, the percentage of radiotherapy alone treatment was higher in non-medical center (14.1% vs 9.8% in medical center) hospitals and low volume (13.4% in Q-2 vs 8.7% in Q3-4; 12.5% in Q1-3 vs 7.6% in Q4) hospitals.
Table 3.
Patient characteristics according to hospital type and hospital volume in non-surgical treatment patients (n = 3955) n (%)
| Variables |
Medical center |
Hospital volume |
Hospital volume |
||||||
| Yes | No | P value | Q1-2 | Q3-4 | P value | Q1-3 | Q4 | P value | |
| Sex | NS | NS | NS | ||||||
| Male | 2523 (95.0) | 1220 (94.0) | 1917 (94.2) | 1826 (95.1) | 2845 (94.8) | 898 (94.2) | |||
| Female | 133 (5.0) | 79 (6.0) | 118 (5.8) | 94 (4.9) | 157 (5.2) | 55 (5.8) | |||
| Age (yr) | < 0.05a | NS | NS | ||||||
| < 40 | 84 (3.2) | 29 (2.2) | 50 (2.5) | 63 (3.3) | 83 (2.8) | 30 (3.1) | |||
| 40-49 | 536 (20.2) | 315 (24.2) | 425 (20.1) | 426 (22.2) | 636 (21.2) | 215 (22.6) | |||
| 50-59 | 957 (36.0) | 433 (33.3) | 723 (35.5) | 667 (34.7) | 1052 (35.0) | 338 (35.5) | |||
| 60-69 | 587 (22.1) | 255 (19.6) | 417 (20.5) | 425 (22.1) | 645 (21.5) | 197 (20.7) | |||
| 70-79 | 304 (11.4) | 187 (14.4) | 270 (13.3) | 221 (11.5) | 371 (12.4) | 120 (12.6) | |||
| ≥ 80 | 188 (7.1) | 80 (6.2) | 150 (7.4) | 118 (6.1) | 215 (7.2) | 53 (5.6) | |||
| Tumor location | NS | < 0.01d | NS | ||||||
| Upper third | 759 (28.6) | 430 (33.1) | 680 (33.4) | 509 (26.5) | 642 (21.4) | 174 (18.3) | |||
| Middle third | 841 (31.7) | 501 (38.6) | 773 (38.0) | 569 (29.6) | 1079 (35.9) | 263 (27.6) | |||
| Lower third | 336 (12.7) | 169 (13.0) | 222 (10.9) | 283 (14.7) | 694 (23.1) | 184 (19.3) | |||
| Unknown | 720 (27.1) | 199 (15.3) | 360 (17.7) | 559 (29.1) | 587 (19.6) | 332 (34.8) | |||
| Tumor differentiation | NS | < 0.05c | < 0.01d | ||||||
| Well | 50 (1.9) | 27 (2.1) | 43 (2.1) | 34 (1.8) | 66 (2.2) | 11 (1.1) | |||
| Moderate | 985 (37.1) | 577 (44.4) | 910 (44.7) | 652 (34.0) | 1176 (39.1) | 386 (40.5) | |||
| Poorly | 547 (20.1) | 264 (20.3) | 428 (21.0) | 383 (19.9) | 534 (17.8) | 277 (29.1) | |||
| Unknown | 1074 (40.4) | 431 (33.2) | 654 (32.1) | 851 (44.3) | 1226 (40.8) | 279 (29.3) | |||
| Clinical stage | < 0.01b | < 0.01d | < 0.05c | ||||||
| 0/I | 70 (2.7) | 67 (5.2) | 77 (3.6) | 60 (3.3) | 111 (3.9) | 26 (2.5) | |||
| II | 272 (10.2) | 200 (15.4) | 298 (14.1) | 174 (9.5) | 375 (12.9) | 97 (9.3) | |||
| III | 1184 (44.6) | 509 (39.2) | 865 (40.9) | 828 (45.0) | 1233 (42.4) | 460 (43.9) | |||
| IV | 1071 (40.3) | 507 (39.0) | 841 (39.8) | 737 (40.0) | 1138 (39.1) | 440 (42.0) | |||
| Unknown | 59 (2.2) | 16 (1.2) | 32 (1.5) | 43 (2.3) | 51 (1.8) | 24 (2.3) | |||
| Treatment modality | < 0.05a | < 0.01d | < 0.01d | ||||||
