Abstract
Mixed adenoneuroendocrine carcinoma (MANEC) is a rare tumor of the gastrointestinal tract that consists of a dual adenocarcinomatous and neuroendocrine differentiation, each component representing at least 30% of the tumor. To date, only seven cases have been reported in the cecum, and less than 40 in the stomach. Our first case was diagnosed in a 74-years-old female as a polypoid lesion of the cecum with direct invasion in the transverse colon, without lymph node metastases. The second case was diagnosed in the stomach of a 46-years-old male as a polypoid tumor of the antral region that invaded the pancreas and presented metastases in 22 regional lymph nodes. The metastatic tissue was represented by the glandular component. In both cases, the tumor consisted of a moderately-differentiated tubular adenocarcinoma (with mucinous component in Case 1) intermingled with neuroendocrine carcinoma. Ki67 index was lower than 20% in Case 1, respectively higher than 20% in Case 2. The neuroendocrine component was marked by synaptophysin and neuron specific enolase, being negative for Keratins 7/20. The neuroendocrine component represented 60% in Case 1, and 40% in Case 2, respectively. The glandular components were marked by carcinoembryonic antigen, maspin and keratin 20/7 (Case 1/2). Both cases were proved to be microsatellite stable. Independently by the localization and tumor stage, MANECs appear to be highly malignant tumors, with high risk for distant metastases. The aggressiveness seems to depend on the endocrine component, independent of its proportion. The neuroendocrine component could be a dedifferentiated adenocarcinoma with a neuroendocrine phenotype.
Keywords: Mixed adenoneuroendocrine carcinoma, Composite tumor, Mixed tumor, Colorectal, Stomach, Cecum, Maspin, Carcinoembryonic antigen, Keratin 7, Keratin 20
Core tip: The aim of this paper was to report the clinicopathological data of two cases of mixed adenoneuroendocrine carcinomas (MANECs), one in the cecum and one in the stomach. MANEC is a rare tumor of the gastrointestinal tract that consists of a dual adenocarcinomatous and neuroendocrine differentiation. To date, only seven cases have been reported in the cecum, and less than 40 in the stomach. The characteristics of these cases, in correlation with data from literature, proved that MANEC is a highly malignant tumor, its aggressiveness being related to the endocrine component, independent of its proportion.
INTRODUCTION
The term mixed adenoneuroendocrine carcinoma (MANEC) was introduced by the World Health Organization in 2010 referring to a neoplasm with dual adenocarcinomatous and neuroendocrine differentiation, each component representing at least 30% of the tumor[1]. Before 2010, this tumor was reported as a mixed or composite tumor[2]. It should be distinguished from the collision tumor, in which the two components are closely juxtaposed but not admixed, and the amphicrine tumor with dual endo- and exocrine differentiation within the same cell[1,2]. Diagnosis is mainly based on the tumor architecture, being completed by the immunostains with specific neuroendocrine markers such as chromogranin, synaptophysin, CD56, and neuron-specific enolase (NSE), combined with the markers on non-endocrine differentiation such as keratin 7 (for gastric tumors) and Keratin 20, CDX2, and carcinoembryonic antigen (CEA), respectively, for colorectal segments.
MANECs have been described in several organs. Beside gastrointestinal segments, it was also reported in the pancreas, gallbladder, and uterine cervix[2]. However, this is an extremely rare tumor, with majority being presented as a case report. To date, in the English literature, only seven cases were reported to have occured in the cecum and about 35 cases in the stomach. Due to its rarity, few aspects regarding the origin and best therapeutic options are known.
In this paper, we present two unusual MANECs of the cecum and the stomach and a pertinent review of the literature.
