Abstract
Galactosyl-transferase knock-out (GalT-KO) pigs represent a potential solution to xenograft rejection, particularly in the context of additional genetic modifications. We have performed life supporting kidney xenotransplantation into baboons utilizing GalT-KO pigs transgenic for human CD55/CD59/CD39/HT. Baboons received tacrolimus, mycophenolate mofetil, corticosteroids and recombinant human C1 Inhibitor combined with cyclophosphamide or bortezomib with or without 2–3 plasma exchanges. One baboon received a control GalT-KO xenograft with the latter immunosuppression. All immunosuppressed baboons rejected the xenografts between days 9 to 15 with signs of acute humoral rejection, in contrast to untreated controls (n=2) which lost their grafts on day 3 and 4. Immunofluorescence analyses showed deposition of IgM, C3, C5b-9 in rejected grafts, without C4d staining, indicating classical complement pathway blockade but alternate pathway activation. Moreover, rejected organs exhibited predominantly monocyte/macrophage infiltration with minimal lymphocyte representation. None of the recipients showed any signs of PERV transmission but some showed evidence of PCMV replication within the xenografts. Our work indicates that the addition of bortezomib and plasma exchange to the immunosuppressive regimen did not significantly prolong the survival of multi-transgenic GalT-KO renal xenografts. Non-Gal antibodies, the alternative complement pathway, innate mechanisms with monocyte activation and PCMV replication may have contributed to rejection.
Introduction
Xenotransplantation of wild type (WT) porcine vascularized organs in unmodified non-human-primates (NHP) leads to hyperacute rejection (HAR), mainly due to preformed natural xeno-antibodies (XNA). Since these XNA activate the complement cascade, genetically modified pigs expressing human complement regulatory proteins (hCRP) have been generated (1–3) and the organs of these mutant swine were efficiently protected against HAR (1). After the identification of the major xenoantigen Galactose-α-1,3-Galactose epitope (Gal) (4), other pigs lacking Gal expression were generated by knocking out the corresponding galactosyl-transferase gene (5–9). The use of these GalT-KO organs in pig-to-NHP heart (10, 11) and kidney (11–16) xenotransplantation resulted in prevention of HAR and modest prolongation of graft survival using standard immunosuppression. The survival of xenogeneic kidneys was inferior to that of heterotopic hearts, with graft losses characterized predominantly by thrombotic microangiopathy and acute humoral rejection (AHXR) involving Ig and complement deposition (10, 11, 16). However, in one series using a protocol directed toward T cell tolerance, Yamada et al. (15) achieved survival of composite thymo-kidney transplants up to 3 months, without substantive features of rejection and with no porcine cytomegalovirus (PCMV) infection evident (11, 15).
More recently, new strains of pigs combining multiple genetic modifications have been generated. Hearts from GalT-KO pigs transgenic for hCD55 (17) or hCD46 (18, 19) grafted heterotopically into baboons survived from 15 to 52 days (17), and up to one year with recipient B cell-depletion (18, 19). In the present study, we performed, for the first time, xenotransplantation of kidneys from GalT-KO pigs multi-transgenic for human CD55, CD59 to target complement, CD39 to modulate purinergic signalling and thrombosis and H-transferase (α1,2-fucosyl-transferase, HT, initially to diminish Gal expression), in a life-supporting model in baboons in order to evaluate whether these additional genetic modifications would confer further advantage to GalT-KO pig organs. As adjuncts to conventional immunosuppression, recipients also received combined experimental regimens including plasma exchanges, recombinant human C1 inhibitor (rhC1-INH) and bortezomib to block complement activation and XNA producing B/plasma cells respectively. The potential for porcine endogenous retrovirus (PERV) transmission and the possible PCMV/BCMV activation were also tested.
Materials and Methods
Animals, xenotransplantation procedure and immunosuppressive treatments
Genetically modified pig (Sus scrofa) kidneys were grafted into baboon recipients (Papio anubis, originating from the Centre National de la Recherche Scientifique (CNRS)- primatology centre, Rousset, France) as described (13). All pigs studied were generated by Somatic Cell Nuclear Transfer (SCNT) (20).
Four experimental groups were studied according to the treatment and/or the donor source. All recipients were splenectomized at the time of transplant, except for 2 baboons from group#1 (Table 1). Clinical parameters and renal function were monitored daily by measurement of plasma creatinine, BUN, blood biochemistry and diuresis.
Table 1.
Summary of experimental conditions for the pig kidney to baboon study. Baboon recipient immunosuppression consisted of splenoctomy, plasma exchange and pharmacological treatment as outlined.
| Groups | Recipients | Pig donors | Genetic modifications of donors | Splenectomy | Plasma exchanges | Pharmacological Treatment | Graft survival (days) | Diagnosis | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Control group | V9910C | #007 | GT-KO, tg hCD55, hCD59, hCD39, hHT | + | − | − | 4 | AXHR & AXCR | ||
| V857I | #007 | Id. | + | − | − | 3 | Id. | |||
|
| ||||||||||
| Group #1 | V893AA | #005 | GT-KO, tg hCD55, hCD59, hCD39, hHT | − | − | MMF, CS, Tacrolimus | Cyclophosphamid | C1INH 200U/Kg d0 to d5 | 15 | AXHR & AXCR |
| PA956E | #005 | Id. | − | − | Id. | Id. | Id. | 13 | Id. | |
| V907J | #006 | Id. | − | − | Id. | Id. | Id. | 12 | Id. | |
|
| ||||||||||
| Group #2 | PA997C | #089 | GT-KO, tg hCD55, hCD59, hCD39, hHT | + | d-4, −1 | MMF, CS, Tacrolimus | Bortezomib | C1INH 200U/Kg d5 to d9 | 9 | AXHR & AXCR |
| V9912B | #089 | Id. | + | Id. | Id. | Id. | Id. | 9 | Id. | |
| 022VB | #087 | Id. | + | Id. | Id. | Id. | C1INH 400U/Kg d5 to d9 | 11 | Id. | |
|
| ||||||||||
| Group #3 | PA936G | #095 | GT-KO, tg hCD55, hCD59, hCD39, hHT | + | d-4, −1, 3 | MMF, CS, Tacrolimus | Bortezomib | C1INH 400U/Kg d4 to d8 | 12 | AXHR & AXCR |
| PA936F | #095 | Id. | Id. | Id. | Id. | Id. | Id. | 14 | Id. | |
| K24E | #094 | Id. | Id. | Id. | Id. | Id. | Id. | 12 | Id. | |
| K921F | #094 | Id. | Id. | Id. | Id. | Id. | Id. | 14 | Id. | |
|
| ||||||||||
| Group #4 | K13F | #195 | GT-KO | + | d-4, −1, 3 | MMF, CS, Tacrolimus | Bortezomib | C1INH 400U/Kg d4 to d8 | 11 | AXHR & AXCR |
GT-KO: Galactosyl-transferase knock-out tg: transgenic, HT: Fucosyl-transferase, MMF: Mycophenolate Mofetil, CS: corticosteroids, Id.: Idem, rhC1INH: recombinant human C1 Inhibitor, given every 8 hours, AHXR: Acute Humoral Xenogeneic Rejection, ACXR: Acute Cellular Xenogeneic Rejection.
The NHP groups are described in detail in Table1. In brief, NHP from the control group and groups#1–3 received kidney transplants from GalT-KO.hCD55.hCD59.hCD39.hHTTg pigs, originally generated by Cowan et al. (2, 9, 21, 22), (the triple-transgenic pigs were made before it was possible to knock out Gal, and HT was included in an attempt to reduce Gal; the HT transgene cannot be separated from CD55 and CD59 because they are co-inherited), whereas kidneys grafted in baboons from group#4 came from GalT-KO pigs initially generated with the genetic background of MGH pigs (8) (Table 1). To study anti-pig immunization, 2 untreated recipients were kept alive after rejection: one autologous kidney was kept during surgery with a clamped ureter (under a powerful analgesia) to be unclamped at rejection and graft removal. All other recipients underwent a bilateral nephrectomy prior to transplantation.
Group #1 recipients (n=4), received a combined treatment modified from our previous studies using hCD55 transgenic donors (13, 23), consisting of rhC1-INH (kindly provided by Pharming (the Netherlands) already used in NHP (24)) at 200U/Kg/8hrs for 5 days, cyclophosphamide at 40 mg/Kg at days (d) −1 and d0, 20 mg/Kg at d2 and 10 mg/kg at d4, mycophenolate mofetil at 70 mg/kg/day, tacrolimus at 0.2 mg/Kg/day, and corticosteroids at 1 mg/Kg/day for the duration of the xenotransplantation. In group #2 (n=4), two plasma exchanges (PE) were added at d-4 and d-1 to remove preformed circulating non-Gal XNA, then plasma was replaced by human serum albumin. In addition, bortezomib, 1.3 mg/m2 (Janssen Cilag Int.), was given instead of cyclophosphamide at d-17, −14, −10, −7, −4, −1 and d7 (dose and timing were determined according preliminary pharmacodynamics experiments shown in supplementary data). These recipients received also rhC1-INH at 200U/Kg/8hrs (n=2) or 400U/Kg/8hrs (n=2) from d5 to d9. Group #3 (n=4) and #4 (n=2) recipients received the same treatment as those of group #2 with minor modifications: rhC1-INH was given at 400U/Kg/8hrs from d4 to d8 and an additional PE was performed at d3 (Table1). All treatment modifications between the groups were introduced on the basis of results obtained from the previous group (see results and discussion).
