Abstract
AIM: To study the frequency of vitamin D deficiency in patients with hepatitis C virus (HCV) infection and to evaluate the role of vitamin D supplementation in improving antiviral therapy.
METHODS: Sixty-six children aged from 7-14 years (mean ± SD, 11.17 ± 2.293) diagnosed with HCV infection were matched to 28 healthy controls. Serum levels of 25 (OH) D3, calcium, phosphorus, alkaline phosphatase and plasma level of parathormone were measured. Quantitative PCR for HCV was performed Bone density was determined by dual energy X-ray absorptiometry. All cases received conventional therapy, and only 33 patients received vitamin D supplementation.
RESULTS: Children with HCV showed significantly increased levels of HCV RNA (P < 0.001), parathormone (P < 0.01) and decreased vitamin D levels (P < 0.05) (33.3% deficient and 43.3% insufficient) compared with controls. Abnormal bone status (Z score -1.98 ± 0.75) was found in ribs, L-spine, pelvis and total body. Cases treated with vitamin D showed significant higher early (P < 0.04) and sustained (P < 0.05) virological response. There was a high frequency of vitamin D deficiency among the Egyptian HCV children, with significant decrease in bone density. The vitamin D level should be assessed before the start of antiviral treatment with the correction of any detected deficiency.
CONCLUSION: Adding vitamin D to conventional Peg/RBV therapy significantly improved the virological response and helped to prevent the risk of emerging bone fragility.
Keywords: Vitamin D, Viral response, Hepatitis C, Children
Core tip: Does vitamin D supplementation improves the viral response in hepatitis C infection? Previous studies raised the possibility that disease progression is associated with higher levels of vitamin D, and thus vitamin D supplementation does not have a role in chronic hepatitis C patients. This study aimed to investigate the frequency of vitamin D deficiency among Egyptian hepatitis C virus-infected children, with assessment of bone status by measuring calcium, parathormone and alkaline phosphatase levels, and bone mineral density and to evaluate the role of vitamin D supplementation in improving the viral response of these patients.
INTRODUCTION
Hepatitis C virus (HCV) infection remains an evolving cause of morbidity and mortality worldwide. Despite limited epidemiological data, a higher prevalence is found in Eastern Europe and in the Middle East[1]. Egypt has the highest prevalence of HCV infection; about 9% country wide and up to 50% in certain rural areas[2].
Vitamin Dis a potent immunomodulator that improves insulin sensitivity, suppresses proinflammatory cytokines, increases anti-inflammatory cytokines, and improves CD4 T cell hyper-responsiveness[3]. Recently, specific vitamin D receptors (VDRs) were observed to be expressed in liver cells and this expression of VDRs is reduced in chronic Hepatitis C patients. In addition, an inverse relationship between the liver VDR expression and inflammation severity was observed[4,5].
Abnormal bone metabolism, vitamin D axis, calcium (Ca) and parathormone (PTH) dysfunction have been reported in cholestatic children[6] with the disturbance in vitamin D metabolism, gonads, or chronic inflammation[7]. The role of chronic HCV infection in osteoporosis is supported with the decreased fracture risk in HCV children with successful antiviral treatment[8]. However, other studies demonstrated osteosclerosis in HCV infection[9].
The relation between vitamin D and the antiviral therapy response remains unclear, with previous studies raising the possibility of inconsistent results and failure of the reference methodology[10].
The present study aimed to investigate the frequency of vitamin D deficiency among Egyptian HCV infected children, and to assess bone status by measuring calcium, parathormone, alkaline phosphatase and bone mineral density. We also evaluated the role of vitamin D supplementation in improving the virological response of these patients.
MATERIALS AND METHODS
This prospective study included 66 cases (43 male and 23 female) aged from 7-14 years with HCV infection. They were admitted to the Gastroenterology and Hepatology unit at Assiut Children University Hospital from June 2010 to December 2012. The diagnosis of HCV was based on the quantitation of HCV RNA by quantitative real-time polymerase chain reaction[11].
Exclusion criteria
Patients with chronic liver disease other than HCV, patients with immunodeficiency, malignancy, decompensated liver cirrhosis, and patients on vitamin D or calcium therapy in the previous 3 mo were excluded.
Another 28 apparently healthy volunteer children drawn from Assiut Children University Hospital, of matched age and sex, were recruited as controls. The medical ethical committee of the Faculty of Medicine, Assuit University approved the study, and informed written consent was obtained from the parents or legal guardians.
A full history was taken and a thorough clinical examination was performed for all members of both study groups. All cases were subjected to the following investigations: (1) serum liver enzymes (AST and ALT), Ca, phosphorus, alkaline phosphatase, 25 (OH) D 3 and PTH levels; (2) PCR for HCV RNA; and (3) bone mineral density (BMD) as measured by the dual energy X-ray absorptiometry method (Hologic Model Delphi, CT, United States). BMD (g/cm2 and Z-score) of the ribs, arms, head, lumbar spine, pelvis, legs and total body were measured. The BMD values were compared with those of the healthy controls. Osteoporosis was considered with a Z-score of -2 standard deviations (SDs) and osteopenia between -1.0 and 2.0 SDs in relation to the patient’s age, as suggested by the World Health Organization[12].
