Abstract
A host of diabetes-related insults to the central nervous system (CNS) have been clearly documented in type-1 and -2 diabetic patients as well as experimental animal models. These host of neurological disorders encompass hemodynamic impairments (e.g., stroke), vascular dementia, cognitive deficits (mild to moderate), as well as a number of neurochemical, electrophysiological and behavioral alterations. The underlying causes of diabetes-induced CNS complications are multifactorial and are relatively little understood although it is now evident that blood-brain barrier (BBB) damage plays a significant role in diabetes-dependent CNS disorders. Changes in plasma glucose levels (hyper- or hypoglycemia) have been associated with altered BBB transport functions (e.g., glucose, insulin, choline, amino acids, etc.), integrity (tight junction disruption), and oxidative stress in the CNS microcapillaries. Last two implicating a potential causal role for upregulation and activation of the receptor for advanced glycation end products (RAGE). This type I membrane-protein also transports amyloid-beta (Aβ) from the blood into the brain across the BBB thus, establishing a link between type 2 diabetes mellitus (T2DM) and Alzheimer’s disease (AD, also referred to as “type 3 diabetes”). Hyperglycemia has been associated with progression of cerebral ischemia and the consequent enhancement of secondary brain injury. Difficulty in detecting vascular impairments in the large, heterogeneous brain microvascular bed and dissecting out the impact of hyper- and hypoglycemia in vivo has led to controversial results especially with regard to the effects of diabetes on BBB. In this article, we review the major findings and current knowledge with regard to the impact of diabetes on BBB integrity and function as well as specific brain microvascular effects of hyper- and hypoglycemia.
Keywords: Diabetes mellitus, Blood–brain barrier, Glucose transporter, P-glycoprotein, Oxidative stress, Permeability, Tight junctions, in vitro
Introduction
Diabetes mellitus (DM) is a multi-faceted metabolic syndrome and currently one of the major health concerns in public health across the globe. DM is characterized by high rates of mortality and morbidity, especially in relation to T2DM [1]. Both, insulin-dependent (type 1) and independent (type 2) DM, have detrimental effects on the structure integrity and function of vascular beds, underlying the pathophysiology and development of various peripheral and CNS disorders. Hyperglycemia-elicited complications at the microvascular level include low perfusion rates, thickening of capillary walls and abnormal proliferation of endothelial cells with increased vascular permeability (both in vitro and in vivo including DM patients). The pathophysiology of microvascular complications in diabetes encompasses major biochemical pathways while the common endpoint appears to be mitochondrial superoxide overproduction in the endothelial cells lining the vascular walls of the blood vessels. The increased superoxide production causes the activation of four major pathways involved in the pathogenesis of complications: increase in polyol and hexosamine pathways flux, activation of Protein Kinase C (PKC) and increased formation of advanced glycation end product (AGE) ligands originating from proteins, lipids and nucleic acids (e.g., LDL) [2,3]. RAGE activation initiates a vicious cycle eliciting more oxidative stress generation [3,4] and subsequently evoking vascular inflammation [5] and thrombosis [6], thereby implicating a potential vascular damage [7,8]. Furthermore, the overproduction of reactive oxygen species (ROS) inactivates endothelial nitric oxide synthase (eNOS) and prostacyclin synthase, thereby impairing the vascular tone [2,9,10].
A growing body of evidence from recent clinical and experimental studies suggests that prolonged hyperglycemic conditions, particularly in type 2 DM, elicit a progressive impairment of neuronal function in the brain [10]. Stroke and cerebral ischemia are typical CNS complications related to diabetes due to the impairments in cerebral vascular supply [11]. Diabetic patients are also at higher risk of experiencing stroke than normal population [11-13] and 50% of stroke-affected individuals have been diagnosed with hyperglycemia [14]. It is also reported that subjects with type 2 DM have significantly lower brain volume and are more likely to have single or multiple cerebral infarcts compared to normoglycemic individuals [13]. In addition, preclinical studies in mice suggest that vascular injury occurring in response to an ischemic insult following stroke is significantly exacerbated in diabetic subjects [15] and the situation is further worsened by recurrent hypoglycemia [16]. Type 2 diabetes can negatively impact the outcome of stroke (ischemic brain damage); in fact increases the risk of stroke, as demonstrated in vivo in type 2 diabetic mice [15]. Conversely, hyperglycemia is also associated with high levels of mortality and morbidity during cerebral ischemia, perhaps, caused by increased cerebral hematoma expansion [14] and higher risk of cerebral hemorrhage due to tissue Plasminogen Activator (tPA) activation and superoxide production damaging the BBB [17] Recent studies also evoke a role for the AGE-RAGE system activated by hyperglycemia leading to a further enhancement of oxidative stress and amplification of inflammatory signals from nearby leukocytes [18,19]. Improved glycemic control in these patients seem to ameliorate these pathological conditions [10] however, rapid normalization of plasma glucose levels in hyperglycemic subjects can lead to cerebral hypoglycemia thus favoring cognitive decline [20-25]. Other studies have demonstrated an association between altered glycemic conditions and alterations of the electrophysiological, structural and neurochemical profiles of brain function [26] which can impair neuronal plasticity and synaptic transmission [9,10].
T2DM has been strongly associated with mild cognitive impairments [24,27] and is considered a predisposing factor for developing vascular dementia [28] and Alzheimer disease [22,29]. Furthermore, DM has also been associated with increased severity of epileptic seizures [30] and risk of mortality following traumatic brain injury (TBI) [31]. Frequent co-occurrence of psychiatric disorders (such anxiety and depression) [32] have also been recorded in DM patients. These large onset of CNS comorbidities in diabetic patients is not entirely surprising considering the cerebrovascular (such as reduced vascular tone, BBB leakage, inflammation, thrombosis [4,33-38]) and metabolic (hyper/hypoglycemia and hyperinsulinemia [34,39,40]) abnormalities that characterize DM.
Altered glycemic conditions such as those observed in diabetic patients are prodromal to blood-brain barrier (BBB) impairment [34,41-46]. This is of paramount relevance for the pathogenesis of brain disorders in DM since the BBB act as a gate keeper of the brain with a range of interrelated functions including protecting the CNS from potentially harmful substances, regulate transport of essential molecules, maintain the brain homeostasis, and provide immune regulatory functions [47].
Unfortunately, despite the existence of a large body of data regarding DM-induced microvascular complications of the kidney and retina, the impact of DM at the level of the cerebrovascular system is still poorly understood from a mechanistic point of view and under-investigated. Thus, advancements in this specific field can reveal unique pharmacological targets for the development of novel and more effective drugs for the treatment and/or prevention of diabetes-associated CNS complications.
BBB: Structure, Function and Glucose Transport
The BBB has been described in detail both physiologically and morphologically [48]. At the cellular level, the BBB is constituted by vascular endothelium lining the cerebral microvessels with the closely apposed astrocytic end-feet processes (Figure 1) [49]. The BBB endothelium is characterized by distinctive expression patterns of trans-membrane transport systems to regulate traffic of substances in and out of the brain parenchyma [50,51]. In addition, expression of inter-endothelial tight junctions, lack of fenestrations and minimal pinocytic transport which concur in the regulation and maintenance of the brain microenvironment are the unique features of the BBB endothelium. Tight junctions between adjacent endothelial cells form a diffusion barrier, which selectively excludes most blood-borne (including electrolytes and other water soluble compounds) and xenobiotic polar substances from entering the brain through paracellular routes. Specialized efflux transport mechanisms (e.g., P-glycoprotein, breast cancer resistant protein/BCRP, multidrug resistance protein-4/MRP-4 etc.) are also in place to regulate the passage of amphipathic and hydrophobic molecules and protect the brain from potentially harmful substances.
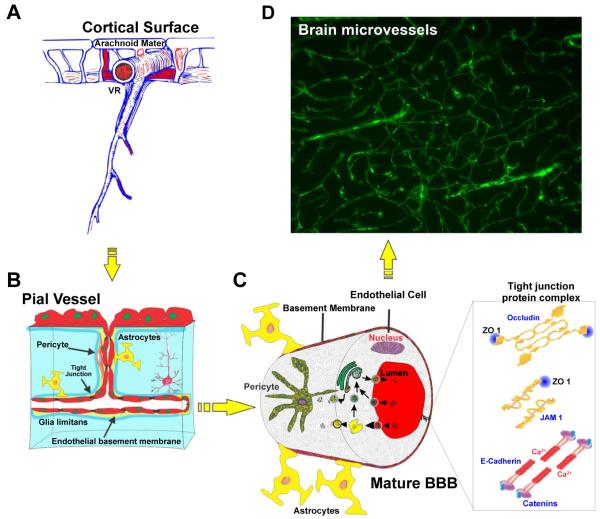
Figure 1.
Structure and location of brain microvessels. (A) Gross view of brain microvessels crossing through brain parenchyma, (B and C) Schematics of the inner view of the brain microvessels lined with closely associated endothelial cells together with pericytes and astrocytic foot processes, (D) FITC albumin stained brain section showing the overall network of brain microcapillaries. Note: VR (Virchow-Robin space).