The French regional ethical committee for animal experimentation approved the study. The pig SCNT work was conducted in conformity with the Italian legislation DLg n116/92 and approved by the local ethics committee for animal experimentation of Avantea.
Histological analyses
All tissue sections were stained with H&E and examined in a blinded manner. Immuno-fluorescence (IF) was performed on biopsy and graftectomy sections of 10 μm fixed in acetone. Sections were incubated overnight at 4°C with Bandeiraea simplicifolia- Isolectin B4 (IB4)-FITC (Sigma, Saint-Louis, MI, USA), mouse anti-hCD55 (67), mouse anti-hCD59 (MEM-43), mouse anti-hCD39 (A1), rabbit polyclonal anti-hIgM all from AbDSerotec (Oxford, UK), rabbit anti-hCD3, mouse anti-hCD20 (L26), mouse anti-hCD68 (KP1), rabbit anti-hC3c all from Dako (Glostrup, Denmark), mouse anti-hCD4 (13B8.2) mouse anti-hCD8 (B9.11), mouse anti-hCD11b (BEAR1) all from Beckman Coulter (Fullerton, CA, USA), mouse anti-hC4d (Quidel, San Diego, CA, USA) and mouse anti-hC5b-9 (aE11, Diatec, Oslo, Norway). After washing, sections were incubated with either AlexaFluor®568-labelled goat anti-mouse IgG (Invitrogen, Carlsbad, CA, USA) or FITC-labelled donkey anti-rabbit IgG (Jackson ImmunoResearch, West Grove, PA, USA) for 90 min. at room temperature (RT). Sections were analyzed using an Axioskop2 plus microscope and Axion Vision software (Zeiss, Le Pecq, France).
Thrombin generation measurements
Thrombin generation (TG) was measured by the Calibrated Automated Thrombogram method in Platelet Poor Plasma (PPP) (25). Briefly, PPP was mixed at 4:1 in PPP-Reagent (Thrombinoscope BV, Maastricht, The Netherlands), with Tissue Factor and synthetic phospholipids (respectively 5pM and 4μM final concentration). The reaction was initiated by adding a fluorogenic substrate (Z-Gly-Gly-Arg-AMC, Thrombinoscope BV) and CaCl2, and fluorescence was read in a Fluoroscan Ascent reader (Thermo Labsystems, Helsinki, Finland). TG curves were calculated using the Thrombinoscope Software and Thrombin Calibrator (Thrombinoscope BV). Prolongation of Lag-times and lower values of both peak thrombin and ETP are indexes of hypocoagulability.
Donor Screening and XenoAntibody Monitoring by FlowCytometry
Confirmation of gene inactivation (KO) and transgene expression were performed on resting human (HAEC), WT and donor porcine aortic endothelial cells (PAEC) isolated from aorta (26, 27). Briefly, 1 to 2×105 PAEC after 2 washes in cold FACS buffer and incubated for 30 min. at 4°C with IB4-FITC, mouse anti-hCD55 (67), mouse anti-hCD59 (MEM-43) and mouse anti-hCD39 (A1) (AbDSerotec). Thereafter, cells were washed twice and incubated with anti-mouse IgG-FITC (for 30 min. at 4°C).
The baboon serum xenoreactivity was assessed by flow endothelial cross-matches in donor PAEC. Recipient sera were harvested at d-17, d-4 pre- and post-PE, d-1 pre- and post-PE, d0, d1, d2, d3 pre- and post-PE, d4 and weekly thereafter. Heat inactivated sera were incubated for 30 min at RT with 1 to 2×105 PAEC, thereafter cells were washed twice in cold FACS buffer and incubated separately for 30 min at 4°C with rabbit polyclonal anti-hIgM or anti-monkey IgG-FITC (AbDSerotec). The anti-pig IgM was revealed using donkey anti-rabbit IgG-FITC (Jackson ImmunoResearch).
Baboon serum cytotoxicity was measured by Flow Cytometry Complement-Mediated Cytotoxicity Assay. Briefly, heat inactivated sera were incubated for 30 min. at 4°C with 1 to 2×105 donor PAEC, thereafter the cells were washed twice in FACS buffer and incubated for 30 min at 37°C with 1:3 diluted rabbit complement. Then, cells were washed and propidium iodide was added to detect dead cells.
Cells were washed, resuspended in FACS buffer and analyzed using a Canto-II Flow cytometer with DIVA (Becton Dickinson, San Diego, CA, USA) and FlowJo software (Tree stars, Ashland, OR, USA).
Serum Complement monitoring
Classical hemolytic complement (CH50), alternative pathway complement (AP50), mannose-binding lectin complement (MBL50) activities, some complement components (C3, C4 and MBL) and C1-Inhibitor were measured in pre- and post-xenotransplantation plasma samples from control and groups#1–3 recipients. Due to the cross-reactivity of human reagents with baboons, we used human activation and detection systems (28–31).
PERV, baboon and porcine CMV detection
Serum samples were taken from control animals pre-xenograft, day 41, and month 3 (euthanasia); and treated animals prior xenograft and at euthanasia. Viral RNA (vRNA) was extracted from serum using the QIAmp viral RNA mini kit (Qiagen, Crawley, UK). The presence of PERV RNA was detected by RT-PCR using a method modified from that described by Paradis et al (32). To determine if organ recipients presented microchimerism, PBMC isolated from the recipient and donor’s blood and tested for the presence of porcine centromeric DNA and PERV as described previously (32). A PERV:centromeric ratio higher in the recipient than donor would indicate PERV integration and thus possible infection.
DNA was isolated from donor PBMC, donor spleen, recipient baseline PBMC, recipient date of death PBMC and the xenograft, using the DNeasy mini kit (Qiagen). DNA isolated from recipient and xenograft tissue was screened by qPCR for the presence of BCMV. DNA isolated from donor animal, recipients and xenografts were screened by qPCR for the presence of PCMV. Both the PCMV and BCMV qPCR assays were modified from the methods described by Mueller et al (33). The sensitivity of the assay is 10 (BCMV) and 1 (PCMV) copies per reaction (Figure S1).
Results
Knockout and transgene evidence in pig kidneys
The lack of Gal and the expression of hCD55, hCD59 and hCD39 transgenes were assessed by pig kidney IF staining (Fig. 1A) and by FACS on resting PAEC and HAEC (Fig. 1B). Both analyses confirmed the absence of Gal, strong expression of hCD55 and hCD59, and weak levels of expression of hCD39 in PAEC and pig kidneys.
Figure 1.
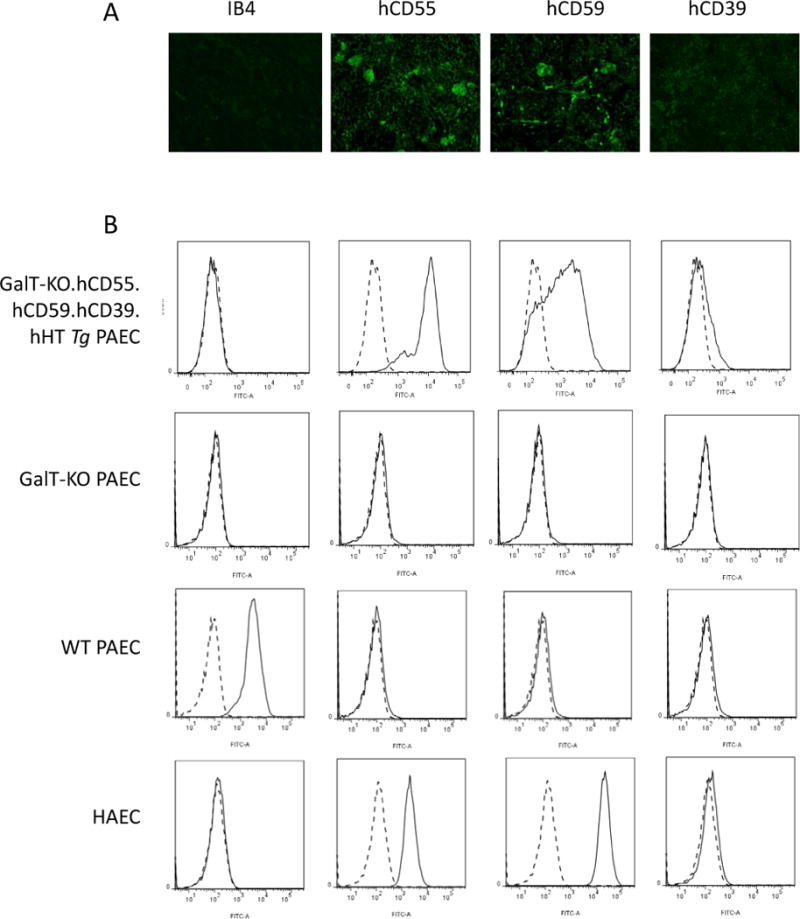
Transgene expression and knock-out in pig cells and tissue. The expression of Gal epitope (IB4), hCD55, hCD59 and hCD39 was assessed by immunofluorescence staining on GalT-KO.hCD55.hCD59.hCD39.hHTTg kidney (1A) and by FACS on human (HAEC) and porcine aortic endothelial cells (PAEC) (1B): first row: GalT-KO.hCD55.hCD59.hCD39.hHTTg PAEC (donor from Control group and groups #1–3), second row: GalT-KO PAEC (donor from group #4), third row: PAEC WT and fourth row: HAEC. Histograms show negative control (cells without Ab, dashed line) and endothelial cells with Ab (plain line).