The patients were classified randomly by a simple randomization method into two groups: Group A received PEG-alpha-2b interferon (60 μg/kg per week) SC and ribavirin (15 mg/kg per day) orally for 48 wk together with vitamin D3 2000 IU/d orally. Group B received the same therapy without vitamin D supplementation. Vitamin D insufficiency, deficiency and sufficiency were defined according to 25OHD levels as > 75 nmol/L, from 75-30 nmol/L, and < 30 nmol/L, respectively[13].
Follow up for all patients was performed at 12 and 24 wk from the beginning of the therapy and at 24 wk after cessation of therapy by measuring PCR for HCV RNA and liver functions. All complications and side effects during the course of treatment were recorded. Six cases (two from group A and four from group B) were excluded from the study after 12 wk of treatment because of a lack of response. Non-responders were defined as those who failed to clear HCV RNA from serum after 24 wk of therapy.
Discontinuation of therapy occurred if HCV RNA level after 12 wk < 2 log unit compared to baseline or neutropenia < 500/cm, platelets < 5000 mm³ and Hb < 8 gm/dL. Sustained virological response (SVR) was considered when an undetectable HCV-RNA was found at 24 wk after therapy.
Sample collection and laboratory investigations
Venous blood samples were collected from patients under standardized conditions. Samples were centrifuged (3000 × g for 10 min); serum and plasma samples were divided and stored in aliquots at -20 °C until analysis. Serum calcium, phosphorus and alkaline phosphatase were measured by conventional methods using a COBAS INTEGRA 400 autoanalyzer. Serum 25-OH Vitamin D measurement was performed by Immundiagnostik AG kit (Bensheim; Germany; Ref.K2110), using an enzyme immunoassay technique, based on competition of 25 (OH) D present in the sample with 20(OH) D tracer for the binding pocket of vitamin D binding protein. Assay of plasma levels of parathyroid hormone using MAGLUMI Intact PTH (Shenzhen; China) Ref. 13330211001M (Sandwich immunoluminometric assay) Quantitative PCR for HCV was performed using an AB Applied Biosystems 7500 Fast Real-Time PCR System.
Statistical analysis
Statistical analysis was carried out using SPSS (version 16, SPSS Inc., Chicago, IL, United States). Quantitative data were expressed as mean ± SD and categorical data were presented in the form of frequency and percent (%), as appropriate. Student’s t-test was used for parametric data and non-parametric χ2 was used for independent variables when comparing the two groups. Multiple groups were compared using the one-way ANOVA test. Linear correlations were performed by Pearson’s test. For all tests, a difference was considered significant if the probability (P) was < 0.05.
RESULTS
Positive family history of HCV was significantly different between group A and B (P < 0.05) (Table 1).
Table 1.
Demographic and clinical data for the studied groups and control
| A | B | Total | Control | P value | |
| (n = 31) | (n = 29) | (n = 60) | (n = 28) | ||
| Age (yr) | 11.1 ± 2.178 | 11.043 ± 2.882 | 11.17 ± 2.293 | 9.35 ± 3.24 | 0.445 |
| Duration of illness (yr) | 3.4 ± 1.984 | 3.123 ± 1.688 | 3.262 ± 1.832 | - | 0.563 |
| BMI (kg/m2) | 20.81 ± 4.29 | 20.28 ± 3.13 | 20.54 ± 3.73 | 21.21 ± 2.34 | 0.588 |
| Male/female | 23/8 (74.1%/25.8%) | 20/9 (68.9%/31.1%) | 43/17 (71.6%/28.3 %) | 17/13 (60.7%/46.4%) | 0.284 |
| Blood transfusion, n (%) | 16 (51.6) | 16 (55.1) | 32 (53.3) | - | 0.602 |
| Positive family history of HCV | 8 (25.8) | 1 (3.4) | 9 (15.0) | - | 0.051 |
| Jaundice, n (%) | 19 (61.2) | 13 (44.8) | 32 (53.3) | - | 0.098 |
| Fever, n (%) | 9 (29.0) | 9 (31.0) | 18 (30.0) | - | 0.611 |
| History of bleeding, n (%) | 17 (54.8) | 16 (55.1) | 33 (55.0) | - | 0.501 |
Significance total vs control. No significant difference between group A and B. Data are presented as the mean ± SD or number (%) as appropriate. BMI: Body mass index.
Complications during the course of treatment were detected in 10 cases, with mild symptoms in the form of fever, nausea, itching, headache, anemia < 9 gm/dL, neutropenia < 5000/cm³ or thrombocytopenia < 100000 mm³, where the antiviral dose were reduced to half the dose.
Significant decrease in serum vitamin D levels (P < 0.05) and significant increases in plasma PTH (P < 0.01) and HCV RNA (P < 0.001) were detected in the studied cases compared with the controls, despite no significant differences being found regarding liver enzymes, albumin, Phosphorus and ALP between the two groups (Table 2).
Table 2.