Glucose is the primary brain bioenergetic source. While the CNS utilizes approximately 25% of the total glucose, it only accounts for ~2% of the total body mass [52]. Besides glucose, lactate (from astrocytes) also provides energy to neurons [52,53]. Glucose crosses the BBB through two independent groups of transporter proteins: facilitative sodium independent transporters (GLUT) and sodium dependent glucose co-transporters (SGLT) [54,55]. Facilitative glucose transporters (GLUT) include 14 proteins [56] of which GLUT-1 is the first identified member and a major transporter of glucose across BBB [55,57,58]. This is evident from the clinical reports in which patients with GLUT-1 deficiency syndrome had CSF/blood glucose ratio of 0.19 - 0.35 (vs. the normal value of 0.65), developed seizures and other development disorders [57]. In contrast to SGLT, GLUT proteins are saturable transporters and help in movement of glucose along the concentration gradient [53,59]. While GLUT-1 transporters in brain microvascular endothelial cells and astrocytes carry glucose across the BBB, GLUT-3 is considered the main (although not the exclusive) neuronal glucose transporters [57]. GLUT-1 transporters in mature and fully differentiated BBB endothelium are heterogeneously distributed with studies reporting higher luminal to abluminal ratio in human brain microvessels [60,61], while the opposite holds true in rats and other species [62,63]. This heterogeneity correlates to the variable energy demands, cerebral glucose utilization and maintenance of normal glucose level [64,65] with reports suggesting an increase in their local densities, due to increase in local cerebral glucose utilization [53]. More specifically, increased glucose transport across BBB correlated with increased luminal density of GLUT-1. On the other hand, higher abluminal expression (lower luminal/abluminal ratio) was observed when GLUT-1 is down-regulated [66] suggesting that luminal-abluminal redistribution and/or expression of GLUT-1 modulates glucose entry into the brain (Figure 2). SGLT-1 plays a major role in transport of glucose across intestinal membrane and renal proximal tubule. Although the role of SGLT-1 in CNS glucose transport has not been fully investigated, their presence on brain microvascular endothelial cells and involvement in transporting glucose in certain pathophysiological conditions (e.g., oxygen glucose deprivation-ischemia) has been observed [59]. Unpublished data from our group revealed up-regulation of SGLT-1 expression in a well-established human BBB endothelia cells line (hCMEC/D3 [67]) following either acute or chronic exposure to hypoglycemia.
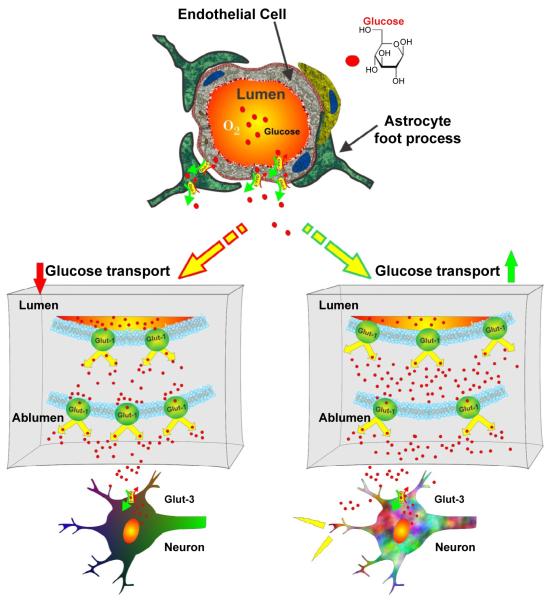
Figure 2. Glucose transport across the BBB.
Glut1 transporters located on microvascular endothelial cells and astrocytes transport glucose across the BBB, while Glut-3 transports glucose in neurons. Heterogeneous distribution of Glut-1 transporters is a typical hallmark of a fully mature BBB and correlates to the variable energy demands, glucose utilizations and maintenance of normal glucose level.
Insulin-sensitive glucose transporters (GLUT-4) and insulin receptors on BBB endothelial cells also may play a central role in regulating glucose transport into CNS. Very little or no insulin is synthesized by the brain in normal conditions; although it has been shown that DM increases its transport across the BBB [39,68-70]. in vivo, insulin can access the brain cells both via the cerebrospinal fluid reaching through regions lacking proper BBB (e.g. circumventricular organs), and directly through the BBB via specific insulin receptors that can act as transporters [71,72]. The effect of insulin on CNS is of particular interest in view of a recent body of evidence suggesting that DM could affect the pathogenesis of AD through mechanisms involving alterations in downstream signaling of the insulin receptor at BBB. Although additional studies are required to shed full light on the mechanisms involved, insulin may have a role in regulating phosphorylation of tau in tauopathies - via activation of glycogen synthase kinase-3 and amyloid-β peptides [73]) as well as the expression (up-regulation) of soluble receptors for advanced glycation end products (sRAGE) [74]. High levels of sRAGE have been associated with incident cardiovascular disease and all-cause mortality in type 1 [75] and 2 diabetes [76] and may also reflect tissue RAGE expression in DM [77]. Thus, according to recent studies, activation of the AGE-RAGE system seems to plays a central role in the pathogenesis of vascular abnormalities and thrombotic insult observed in DM patients [4,6].
Ultimately, alteration of BBB function and integrity can have a profound impact on the CNS being prodromal to the pathogenesis and progression of major neurological disorders. Thus diabetes-dependent impairment of BBB function can severely impact the CNS.
Effect of hyperglycemia on BBB glucose transport in DM
On the basis of common understanding it is believed that when a substrate is in excess, body will down-regulate the receptor expression for it, in order to balance its demand and supply cycle. This suggests that the biological answer to chronic excess of glucose in the systemic circulation should be down regulation of its transporters. In this line, many experimental studies report a down-regulation of BBB glucose transporters in hyperglycemic animals [63,78,79], indicating the measures taken by brain from preventing excessive glucose intake. However, this is not a universal scientific consensus since other studies (in both animals and humans) did not reveal significant changes in the BBB glucose transporters expression in DM [41,80-83].
Glucose transporter expression studies performed in streptozotocin (STZ)- induced diabetic rats showed that chronic hyperglycemia down-regulates both mRNA and protein expression of GLUT-1 and GLUT-3. Down-regulation of glucose transporters was independent of the method used to induce DM in vivo [78]. Local cerebral glucose utilization during chronic but not acute hyperglycemia was increased in DM Sprague–Dawley rat models. This was paralleled by a moderate (yet significant) decrease in expression of GLUT-1 in brain vessels although no changes in GLUT-3 were observed [63].
in vivo studies in pig models undergoing continuous glucose monitoring showed alteration of brain glucose similar to that measured locally in muscle and subcutaneous fat tissue in response to hyperglycemia (although in hypoglycemic conditions the glucose levels in the brain decreased on a similar time scale as the other tissues but to a much lesser degree) [82]. On the other hand, no significant changes in glucose uptake and GLUT-1 expression were observed in STZ-induced diabetic rats [41,84]. Further, high-field magnetic resonance spectroscopy study in humans by Seaquist and colleagues did not reveal major changes in global cerebral blood flow (CBF) or regional glucose metabolism (including maximal transport velocity of glucose) after acute hyperglycemia [83]. Nevertheless, such conflicting in vivo data with regard to BBB GLUT expression can be plausibly explained by differences in experimental approaches, animal models and methods of analyses. Further investigation based on common experimental ground with regard to model platforms, method, and analysis will be required to validate the results and reach a consensus over the final conclusions. To this end, in vitro human models could certainly help dissecting out specific aspects of DM-related alteration of glucose transport at the BBB which are currently impractical or unfeasible in in vivo or in human studies.
Hypoglycemia and glucose transport across the BBB
Hypoglycemia is of major concern in diabetes as it leads to severe impairment of CNS function. Frequent treatment regimen to reduce glucose plasma concentration in DM patients, triggers repetitive hypoglycemic insults to the brain cells and induce hypoglycemia-associated autonomic failure (HAAF). The general concept underlying HAAF is that defective glucose counter-regulation (lack of adrenomedullary epinephrine response which under normal circumstances would reduce insulin concentration and increase glucagon) and hypoglycemia unawareness lead to a vicious cycle of recurrent hypoglycemia and further impairment of glucose counter-regulation. The clinical relevance of this phenomenon is now well established, but the precise mechanisms and mediators remain largely unknown [85,86].
Majority of diabetic patients face a great difficulty in maintaining normal blood glucose level due to HAAF and hypoglycemia unawareness. Using [1H] nuclear magnetic resonance (NMR) spectroscopy, Criego et al. [87] measured the in vivo steady state brain glucose concentrations under controlled metabolic conditions to understand whether subjects with hypoglycemia unawareness would have higher brain glucose concentrations than control subjects. They concluded that brain glucose concentration was higher in the hypoglycemia unaware group by 17 ± 6% in comparison to the control group [87]. However, Segel et al. reported contradictory data (measured by using 1-11C glucose Positron Emission Tomography) showing no changes in glucose transport from blood to brain, blood flow to brain and cerebral glucose metabolism between healthy subjects and patients subjected to 24 hrs interprandial hypoglycemia [88]. Brain glucose concentration in sixteen human subjects subjected to recurrent hypoglycemia (three hypoglycemic clamps for 30 min at 0 hr, 9 hr, and 24 hr intervals) was not different from the results obtained from the same subjects during a control study [89]. Even, prior exposure to recurrent hypoglycemia in diabetic rats did not result in an increase in extra cellular fluid glucose concentration in the inferior colliculus [90]; thereby suggesting short episodes of recurrent hypoglycemia do not alter transport or metabolism of brain glucose. However, increased expression of GLUT-1 mRNA and protein at the BBB in rodent model of chronic hypoglycemia suggest the existence of a compensatory mechanism to increase glucose transport activity across the BBB in response to chronically low circulating blood glucose levels [91].