Survival, histological analyses and coagulation data
In the untreated control group, no HAR occurred but graft survival did not exceed d4, because of biopsy proven acute vascular rejection (Fig. 2a) indicating persistent roles for XNA despite expression of hCRP transgenes. Three grafts (one each within groups #1, 2 and 4) were lost due to technical failure within 4 days, whereas the remaining eleven recipients rejected their xenografts between d9 and d15 (median 12 days; mean 12 days) (Table 1).
Figure 2.
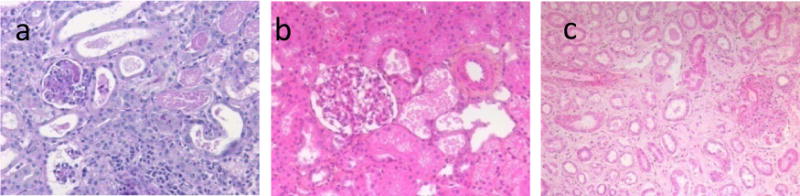
Histological analyses of animals from control group (a: recipient #V857I) and from group#3 (recipient #K921F:b, c). a (×20), c (×10) showed kidney biopsies at rejection respectively at day 3 and d14 whereas b (×20) showed protocol biopsies at d4 in functioning graft.
The graft survival of the remaining GalT-KO kidney (group #4) was 11 days and did not differ from GalT-KO.hCD55.hCD59.hCD39.hHTTg kidneys survival in groups #1–3.
In all rejected grafts from groups #1–4, histology showed signs of acute vascular rejection with focal interstitial haemorrhage, glomerular and capillary thrombi, interstitial cellular infiltrate and oedema qualified as AHXR and ACXR (Fig. 2).
Usually animals showed an immediate graft function (assessed by the creatininemia), except the ones with surgical complications (V893AA, O22VB, PA936F, PA936G & K24E) that could be fixed (Fig. 3).
Figure 3.
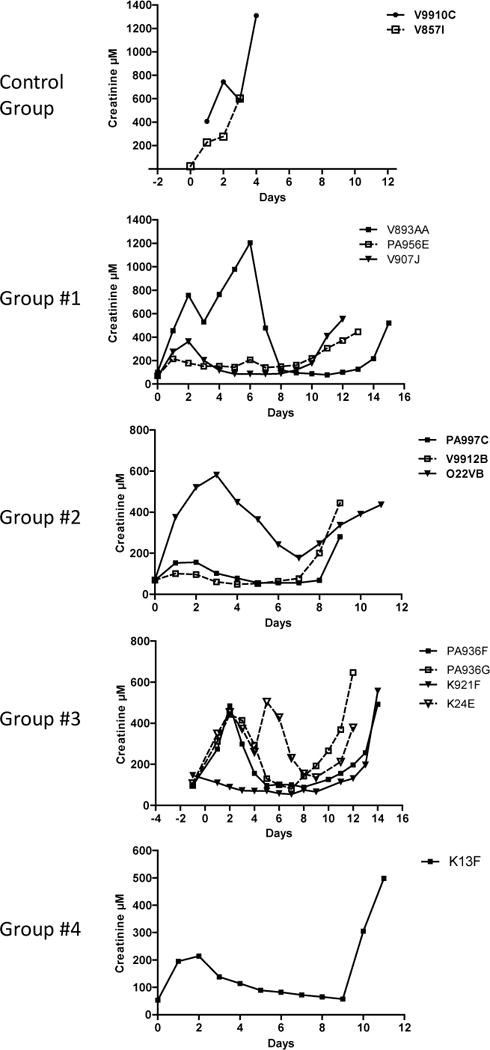
Creatininemia of recipients was measured daily (μM).
Global coagulation data showed at ultimate time point of rejection in groups #2–3 (Fig. 4A), a reduction of thrombin generation presumably due to coagulation factors consumption secondary to the immune microvascular aggression, as already described (34). Related thrombocytopenia were also noted at the same time (Fig. 4B), suggesting a possible ultimate rising consumptive coagulopathy although no diffuse haemorrhages were noted at the autopsy.
Figure 4.
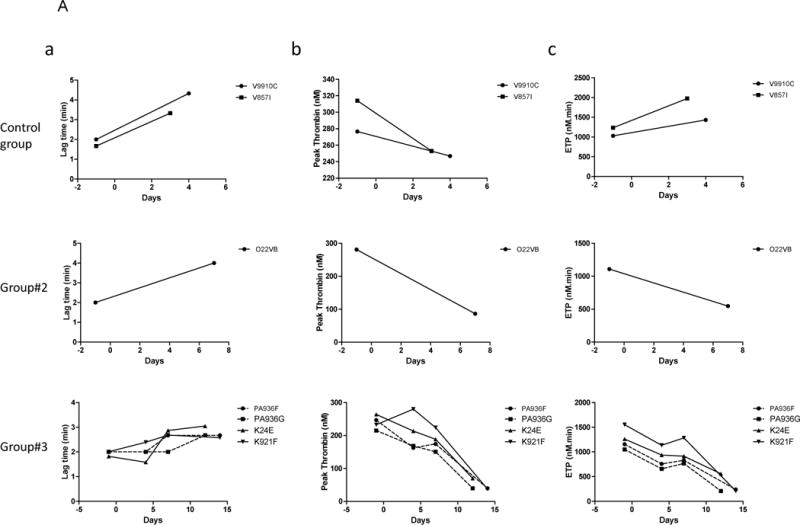
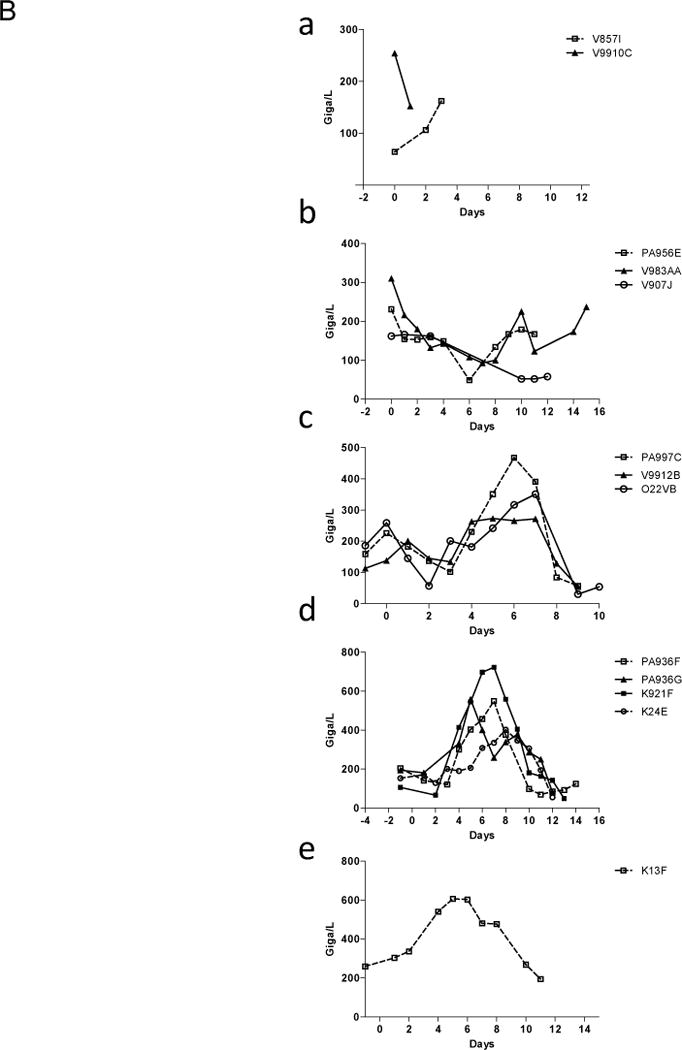
Coagulation. Thrombin generation curves (4A) were calculated and evaluated by measuring (a) Lag time (min), (b) Peak thrombin (nM) and (c) area under the curve (ETP, nM.min) first row: in control group (recipients #V9910C and #V857I), second row: in group #2 (recipient #O22VB) and third row: in group #3 (recipients #PA936F, #PA936G, #K24E and #K921F). Prolongation of Lag-times and lower values of both peak thrombin and ETP are indexes of hypocoagulability. Platelets level was expressed in Giga/L (4B), in recipient from control group (a), group #1 (b), group #2 (c), group #3 (d) and group #4 (e).
Immuno-staining analyses
Immuno-staining showed IgM deposition within the graft from d3 post-transplantation in both vascular and glomerular areas in control animals (Fig. 5Aa) as well as in immune-suppressed animals, without (Fig. 5Ab) or with (Fig. 5Ac, d, e & f) multiple PE. This suggests that even a small amount of preformed circulating anti-non-Gal Ab rapidly bound microvascular structures of the xenograft and induced severe and early damage. C3c, C4d and C5b-9 deposits were observed, with a similar Ig deposition kinetics in animals before (Fig. 5Aa) and after the end of rhC1-INH treatment (Fig. 5Ab: day 15; e: day12; f: day11). Interestingly, we observed a C3c and C5b-9 deposition, without C4d staining, at d4 in a functioning graft (Fig. 5Ad) while the recipient #PA936G was still under rhC1-INH, suggesting an efficient complement classical pathway blockade as long as the rhC1-INH was given but a persistent alternative pathway.
Figure 5.