Mean ± SD values of laboratory parameters in the studied groups and control
| Group A | Group B | Total | Control (n = 28) | P value | |
| (n = 31) | (n = 29) | (n = 60) | |||
| ALT (IU/L) | 75.46 ± 18.18 | 87.96 ± 14.11 | 81.71 ± 18.25 | 21.35 ± 6.21 | 0.148 |
| AST (IU/L) | 75.5 ± 24.95 | 104 ± 11.204 | 89.75 ± 23.41 | 35.74 ± 4.92 | 0.24 |
| Calcium (mg/dL) | 6.733 ± 1.52 | 6.657 ± 1.24 | 6.69 ± 1.38 | 8.2 ± 0.91 | 0.83 |
| Phosphorus (mg/dL) | 4.24 ± 0.42 | 4.21 ± 0.39 | 4.25 ± 0.41 | 3.1 ± 0.65 | 0.68 |
| Alkaline phos (IU/L) | 140.97 ± 10.98 | 99.12 ± 24.94 | 120.05 ± 27.66 | 78.48 ± 21.10 | 0.20 |
| HCV RNA (IU/mL) | 1393290 ± 52580 | 967371 ± 56534 | 1180334 ± 79910 | 10.21 ± 0.74 | 0.0011 |
| Vitamin D (nmol/L) | 65.26 ± 22.71 | 57.9 ± 16.17 | 61.58 ± 17.05 | 98.31 ± 3.50 | 0.051 |
| PTH (pg/mL) | 179.10 ± 10.25 | 188.03 ± 14.96 | 186.56 ± 25.67 | 65.71 ± 12.05 | 0.011 |
Significance total vs control. No significant difference between group A and B. AST: Aspartate transaminase; ALT: Alanine transaminase; PTH: Parathormone.
Table 3 represents the mean values of Bone density parameters in the studied groups with HCV infection. There were significant differences in Z- score regarding ribs (P < 0.04), pelvis (P < 0.04), L-spine (P < 0.05) and total BMD (P < 0.03) between the two groups (Table 3).
Table 3.
Mean ± SD values of bone density parameters in the studied groups with hepatitis C virus infection
| A (n = 31) | B (n = 29) | Total (n = 60) | Z- score | P value | |
| Head | 1.226 ± 0.394 | 1.12 ± 0.383 | 1.173 ± 0.389 | -0.16 ± 0.42 | 0.297 |
| Arm | 1.063 ± 1.223 | 0.89 ± 0.277 | 0.976 ± 0.883 | -0.916 ± 1.2 | 0.453 |
| Leg | 0.912 ± 0.225 | 0.969 ± 0.38 | 0.94 ± 0.311 | -1.13 ± 1.34 | 0.482 |
| Rib | 0.688 ± 0.248 | 0.869 ± 0.411 | 0.778 ± 0.349 | -1.31 ± 0.78 | 0.0431 |
| Pelvis | 0.839 ± 0.187 | 0.92 ± 0.238 | 0.879 ± 0.216 | -2.8 ± 1.53 | 0.051 |
| L Spine | 0.77 ± 0.207 | 0.901 ± 0.294 | 0.835 ± 0.261 | -2.43 ± 1.7 | 0.0511 |
| Total | 0.834 ± 0.281 | 0.913 ± 0.381 | 0.874 ± 0.334 | -1.98 ± 0.75 | 0.0361 |
Significance between total BMD of HCV patients vs control. BMD: Bone mineral density (g/cm2). HCV: Hepatitis C virus.
Vitamin D sufficiency was present in 23.3%, insufficiency in 43.3% and deficiency in 33.3% of cases. There were significant decreases in serum calcium levels in the deficient group vs the sufficient group. Serum vitamin D showed a statistically significant difference between the sufficient, insufficient and deficient groups compared with the control. PCR for HCV RNA was significantly different in the sufficient and insufficient versus the deficient groups and also compared with control (Table 4).
Table 4.
Mean ± SD values of laboratory parameters in relation to Vitamin D status in the studied groups
|
Vitamin D status |
Control (n = 28) | Suff vs Insuff | Suff vs Def | Insuff vsDef | Total P value | |||
| Sufficient (n = 14) | Insufficient (n = 26) | Deficient (n = 20) | ||||||
| ALT (IU/L) | 97.643 ± 18.06 | 75.654 ± 15.954 | 78.45 ± 17.824 | 21.35 ± 6.21 | 0.327 | 0.475 | 0.885 | 0.610 |
| AST (IU/L) | 97.786 ± 24.215 | 94.923 ± 13.151 | 77.4 ± 13.363 | 35.74 ± 4.92 | 0.934 | 0.453 | 0.551 | 0.772 |
| Calcium (mg/dL) | 7.993 ± 1.183 | 7.023 ± 1.396 | 6.13 ± 1.185 | 8.2 ± 0.91 | 0.769 | 0.031 | 0.090 | 0.077 |
| Phosphorus (mg/dL) | 3.31 ± 0.25 | 4.21 ± 0.31 | 4.24 ± 0.61 | 3.1 ± 0.65 | 0.354 | 0.412 | 0.614 | 0.456 |
| Alkaline phosphatase (IU/L) | 82.92 ± 15.62 | 109.34 ± 13.08 | 159.96 ± 14.64 | 78.48 ± 21.10 | 0.493 | 0.068 | 0.233 | 0.192 |
| HCV RNA(IU/mL) | 1300824 ± 664010 | 1417943 ± 137873 | 787100 ± 65915 | 10.21 ± 0.74 | 0.705 | 0.031 | 0.0221 | 0.0432 |
| Vitamin D (nmol/L) | 84.21 ± 17.821 | 49.23 ± 15.07 | 22.6 ± 3.858 | 98.31 ± 3.50 | 0.0001 | 0.001 | 0.0001 | 0.0012 |
| PTH (pg/mL) | 128.53 ± 20.37 | 164.05 ± 12.63 | 179.46 ± 19.24 | 65.71 ± 12.05 | 0.181 | 0.322 | 0.607 | 0.296 |
Significance between groups vs control;
Significance total vs control. AST: Aspartate transaminase; ALT: Alanine transaminase; PTH: Parathormone; Suff: sufficient; Insuff: Insufficient; Def: Deficient.