Increased brain glucose uptake under hypoglycemic condition seems to be due to both increased GLUT-1 synthesis and redistribution across BBB [91]. Brain glucose extraction [measured by Brain Uptake Index] increased in experimental hypoglycemia group versus controls (independently from the method used to induce hypoglycemia in vivo such as implanting insulin secreting tumors, insulin osmotic mini-pumps or repeated injections [92]). Similar observations were made by Lei et al. in rats following 12-14 days of hypoglycemia. 48% increase in brain glucose levels was observed, while brain glycogen concentration remained unaffected (although, others have reported decreased brain glycogen level in hypoglycemic conditions) [93]. Further, hypoglycemia resulted in 25-45% increase in glucose uptake, 23% increase in total GLUT-1 expression and redistribution to the luminal (vascular) side of BBB endothelium [41]. Increased GLUT-1 expression and luminal redistribution further aggravates the condition by increasing the transport of glucose across the BBB. In addition, acute (or mild) hypoglycemia was shown to up-regulate, GLUT-1, GLUT-4, angiotensinogen and mitogen-activated protein kinase phosphatase-1 [94]. Increase in angiotensinogen expression can favor vasodilation, thereby increasing local blood flow. Alternatively, increased local blood flow raises glucose level locally leading to hypothalamic overestimation of cerebral blood glucose which results in counter regulatory imbalance [94]. See Table 1 for a summary of glycemia-dependent alteration of glucose transport.
Table 1.
Effects of hyperglycemia and hypoglycemia on BBB glucose transporter expression.
Transport of amino acids across the BBB in DM
Choline is the precursor for the neurotransmitter acetylcholine, which is involved in variety of functions including memory and muscle control. Choline enters the brain through a saturable transport mechanism at the BBB. Reduced choline transport across the BBB was observed in streptozocin-induced diabetic rats when the kinetics of transport was compared to age-matched vehicle-injected controls [95]. The effect was statistically significant only in long standing diabetic animals (9 wks) thus, suggesting a time dependent effect of diabetes on choline transport. In parallel studies using continuous infusion quantitative autoradiograph methods, Mans and colleagues determined the regional permeability-times-surface area (PS) product and influx for several plasma amino acids in streptozotocin-diabetic rats. Transport of branched chain neutral amino acids was increased, whereas that of all basic amino acids and some essential amino acids (such as tryptophan, phenylalanine, methionine, and lysine) was decreased. Interestingly, alterations in the plasma concentrations of these amino acid rather than alteration of the transport mechanisms at the BBB were primarily responsible for the observed effects [96].
Impact of diabetes on BBB integrity and other pathophysiological changes
Experimental evidence from in vitro and in vivo studies has shown that BBB integrity in diabetes is somewhat compromised resulting in increased barrier permeability [34,45,46,97]; although current data do not provide conclusive results and outcomes remain controversial. in vitro BBB studies using co-culture of human brain microvascular endothelial cell (HBMEC) with juxtaposed human astrocyte (HA) showed loss of BBB integrity (measured in terms of trans-endothelial electrical resistance, TEER) under hyperglycemic culture conditions (25 mM D-glucose) maintained for five days. BBB integrity normalized upon re-establishment of normoglycemic conditions (5mM D-Glucose) or upon treatment with antioxidants [98]. A significant increase in expression of pro-inflammatory cytokines (TNF-α, IL-6, IL-1, IL-4), followed by activation of Nuclear Factor kappa-light chain-enhancer of activated B cells (NF-κB) and Signal Transducer Activator of transcription 3 (STAT3) inflammatory pathways was reported in other studies investigating the impact of hyperglycemia on human astrocytes (HA) [99]. Closely associated astrocytic end-feet processes contribute to modulate ECs differentiation in addition to induction and maintenance of the BBB properties. There is also evidence showing inhibition of astrocytic gap junctional communication in tissue culture and brain slices obtained from diabetic rats. Increased production of reactive oxygen-nitrogen species was also noted although the exact mechanism remains unclear [100,101]. Further, increased level of vascular endothelial growth factor (VEGF; a pro-angiogenic factor) in response to advanced glycation end-products was also reported [102].
Protein expression and transcriptional activity of hypoxia-inducible factor-1α (HIF-1α) was up-regulated in endothelial cell cultures by high glucose levels (30mM). In addition, the expression level of VEGF - a downstream vascular effector of HIF-1α was also increased. VEGF enhances and supports the translocation of GLUT-1 to the cell surface at the BBB besides promoting angiogenesis and decreasing expression of inter-endothelial tight junction proteins (e.g., ZO-1 and occludin [103]), thus increasing BBB permeability. Morphometric analysis using colloidal gold particles (GPs), showed that the immunosignal density for occludin was significantly lower in the brain microvessels of diabetic mice in comparison to controls [104].
On similar lines, inhibition of VEGF expression improved occludin and ZO-1 expression thereby attenuating inter-endothelial leakage. In accordance, down-regulation of the HIF-1α activity by the use of inhibitors improved BBB integrity and tightness [105].
Experimental studies with contrasting results have been reported in humans where plasma VEGF levels decreased during hyperglycemia and increased during hypoglycemia; with a convincible explanation that this is probably a neuroprotective mechanism to maintain a constant cerebral glucose supply [106]. Overall, these findings implicate the critical role for VEGF, as a vascular permeability factor, in hyper and hypoglycemia-induced BBB dysfunction.
In addition to VEGF, recent studies provide evidence for a major role of matrix metalloproteinases (MMP) in altered glycaemia-induced loss of BBB integrity. For example, BBB permeability to 14C sucrose (a well-known paracellular marker) increased in response to increased expression of MMP-2 (also paralleled by down-regulation of occludin and ZO-1) in diabetic rats [34,107-109]. Similar to VEGF, advanced glycation end-products were shown to stimulate the release of MMP-2 [102]. See Table 2 for a summary of glycemia-dependent alteration of BBB tight junction and EC adhesion molecules.
Table 2.
Effects of DM on BBB TJ proteins.
Acute transient hyperglycemia has also been reported to cause early inflammation and endothelial injury. This was clearly outlined in middle cerebral carotid artery occlusion (MCAO) rat model of ischemia-reperfusion injury. Increase in high-mobility group box 1 (HMGB1) and intercellular adhesion molecule-1 (ICAM-1) levels during ischemia-reperfusion were observed in both mild hyperglycemia (blood glucose ~150 mg/dL) and transient severe hyperglycemia (blood glucose ~400 mg/dL) with significant impact on BBB integrity [110]. These results were further reiterated by subsequent studies showing a marked increase of ICAM-1 positive stained cortex microvessels in diabetic rats after 3 days of reperfusion which was also paralleled by increase in IL-1β expression [109]. It is possible that the increased HIF-1α and VEGF expression in response to hyperglycemia synergistically adds to the detrimental BBB response elicited by flow-cessation [111], thus further increasing the loss of barrier integrity observed during reperfusion. However this hypothesis needs to be confirmed.
Regional as well as whole brain permeability increased by more than 100% in diabetic ketoacidosis patients (13 children) under observation right from initiation of study through the treatment period. Level of inflammatory cytokines also significantly increased in these patients. Diabetic ketoacidosis is a result of type 1 diabetes in children wherein increased BBB permeability results in complications like cerebral edema [112]. Absence of tight junction proteins like occludin, claudin-5, ZO-1 and JAM-1; albumin extravasation; and presence of increased neuroinflammatory markers chemokine CC ligand 2 (CCL2), NF-κB and nitrotyrosine were also observed in a similar study. This clearly suggests that neuroinflammation combined with loss of BBB integrity (TJ proteins) plays a primary role in the pathogenesis of brain edema in these patients [108]. All together, these data strongly support a major impairing role of diabetes on BBB integrity and maintenance of brain homeostasis prodromal to the onset of major neurological disorders.
Hyperglycemia also hinders the supply of vitamin C (ascorbic acid) to both retina and brain. Vitamin C is mainly transported across the BBB by GLUT-1 as dehydroascorbic acid (DHA) and is accumulated in the form of ascorbic acid. Vitamin C is involved in the biosynthesis of collagen, catecholamine and peptide neurohormones. It acts as an antioxidant and scavenges the free radicals, thereby detoxifying the brain [113-115]. 14C DHA transport was reduced by 84.1% in streptozotocin-induced diabetic rats. In addition, P-glycoprotein (P-gp: a member of ATP-binding cassette transporter glycoprotein) expression levels in diabetic mice have been contrastingly reported to decrease [116,117], increase [118] or remain unaffected [119] by others. Further, Insulin therapy was reported to restore the increased P-gp levels in STZ-induced diabetic rats [117]. Additional histological changes and vascular abnormalities observed at the level of the brain microcirculation in diabetes include thickening of the capillary basal membrane, collagen deposition, accumulation of lipid peroxidation by-products and endothelial degeneration at the level of the cerebral microvasculature [120]. Ultimately diabetes can lead to abnormal cerebral neovascularization and remodeling [121], which may further contribute to vascular damage and risk of hemorrhage associated with stroke or neurodegenerative processes in diabetes.
Oxidative stress at BBB in DM
Understanding the brain metabolic pathways involved in energy production is imperative to unravel how diabetes ultimately causes oxidative stress which can lead to early inflammation and endothelial injury.
Glucose metabolism (Figure 3) and energy production starts with glycolysis where glucose undergoes a series of metabolic steps to produce lactate (anaerobic metabolism end product) or pyruvate which can be further processed to extract more energy. Under normal condition the extensive bioenergetic demand of the BBB machinery is met by the conversion of pyruvate into carbon dioxide (CO2) and water (H2O) in an oxygen-dependent 8-step enzymatic process along the tricarboxylic acid cycle (TCA cycle). The theoretical energetic yield of the process from the complete oxidation of one glucose molecule to the end product generation of CO2 and H2O is of 6X NADH, 2X FADH2, and 2X ATP (equivalent to 36X ATP molecules). The aerobic pathway also provides reducing equivalents (such as NADH and NADPH) to counteract oxidative stress caused by both endogenous and exogenous reactive oxidative species (ROS) [2,122,123].