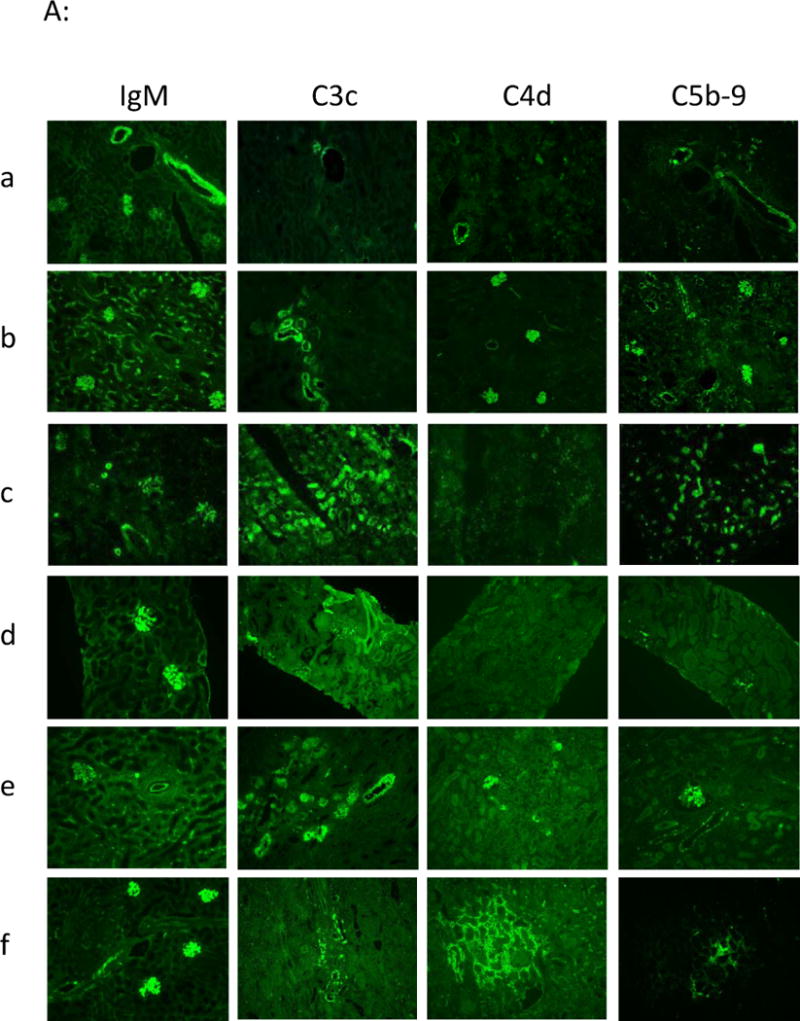
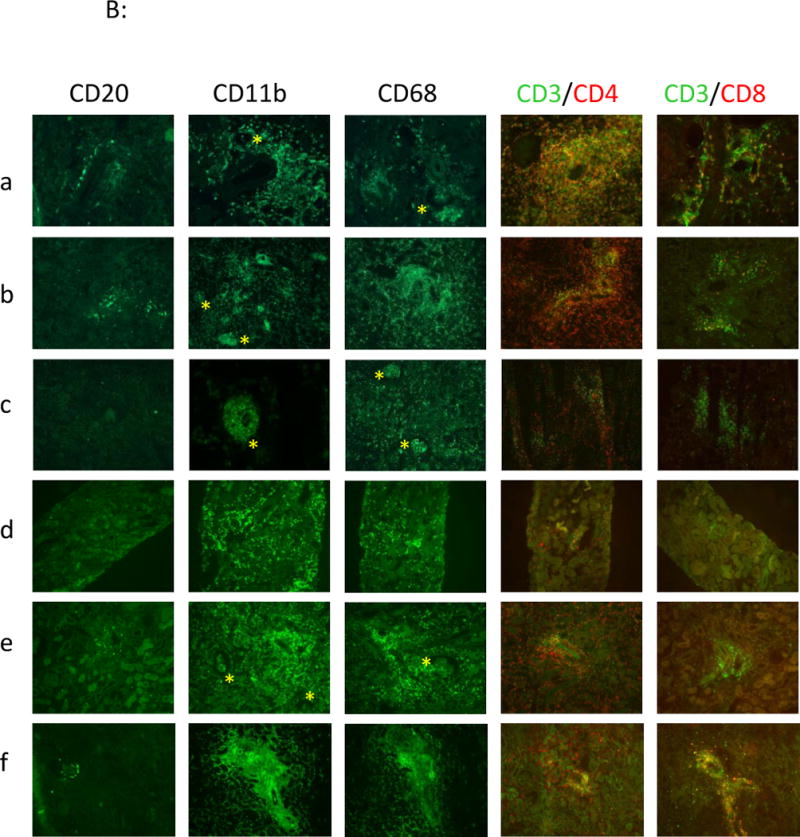
Immuno-histofluorescence analyses. IgM, C3c, C4d, C5b-9 staining (4A) and cellular staining: B cells (CD20), monocytes/macrophages (CD11b, CD68): yellow star is emphasizing the presence of monocytes/macrophages in glomerular capillaries, CD4 and CD8 T cells (4B), were performed in frozen kidney biopsies from recipients a: control group (recipient #V9910C) at rejection at d3, b: from group#1 at rejection at d15 (recipient #V893AA), c: from group# 2 at rejection at d9 (recipient #PA997C) and d & e: from group#3 (recipient #PA936G) respectively at d4 in functioning graft (d) and at rejection at d12 (e), and f: from group#4 (recipient #V906F) at rejection at d11.
At the cellular level, we observed a modest B cell infiltration (CD20) in perivascular areas in control and group #1 animals (Fig. 5Ba & b), whereas this infiltrate was almost abrogated in biopsies of animals from groups #2 (Fig. 5Bc), #3 (Fig. 5Bd & e), and #4 (Fig. 5Bf) suggesting an efficient B cell blockade by bortezomib. A marked CD4+ T cell infiltrate in perivascular areas was observed in controls, as opposed to treated animals for which it remained weak. CD8+ T cell infiltrate was minor in all groups. Interestingly, we observed in all groups an early intense and dominant monocyte/macrophage infiltration (CD11b, CD68) in perivascular areas as well as inside glomeruli (Fig. 5Ba, b, c & e), proving an important innate cellular response but also suggesting a possible direct or indirect binding to the porcine endothelium in GalT-KO xenotransplantation rejection.
Serum xenoreactivity, cytotoxicity and complement monitoring
The two control animals, kept alive after rejection, were followed to monitor their long term immunization. The low level of preformed anti-non-Gal IgM XNA decreased further during the first days post-transplant, most likely due to binding to the graft. Thereafter, anti-non-Gal IgM and IgG increased respectively from d6 and d9 to reach a maximum around d21 and d28 and then decreased slowly (Fig. 6A). These kinetics were similar to group #1 recipients, with a maximum of immunization at the time of rejection. The use of bortezomib in the pre-transplant period (groups #2–4) induced a modest reduction of preformed IgM and IgG (data not shown). Because of the evidence of preformed anti-non-Gal Ab, PE were then added to bortezomib (groups #2–4) and efficiently decreased these antibodies, followed by a fast rebound, justifying repetition until an optimized Ab depletion; thereafter, half of the baboons showed no re-increase of XNA (d-1 group #2, d3 groups #3–4). Serum complement dependent cytotoxicity (CDC) persisted and increased at the time of rejection in most cases (Fig. 6B), suggesting a possible role of high affinity anti-non-Gal Ab in the early graft damage leading to AHXR. However, other than early alternative pathway activation, plasma complement monitoring showed no major change of the classical and MBL pathways (data not shown), suggesting that the complement activation took place in the xenografts.
Figure 6.
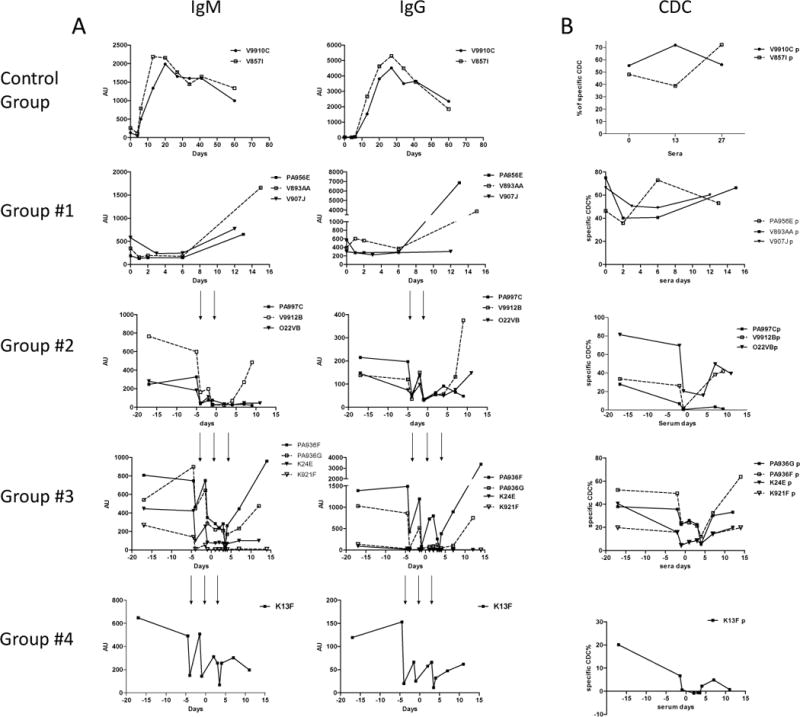
Baboon serum xenoreactivity (6A) and cytotoxicity (6B) were assessed by FACS. A: IgM and IgG anti-nonGal antibodies were assessed by crossmatch using donor PAEC by FACS. Arrows show the decrease of circulating XNA after each PE. B: serum complement dependant cytotoxicity was assessed by a Flow cytometry Complement-mediated Cytotoxic Assay on donor PAEC.