There were significant difference in ribs (P < 0.04), pelvis (P < 0.05), L spine (P < 0.05) and head (P < 0.005) in BMD between the sufficient and deficient groups (Table 5).
Table 5.
Mean ± SD values of bone mineral density in relation to Vitamin D status in the studied groups
|
Vitamin D status |
Suff vs Insuff | Suff vs Def | Insuff vs Def | Total | |||
| Sufficient (n = 14) | Insufficient (n = 26) | Deficient (n = 20) | P value | ||||
| Head | 0.921 ± 0.343 | 1.237 ± 0.415 | 1.267 ± 0.317 | 0.0201 | 0.0051 | 0.790 | 0.0182 |
| Arm | 1.03 ± 0.901 | 0.879 ± 0.259 | 1.065 ± 1.325 | 0.428 | 0.931 | 0.487 | 0.759 |
| Leg | 0.873 ± 0.28 | 1.001 ± 0.321 | 0.978 ± 0.291 | 0.341 | 0.428 | 0.798 | 0.067 |
| Rib | 0.856 ± 0.34 | 0.749 ± 0.358 | 0.662 ± 0.353 | 0.365 | 0.0431 | 0.910 | 0.637 |
| Pelvis | 0.996 ± 0.234 | 0.895 ± 0.219 | 0.748 ± 0.107 | 0.990 | 0.051 | 0.469 | 0.738 |
| L- Spine | 0.959 ± 0.115 | 0.838 ± 0.24 | 0.716 ± 0.258 | 0.808 | 0.0591 | 0.766 | 0.892 |
| Total | 0.918 ± 0.444 | 0.897 ± 0.11 | 0.812 ± 0.28 | 0.863 | 0.398 | 0.340 | 0.596 |
| Z score | 1.121 ± 0.577 | 0.927 ± 0.25 | -1.875 ± 0.441 | 0.619 | 0.0421 | 0.431 | 0.493 |
Significance between groups;
Significance total vs control. BMD: Bone mineral density (g/cm2). Suff: Sufficient; Insuff: Insufficient; Def: Deficient.
At 12 wk, there were no significance in the early virological response (EVR) between the two groups, where 10/31 patients (32.3%) from group A and 6/29 (20.6%) from group B were HCV-RNA negative. At 24 wk, there were significant differences in virological response (P value < 0.04),where 24/31 patients (77.4%) from group A and 17/29 (58.6%) from group B were negative for HCV-RNA. A significant difference in SVR was detected at 24 wk after treatment, between group A and B (P < 0.05). 22/31 patients (70.9%) from group A and 15/29 (51.7%) from group B were HCV-RNA negative.
DISCUSSION
Egypt has the highest worldwide prevalence of HCV infection, about 9% countrywide and up to 50% in rural areas[2]. This goes with our results where 15% of patients had positive family history of HCV, with a significant higher proportion in group A than in group B.
The baseline of PCR HCV RNA (> 400000 IU/mL) was significantly higher compared with the controls in this study, with no significance between group A and B. Assy et al[13] showed that patients supplemented with vitamin D had a significantly higher baseline of HCV RNA (60%) than those treated without vitamin D (40%). While Abu-Mouch et al[14], found that the baseline of HCV RNA with high viral load (> 800000 IU/mL) in patients treated with vitamin D (50%) showed higher significant results than those without vitamin D treatment (42%).
In this study, vitamin D sufficiency was present in 23.3%, insufficiency in 43.3% and deficiency in 33.3% of cases with no significant difference between the two groups. This agrees with the results of previous studies[13,14], which found no significant difference regarding the baseline vitamin D between cases supplemented with vitamin D and those without. Highleyman[15] stated the importance of vitamin D in the immune response against HCV infection: 84% of adult patients with HCV infection have low vitamin D levels and 1/3 of patients had severe deficiency.
Mandorfer et al[16] also found sufficient levels of vitamin D in 1/5 of their participants and 57% were insufficient and 23% were deficient. Ladero et al[17], found vitamin D insufficiency in 40% and deficiency in 36% of adult patients.
Vitamin D status may differ across different geographical locations and environments[13]. Consequently, its influence on disease pathogenesis is likely to vary from one location to the other. Vitamin. D deficiency could be related to lack of exposure to sunlight, although Africa is sunny, and/or to the lack of vitamin D in the diet[13].
Vitamin D is an important modulator of inflammatory responses and fibrosis in HCV infection by inhibiting TNF-α, which regulates the immune response and inhibits the fibrosis process by suppressing TGF-β, which affects fibrosis progression[18]. Ladero et al[17], concluded that vitamin D deficiency is more common in Spanish patients with HCV infection, but it is neither related to biochemical and virological variations, nor to fibrosis stage or IL 28B polymorphisms.
On the contrary, other studies[19], raised the possibility that the disease progression is associated with higher levels of vitamin D, and thus vitamin D supplementation does not have a role in chronic hepatitis C patients. Terrier et al[20], showed that were no associations between serum 25 OH D3 levels and viral response to therapy or the severity of immunodeficiency in HCV patients.