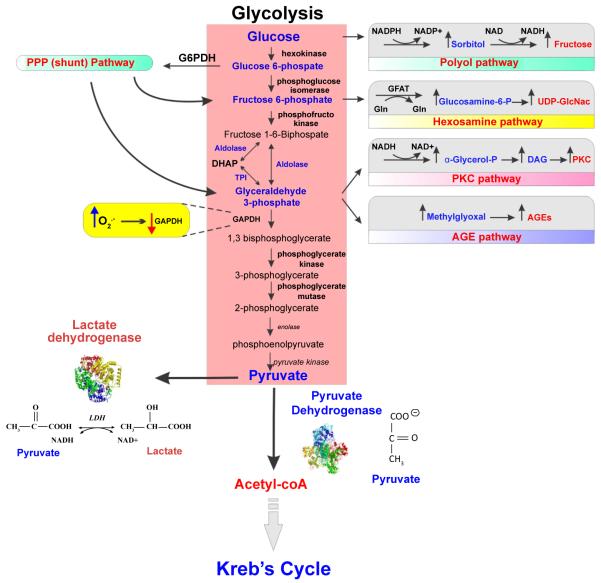
Figure 3. Glucose metabolism and oxidative stress.
Glucose metabolism and energy production starts with glycolysis where glucose undergoes a series of metabolic steps to produce pyruvate. Pyruvate can either enter Kreb’s cycle for further metabolism processing and energy production via aerobic pathways or get converted to lactate (ending its metabolic transformation). The intermediates formed during glycolysis can enter into different pathways of metabolism such as Pentose phosphate pathway (PPP), Hexosamine biosynthetic pathway (HBP), Protein kinase C pathway and advanced glycation end-product pathway (AGE). Glucose itself can also enter polyol pathway instead of glycolysis to form fructose. Altered glycaemia can hamper/affect any of these other pathways of glucose metabolism, leading to oxidative stress and potential cell damage.
Neurons and astrocytes interact to fulfill the energy requirements, with glycolysis occurring in astrocytes while TCA cycle taking place in neurons. Each cell type contains both sets of enzymes, thereby making it a subject for validation. During glycolysis itself, the intermediates formed can enter into different pathways of metabolism (Figure 3) - namely pentose phosphate pathway (PPP), hexosamine biosynthetic pathway (HBP), protein kinase C pathway (PKC) and AGE pathway. Glucose itself can also enter polyol pathway instead of glycolysis to form fructose [2,122,123]. We have subsequently discussed how these pathways abnormally get regulated in DM.
Hyperglycemia aids in production of ROS which leads to variety of microvascular and macrovascular complications [2,122]. Although preliminary studies have shown that human brain endothelial cells exhibit vulnerability to hyperglycemic stress (associated with notable cytosolic and mitochondrial redox shifts) [124,125] characterization of molecular and physiological responses of the BBB to DM-associated oxidative stress (ROS generation) has been barely initiated and is still under-investigated. This is extremely important since oxidative stress has been associated with the pathogenesis and progression of many neurodegenerative diseases including Alzheimer’s disease (AD), Parkinson’s disease (PD) and amyotrophic lateral sclerosis (ALS) [126,127].
Takahashi et al. [128] reported that acutely and chronically induced hyperglycemia increased PPP activity and glutathione (GSH) levels in astrocytic culture, in turn decreasing ROS levels. Higher PPP activity facilitates the regeneration of GSH such that cells are able to combat the higher oxidative stress level (protective role). To this end, nuclear translocation of Nrf2 (nuclear factor-erythroid 2 p45 subunit-related factor 2) in association with BiP (immunoglobulin heavy-chain-binding protein) was also observed. Nrf2 (the primary cellular defense against the cytotoxic effects of oxidative stress) normally lies in the cell cytoplasm but translocates into nucleus under oxidative stress to initiate the Nrf2 antioxidant response pathway of transcription of anti-oxidant genes and proteins (e.g., NAD(P)H quinone oxidoreductase 1, Heme oxygenase-1, glutathione S-transferase, etc.).
Generation of excess superoxide during hyperglycemia suppresses GAPDH, thereby promoting glucose utilization by alternative pathways (e.g., processing of Glyceraldehyde-3-P through PKC pathway and AGE pathway) [2]. Pronounced vasogenic edema that occurs during hyperglycemic stroke has been investigated to be mainly due to PKCβ activation. Further PKC activation affects BBB permeability through ZO-1 phosphorylation, TJ disruption and increase in VEGF expression [129]. Increased intracellular levels of AGE products damage the cells by altering the function of various proteins (modified by AGEs), including their interaction with surface membrane components (e.g., integrins) and AGE receptors. This occurs in macrophages, vascular endothelial and smooth muscle cells. Activation of AGE receptors (RAGE) increases ROS formation and leads to activation of NF-κB pathway. This ultimately promotes the expression of a variety of pro-inflammatory mediators [2,122,130,131] thus adjuvanting/strengthening the immune and pro-inflammatory responses. In fact increased accumulation of AGEs in Alzheimer’s patients has proven to cause neuronal death and degeneration, thereby supporting the fact that diabetes increases the risk of AD and any shift in normal glucose metabolism is deleterious for BBB integrity. In addition, oxidative stress has been shown to activate matrix metalloproteinases (MMP-1, -2, and -9) while decreasing tissue inhibitors of MMPs (TIMP-1 and -2) in a protein tyrosine kinase (PTK)-dependent manner [126].
Even though all cells are exposed to elevated glucose level in DM, hyperglycemic damage is restricted to only certain subtypes (retinal cells, endothelial cells) that are unable to down-regulate glucose transporter expression. As previously discussed, five major mechanisms are believed to be responsible for hyperglycemic damage: 1) Increased polyol pathway flux; 2) increased intracellular production of advanced glycation end-products; 3) increased expression of AGEs receptors and its activating ligands; 4) activation of protein kinase C; 5) increased flux through the hexosamine pathway. The upstream triggering event shared by all these mechanism is mitochondrial overproduction of ROS [2,132].
Conclusions
Although the DM-dependent vascular damage has been clearly associated to downstream mechanisms generating oxidative stress and inciting inflammation, the impact of DM on the blood-brain barrier has only been marginally addressed. Due to the unique characteristics of the BBB the effects of DM on the brain microcapillaries are, perhaps, different from other microvascular beds such as the retina and peripheral nerves. Most of these adverse effects can build over time with each insult being clinically unnoticeable until neurological damage has occurred.
Diabetes effects on BBB function have been approached and documented with respect to transport of glucose across the BBB in pathophysiological conditions like hyperglycemia and hypoglycemia. BBB regulates the efflux of metabolic byproducts of the CNS metabolism thereby allowing the transport functions to adapt to alterations in blood glucose levels. Chronic alteration in blood glucose content (hyper- and hypoglycemia) is a characteristic hallmark of diabetes and has been shown to trigger corresponding/compensatory alteration in the expression of glucose transporters at the BBB. Specifically, up-regulation of glucose transporters has been correlated to hypoglycemia suggesting an increase in BBB glucose extraction [83,92,93]. By contrast, hyperglycemia appears to down-regulate glucose transporters [63,78], although in vivo studies so far have provided controversial results. Alteration of glucose metabolism has also been suggested as having a role in the pathophysiology of diabetes at the BBB. Unfortunately, inconsistency and often controversial results between experimental and clinical studies do not offer a compounding final answer to this riddle. Therefore, whether diabetes and more specifically hypo- and hyperglycemia alter glucose transport and metabolism at the BBB (and the extent of these changes) is still unclear. Without a common denominator (study platform and methods) interpretation of current data (either in vivo or in vitro) becomes very difficult and translational relevance of such findings is somewhat questionable. The appropriateness of these studies (long versus short-term, in vivo versus in vitro, etc.) performed so far also need to be addressed to find a common experimental ground. On the other hand, there are ample experimental (both in vivo and in vitro) evidences supporting an oxidative and pro-inflammatory effect of diabetes on brain microcapillaries and the BBB for which RAGE is gaining an increasingly prominent role as a prodromal factor for many of the vascular pathophysiological changes associated with diabetes. RAGE activation promotes vascular dysfunction by impairing endothelial nitric oxide bioavailability, increasing the expression of adhesion molecules as well as the release of proinflammatory cytokines, chemoattractant mediators, matrix metalloproteinases and prothrombotic factors [133] (Figure 4). This inflammatory and oxidant milieu promoted by RAGE activation enhances the generation of its own ligands, thus sustaining a harmful vicious cycle enhancing vascular inflammation and BBB impairment.
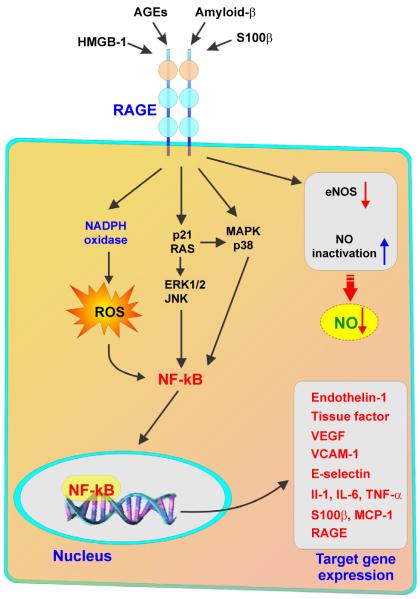
Figure 4. Simplified RAGE signal transduction pathways.