PERV, baboon and porcine CMV detection
PERV vRNA levels found in the recipient’s serum along with the recipients and donors PERV:centromeric ratios are shown in Table 2. With the exception of animals #PA997C and #PA936G, all recipients produced PERV:centromeric ratios lower than that of their donor animal. This would indicate that PERV vRNA detected in their serum and PERV DNA detected in their PBMC preparations was a result of microchimerism (circulating donor cells) and not PERV infection. The PERV:centromeric ratios observed in animals #PA997C and #PA936G are slightly higher than their donors’ PERV:centromeric ratios. However, these values were not significantly different (p=0.315 and p=0.227 respectively).
Table 2.
PERV analysis.
| Groups | Recipients | PERV vRNA in serum (copies/ml) | PERV: centromeric ratio | Donor PERV: centromeric ratio |
|---|---|---|---|---|
| Group #1 | V893AA | Neg | 0.0188 | 0.032 |
| PA956E | Neg | 0.0285 | 0.032 | |
| V907J | 5.9*102 | 0.0375 | 0.0604 | |
|
| ||||
| Group #2 | PA997C | 4.8*104 | 0.0267† | 0.0221 |
| V9912B | 2.7*104 | 0.0148 | 0.0221 | |
| O22VB | 3.4*104 | 0.0144 | 0.0195 | |
|
| ||||
| Group #3 | PA936G | 4.5*105 | 0.0884‡ | 0.0593 |
| PA936F | 9.9*105 | 0.0182 | 0.0593 | |
| K24E | 2.7*104 | 0.0281 | 0.0285 | |
| K921F | 1.3*104 | 0.0238 | 0.0285 | |
|
| ||||
| Group #4 | K13F | 2.9*103 | 0.0211 | 0.0236 |
p=0.315
p=0.227
All recipient baboons testing positive for BCMV at baseline (n=3) showed an increase in BCMV copy number at time of rejection; (23–253 copies/500ng DNA (mean 107.3 copies/500ng DNA) at baseline vs. 79–3194 copies/500ng DNA (mean1181 copies/500ng DNA) at rejection). In addition 2 baboons (#V907J and #PA997C), which tested negative for BCMV at baseline tested positive for BCMV at rejection. No BCMV infection was detected in any of the xenografts (Table 3).
Table 3.
Summary of BCMV and PCMV copy number per 500ng DNA in donor graft and recipients.
| Group | Graft Survival (days) | NHP ID | Pig donors | BCMV | PCMV | |||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Recipient PBMC Pre-XT | Recipient PBMC Post-XT | Xenograft | Donor PBMC | Donor Spleen | Recipient Post-XT | Xenograft | ||||
| Group #1 | 15 | V893AA | #005 | NEG | NEG | NEG | NEG | NEG | NEG | NEG |
| 13 | PA956E | #005 | 46 ± 15 | 270 ± 46 | NEG | NEG | NEG | NEG | NEG | |
| 12 | V907J | #006 | NEG | 238 ± 8 | NEG | NEG | NEG | NEG | NEG | |
|
| ||||||||||
| Group #2 | 9 | PA997C | #089 | NEG | 117 ± 5 | NEG | 7 ± 1 | 2 ± 1 | NEG | 153 ± 1 |
| 9 | V9912B | #089 | NEG | NEG | NEG | 7 ± 1 | 2 ± 1 | 27 ± 1 | 566 ± 7 | |
| 11 | O22VB | #087 | nd | Nd | NEG | NEG | 9 ± 1 | nd | 571 ± 17 | |
|
| ||||||||||
| Group #3 | 12 | PA936G | #095 | NEG | NEG | NEG | 5 ± 2 | 6 ± 1 | 370 ± 3 | 4469 ± 31 |
| 14 | PA936F | #095 | NEG | NEG | NEG | 5 ± 2 | 6 ± 1 | 41 ± 1 | 7646 ± 13 | |
| 12 | K24E | #094 | 23 ± 4 | 79 ± 7 | NEG | 4 ± 2 | 9 ± 2 | 450 ± 16 | 47355 ± 3538 | |
| 14 | K921F | #094 | NEG | NEG | NEG | 4 ± 2 | 9 ± 2 | 373 ± 2 | 92332 ± 803 | |
|
| ||||||||||
| Group #4 | 11 | K13F | #195 | 253 ± 15 | 3194 ± 329 | NEG | NEG | NEG | NEG | NEG |
Of the 7 donor animals used, PCMV was detected in the blood (n=3) and in the spleen (n=4). PCMV detection in these animals was also noted in the donor kidney xenograft (Table 3). The two donor animals for group #3 were positive at time of graft and PCMV was also detected in the blood of the recipient animals at the time of rejection. Of the recipients of positive donors used for group#2, only 1 animal was shown to have a low copy number of PCMV at the time of rejection and one was negative (Table 3). In most of PCMV positive kidney xenograft, we have observed early tubular cell nuclear dystrophia potentially related to this viral replication, but in any case we observed specific signs of CMV kidney aggression, such as Cowdry inclusions (data not shown).
Finally, the Table 4 summarized most results obtained in this study, indicating for each group the graft survival (days), the cause of graft failure, Immuno-histology at rejection, the nature and cytotoxicity of peripheral elicited anti-nonGal Ab, and the viral PERV, BCMV and PCVM status.
Table 4.
Summary of experimental results according to recipients’ groups.
| Groups | Recipients | Graft survival (days) |
Diagnosis | Immuno-histology at rejection |
Peripheral anti-nonGal Ab | Virology | ||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Humoral staining |
Cellular infiltrate |
Preformed at XT |
Elicited at rejection |
PERV: R/D centromeric ratio |
Intragraft BCMV |
Intragraft PCMV |
||||
| Control group | V9910C | 4 | AXHR & AXCR | IgM, C3c, C4d, C5b-9 | Monocytes/CD4+ | IgM/IgG CDC | IgM/IgG CDC | ND | ND | ND |
| V857I | 3 | Id. | Id. | Id. | Id. | Id. | ND | ND | ND | |
|
| ||||||||||
| Group #1 | V893AA | 15 | AXHR & AXCR | IgM, C3c, C4d, C5b-9 | Monocytes/CD4+ | IgM/IgG CDC | IgM/IgG CDC | Neg | Neg | |
| PA956E | 13 | Id. | Id. | Id. | Id. | Id | <1 | Neg | Neg | |
| V907J | 12 | Id. | Id. | Id. | Id. | IgM CDC | <1 | Neg | Neg | |
|
| ||||||||||
| Group #2 | PA997C | 9 | AXHR & AXCR | IgM, C3c, C4d, C5b-9 | Monocytes/CD4+ | IgM/IgG CDC | * | >1 (NS) | Neg | +/− |
| V9912B | 9 | Id. | Id. | Id. | Id. | IgM/IgG CDC | <1 | Neg | + | |
| 022VB | 11 | Id. | Id. | Id. | Id. | IgG CDC | <1 | Neg | + | |
|
| ||||||||||
| Group #3 | PA936G | 12 | AXHR & AXCR | IgM, C3c, C4d, C5b-9 | Monocytes/CD4+ | IgM/IgG CDC | IgM/IgG CDC | >1(NS) | Neg | ++ |
| PA936F | 14 | Id. | Id. | Id. | Id. | Id. | <1 | Neg | ++ | |
| K24E | 12 | Id. | Id. | Id. | Id. | * | <1 | Neg | +++ | |
| K921F | 14 | Id. | Id. | Id. | Id. | * | <1 | Neg | +++ | |
|
| ||||||||||
| Group #4 | K13F | 11 | AXHR & AXCR | IgM, C3c, C4d, C5b-9 | Monocytes/CD4+ | IgM/IgG CDC | * | <1 | Neg | Neg |
AHXR: Acute Humoral Xenogeneic Rejection, ACXR: Acute Cellular Xenogeneic Rejection, CDC: Complement Dependant Cytotoxicity
elicited anti-nonGal Ab level and CDC at rejection < preformed anti-nonGal Ab level and CDC at XT, Neg: negative, ND: not done, NS: non significatnt. R/D: recipient/donor, XT: xenotransplantation.
Discussion
This study reports for the first time pig-to-primate renal xenotransplantation using GalT-KO.hCD55.hCD59.hCD39.hHTTg donors. In no case did we observe HAR, confirming the efficiency of the Gal KO strategy (10, 15, 16, 18, 19). Unfortunately, CD39 expression was not noted at high levels and furthermore because of only one control baboon grafted with a GalT-KO donor, we could not assess whether the efficient combined GalT-KO/CRP transgene strategy was of benefit.
In fact, cases of HAR have been reported in GalT-KO cardiac xenotransplantation, but never using GalT-KO-hCD55Tg donor (17), suggesting that anti-non-Gal Ab dependent activation of complement may be beneficially regulated by local CD55 expression only at early times. However, the major issue is that survival times observed in our study are not as encouraging as earlier experience in this area (specifically with GalT-KO thymokidneys in PCMV free studies), irrespective of the modified immunosuppressive strategies. Indeed, complement and Ab producing cell blockade, PE and general immunosuppression all failed to prevent AXHR and AXCR, as noted previously for GalT-KO heart (11, 17, 35) and kidney (15, 16) xenotransplantation.