The present study observed significantly higher plasma PTH levels in HCV patients compared with controls. This may be related to the functioning feedback mechanisms of low serum calcium and 25(OH)D levels. Malabanan et al[21] stated that when the 25(OH)D concentration reaches < 50 nmol/L (20 ng/mL), the PTH concentration increases.
In the present study, plasma PTH levels were negatively associated with BMD and the Z-score of the total body (Figure 1). The increased PTH levels found in our patients may lead to increased bone resorption and decreased BMD. The decrease in sensitivity of the PTH effect on bone resorption[22] or the anabolism of the osteoblasts to PTH could be an explanation[23]. Similarly, Choudhary et al[24] found a negative correlation between PTH and BMD in viral cirrhosis. By contrast, other studies[25,26] revealed normal or low PTH levels in HCV infection. This discrepancy could be explained by the increased prevalence of 25(OH)D deficiency or insufficiency in Egyptian children with HCV infection.
Figure 1.
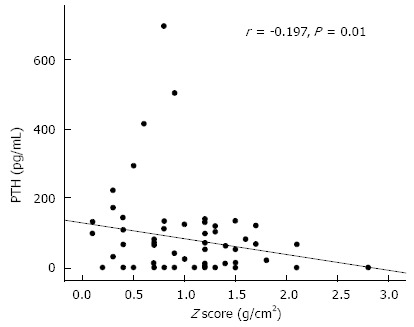
Correlation between plasma parathormone levels and the Z-score of the total body.
In partial agreement with other studies[27], we observed that the peripheral skeleton was less influenced than the axial skeleton in our HCV patients. The accelerated growth in the peripheral skeleton in children could explain this, while during puberty, it occurs in the axial skeleton[27]. In addition, most of our patients had been diagnosed before puberty. Therefore, the highest bone mass was obtained during the disease process, which affects the bone status in these patients. The protective role of puberty on bone tends to be opposite to temporary bone loss, which seems to be a complication of the disease[28].
Regarding bone density in the present study, the Z score was significantly decreased and showed significant difference between the two groups. This is agrees with the results of El Karmouty et al[29], who revealed that BMD, T score and Z score were significantly lower in children with HBV and HCV than HAV infection. The severity of osteoporosis increases with the severity of liver disease. However, no significant correlation could be detected between serum Ca, Phosphorus, vitamin D and the degree of bone loss, which is higher in osteoporosis than in osteomalacia[29]. Kryskiewicz et al[6] observed some risk factors of bone tissue pathology, which included hepatocyte dysfunction, disorders of vitamin D, Ca and Phosphorus metabolism, immunosuppressive therapy and malnutrition. Theoretically disturbance in the endocrine Ca - PTH- vitamin D axis seems to play a role in the pathogenesis of osteo metabolic disturbances.
López-Larramona et al[30] stated that the origin of hepatocyte osteodystrophy is unclear; it may be multifactorial and its etiology and severity vary in accordance with the underlying liver disease. Bone loss occurs as a result of increased bone turnover and/or remodeling in balance, the latter being caused by reduced formation, and increased resorption or a combination of both. Vitamin D metabolism in severe HCV infection and deficiency may cause hyperparathyroidism, increase bone turnover and accelerate the loss of BMD. This regulation of the RANKL/OPG system is activated by cytokines involved in the pathogenesis of chronic liver disease (IL 1, 11, 6, TNF-α1). Previous studies[24,31] found that patients with chronic liver disease have low BMD in 93% of cases. Other factors, including decreased physical activity, decreased body mass, deficiency of vitamin D and low IGF level were considered.
Yurci et al[32] stated that all anti osteoporotic agents in hepatic osteodystrophy seems to be safe and effective. Oral bisphosphonate (anti resorptive drug) was the most effective in preventing both cortical and trabecular bone loss in patients with chronic viral hepatitis, but only limited data are available.
By contrast, Yenice et al[25], stated that hepatitis B and C infection do not pose a risk factor for osteoporosis and bone loss, and that the diagnosis of BMD should be based on multiple parameters. It should be kept in mind that the key osteomalacia is rare and requires bone biopsy. The differences observed between reports may be related to the study design, different methods used for measuring BMD and selection of patients.
Regarding the use of vitamin D in this study, cases in group A showed significantly higher rates of EVR, a greater response after 24 wk and higher SVRs than group B (Figure 2). This agrees with a previous study[14] that found that patients treated with vitamin D in addition to conventional antiviral therapy had significantly higher responses at 4 wk than those treated without vitamin D (44% vs 17%), at 12 wk (94% vs 43%), and at 24 wk after stoppage of therapy (86% vs 42%) with a small relapse rate (8%). Previous studies[13,14] concluded that the addition of vitamin D to the therapy in HCV patients increases the rate of rapid and early SVR. Therefore, assessment of vitamin D levels before combined therapy and correction during the course of therapy may be needed.
Figure 2.
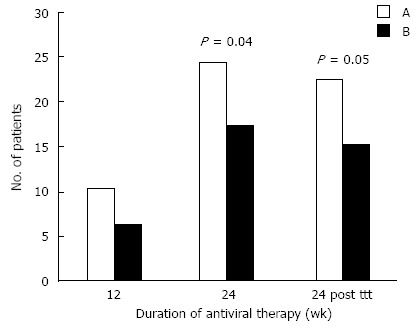
Early and sustained viral response of hepatitis C virus RNA in group A and B. PTH: Parathormone.