Note that the engagement of RAGE stimulates the activation of a diverse array of signaling cascades. These include mitogen activated protein kinases (MAPK), such as extracellular regulated (ERK)-1/2, p38 and c-Jun N-terminal kinase (JNK), Jak/STAT, phosphoinositol 3-kinase (PI3K), and members of the Rho GTPase signaling pathway (Cdc42 and Rac-1). Moreover, RAGE activation enhances the generation of reactive oxygen species (ROS) by activating NAD(P)H oxidase. Conversely, AGE may decrease NO availability by the decreased activity of NOS and by quenching NO. RAGE-dependent responses eventually converge to the activation of nuclear transcription factors (such as nuclear factor (NF-κB) and consequent target gene transcription (including endothelin-1, ICAM-1, E-selectin, and tissue factor) and ultimately triggering inflammatory pathways.
Overall, the current common consensus is that diabetes-dependent alteration of systemic glucose level play a significant role in the pathogenesis and/or progression of major neurological disorders by altering the structural and functional properties of the BBB [2,122,134]. However, this field of research is still severely under-explored. In depth studies aimed at unraveling how DM progressively alters BBB function and structure, the molecular players and mechanisms involved are still very much needed.
Acknowledgement
These studies were supported by A.R.D.F. and in part by NIH/NIDA R01-DA029121-01A1 to Dr. Luca Cucullo.
References
- 1.Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414:782–787. doi: 10.1038/414782a. [DOI] [PubMed] [Google Scholar]
- 2.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 3.Dias IH, Griffiths HR. Oxidative stress in diabetes - circulating advanced glycation end products, lipid oxidation and vascular disease. Ann Clin Biochem. 2014;51:125–127. doi: 10.1177/0004563213508747. [DOI] [PubMed] [Google Scholar]
- 4.Yamagishi S. Role of advanced glycation end products (AGEs) and receptor for AGEs (RAGE) in vascular damage in diabetes. Exp Gerontol. 2011;46:217–224. doi: 10.1016/j.exger.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 5.Toma L, Stancu CS, Botez GM, Sima AV, Simionescu M. Irreversibly glycated LDL induce oxidative and inflammatory state in human endothelial cells; added effect of high glucose. Biochem Biophys Res Commun. 2009;390:877–882. doi: 10.1016/j.bbrc.2009.10.066. [DOI] [PubMed] [Google Scholar]
- 6.Takenaka K, Yamagishi S, Matsui T, Nakamura K, Imaizumi T. Role of advanced glycation end products (AGEs) in thrombogenic abnormalities in diabetes. Curr Neurovasc Res. 2006;3:73–77. doi: 10.2174/156720206775541804. [DOI] [PubMed] [Google Scholar]
- 7.Kook SY, Hong HS, Moon M, Ha CM, Chang S, et al. Aβâ‚â‚‹â‚„â‚‚-RAGE interaction disrupts tight junctions of the blood-brain barrier via Ca²âº-calcineurin signaling. J Neurosci. 2012;32:8845–8854. doi: 10.1523/JNEUROSCI.6102-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wan W, Chen H, Li Y. The potential mechanisms of Aβ-receptor for advanced glycation end-products interaction disrupting tight junctions of the blood-brain barrier in Alzheimer’s disease. Int J Neurosci. 2014;124:75–81. doi: 10.3109/00207454.2013.825258. [DOI] [PubMed] [Google Scholar]
- 9.Mooradian AD. Pathophysiology of central nervous system complications in diabetes mellitus. Clin Neurosci. 1997;4:322–326. [PubMed] [Google Scholar]
- 10.Mooradian AD. Central nervous system complications of diabetes mellitus--a perspective from the blood-brain barrier. Brain Res Brain Res Rev. 1997;23:210–218. doi: 10.1016/s0165-0173(97)00003-9. [DOI] [PubMed] [Google Scholar]
- 11.Li ZG, Britton M, Sima AA, Dunbar JC. Diabetes enhances apoptosis induced by cerebral ischemia. Life Sci. 2004;76:249–262. doi: 10.1016/j.lfs.2004.03.039. [DOI] [PubMed] [Google Scholar]
- 12.Kissela BM, Khoury J, Kleindorfer D, Woo D, Schneider A, et al. Epidemiology of ischemic stroke in patients with diabetes: the greater Cincinnati/Northern Kentucky Stroke Study. Diabetes Care. 2005;28:355–359. doi: 10.2337/diacare.28.2.355. [DOI] [PubMed] [Google Scholar]
- 13.Saczynski JS, Siggurdsson S, Jonsson PV, Eiriksdottir G, Olafsdottir E, et al. Glycemic status and brain injury in older individuals: the age gene/environment susceptibility-Reykjavik study. Diabetes Care. 2009;32:1608–1613. doi: 10.2337/dc08-2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu J, Gao BB, Clermont AC, Blair P, Chilcote TJ, et al. Hyperglycemia-induced cerebral hematoma expansion is mediated by plasma kallikrein. Nat Med. 2011;17:206–210. doi: 10.1038/nm.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sakata A, Mogi M, Iwanami J, Tsukuda K, Min LJ, et al. Female type 2 diabetes mellitus mice exhibit severe ischemic brain damage. J Am Soc Hypertens. 2011;5:7–11. doi: 10.1016/j.jash.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 16.Dave KR, Tamariz J, Desai KM, Brand FJ, Liu A, et al. Recurrent hypoglycemia exacerbates cerebral ischemic damage in streptozotocin-induced diabetic rats. Stroke. 2011;42:1404–1411. doi: 10.1161/STROKEAHA.110.594937. [DOI] [PubMed] [Google Scholar]
- 17.Won SJ, Tang XN, Suh SW, Yenari MA, Swanson RA. Hyperglycemia promotes tissue plasminogen activator-induced hemorrhage by Increasing superoxide production. Ann Neurol. 2011;70:583–590. doi: 10.1002/ana.22538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weil ZM. Ischemia-induced hyperglycemia: consequences, neuroendocrine regulation, and a role for RAGE. Horm Behav. 2012;62:280–285. doi: 10.1016/j.yhbeh.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 19.Dinapoli VA, Benkovic SA, Li X, Kelly KA, Miller DB, et al. Age exaggerates proinflammatory cytokine signaling and truncates signal transducers and activators of transcription 3 signaling following ischemic stroke in the rat. Neuroscience. 2010;170:633–644. doi: 10.1016/j.neuroscience.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Block WM, Putzer GJ, Jaramillo JR. Children with type 2 diabetes mellitus and the prevalence of psychiatric disorders. South Med J. 2010;103:1214–1218. doi: 10.1097/SMJ.0b013e3181f96d5f. [DOI] [PubMed] [Google Scholar]
- 21.Haan MN. Therapy Insight: type 2 diabetes mellitus and the risk of late-onset Alzheimer’s disease. Nat Clin Pract Neurol. 2006;2:159–166. doi: 10.1038/ncpneuro0124. [DOI] [PubMed] [Google Scholar]
- 22.Hölscher C. Diabetes as a risk factor for Alzheimer’s disease: insulin signalling impairment in the brain as an alternative model of Alzheimer’s disease. Biochem Soc Trans. 2011;39:891–897. doi: 10.1042/BST0390891. [DOI] [PubMed] [Google Scholar]
- 23.Humpel C. Chronic mild cerebrovascular dysfunction as a cause for Alzheimer’s disease? Exp Gerontol. 2011;46:225–232. doi: 10.1016/j.exger.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mogi M, Horiuchi M. Neurovascular coupling in cognitive impairment associated with diabetes mellitus. Circ J. 2011;75:1042–1048. doi: 10.1253/circj.cj-11-0121. [DOI] [PubMed] [Google Scholar]
- 25.Summers WK. Alzheimer’s disease, oxidative injury, and cytokines. J Alzheimers Dis. 2004;6:651–657. doi: 10.3233/jad-2004-6609. [DOI] [PubMed] [Google Scholar]
- 26.Wang WT, Lee P, Yeh HW, Smirnova IV, Choi IY. Effects of acute and chronic hyperglycemia on the neurochemical profiles in the rat brain with streptozotocin-induced diabetes detected using in vivo ¹H MR spectroscopy at 9.4 T. J Neurochem. 2012;121:407–417. doi: 10.1111/j.1471-4159.2012.07698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pasquier F, Boulogne A, Leys D, Fontaine P. Diabetes mellitus and dementia. Diabetes Metab. 2006;32:403–414. doi: 10.1016/s1262-3636(07)70298-7. [DOI] [PubMed] [Google Scholar]
- 28.Cheng G, Huang C, Deng H, Wang H. Diabetes as a risk factor for dementia and mild cognitive impairment: a meta-analysis of longitudinal studies. Intern Med J. 2012;42:484–491. doi: 10.1111/j.1445-5994.2012.02758.x. [DOI] [PubMed] [Google Scholar]
- 29.Umegaki H. Neurodegeneration in diabetes mellitus. Adv Exp Med Biol. 2012;724:258–265. doi: 10.1007/978-1-4614-0653-2_19. [DOI] [PubMed] [Google Scholar]
- 30.Huang CW, Tsai JJ, Ou HY, Wang ST, Cheng JT, et al. Diabetic hyperglycemia is associated with the severity of epileptic seizures in adults. Epilepsy Res. 2008;79:71–77. doi: 10.1016/j.eplepsyres.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 31.Ley EJ, Srour MK, Clond MA, Barnajian M, Tillou A, et al. Diabetic patients with traumatic brain injury: insulin deficiency is associated with increased mortality. J Trauma. 2011;70:1141–1144. doi: 10.1097/TA.0b013e3182146d66. [DOI] [PubMed] [Google Scholar]