| Chemotherapy | 234 (8.8) | 99 (7.6) | 175 (8.3) | 158 (8.6) | 228 (7.8) | 105 (10.0) | |||
| Radiotherapy | 259 (9.8) | 183 (14.1) | 282 (13.4) | 160 (8.7) | 362 (12.5) | 80 (7.6) | |||
| Chemoradiation | 2040 (76.8) | 980 (75.4) | 1572 (74.4) | 1448 (78.6) | 2206 (75.9) | 814 (77.8) | |||
| Unknown | 123 (4.6) | 37 (2.9) | 84 (4.0) | 76 (4.1) | 112 (3.9) | 48 (4.6) | |||
| Stage-specific 3-yr survival | |||||||||
| Stage I | 33.44% | 48.35% | 41.81% | 43.03% | 42.23% | 45.86% | |||
| Stage II | 21.1% | 21.12% | 22.53% | 18.74% | 22.60% | 15.76% | |||
| Stage III | 11.84% | 14.11% | 12.61% | 12.32% | 12.61% | 12.03% | |||
| Stage IV | 6.6% | 5.05% | 6.2% | 6.03% | 6.04% | 6.36% | |||
P < 0.05, patients in medical center vs non-medical center hospital;
P < 0.01, patients in medical center vs non-medical center hospital;
P < 0.05, patients in low volume hospitals vs high volume hospitals;
P < 0.01, patients in low volume hospitals vs high volume hospitals. NS: Not significant.
In the survival analysis of 3955 patients with non-surgical treatments, the significant prognostic factors included cT stage, cN stage, cM stage, hospital type and hospital volume (Q4 vs Q1-3) (Table 4). The prognosis of patients without resection seemed to be better in the non-center hospitals (HR = 0.92, 95% CI: 0.86-0.99; P = 0.028). The Kaplan-Meier plot demonstrated that the 1- and 3-year overall survival rates in the non-medical centers (36.2% and 13.2%, respectively) were significantly higher than those in the medical centers (33.5% and 11.3%, respectively; P = 0.027) (Figure 1A). However, after adjustment for clinicopathological factors, the hospital type was not a significant prognostic factor of survival (P = 0.447) (Table 4, model 1). As for the volume-outcome analysis, there was no survival difference when comparing quartiles 3-4 to quartiles 1-2 (P = 0.315) (Table 4). The 1-/3-year overall survival rates were 33.8%/11.3%, respectively, in hospitals in quartiles 3-4 and 35.0%/12.6%, respectively, in hospitals in quartiles 1-2 (P = 0.315) (Figure 1B). However, when comparing hospitals in quartile 4 to quartiles 1-3, a significant survival benefit was noted in quartiles 1-3 (Table 4) (HR = 0.87; 95%CI: 0.80-0.94; P = 0.001). The Kaplan-Meier plot demonstrated that the 1- and 3-year overall survival rates in quartiles 1-3 (35.3% and 12.6%, respectively) were significantly higher than those in quartile 4 (31.1% and 9.4%, respectively; P = 0.001) (Figure 1C). After adjustment for age, sex and clinical tumor-node-metastasis (TNM) stage, hospital volume (Q1-3 vs Q4) remained statistically significant independent prognostic factors (HR = 0.91; 95%CI: 0.83-0.99; P = 0.028) (Table 3, model 4).
Table 4.