Bitetto et al[33] concluded that unfavorable responses to antiviral therapy are predicted in vitamin D deficient patients with RHC. Supplementing vitamin D improves the possibility of achieving an SVR after antiviral treatment. In addition, low levels of vitamin D are associated with significant poor rate of EVR and SVR in co-infection of HIV[16], and this decreased vitamin D is limited to fibrosis and low SVR in interferon-based therapy[34]. The exact mechanism of vitamin D supplementation action on EVR, RVR and SVR is unclear. It may reflect the fact that 1, 25 dihydroxy vitamin D appears to be an immunomodulator via regulation of T cell function through its effect on T cell antigen[35]. T helper cell type I action is intensified when vitamin D is insufficient or when VDR signals are weak[36]. Vitamin D increases the expression of VDR proteins and inhibits viral replication[37].
Gal-Tanamy et al[38], stated that the interferon sparing effect of vitamin D improves the antiviral treatment in patients with HCV infection. 25(OH) D3 is a novel antiviral agent and a better therapeutic option to reduce the enzyme activity in patients with HCV[39].
In conclusion, there was a high frequency of Vitamin D deficiency among the Egyptian HCV children studied, with significant decrease in bone density. Vitamin D levels should be assessed before the start of antiviral treatment, with correction of any detected deficiency. Adding vitamin D to conventional Peg/RBV therapy significantly improved viral response and helped to prevent the risk of emerging bone fragility.
ACKNOWLEDGMENTS
We acknowledge the great support to all staff members and nursing team in the GIT Outpatient Clinic of the Pediatric Department at the Assiut Children University Hospital, Egypt for their help and cooperation in this work.
COMMENTS
Background
Hepatitis C virus (HCV) infection remains an evolving cause of morbidity and mortality worldwide. Despite limited epidemiological data, a higher prevalence is found in Eastern Europe and the Middle East. Abnormal bone metabolism, vitamin D axis, calcium and parathormone dysfunction have been reported in cholestatic children. The relation between vitamin D and antiviral therapy response remains unclear, with previous studies raising the possibility of inconsistent results.
Research frontiers
To investigate the frequency of vitamin D deficiency among Egyptian HCV-infected children, with assessment of bone status by measuring calcium, parathormone and alkaline phosphatase levels, and bone mineral density. We also to evaluated the role of vitamin D supplementation in improving the viral response of these patients.
Innovations and breakthroughs
Previous studies raised the possibility that disease progression is associated with higher levels of vitamin D, and thus vitamin D supplementation does not have a role in chronic hepatitis C patients. In this study, cases supplemented with vitamin D showed a significantly higher percentage of early virological response (EVR), higher response after 24 wk and a higher sustained virological response (SVR) than those without supplementation.
Applications
The addition of vitamin D to the therapy in HCV patients increases the rate of rapid and early SVR. Cases supplemented with vitamin D showed a significantly higher percentage of EVR, higher response after 24 wk and a higher SVR than those without supplementation.
Terminology
The addition of vitamin D to the therapy in HCV patients increases the rate of rapid and early SVR. Assessment of Vitamin D levels before combined therapy and correction during the course of therapy is required.
Peer review
The study investigated the effect of vitamin D in the response to antiviral treatment in chronic infected pediatric patients with HCV. There are several works published in the literature exploring this issue, however the relation between vitamin D status and the antiviral response to therapy remains unclear.
Footnotes
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: June 17, 2014
First decision: July 21 ,2014
Article in press: September 30, 2014
P- Reviewer: Liu EQ, Romero MR, Videla LA S- Editor: Qi Y L- Editor: Stewart G E- Editor: Wang CH
References
- 1.Esteban JI, Sauleda S, Quer J. The changing epidemiology of hepatitis C virus infection in Europe. J Hepatol. 2008;48:148–162. doi: 10.1016/j.jhep.2007.07.033. [DOI] [PubMed] [Google Scholar]
- 2.Kamal SM, Nasser IA. Hepatitis C genotype 4: What we know and what we don’t yet know. Hepatology. 2008;47:1371–1383. doi: 10.1002/hep.22127. [DOI] [PubMed] [Google Scholar]