Our results confirm the presence of circulating preformed cytotoxic anti-pig-non-Gal IgM and IgG in baboons, already demonstrated by others (11, 15–17). The rapid occurrence and the cytotoxic property of these elicited anti-non-Gal Ab also suggest their significant involvement in AHXR. Therefore, we also employed the anti-B/plasma cell agent bortezomib to decrease active bone marrow plasma cells, but only a modest reduction of circulating preformed IgM/G XNA was achieved similar to an earlier report (36). Pre- and post-xenotransplantation PE efficiently reduced circulating preformed XNA, although they were followed consistently by a rebound. Our results suggest that this treatment was efficient at blocking the B/plasma cell reactivity in some animals but that even when it did, it could not control the intra-graft aggression. In this regard, other drug combinations have been reported to result in graft survival of heterotopic heart xenotransplants for up to 1 year without XNA recovery (18, 19) and a combination of drugs and a thymokidney have led to survivals up to 83 days (in PCMV free animals) (37).
Structural identification of non-Gal Ag in the pig-to-baboon combination is also an important objective. In human, recent data argue for a role of anti N-glycolylneuraminic acid (Neu5Gc) antibodies (38, 39) and double GalT and Neu5Gc-KO pig (40) have been generated which are less sensitive to human serum CDC. However, our data (supplementary data) have demonstrated considerable variability in the anti-pig carbohydrate antibody repertoire between primates, limiting the general conclusions about the importance of particular carbohydrate antigens in NHP xenotransplantation trials.
One potential advantage of our pig donors was the combination of hCRP (CD55, CD59). The protection against complement activation seemed to play a role only in the early post-transplant period and justified the use of rhC1-INH thereafter, as shown in alloAb-mediated rejection (24). rhC1-INH blocked efficiently of the classical pathway activation only as long as it was used but failed to prevent eventual xenotransplantation rejection, possibly due to alternative pathway activation. This phenomenon described in xenotransplantation rodent models (41), was not previously demonstrated in NHP and might justify a strategy of wider complement blockade such as anti-C5 mAb.
As shown in GalT-KO heart (11, 35) and kidney (11) xenotransplantation, we observed a strong monocyte/macrophage infiltrate in all groups, regardless of the immunosuppressive treatments. Interestingly, this infiltrate affected glomerular capillaries, participating and/or worsening vessel damage and possibly linked to deposition of complement factors such as iC3b, known to bind to CD11b/18 (CR3). This role of macrophages in the xeno-cellular response may support the use of the inhibitory pathway of CD47-SIRPα through a transgenic approach (42). Obviously in our model, T and B cell infiltrate remained minor, although the majority of animals developed elicited XNA requiring a T-B cooperation in peripheral lymphoid organs, suggesting a suboptimal immunosuppression.
As already known (34), late time points of rejection were associated to coagulation disorders potentially leading to consumptive coagulopathy, suggesting that genetic modified organs did not bring any protection in this regard without ruling out that they could be worse.
The recipients showed no evidence of PERV infection, despite the use of strong immunosuppression. Thus, the potential increased risk of PERV transmission in the Gal-KO context, hypothesized on the basis of the absence of a possible protective effect of Gal Ab toward Gal negative PERV (43), was not observed here.
Similarly, despite an increase in BCMV copy number in the serum of some immunosuppressed animals, none showed signs of BCMV disease and all xenografts were BCMV-negative. The copy number of PCMV, which can play a role in the rejection of porcine renal xenografts in NHP recipients (33, 37, 44, 45), was low in donors pre-transplant, comparable to other studies (46). However, a significant increase in intra-graft copy number was observed post-transplant. PCMV replication was much higher in group#3 than in group#2, potentially due to the additional PE in group#3, although no Cowdry inclusions indicative of active PCMV graft infection were observed. Detection of circulating PCMV in the blood of the recipients at time of rejection could be explained by the presence of porcine cell microchimerism.
One question to be clarified is whether the GalT-KO.hCD55.hCD59.hCD39.hHTTg phenotype may become a disadvantage regarding protection from CMV, specifically CD55 and CD59 and incorporation into viral envelope limiting lysis (47). Another question is whether PCMV infection may have caused graft loss too soon for the benefit of the other genetic modifications to have exerted a measurable effect using the immunosuppressive regimens we have tested. This possibility would be supported by recent studies of Yamada and colleagues showing marked improvement of renal xenograft survival by use of PCMV-free donors, using an immunosuppressive regimen including costimulatory blockade with anti-CD154 mAb (48, 49). Thus, further attention to PCMV reactivation, further genetic modifications and further modifications of immunosuppression may be needed to achieve long-term xenograft survivals.
Supplementary Material
Blood and bone morrow cell monitoring in one baboon (V932H). A: platelets, B: blood leukocytes: leukocytes (dark plain line, with scare), lymphocytes (grey plain line with triangle) and monocytes (dark dashed line with triangle), C: Lymphocytes: T cells (dark plain line with triangle), B cells (grey plain line with full scare), monocytes (dark plain line with empty circle), NK cells (dark dashed line with diamond), D: blood B cell populations: CD20+CD19+ B cells (dark plain line empty scare), CD20+19+38+27− cells (dark plain line with full triangle), CD20+19+38+27+ cells (grey plain line with empty scare) and CD20+19+38−27+ cells (dark dashed line with diamond), F: % of bone marrow plasma cells / of bone morrow CD45+ cells. Arrows in histograms A, B, C, D, E, represent the bortezomib injections (days 0, 3, 7, 10 and 21). Evolution of the reactivity of circulating preformed XNA IgM (F) and IgG (G) on donor PAEC by FACS, in 10 baboons from groups # 2–4 at d-17 (before Bortezomid treatment, circle) and at d-4 (after 4 injections of Bortezomib and before the 1st plasma exchange, scare) before xenotransplantation.
Standard curves for BCMV and PCMV assay.
Thin layer chromatography analysis of neutral and acidic glycolipids isolated from kidneys and hearts from GalT-KO and WT pigs. Top plates (A) were stained with chemical reagents and (B) shows the corresponding immune staining using human purified anti-Gal Ig and human AB serum (2) as well as pre- and post-transplantation baboon sera from animals #PA956E and #K921F and control non-immunosupressed animal #V9910C (Table 1). Glycolipids with ≥3 sugars from WT #285 kidney (lane K WT), GalT-KO #196 kidney (lane K KO), GalT-KO #195 kidney (lane Ka KO) and heart (lane H KO) and WT heart (lane H WT) (7) were separated using chloroform: methanol: water, 60:35:8 for neutral glycolipids and chloroform:methanol: 0.25% KCl in water, 40:40:10 for acidic glycolipids. Reference glycolipid fractions were total neutral glycolipids from sheep small intestine (lane R1, 50 μg) and total gangliosides from pig kidney (lane R2, 4 μg). In the immunostaining experiments, 0.6% of the extracted neutral and acidic glycolipids were loaded per lane together with the Galα3nLc4 (lane R3, 0.1 μg) reference. Time points of serum sample collection are shown on each individual plate (d0 is pre-transplantation). Chemical detection for neutral glycolipids was anisaldehyde (1) and the sialic acid specific resorcinol reagent (8) for gangliosides. Number of sugar residues of neutral glycolipids are shown to the left and Gal5 indicate the mobility of Galα3nLc4 and S mobility of sulphatide.
Acknowledgments
We thank Edwin van Amersfoort (Pharming Technologies BV) for supplying the rhC1INH, Dr. Franco Caffi, (Prosus, Scrl) for the access to pig ovaries, Paola Turini, Gabriella Crotti and Silvia Colleoni (Avantea) for technical support in cloning pigs for this study.
The following reagent was obtained through the NIH Nonhuman Primate Reagent Resource: anti-CD38 Ab (OKT10).
This work was supported by the European Commission’s Sixth Framework Programme, under the priority thematic area Life Sciences, Genomics and Biotechnology for Health, contract N°. LSHB-CT- 2006- 037377, XENOME and by NIH Grant #5P01AI45897.
Abbreviations
- ACXR
Acute Cellular Xenograft Rejection
- AHXR
Acute Humoral Xenograft Rejection
- AlloAb
Allo-antibodies
- AP50
Alternative pathway complement activity
- BCMV
Baboon Cytomegalovirus
- CH50
Total complement activity
- rhC1-INH
recombinant human C1 Inhibitor
- CNRS
Centre National de la Recherche Scientifique
- hCRP
human Complement Regulatory Proteins
- Gal
Galactose-α-1,3-Galactose
- GalT-KO
Galactosyl-transferase knock-out
- HAR
Hyper Acute Rejection
- hHT
human H-transferase (α1.2-Fucosyl-transferase)
- IF
Immunofluorescence
- MBL
Mannose-binding lectin
- MBL50
Mannose-binding lectin pathway complement activity
- NHP
Non Human Primates
- PAEC
Porcine Aortic Endothelial Cells
- PCMV
Porcine Cytomegalovirus
- PE
Plasma Exchange
- PERV
Porcine Endogenous Retrovirus
- PPP
Platelet Poor Plasma
- RT
Room Temperature
- SCNT
Somatic Cell Nuclear Transfer
- TG
Thrombin generation
- WT
Wild Type
- XenoAg
Xenoantigens
- XNA
Xeno Natural Antibodies
Footnotes
Supporting Information: Additional Supporting Information may be found in the online version of this article.