- 32.Maia AC, Braga Ade A, Brouwers A, Nardi AE, Oliveira e Silva AC. Prevalence of psychiatric disorders in patients with diabetes types 1 and 2. Compr Psychiatry. 2012;53:1169–1173. doi: 10.1016/j.comppsych.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 33.Tsuruta R, Fujita M, Ono T, Koda Y, Koga Y, et al. Hyperglycemia enhances excessive superoxide anion radical generation, oxidative stress, early inflammation, and endothelial injury in forebrain ischemia/reperfusion rats. Brain Res. 2010;1309:155–163. doi: 10.1016/j.brainres.2009.10.065. [DOI] [PubMed] [Google Scholar]
- 34.Hawkins BT, Lundeen TF, Norwood KM, Brooks HL, Egleton RD. Increased blood-brain barrier permeability and altered tight junctions in experimental diabetes in the rat: contribution of hyperglycaemia and matrix metalloproteinases. Diabetologia. 2007;50:202–211. doi: 10.1007/s00125-006-0485-z. [DOI] [PubMed] [Google Scholar]
- 35.Taguchi A. Vascular factors in diabetes and Alzheimer’s disease. J Alzheimers Dis. 2009;16:859–864. doi: 10.3233/JAD-2009-0975. [DOI] [PubMed] [Google Scholar]
- 36.Fukasawa R, Hanyu H, Namioka N, Hatanaka H, Sato T, et al. Elevated inflammatory markers in diabetes-related dementia. Geriatr Gerontol Int. 2014;14:229–231. doi: 10.1111/ggi.12140. [DOI] [PubMed] [Google Scholar]
- 37.Honing ML, Morrison PJ, Banga JD, Stroes ES, Rabelink TJ. Nitric oxide availability in diabetes mellitus. Diabetes Metab Rev. 1998;14:241–249. doi: 10.1002/(sici)1099-0895(1998090)14:3<241::aid-dmr216>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 38.McAuley DF, Nugent AG, McGurk C, Maguire S, Hayes JR, et al. Vasoconstriction to endogenous endothelin-1 is impaired in patients with type II diabetes mellitus. Clin Sci (Lond) 2000;99:175–179. [PubMed] [Google Scholar]
- 39.Banks WA, Owen JB, Erickson MA. Insulin in the brain: there and back again. Pharmacol Ther. 2012;136:82–93. doi: 10.1016/j.pharmthera.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shao B, Bayraktutan U. Hyperglycaemia promotes cerebral barrier dysfunction through activation of protein kinase C-β. Diabetes Obes Metab. 2013;15:993–999. doi: 10.1111/dom.12120. [DOI] [PubMed] [Google Scholar]
- 41.Simpson IA, Appel NM, Hokari M, Oki J, Holman GD, et al. Blood-brain barrier glucose transporter: effects of hypo- and hyperglycemia revisited. J Neurochem. 1999;72:238–247. doi: 10.1046/j.1471-4159.1999.0720238.x. [DOI] [PubMed] [Google Scholar]
- 42.Horani MH, Mooradian AD. Effect of diabetes on the blood brain barrier. Curr Pharm Des. 2003;9:833–840. doi: 10.2174/1381612033455314. [DOI] [PubMed] [Google Scholar]
- 43.Muresanu DF, Sharma A, Sharma HS. Diabetes aggravates heat stress-induced blood-brain barrier breakdown, reduction in cerebral blood flow, edema formation, and brain pathology: possible neuroprotection with growth hormone. Ann N Y Acad Sci. 2010;1199:15–26. doi: 10.1111/j.1749-6632.2009.05328.x. [DOI] [PubMed] [Google Scholar]
- 44.Serlin Y, Levy J, Shalev H. Vascular pathology and blood-brain barrier disruption in cognitive and psychiatric complications of type 2 diabetes mellitus. Cardiovasc Psychiatry Neurol. 20112011:609202. doi: 10.1155/2011/609202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Acharya NK, Levin EC, Clifford PM, Han M, Tourtellotte R, et al. Diabetes and hypercholesterolemia increase blood-brain barrier permeability and brain amyloid deposition: beneficial effects of the LpPLA2 inhibitor darapladib. J Alzheimers Dis. 2013;35:179–198. doi: 10.3233/JAD-122254. [DOI] [PubMed] [Google Scholar]
- 46.Starr JM, Wardlaw J, Ferguson K, MacLullich A, Deary IJ, et al. Increased blood-brain barrier permeability in type II diabetes demonstrated by gadolinium magnetic resonance imaging. J Neurol Neurosurg Psychiatry. 2003;74:70–76. doi: 10.1136/jnnp.74.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Daneman R. The blood-brain barrier in health and disease. Ann Neurol. 2012;72:648–672. doi: 10.1002/ana.23648. [DOI] [PubMed] [Google Scholar]
- 48.Bradbury MW. The blood-brain barrier. Exp Physiol. 1993;78:453–472. doi: 10.1113/expphysiol.1993.sp003698. [DOI] [PubMed] [Google Scholar]
- 49.Emmi A, Wenzel HJ, Schwartzkroin PA, Taglialatela M, Castaldo P, et al. Do glia have heart? Expression and functional role for ether-a-go-go currents in hippocampal astrocytes. J Neurosci. 2000;20:3915–3925. doi: 10.1523/JNEUROSCI.20-10-03915.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Davson H, Segal MB. Physiology of the CSF and Blood-Brain Barriers. CRC Press; United States: 1996. [Google Scholar]
- 51.Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57:173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- 52.Naik P, Cucullo L. In vitro blood-brain barrier models: current and perspective technologies. J Pharm Sci. 2012;101:1337–1354. doi: 10.1002/jps.23022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Duelli R, Kuschinsky W. Brain glucose transporters: relationship to local energy demand. News Physiol Sci. 2001;16:71–76. doi: 10.1152/physiologyonline.2001.16.2.71. [DOI] [PubMed] [Google Scholar]
- 54.Thorens B, Mueckler M. Glucose transporters in the 21st Century. Am J Physiol Endocrinol Metab. 2010;298:E141–145. doi: 10.1152/ajpendo.00712.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhao FQ, Keating AF. Functional properties and genomics of glucose transporters. Curr Genomics. 2007;8:113–128. doi: 10.2174/138920207780368187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Augustin R. The protein family of glucose transport facilitators: It’s not only about glucose after all. IUBMB Life. 2010;62:315–333. doi: 10.1002/iub.315. [DOI] [PubMed] [Google Scholar]
- 57.Brockmann K. The expanding phenotype of GLUT1-deficiency syndrome. Brain Dev. 2009;31:545–552. doi: 10.1016/j.braindev.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 58.Klepper J. GLUT1 deficiency syndrome in clinical practice. Epilepsy Res. 2012;100:272–277. doi: 10.1016/j.eplepsyres.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 59.Vemula S, Roder KE, Yang T, Bhat GJ, Thekkumkara TJ, et al. A functional role for sodium-dependent glucose transport across the blood-brain barrier during oxygen glucose deprivation. J Pharmacol Exp Ther. 2009;328:487–495. doi: 10.1124/jpet.108.146589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McAllister MS, Krizanac-Bengez L, Macchia F, Naftalin RJ, Pedley KC, et al. Mechanisms of glucose transport at the blood-brain barrier: an in vitro study. Brain Res. 2001;904:20–30. doi: 10.1016/s0006-8993(01)02418-0. [DOI] [PubMed] [Google Scholar]
- 61.Cornford EM, Hyman S, Swartz BE. The human brain GLUT1 glucose transporter: ultrastructural localization to the blood-brain barrier endothelia. J Cereb Blood Flow Metab. 1994;14:106–112. doi: 10.1038/jcbfm.1994.15. [DOI] [PubMed] [Google Scholar]
- 62.Farrell CL, Pardridge WM. Blood-brain barrier glucose transporter is asymmetrically distributed on brain capillary endothelial lumenal and ablumenal membranes: an electron microscopic immunogold study. Proc Natl Acad Sci U S A. 1991;88:5779–5783. doi: 10.1073/pnas.88.13.5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Duelli R, Maurer MH, Staudt R, Heiland S, Duembgen L, et al. Increased cerebral glucose utilization and decreased glucose transporter Glut1 during chronic hyperglycemia in rat brain. Brain Res. 2000;858:338–347. doi: 10.1016/s0006-8993(00)01942-9. [DOI] [PubMed] [Google Scholar]
- 64.Castaneda-Sceppa C, Castaneda F. Sodium-dependent glucose transporter protein as a potential therapeutic target for improving glycemic control in diabetes. Nutr Rev. 2011;69:720–729. doi: 10.1111/j.1753-4887.2011.00423.x. [DOI] [PubMed] [Google Scholar]
- 65.Leybaert L, De Bock M, Van Moorhem M, Decrock E, De Vuyst E. Neurobarrier coupling in the brain: adjusting glucose entry with demand. J Neurosci Res. 2007;85:3213–3220. doi: 10.1002/jnr.21189. [DOI] [PubMed] [Google Scholar]
- 66.Cornford EM, Hyman S. Localization of brain endothelial luminal and abluminal transporters with immunogold electron microscopy. NeuroRx. 2005;2:27–43. doi: 10.1602/neurorx.2.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Weksler BB, Subileau EA, Perrière N, Charneau P, Holloway K, et al. Blood-brain barrier-specific properties of a human adult brain endothelial cell line. FASEB J. 2005;19:1872–1874. doi: 10.1096/fj.04-3458fje. [DOI] [PubMed] [Google Scholar]