CASE REPORT
Case 1
A 74-years-old female was admitted to the hospital with intestinal obstruction symptoms, and an emergency right hemicolectomy with terminal ileum resection was performed. Gross examination of the surgical specimen revealed a 70 mm × 18 mm polypoid tumor that produced obstruction of the cecum and presented direct invasion in the serosa of the transverse colon, without invasion in the appendix. The proximal and distal resected margins were free from tumor involvement. Histopathological examination of the surgical specimen confirmed the tumor infiltration of the transverse colon, without metastases in the 40 regional lymph nodes. Angiolymphatic invasion, without perineural invasion, was also noted (pT4bN0 stage). The tumor architecture was predominantly (60% of tumor) solid, consisting of clusters of monomorphic tumor cells with abundant cytoplasm and large nuclei, marked by synaptophysin and NSE, which did not display positivity for keratin AE1/AE3, keratins 7/20, CEA, and chromogranin. A low mitotic activity was seen (< 20 mitoses/10 HPFs). Among these tumor clusters, moderately differentiated glandular structures with focal intraluminal Alcian blue-positive mucus were also seen. The glandular component represented about 40% of the tumor, being marked by keratin AE1/AE3, keratin 20, and CEA, without positivity for chromogranin, synaptophysin, NSE, and keratin 7. Ki67 proliferative index was < 20% (G2), without differences among the two components. Maspin expression was cytoplasmic in the glandular component and slightly positive in the neuroendocrine areas (Figure 1). In the ascending colon, an adenomatous polyp without dysplasia was associated.
Figure 1.
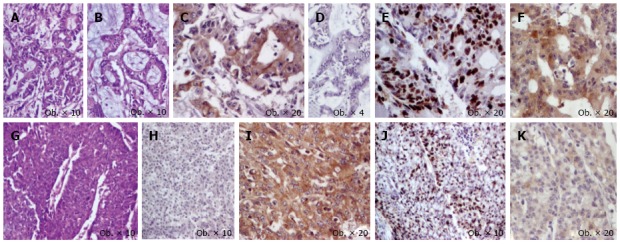
Cecum mixed adenoneuroendocrine carcinoma consists of proliferation of two components. First component is represented by glandular structures with mucinous foci (A-B) and displays positivity for keratin 20 (C) without immunoreactivity for synaptophysin (D), with a high Ki67 index (E) and cytoplasmic expression of maspin (F); The second component is represented by solid tumor islands (G) that are negative for keratin 20 (H) and are immunoreactive for synaptophysin (I), with a lower Ki67 index (J) and slightly cytoplasmic expression of maspin (K).
The molecular examinations, performed with Roche’s LightCycler PCR- and melting-point analysis, revealed a stable microsatellite status. MLH-1 and MSH-2 were positive in both tumor components.
Based on the tumor stage, a classical FOLFOX therapy was recommended. The postoperative course was uneventful, and to date, the patient has survived 10 mo without any evidence of recurrence or metastases.
Case 2
A 46-years-old male was admitted to the hospital with symptoms suggesting gastric cancer: weight loss, hematemesis and melena, without signs of carcinoid syndrome. On upper gastrointestinal endoscopy, a type I-Borrmann’s tumor was described in the antral region, which was surgically removed. Intraoperatively, direct invasion in the pancreas was identified. Total gastrectomy with D2 lymphadenectomy was performed. Gross examination of the surgical specimen revealed a 70 mm × 80 mm polypoid antral tumor that produced direct invasion in the pancreas. The tumor invaded the distal resection margin. Histopathological examination of the surgical specimen confirmed the pancreatic invasion, and the presence of metastases in 22 of the 32 lymph nodes, with peri lymphonodular invasion; celiac and hepatic hilum lymph nodes presented metastases. Angiolymphatic and perineural invasions were also noted (pT4N3b stage). The tumor architecture was predominantly (60% of tumor) glandular, with moderately differentiated glandular structures, without mucinous component, marked by keratin AE1/AE3, keratin 7, and CEA, without positivity for chromogranin, synaptophysin, NSE, HER-2, keratin 20, and vimentin. Among the glandular structures, solid sheets, cords, and islands of monomorphic tumor cells with scant cytoplasm and large pleomorphic nuclei were seen; they were immunoreactive for synaptophysin and NSE, and did not display positivity for keratin AE1/AE3, keratins 7/20, chromogranin, HER-2, CEA, and vimentin. Mitotic activity was high (> 20 mitoses/10 HPFs). Ki67 proliferation index was 80% (G3), without differences among the two components. Maspin expression was nuclear in the glandular component and negative in the neuroendocrine areas (Figure 2). In the metastatic lymph nodes, the glandular component was predominant.