Disclosure: The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Bibliography
- 1.Cozzi E, White DJ. The generation of transgenic pigs as potential organ donors for humans. Nat Med. 1995 Sep;1(9):964–6. doi: 10.1038/nm0995-964. [DOI] [PubMed] [Google Scholar]
- 2.Cowan PJ, Aminian A, Barlow H, Brown AA, Chen CG, Fisicaro N, et al. Renal xenografts from triple-transgenic pigs are not hyperacutely rejected but cause coagulopathy in non-immunosuppressed baboons. Transplantation. 2000 Jun 27;69(12):2504–15. doi: 10.1097/00007890-200006270-00008. [DOI] [PubMed] [Google Scholar]
- 3.Le Bas-Bernardet S, Anegon I, Blancho G. Progress and prospects: genetic engineering in xenotransplantation. Gene Ther. 2008 Sep;15(18):1247–56. doi: 10.1038/gt.2008.119. [DOI] [PubMed] [Google Scholar]
- 4.Winand RJ, Anaraki F, Etienne-Decerf J, Galili U. Xenogeneic thyroid-stimulating hormone-like activity of the human natural anti-Gal antibody. Interaction of anti-Gal with porcine thyrocytes and with recombinant human thyroid-stimulating hormone receptors expressed on mouse cells. J Immunol. 1993 Oct 1;151(7):3923–34. [PubMed] [Google Scholar]
- 5.Dai Y, Vaught TD, Boone J, Chen SH, Phelps CJ, Ball S, et al. Targeted disruption of the alpha1,3-galactosyltransferase gene in cloned pigs. Nat Biotechnol. 2002 Mar;20(3):251–5. doi: 10.1038/nbt0302-251. [DOI] [PubMed] [Google Scholar]
- 6.Lai L, Kolber-Simonds D, Park KW, Cheong HT, Greenstein JL, Im GS, et al. Production of alpha-1,3-galactosyltransferase knockout pigs by nuclear transfer cloning. Science. 2002 Feb 8;295(5557):1089–92. doi: 10.1126/science.1068228. [DOI] [PubMed] [Google Scholar]
- 7.Phelps CJ, Koike C, Vaught TD, Boone J, Wells KD, Chen SH, et al. Production of alpha 1,3-galactosyltransferase-deficient pigs. Science. 2003 Jan 17;299(5605):411–4. doi: 10.1126/science.1078942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kolber-Simonds D, Lai L, Watt SR, Denaro M, Arn S, Augenstein ML, et al. Production of alpha-1,3-galactosyltransferase null pigs by means of nuclear transfer with fibroblasts bearing loss of heterozygosity mutations. Proc Natl Acad Sci U S A. 2004 May 11;101(19):7335–40. doi: 10.1073/pnas.0307819101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nottle MB, Beebe LF, Harrison SJ, McIlfatrick SM, Ashman RJ, O’Connell PJ, et al. Production of homozygous alpha-1,3-galactosyltransferase knockout pigs by breeding and somatic cell nuclear transfer. Xenotransplantation. 2007 Jul;14(4):339–44. doi: 10.1111/j.1399-3089.2007.00417.x. [DOI] [PubMed] [Google Scholar]
- 10.Kuwaki K, Tseng YL, Dor FJ, Shimizu A, Houser SL, Sanderson TM, et al. Heart transplantation in baboons using alpha1,3-galactosyltransferase gene-knockout pigs as donors: initial experience. Nat Med. 2005 Jan;11(1):29–31. doi: 10.1038/nm1171. [DOI] [PubMed] [Google Scholar]
- 11.Ezzelarab M, Garcia B, Azimzadeh A, Sun H, Lin CC, Hara H, et al. The innate immune response and activation of coagulation in alpha1,3-galactosyltransferase gene-knockout xenograft recipients. Transplantation. 2009 Mar 27;87(6):805–12. doi: 10.1097/TP.0b013e318199c34f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cozzi E, Bhatti F, Schmoeckel M, Chavez G, Smith KG, Zaidi A, et al. Long-term survival of nonhuman primates receiving life-supporting transgenic porcine kidney xenografts. Transplantation. 2000 Jul 15;70(1):15–21. [PubMed] [Google Scholar]
- 13.Ashton-Chess J, Roussel JC, Bernard P, Barreau N, Karam G, Dantal J, et al. The effect of immunoglobulin immunadsorptions on delayed xenograft rejection of human CD55 transgenic pig kidneys in baboons. Xenotransplantation. 2003 Nov;10(6):552–61. doi: 10.1034/j.1399-3089.2003.00052.x. [DOI] [PubMed] [Google Scholar]
- 14.Cozzi E, Vial C, Ostlie D, Farah B, Chavez G, Smith KG, et al. Maintenance triple immunosuppression with cyclosporin A, mycophenolate sodium and steroids allows prolonged survival of primate recipients of hDAF porcine renal xenografts. Xenotransplantation. 2003 Jul;10(4):300–10. doi: 10.1034/j.1399-3089.2003.02014.x. [DOI] [PubMed] [Google Scholar]
- 15.Yamada K, Yazawa K, Shimizu A, Iwanaga T, Hisashi Y, Nuhn M, et al. Marked prolongation of porcine renal xenograft survival in baboons through the use of alpha1,3-galactosyltransferase gene-knockout donors and the cotransplantation of vascularized thymic tissue. Nat Med. 2005 Jan;11(1):32–4. doi: 10.1038/nm1172. [DOI] [PubMed] [Google Scholar]
- 16.Chen G, Qian H, Starzl T, Sun H, Garcia B, Wang X, et al. Acute rejection is associated with antibodies to non-Gal antigens in baboons using Gal-knockout pig kidneys. Nat Med. 2005 Dec;11(12):1295–8. doi: 10.1038/nm1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGregor CG, Ricci D, Miyagi N, Stalboerger PG, Du Z, Oehler EA, et al. Human CD55 expression blocks hyperacute rejection and restricts complement activation in Gal knockout cardiac xenografts. Transplantation. 2012 Apr s;93(7):686–92. doi: 10.1097/TP.0b013e3182472850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mohiuddin MM, Corcoran PC, Singh AK, Azimzadeh A, Hoyt RF, Jr, Thomas ML, et al. B-cell depletion extends the survival of GTKO.hCD46Tg pig heart xenografts in baboons for up to 8 months. Am J Transplant. 2012 Mar;12(3):763–71. doi: 10.1111/j.1600-6143.2011.03846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mohiuddin MM, Singh AK, Corcoran PC, Hoyt RF, Thomas ML, 3rd, Lewis BG, et al. One-year heterotopic cardiac xenograft survival in a pig to baboon model. Am J Transplant. 2014 Feb;14(2):488–9. doi: 10.1111/ajt.12562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lagutina I, Lazzari G, Galli C. Birth of cloned pigs from zona-free nuclear transfer blastocysts developed in vitro before transfer. Cloning Stem Cells. 2006 Winter;8(4):283–93. doi: 10.1089/clo.2006.8.283. [DOI] [PubMed] [Google Scholar]
- 21.Dwyer KM, Robson SC, Nandurkar HH, Campbell DJ, Gock H, Murray-Segal LJ, et al. Thromboregulatory manifestations in human CD39 transgenic mice and the implications for thrombotic disease and transplantation. J Clin Invest. 2004 May;113(10):1440–6. doi: 10.1172/JCI19560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Westall GP, Levvey BJ, Salvaris E, Gooi J, Marasco S, Rosenfeldt F, et al. Sustained function of genetically modified porcine lungs in an ex vivo model of pulmonary xenotransplantation. J Heart Lung Transplant. 2013 Nov;32(11):1123–30. doi: 10.1016/j.healun.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 23.Ashton-Chess J, Meurette G, Karam G, Petzold T, Minault D, Naulet J, et al. The study of mitoxantrone as a potential immunosuppressor in transgenic pig renal xenotransplantation in baboons: comparison with cyclophosphamide. Xenotransplantation. 2004 Mar;11(2):112–22. doi: 10.1111/j.1399-3089.2004.00040.x. [DOI] [PubMed] [Google Scholar]
- 24.Tillou X, Poirier N, Le Bas-Bernardet S, Hervouet J, Minault D, Renaudin K, et al. Recombinant human C1-inhibitor prevents acute antibody-mediated rejection in alloimmunized baboons. Kidney Int. 2010 Jul;78(2):152–9. doi: 10.1038/ki.2010.75. [DOI] [PubMed] [Google Scholar]
- 25.Hemker HC, Giesen P, AlDieri R, Regnault V, de Smed E, Wagenvoord R, et al. The calibrated automated thrombogram (CAT): a universal routine test for hyper- and hypocoagulability. Pathophysiol Haemost Thromb. 2002 Sep-Dec;32(5–6):249–53. doi: 10.1159/000073575. [DOI] [PubMed] [Google Scholar]
- 26.Devalliere J, Chatelais M, Fitau J, Gerard N, Hulin P, Velazquez L, et al. LNK (SH2B3) is a key regulator of integrin signaling in endothelial cells and targets alpha-parvin to control cell adhesion and migration. FASEB J. 2012 Jun;26(6):2592–606. doi: 10.1096/fj.11-193383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chatelais M, Devalliere J, Galli C, Charreau B. Gene transfer of the adaptor Lnk (SH2B3) prevents porcine endothelial cell activation and apoptosis: implication for xenograft’s cytoprotection. Xenotransplantation. 2011 Mar-Apr;18(2):108–20. doi: 10.1111/j.1399-3089.2011.00629.x. [DOI] [PubMed] [Google Scholar]