- 68.Bingham EM, Hopkins D, Smith D, Pernet A, Hallett W, et al. The role of insulin in human brain glucose metabolism: an 18fluoro-deoxyglucose positron emission tomography study. Diabetes. 2002;51:3384–3390. doi: 10.2337/diabetes.51.12.3384. [DOI] [PubMed] [Google Scholar]
- 69.Frank HJ, Pardridge WM, Jankovic-Vokes T, Vinters HV, Morris WL. Insulin binding to the blood-brain barrier in the streptozotocin diabetic rat. J Neurochem. 1986;47:405–411. doi: 10.1111/j.1471-4159.1986.tb04516.x. [DOI] [PubMed] [Google Scholar]
- 70.Laron Z. Insulin and the brain. Arch Physiol Biochem. 2009;115:112–116. doi: 10.1080/13813450902949012. [DOI] [PubMed] [Google Scholar]
- 71.Pardridge WM, Eisenberg J, Yang J. Human blood-brain barrier insulin receptor. J Neurochem. 1985;44:1771–1778. doi: 10.1111/j.1471-4159.1985.tb07167.x. [DOI] [PubMed] [Google Scholar]
- 72.Banks WA, Jaspan JB, Huang W, Kastin AJ. Transport of insulin across the blood-brain barrier: saturability at euglycemic doses of insulin. Peptides. 1997;18:1423–1429. doi: 10.1016/s0196-9781(97)00231-3. [DOI] [PubMed] [Google Scholar]
- 73.Li XH, Lv BL, Xie JZ, Liu J, Zhou XW, et al. AGEs induce Alzheimer-like tau pathology and memory deficit via RAGE-mediated GSK-3 activation. Neurobiol Aging. 2012;33:1400–1410. doi: 10.1016/j.neurobiolaging.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 74.Lam JK, Wang Y, Shiu SW, Wong Y, Betteridge DJ, et al. Effect of insulin on the soluble receptor for advanced glycation end products (RAGE) Diabet Med. 2013;30:702–709. doi: 10.1111/dme.12166. [DOI] [PubMed] [Google Scholar]
- 75.Thomas MC, Söderlund J, Lehto M, Mäkinen VP, Moran JL, et al. Soluble receptor for AGE (RAGE) is a novel independent predictor of all-cause and cardiovascular mortality in type 1 diabetes. Diabetologia. 2011;54:2669–2677. doi: 10.1007/s00125-011-2186-5. [DOI] [PubMed] [Google Scholar]
- 76.Fujisawa K, Katakami N, Kaneto H, Naka T, Takahara M, et al. Circulating soluble RAGE as a predictive biomarker of cardiovascular event risk in patients with type 2 diabetes. Atherosclerosis. 2013;227:425–428. doi: 10.1016/j.atherosclerosis.2013.01.016. [DOI] [PubMed] [Google Scholar]
- 77.Yamagishi S, Imaizumi T. Serum levels of soluble form of receptor for advanced glycation end products (sRAGE) may reflect tissue RAGE expression in diabetes. Arterioscler Thromb Vasc Biol. 2007;27:e32. doi: 10.1161/ATVBAHA.107.139923. [DOI] [PubMed] [Google Scholar]
- 78.Hou WK, Xian YX, Zhang L, Lai H, Hou XG, et al. Influence of blood glucose on the expression of glucose trans-porter proteins 1 and 3 in the brain of diabetic rats. Chin Med J (Engl) 2007;120:1704–1709. [PubMed] [Google Scholar]
- 79.Pardridge WM, Triguero D, Farrell CR. Downregulation of blood-brain barrier glucose transporter in experimental diabetes. Diabetes. 1990;39:1040–1044. doi: 10.2337/diab.39.9.1040. [DOI] [PubMed] [Google Scholar]
- 80.Gruetter R, Novotny EJ, Boulware SD, Rothman DL, Shulman RG. 1H NMR studies of glucose transport in the human brain. J Cereb Blood Flow Metab. 1996;16:427–438. doi: 10.1097/00004647-199605000-00009. [DOI] [PubMed] [Google Scholar]
- 81.Hasselbalch SG, Knudsen GM, Capaldo B, Postiglione A, Paulson OB. Blood-brain barrier transport and brain metabolism of glucose during acute hyperglycemia in humans. J Clin Endocrinol Metab. 2001;86:1986–1990. doi: 10.1210/jcem.86.5.7490. [DOI] [PubMed] [Google Scholar]
- 82.Nielsen JK, Djurhuus CB, Gravholt CH, Carus AC, Granild-Jensen J, et al. Continuous glucose monitoring in interstitial subcutaneous adipose tissue and skeletal muscle reflects excursions in cerebral cortex. Diabetes. 2005;54:1635–1639. doi: 10.2337/diabetes.54.6.1635. [DOI] [PubMed] [Google Scholar]
- 83.Seaquist ER, Tkac I, Damberg G, Thomas W, Gruetter R. Brain glucose concentrations in poorly controlled diabetes mellitus as measured by high-field magnetic resonance spectroscopy. Metabolism. 2005;54:1008–1013. doi: 10.1016/j.metabol.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 84.Badr GA, Tang J, Ismail-Beigi F, Kern TS. Diabetes downregulates GLUT1 expression in the retina and its microvessels but not in the cerebral cortex or its microvessels. Diabetes. 2000;49:1016–1021. doi: 10.2337/diabetes.49.6.1016. [DOI] [PubMed] [Google Scholar]
- 85.Cryer PE. Mechanisms of hypoglycemia-associated autonomic failure in diabetes. N Engl J Med. 2013;369:362–372. doi: 10.1056/NEJMra1215228. [DOI] [PubMed] [Google Scholar]
- 86.Bakatselos SO. Hypoglycemia unawareness. Diabetes Res Clin Pract. 2011;93(Suppl 1):S92–96. doi: 10.1016/S0168-8227(11)70020-1. [DOI] [PubMed] [Google Scholar]
- 87.Criego AB, Tkac I, Kumar A, Thomas W, Gruetter R, et al. Brain glucose concentrations in patients with type 1 diabetes and hypoglycemia unawareness. J Neurosci Res. 2005;79:42–47. doi: 10.1002/jnr.20296. [DOI] [PubMed] [Google Scholar]
- 88.Segel SA, Fanelli CG, Dence CS, Markham J, Videen TO, Paramore DS, et al. Blood-to-brain glucose transport, cerebral glucose metabolism, and cerebral blood flow are not increased after hypoglycemia. Diabetes. 2001;50:1911–1917. doi: 10.2337/diabetes.50.8.1911. [DOI] [PubMed] [Google Scholar]
- 89.Criego AB, Tkac I, Kumar A, Thomas W, Gruetter R, et al. Brain glucose concentrations in healthy humans subjected to recurrent hypoglycemia. J Neurosci Res. 2005;82:525–530. doi: 10.1002/jnr.20654. [DOI] [PubMed] [Google Scholar]
- 90.McCrimmon RJ, Jacob RJ, Fan X, McNay EC, Sherwin RS. Effects of recurrent antecedent hypoglycaemia and chronic hyperglycaemia on brainstem extra-cellular glucose concentrations during acute hypoglycaemia in conscious diabetic BB rats. Diabetologia. 2003;46:1658–1661. doi: 10.1007/s00125-003-1231-4. [DOI] [PubMed] [Google Scholar]
- 91.Kumagai AK, Kang YS, Boado RJ, Pardridge WM. Upregulation of blood-brain barrier GLUT1 glucose transporter protein and mRNA in experimental chronic hypoglycemia. Diabetes. 1995;44:1399–1404. doi: 10.2337/diab.44.12.1399. [DOI] [PubMed] [Google Scholar]
- 92.McCall AL, Fixman LB, Fleming N, Tornheim K, Chick W, et al. Chronic hypoglycemia increases brain glucose transport. Am J Physiol. 1986;251:E442–447. doi: 10.1152/ajpendo.1986.251.4.E442. [DOI] [PubMed] [Google Scholar]
- 93.Lei H, Gruetter R. Effect of chronic hypoglycaemia on glucose concentration and glycogen content in rat brain: A localized 13C NMR study. J Neurochem. 2006;99:260–268. doi: 10.1111/j.1471-4159.2006.04115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mastaitis JW, Wurmbach E, Cheng H, Sealfon SC, Mobbs CV. Acute induction of gene expression in brain and liver by insulin-induced hypoglycemia. Diabetes. 2005;54:952–958. doi: 10.2337/diabetes.54.4.952. [DOI] [PubMed] [Google Scholar]
- 95.Mooradian AD. Blood-brain barrier choline transport is reduced in diabetic rats. Diabetes. 1987;36:1094–1097. doi: 10.2337/diab.36.10.1094. [DOI] [PubMed] [Google Scholar]
- 96.Mans AM, DeJoseph MR, Davis DW, Hawkins RA. Regional amino acid transport into brain during diabetes: effect of plasma amino acids. Am J Physiol. 1987;253:E575–583. doi: 10.1152/ajpendo.1987.253.5.E575. [DOI] [PubMed] [Google Scholar]
- 97.Huber JD, VanGilder RL, Houser KA. Streptozotocin-induced diabetes progressively increases blood-brain barrier permeability in specific brain regions in rats. Am J Physiol Heart Circ Physiol. 2006;291:H2660–2668. doi: 10.1152/ajpheart.00489.2006. [DOI] [PubMed] [Google Scholar]
- 98.Allen CL, Bayraktutan U. Antioxidants attenuate hyperglycaemia-mediated brain endothelial cell dysfunction and blood-brain barrier hyperpermeability. Diabetes Obes Metab. 2009;11:480–490. doi: 10.1111/j.1463-1326.2008.00987.x. [DOI] [PubMed] [Google Scholar]
- 99.Wang J, Li G, Wang Z, Zhang X, Yao L, et al. High glucose-induced expression of inflammatory cytokines and reactive oxygen species in cultured astrocytes. Neuroscience. 2012;202:58–68. doi: 10.1016/j.neuroscience.2011.11.062. [DOI] [PubMed] [Google Scholar]