Figure 2.
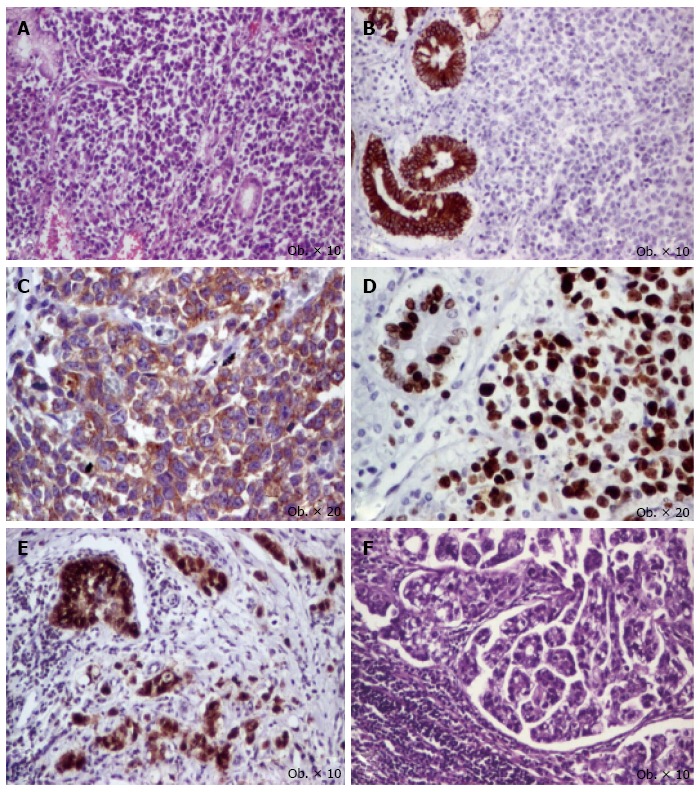
Gastric mixed adenoneuroendocrine carcinoma consists of mixed proliferation of glandular with specific immunoexpression. Solid tumor areas (A); The glandular structures are reactive for keratin AE1/AE3 (B) and solid structures express synaptophysin (C); A high Ki67 index can be seen in both tumor components (D); The nuclear expression of maspin in the glandular component (E) reflects the tumor aggressiveness that is confirmed by predominance of this component in lymph node metastases (F).
The molecular examinations, performed with Roche’s LightCycler PCR- and melting-point analysis, revealed a stable microsatellite status. MLH-1 and MSH-2 were positive in both components. The patient refused chemotherapy and died 5 mo after surgery.
DISCUSSION
The cecum MANEC was first described by Cardier in 1924, and another six cases have been reported later[3]. In the colorectal segments, independently by localization, a polypoid lesion has been reported arising in patients aged around 60 years, with the male/female ratio being about 1.5:1. All seven cases of cecum MANEC were reported in women. In majority of the cases, MANEC presents with an aggressive behavior and a high risk for liver metastases[3,4]. Independent from the proportion of the neuroendocrine component, the associated-carcinoid syndrome was not reported yet in the literature; the serum level of tumor markers such as CEA, CA125, and CA19-9 are also normal[1]. The clinical behavior seems to be influenced by the neuroendocrine component[1]. However, identification of the neuroendocrine component in tubular adenocarcinomas is not easily performed because the neuroendocrine cells are not always immunoreactive for specific markers, with the reported rate of positivity being 60%-70% for chromogranin, 75%-90% for synaptophysin, and 50% for CD56[1,5,6]. In our case, the neuroendocrine component was diffusely positive for synaptophysin and negative for chromogranin. The cytoplasmic expression of maspin in the glandular structures indicates an indolent behavior of this component[7], it’s negativity in the neuroendocrine areas confirming that the clinical outcome depends on the characteristics of the neuroendocrine carcinoma. Angiolymphatic invasion was noted in our cases without any lymph node metastases.