- 3.Abu-Mouch S, Fireman Z, Jarchovsky J, Zeina AR, Assy N. Vitamin D supplementation improves sustained virologic response in chronic hepatitis C (genotype 1)-naïve patients. World J Gastroenterol. 2011;17:5184–5190. doi: 10.3748/wjg.v17.i47.5184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barchetta I, Carotti S, Labbadia G, Gentilucci UV, Muda AO, Angelico F, Silecchia G, Leonetti F, Fraioli A, Picardi A, et al. Liver vitamin D receptor, CYP2R1, and CYP27A1 expression: relationship with liver histology and vitamin D3 levels in patients with nonalcoholic steatohepatitis or hepatitis C virus. Hepatology. 2012;56:2180–2187. doi: 10.1002/hep.25930. [DOI] [PubMed] [Google Scholar]
- 5.Mahon BD, Gordon SA, Cruz J, Cosman F, Cantorna MT. Cytokine profile in patients with multiple sclerosis following vitamin D supplementation. J Neuroimmunol. 2003;134:128–132. doi: 10.1016/s0165-5728(02)00396-x. [DOI] [PubMed] [Google Scholar]
- 6.Kryskiewicz E, Pawlowska J, Pludowski P, Ismail H, Karczmarewicz E, Teisseyre M, Skorupa E, Ryzko J, Kalicinski P, Socha J, et al. Bone metabolism in cholestatic children before and after living-related liver transplantation--a long-term prospective study. J Clin Densitom. 2012;15:233–240. doi: 10.1016/j.jocd.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 7.Huang WH, Yu MC, Huang JY, Lai PC. Impact of hepatitis C virus infection on bone mineral density in renal transplant recipients. PLoS One. 2013;8:e63263. doi: 10.1371/journal.pone.0063263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arase Y, Suzuki F, Suzuki Y, Akuta N, Kobayashi M, Sezaki H, Hosaka T, Kawamura Y, Yatsuji H, Hirakawa M, et al. Virus clearance reduces bone fracture in postmenopausal women with osteoporosis and chronic liver disease caused by hepatitis C virus. J Med Virol. 2010;82:390–395. doi: 10.1002/jmv.21691. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka T, Oki S, Muro S, Tanaka K, Hashimoto J. A case of hepatitis C-associated osteosclerosis in an elderly Japanese man. Endocr J. 2006;53:393–399. doi: 10.1507/endocrj.k04-131. [DOI] [PubMed] [Google Scholar]
- 10.Kitson MT, Dore GJ, George J, Button P, McCaughan GW, Crawford DH, Sievert W, Weltman MD, Cheng WS, Roberts SK. Vitamin D status does not predict sustained virologic response or fibrosis stage in chronic hepatitis C genotype 1 infection. J Hepatol. 2013;58:467–472. doi: 10.1016/j.jhep.2012.11.017. [DOI] [PubMed] [Google Scholar]
- 11.Chevaliez S, Pawlotsky JM. Diagnosis and management of chronic viral hepatitis: antigens, antibodies and viral genomes. Best Pract Res Clin Gastroenterol. 2008;22:1031–1048. doi: 10.1016/j.bpg.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 12.Eren E, Yilmaz N. Biochemical markers of bone turnover and bone mineral density in patients with beta-thalassaemia major. Int J Clin Pract. 2005;59:46–51. doi: 10.1111/j.1742-1241.2005.00358.x. [DOI] [PubMed] [Google Scholar]
- 13.Assy MH, Abd El-Rahman HS, Esh SS, Gaballah BA. Vitamin D in type 1 diabetes mellitus: Possible interrelationship. Z U M J. 2012;18:974–980. [Google Scholar]
- 14.Abu-Mouch S, Fireman Z, Jarchovsky J, Assy N. The Beneficial Effect of Vitamin D with Combined Peg Interferon and Ribavirin for Chronic HCV Infection. 60th Annual Meeting of the AASLD. Oct 30-Nov 3, 2009. Boston, MA: Hynes Convention Center; 2009. [Google Scholar]
- 15.Highleyman L. Vitamin D Increases Sustained Response to Interferon-based Therapy for Hepatitis C, May Improve Liver Fibrosis. 45th Annual Meeting of the European Association for the Study of the Liver (EASL 2010), April 14-18, 2010. Vienna, Austria: EASL; 2010. [Google Scholar]
- 16.Mandorfer M, Reiberger T, Payer BA, Ferlitsch A, Breitenecker F, Aichelburg MC, Obermayer-Pietsch B, Rieger A, Trauner M, Peck-Radosavljevic M. Low vitamin D levels are associated with impaired virologic response to PEGIFN + RBV therapy in HIV-hepatitis C virus coinfected patients. AIDS. 2013;27:227–232. doi: 10.1097/QAD.0b013e32835aa161. [DOI] [PubMed] [Google Scholar]
- 17.Ladero JM, Torrejón MJ, Sánchez-Pobre P, Suárez A, Cuenca F, de la Orden V, Devesa MJ, Rodrigo M, Estrada V, López-Alonso G, et al. Vitamin D deficiency and vitamin D therapy in chronic hepatitis C. Ann Hepatol. 2013;12:199–204. [PubMed] [Google Scholar]
- 18.Tan X, Li Y, Liu Y. Therapeutic role and potential mechanisms of active Vitamin D in renal interstitial fibrosis. J Steroid Biochem Mol Biol. 2007;103:491–496. doi: 10.1016/j.jsbmb.2006.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Corey KE, Zheng H, Mendez-Navarro J, Delgado-Borrego A, Dienstag JL, Chung RT. Serum vitamin D levels are not predictive of the progression of chronic liver disease in hepatitis C patients with advanced fibrosis. PLoS One. 2012;7:e27144. doi: 10.1371/journal.pone.0027144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Terrier B, Carrat F, Geri G, Pol S, Piroth L, Halfon P, Poynard T, Souberbielle JC, Cacoub P. Low 25-OH vitamin D serum levels correlate with severe fibrosis in HIV-HCV co-infected patients with chronic hepatitis. J Hepatol. 2011;55:756–761. doi: 10.1016/j.jhep.2011.01.041. [DOI] [PubMed] [Google Scholar]