- 28.Seelen MA, Roos A, Wieslander J, Mollnes TE, Sjoholm AG, Wurzner R, et al. Functional analysis of the classical, alternative, and MBL pathways of the complement system: standardization and validation of a simple ELISA. J Immunol Methods. 2005 Jan;296(1–2):187–98. doi: 10.1016/j.jim.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 29.Siezenga MA, Chandie Shaw PK, van der Geest RN, Mollnes TE, Daha MR, Rabelink TJ, et al. Enhanced complement activation is part of the unfavourable cardiovascular risk profile in South Asians. Clin Exp Immunol. 2009 Jul;157(1):98–103. doi: 10.1111/j.1365-2249.2009.03959.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reddingius RE, Schroder CH, Daha MR, Monnens LA. The serum complement system in children on continuous ambulatory peritoneal dialysis. Perit Dial Int. 1993;13(3):214–8. [PubMed] [Google Scholar]
- 31.Berger SP, Roos A, Mallat MJ, Schaapherder AF, Doxiadis II, van Kooten C, et al. Low pretransplantation mannose-binding lectin levels predict superior patient and graft survival after simultaneous pancreas-kidney transplantation. J Am Soc Nephrol. 2007 Aug;18(8):2416–22. doi: 10.1681/ASN.2007030262. [DOI] [PubMed] [Google Scholar]
- 32.Paradis K, Langford G, Long Z, Heneine W, Sandstrom P, Switzer WM, et al. Search for cross-species transmission of porcine endogenous retrovirus in patients treated with living pig tissue. The XEN 111 Study Group. Science. 1999 Aug 20;285(5431):1236–41. doi: 10.1126/science.285.5431.1236. [DOI] [PubMed] [Google Scholar]
- 33.Mueller NJ, Barth RN, Yamamoto S, Kitamura H, Patience C, Yamada K, et al. Activation of cytomegalovirus in pig-to-primate organ xenotransplantation. J Virol. 2002 May;76(10):4734–40. doi: 10.1128/JVI.76.10.4734-4740.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cowan PJ, Cooper DK, d’Apice AJ. Kidney xenotransplantation. Kidney Int. 2014 Feb;85(2):265–75. doi: 10.1038/ki.2013.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hisashi Y, Yamada K, Kuwaki K, Tseng YL, Dor FJ, Houser SL, et al. Rejection of cardiac xenografts transplanted from alpha1,3-galactosyltransferase gene-knockout (GalT-KO) pigs to baboons. Am J Transplant. 2008 Dec;8(12):2516–26. doi: 10.1111/j.1600-6143.2008.02444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liang F, Wamala I, Scalea J, Tena A, Cormack T, Pratts S, et al. Increased levels of anti-non-Gal IgG following pig-to-baboon bone marrow transplantation correlate with failure of engraftment. Xenotransplantation. 2013 Nov;20(6):458–68. doi: 10.1111/xen.12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamada K, Tasaki T, Sekijima M, Wilkinson RA, Villani V, Moran SG, et al. Porcine CMV Infection Is Associated with Early Rejection of Kidney Grafts in a Pig to Baboon Xenotransplantation Model. Transplantation. 2014 Apr; doi: 10.1097/TP.0000000000000232. accepted; in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu A, Hurst R. Anti-N-glycolylneuraminic acid antibodies identified in healthy human serum. Xenotransplantation. 2002 Nov;9(6):376–81. doi: 10.1034/j.1399-3089.2002.02138.x. [DOI] [PubMed] [Google Scholar]
- 39.Scobie L, Padler-Karavani V, Le Bas-Bernardet S, Crossan C, Blaha J, Matouskova M, et al. Long-Term IgG Response to Porcine Neu5Gc Antigens without Transmission of PERV in Burn Patients Treated with Porcine Skin Xenografts. J Immunol. 2013 Aug 14; doi: 10.4049/jimmunol.1301195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lutz AJ, Li P, Estrada JL, Sidner RA, Chihara RK, Downey SM, et al. Double knockout pigs deficient in N-glycolylneuraminic acid and Galactose alpha-1,3-Galactose reduce the humoral barrier to xenotransplantation. Xenotransplantation. 2013 Jan-Feb;20(1):27–35. doi: 10.1111/xen.12019. [DOI] [PubMed] [Google Scholar]
- 41.Romanella M, Aminian A, Adam WR, Pearse MJ, d’Apice AJ. Involvement of both the classical and alternate pathways of complement in an ex vivo model of xenograft rejection. Transplantation. 1997 Apr 15;63(7):1021–5. doi: 10.1097/00007890-199704150-00020. [DOI] [PubMed] [Google Scholar]
- 42.Ide K, Wang H, Tahara H, Liu J, Wang X, Asahara T, et al. Role for CD47-SIRPalpha signaling in xenograft rejection by macrophages. Proc Natl Acad Sci U S A. 2007 Mar 20;104(12):5062–6. doi: 10.1073/pnas.0609661104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Quinn G, Wood JC, Ryan DJ, Suling KM, Moran KM, Kolber-Simonds DL, et al. Porcine endogenous retrovirus transmission characteristics of galactose alpha1-3 galactose-deficient pig cells. J Virol. 2004 Jun;78(11):5805–11. doi: 10.1128/JVI.78.11.5805-5811.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sekijima M, Waki S, Sahara H, Tasaki M, Wilkinson RA, Villani V, et al. Results of Life-Supporting GalT-KO kidneys in Cynomolgus Monkeys Using Two Different Sources of GalT-KO Swine. Transplantation. 2014 Apr; doi: 10.1097/TP.0000000000000314. accepted in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gollackner B, Mueller NJ, Houser S, Qawi I, Soizic D, Knosalla C, et al. Porcine cytomegalovirus and coagulopathy in pig-to-primate xenotransplantation. Transplantation. 2003 Jun 15;75(11):1841–7. doi: 10.1097/01.TP.0000065806.90840.C1. [DOI] [PubMed] [Google Scholar]
- 46.Clark DA, Fryer JF, Tucker AW, McArdle PD, Hughes AE, Emery VC, et al. Porcine cytomegalovirus in pigs being bred for xenograft organs: progress towards control. Xenotransplantation. 2003 Mar;10(2):142–8. doi: 10.1034/j.1399-3089.2003.01128.x. [DOI] [PubMed] [Google Scholar]
- 47.Stoermer KA, Morrison TE. Complement and viral pathogenesis. Virology. 2011 Mar 15;411(2):362–73. doi: 10.1016/j.virol.2010.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Waki S, Sahara H, Miura K, Kawai A, Sekijima M, Nakano K, et al. Porcine CMV may be the causative agent of porcine kidney rejection in GalT-KO pig to nonhuman primate preclinical xenotransplantation. Xenotransplantation. 2013;20:328. [Google Scholar]
- 49.Villani V, Sekijima M, Tasaki M, Torabi R, Cormack T, Torabi R, et al. Porcine CMV infection is associated with early rejection of kidney grafts in a pig-to-baboon xenotransplantation model. Xenotransplantation. 2013;20:366. doi: 10.1097/TP.0000000000000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Blood and bone morrow cell monitoring in one baboon (V932H). A: platelets, B: blood leukocytes: leukocytes (dark plain line, with scare), lymphocytes (grey plain line with triangle) and monocytes (dark dashed line with triangle), C: Lymphocytes: T cells (dark plain line with triangle), B cells (grey plain line with full scare), monocytes (dark plain line with empty circle), NK cells (dark dashed line with diamond), D: blood B cell populations: CD20+CD19+ B cells (dark plain line empty scare), CD20+19+38+27− cells (dark plain line with full triangle), CD20+19+38+27+ cells (grey plain line with empty scare) and CD20+19+38−27+ cells (dark dashed line with diamond), F: % of bone marrow plasma cells / of bone morrow CD45+ cells. Arrows in histograms A, B, C, D, E, represent the bortezomib injections (days 0, 3, 7, 10 and 21). Evolution of the reactivity of circulating preformed XNA IgM (F) and IgG (G) on donor PAEC by FACS, in 10 baboons from groups # 2–4 at d-17 (before Bortezomid treatment, circle) and at d-4 (after 4 injections of Bortezomib and before the 1st plasma exchange, scare) before xenotransplantation.
Standard curves for BCMV and PCMV assay.
Thin layer chromatography analysis of neutral and acidic glycolipids isolated from kidneys and hearts from GalT-KO and WT pigs. Top plates (A) were stained with chemical reagents and (B) shows the corresponding immune staining using human purified anti-Gal Ig and human AB serum (2) as well as pre- and post-transplantation baboon sera from animals #PA956E and #K921F and control non-immunosupressed animal #V9910C (Table 1). Glycolipids with ≥3 sugars from WT #285 kidney (lane K WT), GalT-KO #196 kidney (lane K KO), GalT-KO #195 kidney (lane Ka KO) and heart (lane H KO) and WT heart (lane H WT) (7) were separated using chloroform: methanol: water, 60:35:8 for neutral glycolipids and chloroform:methanol: 0.25% KCl in water, 40:40:10 for acidic glycolipids. Reference glycolipid fractions were total neutral glycolipids from sheep small intestine (lane R1, 50 μg) and total gangliosides from pig kidney (lane R2, 4 μg). In the immunostaining experiments, 0.6% of the extracted neutral and acidic glycolipids were loaded per lane together with the Galα3nLc4 (lane R3, 0.1 μg) reference. Time points of serum sample collection are shown on each individual plate (d0 is pre-transplantation). Chemical detection for neutral glycolipids was anisaldehyde (1) and the sialic acid specific resorcinol reagent (8) for gangliosides. Number of sugar residues of neutral glycolipids are shown to the left and Gal5 indicate the mobility of Galα3nLc4 and S mobility of sulphatide.