The reported clinicopathologic characteristics of gastric MANEC are similar with those of colorectal segments, the non-endocrine component being rather an intestinal type rather than a diffuse-type gastric carcinoma. The mean age is 58 years old (range: 30 to 80 years old), and the polypoid lesions are usually located in upper, middle, or lower third of the stomach[6]. Although some authors sustain that clinical behavior depends on the grade of the neuroendocrine component, some of them reveal that the characteristics of adenocarcinomatous part influence the outcome in well-differentiated neuroendocrine components[6,8]. In our case, the glandular component was predominant in lymph node metastases and nuclear expression of maspin in glandular structures compared with its negativity in the neuroendocrine part confirmed the higher aggressiveness of the glandular part, compared to the poorly differentiated neuroendocrine one[7].
Due to the rarity of this tumor, few aspects are known about its histogenesis, with most of the authors admitting its origin in a multipotent stem cell with bidirectional differentiation, opposite to collision tumor, in which a separate origin of the two components is supposed[1-3,6]. Because the poorly differentiated areas can also display positivity for synaptophysin in colorectal carcinomas[9], we tend to believe that it is not about a real multidirectional differentiation of a single neoplasm but rather a neuroendocrine phenotype of dedifferentiated areas of tubular adenocarcinoma. To sustain our hypothesis, it is necessary to also take into account the expression of CD133, the marker of cancer stem cells. Recently, it was reported that CD133 was expressed in 30% of well-differentiated neuroendocrine tumors, in 26% of poorly differentiated neuroendocrine carcinomas, and in 64% of MANEC of the digestive tract[10]. On the other hand, the proportion of CD133 positivity in colorectal adenocarcinomas was 47%, without correlation with the tumor grade but with a strong correlation with the tumor aggressiveness[11]. In gastric adenocarcinoma, the CD133 positivity was 42%, being also correlated with the clinical outcome[12]. Comparing the proportion of CD133 positivity, we can also support that the neuroendocrine component of MANEC, independent of its location, is rather a dedifferentiated area of a classic adenocarcinoma.
Clarifying this hypothesis could help identify of the proper therapeutic management of these rare, highly malignant tumors, similar to that of ordinary adenocarcinomas or for neuroendocrine tumors.
COMMENTS
Case characteristics
A 74-years-old female with a locally advanced tumor of the cecum (Case 1) and an 46-years-old male with a node positive gastric cancer (Case 2).
Clinical diagnosis
Intestinal obstruction in Case 1, and weight loss, hematemesis, and melena, in Case 2.
Differential diagnosis
Intestinal infarction in Case 1, chronic peptic ulcer in Case 2.
Laboratory diagnosis
Non-specific - slight anemia in both of the cases (low hemoglobin and hematocrit); liver function tests were within normal limits.
Imaging diagnosis
Case 1-an emergency hemicolectomy was performed without supplementary examinations. Case 2-CT and MRI examinations were not performed. The upper gastrointestinal endoscopy revealed a type I - Borrmann’s tumor in the antral region.
Pathological diagnosis
Examination of surgical specimens from cecum (case 1) and stomach (case 2) revealed a mixed adenoneuroendocrine carcinoma (MANEC) in both of the cases, that was immunohistochemically confirmed.
Treatment
Case 1 - the patient was treated with FOLFOX regimen; Case 2 - the patient refused the chemotherapy.
Experiences and lessons
This case report shows two microsatellite-sable MANECs of the gastrointestinal tract, tumors that are not only rare but also seems to have an aggressive behavior that depends on the characteristics of the neuroendocrine component, independend of its proportion. Because this component seems to be a deddiferentiated adenocarcinoma with acquired neuroendocrine properties, a very attentive evaluation should be microscopically performed in adenocarcinomas with dedifferentiated areas. Identification of the neuroendocrine components could have therapeutic relevance.
Peer review
This article applies a large panel of molecular markers, including the prognostic marker maspin, to explore the origins and behavior of gastrointestinal MANEC in two cases with distinct characteristics.