- 21.Malabanan A, Veronikis IE, Holick MF. Redefining vitamin D insufficiency. Lancet. 1998;351:805–806. doi: 10.1016/s0140-6736(05)78933-9. [DOI] [PubMed] [Google Scholar]
- 22.Kleerekoper M, Nelson DA, Peterson EL, Flynn MJ, Pawluszka AS, Jacobsen G, Wilson P. Reference data for bone mass, calciotropic hormones, and biochemical markers of bone remodeling in older (55-75) postmenopausal white and black women. J Bone Miner Res. 1994;9:1267–1276. doi: 10.1002/jbmr.5650090817. [DOI] [PubMed] [Google Scholar]
- 23.Poole KE, Reeve J. Parathyroid hormone - a bone anabolic and catabolic agent. Curr Opin Pharmacol. 2005;5:612–617. doi: 10.1016/j.coph.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 24.Choudhary NS, Tomar M, Chawla YK, Bhadada SK, Khandelwal N, Dhiman RK, Duseja A, Bhansali A. Hepatic osteodystrophy is common in patients with noncholestatic liver disease. Dig Dis Sci. 2011;56:3323–3327. doi: 10.1007/s10620-011-1722-y. [DOI] [PubMed] [Google Scholar]
- 25.Yenice N, Gümrah M, Mehtap O, Kozan A, Türkmen S. Assessment of bone metabolism and mineral density in chronic viral hepatitis. Turk J Gastroenterol. 2006;17:260–266. [PubMed] [Google Scholar]
- 26.Miroliaee A, Nasiri-Toosi M, Khalilzadeh O, Esteghamati A, Abdollahi A, Mazloumi M. Disturbances of parathyroid hormone-vitamin D axis in non-cholestatic chronic liver disease: a cross-sectional study. Hepatol Int. 2010;4:634–640. doi: 10.1007/s12072-010-9194-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bass S, Delmas PD, Pearce G, Hendrich E, Tabensky A, Seeman E. The differing tempo of growth in bone size, mass, and density in girls is region-specific. J Clin Invest. 1999;104:795–804. doi: 10.1172/JCI7060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saggese G, Bertelloni S, Baroncelli GI, Federico G, Calisti L, Fusaro C. Bone demineralization and impaired mineral metabolism in insulin-dependent diabetes mellitus. A possible role of magnesium deficiency. Helv Paediatr Acta. 1989;43:405–414. [PubMed] [Google Scholar]
- 29.El Karmouty KZ, Keddeas MW, ElSayed EY. Osteodystrophy in Hepatitis C virus Related Cirrhosis. Nature Sci. 2010;8:158–163. [Google Scholar]
- 30.López-Larramona G, Lucendo AJ, González-Castillo S, Tenias JM. Hepatic osteodystrophy: An important matter for consideration in chronic liver disease. World J Hepatol. 2011;3:300–307. doi: 10.4254/wjh.v3.i12.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.George J, Ganesh HK, Acharya S, Bandgar TR, Shivane V, Karvat A, Bhatia SJ, Shah S, Menon PS, Shah N. Bone mineral density and disorders of mineral metabolism in chronic liver disease. World J Gastroenterol. 2009;15:3516–3522. doi: 10.3748/wjg.15.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yurci A, Kalkan AO, Ozbakir O, Karaman A, Torun E, Kula M, Baskol M, Gursoy S, Yucesoy M, Bayram F. Efficacy of different therapeutic regimens on hepatic osteodystrophy in chronic viral liver disease. Eur J Gastroenterol Hepatol. 2011;23:1206–1212. doi: 10.1097/MEG.0b013e32834cd6f6. [DOI] [PubMed] [Google Scholar]
- 33.Bitetto D, Fabris C, Fornasiere E, Pipan C, Fumolo E, Cussigh A, Bignulin S, Cmet S, Fontanini E, Falleti E, et al. Vitamin D supplementation improves response to antiviral treatment for recurrent hepatitis C. Transpl Int. 2011;24:43–50. doi: 10.1111/j.1432-2277.2010.01141.x. [DOI] [PubMed] [Google Scholar]
- 34.Petta S, Cammà C, Scazzone C, Tripodo C, Di Marco V, Bono A, Cabibi D, Licata G, Porcasi R, Marchesini G, et al. Low vitamin D serum level is related to severe fibrosis and low responsiveness to interferon-based therapy in genotype 1 chronic hepatitis C. Hepatology. 2010;51:1158–1167. doi: 10.1002/hep.23489. [DOI] [PubMed] [Google Scholar]
- 35.Müller K, Bendtzen K. 1,25-Dihydroxyvitamin D3 as a natural regulator of human immune functions. J Investig Dermatol Symp Proc. 1996;1:68–71. [PubMed] [Google Scholar]
- 36.Hewison M. Vitamin D and the intracrinology of innate immunity. Mol Cell Endocrinol. 2010;321:103–111. doi: 10.1016/j.mce.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gutierrez JA, Jones KA, Fitzgerald RL, Branch AD, Schooley RT. Vitamin D metabolites inhibit replication of the hepatitis C virus. Hepatol Suppl. 2010;52:A803. [Google Scholar]
- 38.Gal-Tanamy M, Bachmetov L, Ravid A, Koren R, Erman A, Tur-Kaspa R, Zemel R. Vitamin D: an innate antiviral agent suppressing hepatitis C virus in human hepatocytes. Hepatology. 2011;54:1570–1579. doi: 10.1002/hep.24575. [DOI] [PubMed] [Google Scholar]
- 39.Matsumura T, Kato T, Sugiyama N, Tasaka-Fujita M, Murayama A, Masaki T, Wakita T, Imawari M. 25-Hydroxyvitamin D3 suppresses hepatitis C virus production. Hepatology. 2012;56:1231–1239. doi: 10.1002/hep.25763. [DOI] [PubMed] [Google Scholar]