Cox regression analysis of the relationship between prognostic factors and outcome in patients without esophagectomy
| Variables | Univariate analysis |
Multivariate analysis |
||||||||||
|
Model 1 |
Model 2 |
Model 3 |
||||||||||
| HR | 95%CI | P value | HR | 95%CI | P value | HR | 95%CI | P value | HR | 95%CI | P value | |
| Age (yr) | ||||||||||||
| < 55 | 1 | - | 1 | - | ||||||||
| ≥ 55 | 0.94 | 0.88-1.01 | 0.091 | 0.96 | 0.90-1.03 | 0.288 | 0.96 | 0.90-1.03 | 0.297 | 0.96 | 0.90-1.04 | 0.322 |
| Sex | ||||||||||||
| Male | 1 | - | 1 | - | ||||||||
| Female | 0.86 | 0.73-1.00 | 0.053 | 0.9 | 0.76-1.06 | 0.19 | 0.9 | 0.76-1.05 | 0.185 | 0.89 | 0.76-1.05 | 0.176 |
| cT stage | ||||||||||||
| T1/2 | 1 | - | 1 | - | ||||||||
| T3/4 | 1.77 | 1.61-1.96 | < 0.001 | 1.55 | 1.40-1.73 | < 0.001 | 1.56 | 1.40-1.73 | < 0.001 | 1.55 | 1.39-1.73 | < 0.001 |
| cN stage | ||||||||||||
| N negative | 1 | - | 1 | - | ||||||||
| N positive | 1.64 | 1.48-1.81 | < 0.001 | 1.22 | 1.09-1.37 | 0.001 | 1.22 | 1.09-1.37 | 0.001 | 1.21 | 1.08-1.36 | 0.001 |
| cM stage | ||||||||||||
| 0 | 1 | - | 1 | - | ||||||||
| 1 | 1.67 | 1.56-1.80 | < 0.001 | 1.52 | 1.41-1.64 | < 0.001 | 1.52 | 1.41-1.64 | < 0.001 | 1.52 | 1.41-1.64 | < 0.001 |
| Hospital type | ||||||||||||
| Medical center | 1 | - | 1 | - | ||||||||
| Not center | 0.92 | 0.86-0.99 | 0.028 | 0.97 | 0.90-1.05 | 0.447 | ||||||
| Hospital volume | ||||||||||||
| Q3-4 | 1 | - | 1 | - | ||||||||
| Q1-2 | 0.97 | 0.90-1.03 | 0.315 | 0.99 | 0.92-1.06 | 0.694 | ||||||
| Hospital volume | ||||||||||||
| Q4 | 1 | - | ||||||||||
| Q1-3 | 0.87 | 0.80-0.94 | 0.001 | 0.91 | 0.83-0.99 | 0.028 | ||||||
Model 1: Impact of hospital type adjusted for age, sex and clinical tumor-node-metastasis (TNM) stages; Model 2: Impact of hospital volume (Q3-4 vs Q1-2) adjusted for age, sex and clinical TNM stages; Model 3: Impact of hospital volume (Q4 vs Q1-3) adjusted for age, sex and clinical TNM stages.
Figure 1.
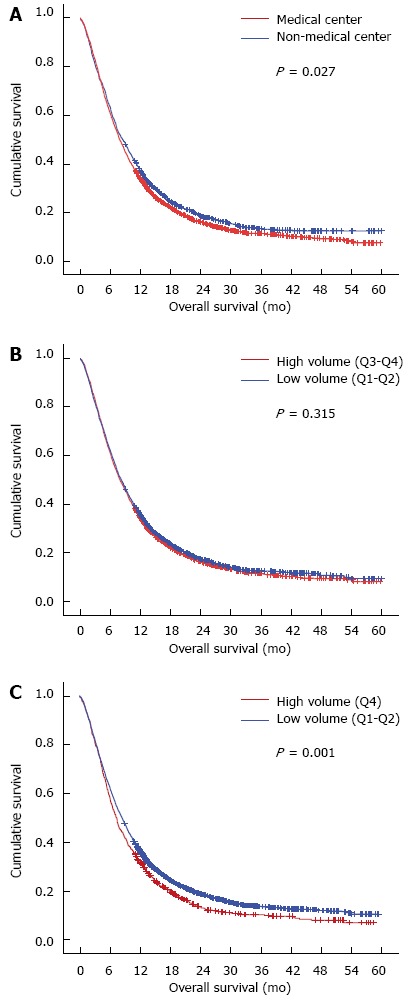
Kaplan-Meier plot. A: Showing that the overall survival was better in the non-medical centers (blue line) compared with the medical centers (red line) (P = 0.027, non-medical centers vs medical centers ); B: Showing that no overall survival difference was noted between the hospitals in Q3-4 (red line) and Q1-2 (blue line) (P = 0.315, Q3-4 vs Q1-2); C: Showing that the overall survival was better in the low-volume hospitals (Q1-3, blue line) compared with the very high-volume hospitals [Q4, red line (P = 0.001), low volume hospitals vs high volume hospitals].