- 100.Ball KK, Harik L, Gandhi GK, Cruz NF, Dienel GA. Reduced gap junctional communication among astrocytes in experimental diabetes: contributions of altered connexin protein levels and oxidative-nitrosative modifications. J Neurosci Res. 2011;89:2052–2067. doi: 10.1002/jnr.22663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gandhi GK, Ball KK, Cruz NF, Dienel GA. Hyperglycaemia and diabetes impair gap junctional communication among astrocytes. ASN Neuro. 2010;2:e00030. doi: 10.1042/AN20090048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shimizu F, Sano Y, Tominaga O, Maeda T, Abe MA, et al. Advanced glycation end-products disrupt the blood-brain barrier by stimulating the release of transforming growth factor-β by pericytes and vascular endothelial growth factor and matrix metalloproteinase-2 by endothelial cells in vitro. Neurobiol Aging. 2013;34:1902–1912. doi: 10.1016/j.neurobiolaging.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 103.Argaw AT, Gurfein BT, Zhang Y, Zameer A, John GR. VEGF-mediated disruption of endothelial CLN-5 promotes blood-brain barrier breakdown. Proc Natl Acad Sci U S A. 2009;106:1977–1982. doi: 10.1073/pnas.0808698106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Vorbrodt AW, Dobrogowska DH, Tarnawski M, Meeker HC, Carp RI. Immunogold study of altered expression of some interendothelial junctional molecules in the brain blood microvessels of diabetic scrapie-infected mice. J Mol Histol. 2006;37:27–35. doi: 10.1007/s10735-006-9026-9. [DOI] [PubMed] [Google Scholar]
- 105.Yan J, Zhang Z, Shi H. HIF-1 is involved in high glucose-induced paracellular permeability of brain endothelial cells. Cell Mol Life Sci. 2012;69:115–128. doi: 10.1007/s00018-011-0731-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Oltmanns KM, Melchert UH, Scholand-Engler HG, Schultes B, Schweiger U, et al. Divergent effects of hyper- and hypoglycemia on circulating vascular endothelial growth factor in humans. Metabolism. 2008;57:90–94. doi: 10.1016/j.metabol.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 107.Chehade JM, Haas MJ, Mooradian AD. Diabetes-related changes in rat cerebral occludin and zonula occludens-1 (ZO-1) expression. Neurochem Res. 2002;27:249–252. doi: 10.1023/a:1014892706696. [DOI] [PubMed] [Google Scholar]
- 108.Hoffman WH, Stamatovic SM, Andjelkovic AV. Inflammatory mediators and blood brain barrier disruption in fatal brain edema of diabetic ketoacidosis. Brain Res. 2009;1254:138–148. doi: 10.1016/j.brainres.2008.11.100. [DOI] [PubMed] [Google Scholar]
- 109.Ding C, He Q, Li PA. Diabetes increases expression of ICAM after a brief period of cerebral ischemia. J Neuroimmunol. 2005;161:61–67. doi: 10.1016/j.jneuroim.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 110.Ennis SR, Keep RF. Effect of sustained-mild and transient-severe hyperglycemia on ischemia-induced blood-brain barrier opening. J Cereb Blood Flow Metab. 2007;27:1573–1582. doi: 10.1038/sj.jcbfm.9600454. [DOI] [PubMed] [Google Scholar]
- 111.Ergul A, Elgebaly MM, Middlemore ML, Li W, Elewa H, et al. Increased hemorrhagic transformation and altered infarct size and localization after experimental stroke in a rat model type 2 diabetes. BMC Neurol. 2007;7:33. doi: 10.1186/1471-2377-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Vavilala MS, Richards TL, Roberts JS, Chiu H, Pihoker C, et al. Change in blood-brain barrier permeability during pediatric diabetic ketoacidosis treatment. Pediatr Crit Care Med. 2010;11:332–338. doi: 10.1097/PCC.0b013e3181c013f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Agus DB, Gambhir SS, Pardridge WM, Spielholz C, Baselga J, et al. Vitamin C crosses the blood-brain barrier in the oxidized form through the glucose transporters. J Clin Invest. 1997;100:2842–2848. doi: 10.1172/JCI119832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Huang J, Agus DB, Winfree CJ, Kiss S, Mack WJ, et al. Dehydroascorbic acid, a blood-brain barrier transportable form of vitamin C, mediates potent cerebroprotection in experimental stroke. Proc Natl Acad Sci U S A. 2001;98:11720–11724. doi: 10.1073/pnas.171325998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Minamizono A, Tomi M, Hosoya K. Inhibition of dehydroascorbic acid transport across the rat blood-retinal and -brain barriers in experimental diabetes. Biol Pharm Bull. 2006;29:2148–2150. doi: 10.1248/bpb.29.2148. [DOI] [PubMed] [Google Scholar]
- 116.Liu H, Xu X, Yang Z, Deng Y, Liu X, et al. Impaired function and expression of P-glycoprotein in blood-brain barrier of streptozotocin-induced diabetic rats. Brain Res. 2006;1123:245–252. doi: 10.1016/j.brainres.2006.09.061. [DOI] [PubMed] [Google Scholar]
- 117.Liu H, Liu X, Jia L, Liu Y, Yang H, et al. Insulin therapy restores impaired function and expression of P-glycoprotein in blood-brain barrier of experimental diabetes. Biochem Pharmacol. 2008;75:1649–1658. doi: 10.1016/j.bcp.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 118.Maeng HJ, Kim MH, Jin HE, Shin SM, Tsuruo T, et al. Functional induction of P-glycoprotein in the blood-brain barrier of streptozotocin-induced diabetic rats: evidence for the involvement of nuclear factor-kappaB, a nitrosative stress-sensitive transcription factor, in the regulation. Drug Metab Dispos. 2007;35:1996–2005. doi: 10.1124/dmd.107.015800. [DOI] [PubMed] [Google Scholar]
- 119.Reichel V, Burghard S, John I, Huber O. P-glycoprotein and breast cancer resistance protein expression and function at the blood-brain barrier and blood-cerebrospinal fluid barrier (choroid plexus) in streptozotocin-induced diabetes in rats. Brain Res. 2011;1370:238–245. doi: 10.1016/j.brainres.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 120.McCuskey PA, McCuskey RS. in vivo and electron microscopic study of the development of cerebral diabetic microangiography. Microcirc Endothelium Lymphatics. 1984;1:221–244. [PubMed] [Google Scholar]
- 121.Prakash R, Johnson M, Fagan SC, Ergul A. Cerebral neovascularization and remodeling patterns in two different models of type 2 diabetes. PLoS One. 2013;8:e56264. doi: 10.1371/journal.pone.0056264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107:1058–1070. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Naik P, Prasad S, Cucullo L. Role and Function of Dehydrogenases in CNS and Blood-Brain Barrier Pathophysiology: In Dehydrogenases. Intech; United Kingdom: 2013. [Google Scholar]
- 124.Takahashi S, Abe T, Izawa Y, Suzuki N. Effects of fluctuating glucose concentrations on oxidative metabolism of glucose in cultured neurons and astroglia. Journal of Diabetes Mellitus. 2012;2:19–26. [Google Scholar]
- 125.Okouchi M, Okayama N, Alexander JS, Aw TY. NRF2-dependent glutamate-L-cysteine ligase catalytic subunit expression mediates insulin protection against hyperglycemia-induced brain endothelial cell apoptosis. Curr Neurovasc Res. 2006;3:249–261. doi: 10.2174/156720206778792876. [DOI] [PubMed] [Google Scholar]
- 126.Haorah J, Ramirez SH, Schall K, Smith D, Pandya R, et al. Oxidative stress activates protein tyrosine kinase and matrix metalloproteinases leading to blood-brain barrier dysfunction. J Neurochem. 2007;101:566–576. doi: 10.1111/j.1471-4159.2006.04393.x. [DOI] [PubMed] [Google Scholar]
- 127.Barnham KJ, Masters CL, Bush AI. Neurodegenerative diseases and oxidative stress. Nat Rev Drug Discov. 2004;3:205–214. doi: 10.1038/nrd1330. [DOI] [PubMed] [Google Scholar]
- 128.Takahashi S, Izawa Y, Suzuki N. Astroglial pentose phosphate pathway rates in response to high-glucose environments. ASN Neuro. 2012;4 doi: 10.1042/AN20120002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cipolla MJ, Huang Q, Sweet JG. Inhibition of protein kinase Cβ reverses increased blood-brain barrier permeability during hyperglycemic stroke and prevents edema formation in vivo. Stroke. 2011;42:3252–3257. doi: 10.1161/STROKEAHA.111.623991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Byun K, Bayarsaikhan E, Kim D, Son M, Hong J, et al. Activated microglial cells synthesize and secrete AGE-albumin. Anat Cell Biol. 2012;45:47–52. doi: 10.5115/acb.2012.45.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Guerin-Dubourg A, Catan A, Bourdon E, Rondeau P. Structural modifications of human albumin in diabetes. Diabetes Metab. 2012;38:171–178. doi: 10.1016/j.diabet.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 132.Lyons TJ, Basu A. Biomarkers in diabetes: hemoglobin A1c, vascular and tissue markers. Transl Res. 2012;159:303–312. doi: 10.1016/j.trsl.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Vazzana N, Santilli F, Cuccurullo C, Davì G. Soluble forms of RAGE in internal medicine. Intern Emerg Med. 2009;4:389–401. doi: 10.1007/s11739-009-0300-1. [DOI] [PubMed] [Google Scholar]
- 134.Mooradian AD, Thurman JE. Glucotoxicity: potential mechanisms. Clin Geriatr Med. 1999;15:255. [PubMed] [Google Scholar]