Footnotes
Supported by Romanian government, the research project frame POSDRU/159/1.5/S/136893, and University of Medicine and Pharmacy of Tirgu-Mures, Romania, the team research project POS-UMFTGM-CC-13-01-V01, No. 15/16189/2013.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: June 6, 2014
First decision: July 21, 2014
Article in press: September 30, 2014
P- Reviewer: Lorenzo-Zuniga V, Wang RZ S- Editor: Qi Y L- Editor: A E- Editor: Wang CH
References
- 1.Kitajima T, Kaida S, Lee S, Haruta S, Shinohara H, Ueno M, Suyama K, Oota Y, Fujii T, Udagawa H. Mixed adeno(neuro)endocrine carcinoma arising from the ectopic gastric mucosa of the upper thoracic esophagus. World J Surg Oncol. 2013;11:218. doi: 10.1186/1477-7819-11-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Faggioni R, Streiff EB. [Treatment of sequelace of corneal wounds] Annee Ther Clin Ophtalmol. 1977;28:33–47. [PubMed] [Google Scholar]
- 3.Jain A, Singla S, Jagdeesh KS, Vishnumurthy HY. Mixed adenoneuroendocrine carcinoma of cecum: a rare entity. J Clin Imaging Sci. 2013;3:10. doi: 10.4103/2156-7514.107995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ito H, Kudo A, Matsumura S, Ban D, Irie T, Ochiai T, Nakamura N, Tanaka S, Tanabe M. Mixed adenoneuroendocrine carcinoma of the colon progressed rapidly after hepatic rupture: report of a case. Int Surg. 2014;99:40–44. doi: 10.9738/INTSURG-D-13-00161.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang YH, Lin Y, Xue L, Wang JH, Chen MH, Chen J. Relationship between clinical characteristics and survival of gastroenteropancreatic neuroendocrine neoplasms: A single-institution analysis (1995-2012) in South China. BMC Endocr Disord. 2012;12:30. doi: 10.1186/1472-6823-12-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim JJ, Kim JY, Hur H, Cho YK, Han SU. Clinicopathologic significance of gastric adenocarcinoma with neuroendocrine features. J Gastric Cancer. 2011;11:195–199. doi: 10.5230/jgc.2011.11.4.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gurzu S, Szentirmay Z, Popa D, Jung I. Practical value of the new system for Maspin assessment, in colorectal cancer. Neoplasma. 2013;60:373–383. doi: 10.4149/neo_2013_049. [DOI] [PubMed] [Google Scholar]
- 8.Lee JH, Kim HW, Kang DH, Choi CW, Park SB, Kim SH. A gastric composite tumor with an adenocarcinoma and a neuroendocrine carcinoma: a case report. Clin Endosc. 2013;46:280–283. doi: 10.5946/ce.2013.46.3.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gurzu S, Serester O, Jung I. Possible neuroendocrine phenotype of poorly differentiated cell clusters in colorectal carcinoma, as a prognostic parameter. Am J Surg Pathol. 2014;38:143–144. doi: 10.1097/PAS.0000000000000118. [DOI] [PubMed] [Google Scholar]
- 10.Mia-Jan K, Munkhdelger J, Lee MR, Ji SY, Kang TY, Choi E, Cho MY. Expression of CD133 in neuroendocrine neoplasms of the digestive tract: a detailed immunohistochemical analysis. Tohoku J Exp Med. 2013;229:301–309. doi: 10.1620/tjem.229.301. [DOI] [PubMed] [Google Scholar]
- 11.Zhou F, Mu YD, Liang J, Liu ZX, Chen HS, Zhang JF. Expression and prognostic value of tumor stem cell markers ALDH1 and CD133 in colorectal carcinoma. Oncol Lett. 2014;7:507–512. doi: 10.3892/ol.2013.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen S, Hou JH, Feng XY, Zhang XS, Zhou ZW, Yun JP, Chen YB, Cai MY. Clinicopathologic significance of putative stem cell marker, CD44 and CD133, in human gastric carcinoma. J Surg Oncol. 2013;107:799–806. doi: 10.1002/jso.23337. [DOI] [PubMed] [Google Scholar]