DISCUSSION
Since Luft et al[9] published the first study on volume-outcome relationship in surgery in 1987, subsequent studies have investigated the volume-outcome relationship in esophageal cancer surgery[10-13]. The results of meta-analyses demonstrated an inverse correlation between hospital volume and short-term postoperative outcomes, including mortality and complication rates in esophageal cancer surgery[10-13]. Although the impact of hospital volume on long-term survival after esophagectomy is controversial, the recent meta-analysis by Brusselaer et al[8] showed that high-volume surgery results in better long-term survival than low-volume surgery. To promote high-value health care, the Leapfrog Group has advocated that esophagectomy be performed only in institutions with an annual caseload of at least 13[14]. The research in the Netherlands also provided evidence that the centralization of esophageal cancer patients to specialized care would lead to better outcome. In a retrospective study, van de Poll-Franse et al[2] showed that 63.2% of patients had surgery in high-volume hospitals after the centralization of esophageal cancer patients, whereas only 17.2% of patients still underwent resections in low-volume hospitals. The 3-year survival rates increased from 32.0% to 45.1% for patients who had surgery (P = 0.004), and from 13.1% to 17.9% for all of the patients included in the study (P = 0.026). They concluded that the centralization of patients with esophageal and gastric cardia cancer surgery was associated with improvements in the overall survival rate for surgically as well as non-surgically treated patients. The majority of the volume-outcome relationship studies supported the idea of “practice makes perfect,” which means more experience gained in hospitals that treat a greater number of patients could lead to improvements in the management of patients as well as improvements in the advantages in patient survival; moreover, the volume-outcome relationship studies supported the idea of “selective referral pattern,” which means hospitals with better outcomes receive more referrals, leading to higher volumes. These two theories did not explain our results that patients without resection had worse prognosis when treated in very high-volume hospitals (> 75%, Q4). One likely reason may be due to a higher percentage of patients with advanced stage tumors in high-volume hospitals, which suggests that patients with poor performance or higher-risk for treatments were more likely to be referred to high-volume hospitals. Furthermore, our observation that worse prognosis in high-volume hospitals was compatible with the findings in the report by Rouvelas et al[15], which showed that patients operated by low-volume surgeons had the highest 30-d mortality risk compared with those operated by medium- and high-volume surgeons; however, this risk did not decrease with an additional increase in workload, which indicates that the volume factor is not the only determinant for patient outcome.
As for the hospital type-outcome relationship, the results in the literature were conflicting. Theoretically, the patients who are managed at a higher-level hospital are more likely to receive a wider range of diagnostic investigations, such as PET/CT scans and endoscopic ultrasound scans, which would result in accurate staging of a greater proportion of tumors and the appropriate use of combined oncological treatment modalities. Therefore, the hospital type may be a likely surrogate for quality of care. For example, Dikken et al[5] and Verhoef et al[6] have demonstrated that patients undergoing esophagectomy in university hospitals exhibited better outcome in terms of 3-mo mortality rates and 5-year survival rates. However, several studies have reported opposing results. Viklund et al[16] demonstrated no decreased risk of overall complications at university hospitals compared with nonuniversity hospitals. Similarly, Rodgers et al[17] showed that urban hospitals did not demonstrate better inpatient mortality than did rural hospitals. Although the teaching status appeared to confer benefit in the univariate analysis, this significance was lost once hospital volume was included. Consistent with Rodgers’ results, Bachmann et al[4] found that teaching hospital status was not independently associated with postoperative mortality rate in the case-mix adjusted survival analysis. Whereas the hospital-type relationship remains uncertain in patients undergoing surgical resection, our present study demonstrated that the hospital type, medical center vs non-medical center, did not influence outcome in patients with non-surgical treatments. Instead, the clinical TNM stages were significant prognostic factors. Our findings suggested that among those non-surgically treated patients, due to either unresectable tumors or their unsuitable status for surgery, the nature of the tumor apparently had a greater influence on the likelihood of long-term survival.
In our cohort, there was a higher proportion of patients in medical centers or high-volume hospitals who received surgical resections despite a higher frequency of advanced stage tumors. The population-based study by Bachmann et al[4] showed that patients treated in high-volume hospitals or by high-volume doctors were more likely to undergo surgical resection. In Coupland’s report, increasing resection rates were associated with lower mortality for all of the patients, including with and without surgical resection, with an HR of 0.86 (95%CI: 0.84-0.89) in the highest resection quintile compared with the lowest resection quintile[18]. However, our study has contradictory results: the higher resection rates in medical centers and high-volume hospitals did not translate into better outcomes in those patients without resection in our study.
Our study has both strengths and weaknesses. Its strengths include the population-based design and the large number of patients. We focused on cancers with squamous cell carcinoma histology, which is in contrast to the adenocarcinoma-predominant databases from Western countries. We emphasized the hospital type- and volume-outcome relationships in patients with non-surgical treatments, which has never been exclusively discussed. However, the results of our study may be limited by the nature of the population-based study. No detailed information was collected on the use of diagnostic tools and the protocol of chemotherapy, radiotherapy or any other non-surgical treatments when surgery was not an option.
In conclusion, the present population-based study, including 3955 non-surgically treated patients with esophageal cancer, demonstrated that the hospital type is not a significant prognostic factor. Moreover, the high-volume hospitals are not associated with better survival compared with the low-volume hospitals. For patients with esophageal cancer who receive non-surgical treatments, the nature of the tumor, i.e., the clinical TNM stage, constitutes a significant prognostic factor.
COMMENTS
Background
The prognosis of esophageal cancer is extremely poor. To improve outcome, centralization of care for patients with esophageal cancer has been proposed. Many reports have focused on the hospital type-outcome and volume-outcome relationships in esophageal cancer after esophagectomy. Little is known about the hospital type- and volume-outcome relationships in patients without surgical resection. Therefore, the authors studied the differences in patient and tumor characteristics according to the hospital type and the volume categories in this population-based study. The authors aimed to investigate whether the hospital type or volume would affect the prognosis in patients with esophageal cancer who receive non-surgical treatments.
Research frontiers
Hospital type-outcome analyses have shown better outcome in university hospitals. Previous studies demonstrated a higher 5-year survival rate for surgical patients in university hospitals compared with patients in teaching non-university and non-teaching hospitals. The hospital volume-outcome analyses also support the impact of volume on patient survival. The meta-analysis results have demonstrated a long-term survival benefit after esophageal cancer resection for high-volume hospitals compared with the low-volume counterparts.
Innovations and breakthroughs
Instead of investigating the outcome after esophagectomy, we focused on patients with esophageal cancer who receive non-surgical treatments. The data showed that hospital volume (annual volume, > 94 vs ≤ 94) remained statistically significant (HR = 0.91, 95%CI: 0.83-0.99; P = 0.028) in the multivariate analysis. The authors concluded that receiving non-surgical treatments for esophageal cancer in medical centers or high-volume hospitals is not associated with better survival. The nature of the tumor, i.e., the clinical TNM stages, was a significant prognostic factor.
Applications
For patients with non-surgically treated esophageal cancer, medical centers or high-volume hospitals were not associated with better survival. The disease aggressiveness, i.e., TNM stages, had a greater impact on patient survival.
Terminology
Hospital types were defined as medical centers and non-medical centers according to the Taiwan Joint Commission on Hospital Accreditation (http://www.tjcha.org.tw) based on the quality of process and outcome in healthcare performance. The threshold for high-volume hospitals was based on the median (Q3-4, > 50%; annual volume, > 56 cases) or upper quartile (Q4, > 75%; annual volume, > 94 cases).
Peer review
The present article delivers an important cautionary recommendation that the data regarding surgical patients with esophageal cancer should not be casually extrapolated to non-surgical patients with esophageal cancer.
Footnotes
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: May 7, 2014
First decision: June 10, 2014
Article in press: August 28, 2014
P- Reviewer: Caboclo JLF, Freedberg DE, Park SH, Tajika M S- Editor: Ma YJ L- Editor: A E- Editor: Zhang DN
References
- 1.Hsu PK, Wang BY, Huang CS, Wu YC, Hsu WH. Prognostic factors for post-recurrence survival in esophageal squamous cell carcinoma patients with recurrence after resection. J Gastrointest Surg. 2011;15:558–565. doi: 10.1007/s11605-011-1458-1. [DOI] [PubMed] [Google Scholar]
- 2.van de Poll-Franse LV, Lemmens VE, Roukema JA, Coebergh JW, Nieuwenhuijzen GA. Impact of concentration of oesophageal and gastric cardia cancer surgery on long-term population-based survival. Br J Surg. 2011;98:956–963. doi: 10.1002/bjs.7493. [DOI] [PubMed] [Google Scholar]
- 3.Wouters MW, Karim-Kos HE, le Cessie S, Wijnhoven BP, Stassen LP, Steup WH, Tilanus HW, Tollenaar RA. Centralization of esophageal cancer surgery: does it improve clinical outcome? Ann Surg Oncol. 2009;16:1789–1798. doi: 10.1245/s10434-009-0458-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bachmann MO, Alderson D, Edwards D, Wotton S, Bedford C, Peters TJ, Harvey IM. Cohort study in South and West England of the influence of specialization on the management and outcome of patients with oesophageal and gastric cancers. Br J Surg. 2002;89:914–922. doi: 10.1046/j.1365-2168.2002.02135.x. [DOI] [PubMed] [Google Scholar]
- 5.Dikken JL, Wouters MW, Lemmens VE, Putter H, van der Geest LG, Verheij M, Cats A, van Sandick JW, van de Velde CJ. Influence of hospital type on outcomes after oesophageal and gastric cancer surgery. Br J Surg. 2012;99:954–963. doi: 10.1002/bjs.8787. [DOI] [PubMed] [Google Scholar]
- 6.Verhoef C, van de Weyer R, Schaapveld M, Bastiaannet E, Plukker JT. Better survival in patients with esophageal cancer after surgical treatment in university hospitals: a plea for performance by surgical oncologists. Ann Surg Oncol. 2007;14:1678–1687. doi: 10.1245/s10434-006-9333-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Birkmeyer JD, Sun Y, Wong SL, Stukel TA. Hospital volume and late survival after cancer surgery. Ann Surg. 2007;245:777–783. doi: 10.1097/01.sla.0000252402.33814.dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brusselaers N, Mattsson F, Lagergren J. Hospital and surgeon volume in relation to long-term survival after oesophagectomy: systematic review and meta-analysis. Gut. 2014;63:1393–1400. doi: 10.1136/gutjnl-2013-306074. [DOI] [PubMed] [Google Scholar]
- 9.Luft HS, Hunt SS, Maerki SC. The volume-outcome relationship: practice-makes-perfect or selective-referral patterns? Health Serv Res. 1987;22:157–182. [PMC free article] [PubMed] [Google Scholar]
- 10.Wouters MW, Gooiker GA, van Sandick JW, Tollenaar RA. The volume-outcome relation in the surgical treatment of esophageal cancer: a systematic review and meta-analysis. Cancer. 2012;118:1754–1763. doi: 10.1002/cncr.26383. [DOI] [PubMed] [Google Scholar]
- 11.Metzger R, Bollschweiler E, Vallböhmer D, Maish M, DeMeester TR, Hölscher AH. High volume centers for esophagectomy: what is the number needed to achieve low postoperative mortality? Dis Esophagus. 2004;17:310–314. doi: 10.1111/j.1442-2050.2004.00431.x. [DOI] [PubMed] [Google Scholar]
- 12.Lauder CI, Marlow NE, Maddern GJ, Barraclough B, Collier NA, Dickinson IC, Fawcett J, Graham JC. Systematic review of the impact of volume of oesophagectomy on patient outcome. ANZ J Surg. 2010;80:317–323. doi: 10.1111/j.1445-2197.2010.05276.x. [DOI] [PubMed] [Google Scholar]
- 13.Markar SR, Karthikesalingam A, Thrumurthy S, Low DE. Volume-outcome relationship in surgery for esophageal malignancy: systematic review and meta-analysis 2000-2011. J Gastrointest Surg. 2012;16:1055–1063. doi: 10.1007/s11605-011-1731-3. [DOI] [PubMed] [Google Scholar]
- 14.Birkmeyer JD, Dimick JB. Potential benefits of the new Leapfrog standards: effect of process and outcomes measures. Surgery. 2004;135:569–575. doi: 10.1016/j.surg.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 15.Rouvelas I, Jia C, Viklund P, Lindblad M, Lagergren J. Surgeon volume and postoperative mortality after oesophagectomy for cancer. Eur J Surg Oncol. 2007;33:162–168. doi: 10.1016/j.ejso.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 16.Viklund P, Lindblad M, Lu M, Ye W, Johansson J, Lagergren J. Risk factors for complications after esophageal cancer resection: a prospective population-based study in Sweden. Ann Surg. 2006;243:204–211. doi: 10.1097/01.sla.0000197698.17794.eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodgers M, Jobe BA, O’Rourke RW, Sheppard B, Diggs B, Hunter JG. Case volume as a predictor of inpatient mortality after esophagectomy. Arch Surg. 2007;142:829–839. doi: 10.1001/archsurg.142.9.829. [DOI] [PubMed] [Google Scholar]
- 18.Coupland VH, Lagergren J, Lüchtenborg M, Jack RH, Allum W, Holmberg L, Hanna GB, Pearce N, Møller H. Hospital volume, proportion resected and mortality from oesophageal and gastric cancer: a population-based study in England, 2004-2008. Gut. 2013;62:961–966. doi: 10.1136/gutjnl-2012-303008. [DOI] [PubMed] [Google Scholar]


