Abstract
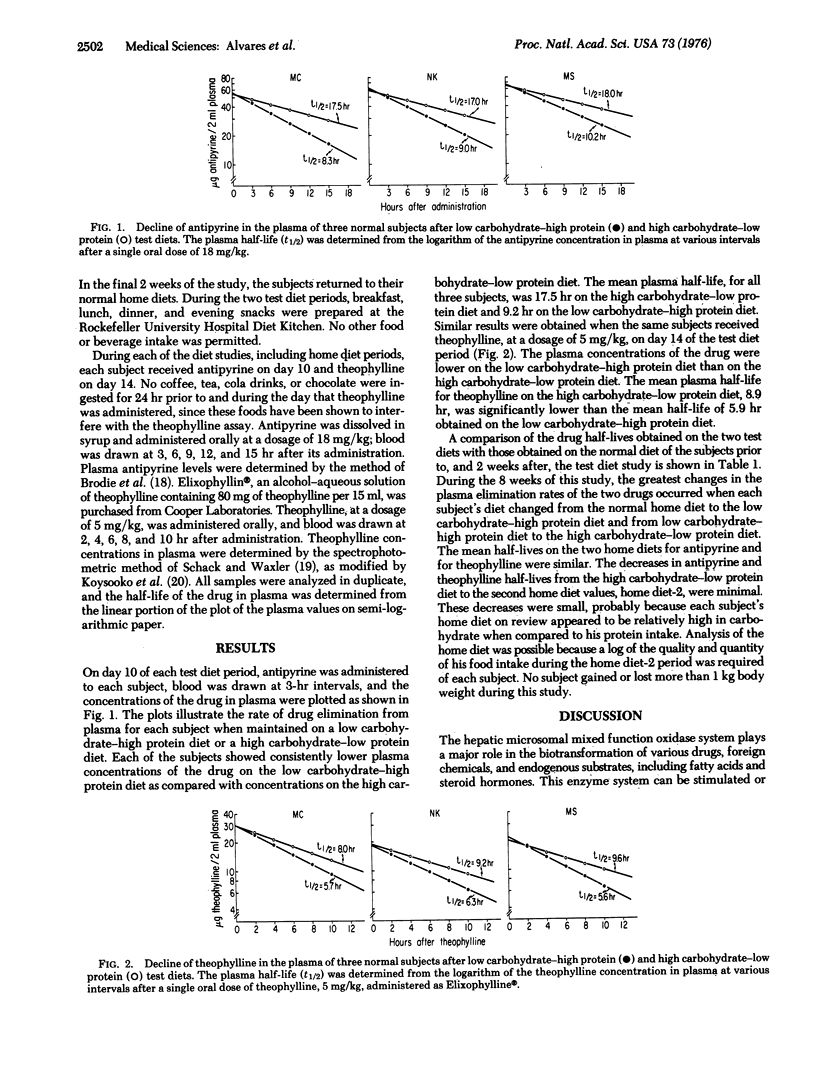
This study was undertaken to examine the influence of nutritional factors on the activity of the mixed function oxidase system in man, which is cytochrome P-450 dependent. Three normal volunteers were fed a low carbohydrate-high protein diet for 2 weeks, followed by a high carbohydrate-low protein diet for the follwoing 2 weeks. At the end of each test diet period, the plasma elimination rates of antipyrine and theophylline were determined. The mean plasma half-life for antipyrine was 17.5 hr on the high carbohydrate-low protein diet and 9.2 hr on the low carbohydrate-high protein diet. The mean plasma half-life for theophylline was 8.9 hr on the high carbohydrate-low protein diet and 5.9 hr on the low carbohydrate-high protein diet. These data demonstrate marked influences of dietary carbohydrate and/or protein ingestion on oxidative biotransformation of drugs in man.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvares A. P., Bickers D. R., Kappas A. Polychlorinated biphenyls: a new type of inducer of cytochrome P-448 in the liver. Proc Natl Acad Sci U S A. 1973 May;70(5):1321–1325. doi: 10.1073/pnas.70.5.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argyris T. S. Additive effects of phenobarbital and high protein diet on liver growth in immature male rats. Dev Biol. 1971 Jun;25(2):293–309. doi: 10.1016/0012-1606(71)90032-7. [DOI] [PubMed] [Google Scholar]
- BRODIE B. B., AXELROD J. The fate of antipyrine in man. J Pharmacol Exp Ther. 1950 Jan;98(1):97–104. [PubMed] [Google Scholar]
- Boyd E. M., Carsky E. Kwashiorigenic diet and diazinon toxicity. Acta Pharmacol Toxicol (Copenh) 1969;27(4):284–294. doi: 10.1111/j.1600-0773.1969.tb00514.x. [DOI] [PubMed] [Google Scholar]
- Boyd E. M., Dobos I. Protein deficiency and tolerated oral doses of endosulfan. Arch Int Pharmacodyn Ther. 1969 Mar;178(1):152–165. [PubMed] [Google Scholar]
- Campbell T. C., Hayes J. R. Role of nutrition in the drug-metabolizing enzyme system. Pharmacol Rev. 1974 Sep;26(3):171–197. [PubMed] [Google Scholar]
- Century B. A role of the dietary lipid in the ability of phenobarbital to stimulate drug detoxification. J Pharmacol Exp Ther. 1973 May;185(2):185–194. [PubMed] [Google Scholar]
- Conney A. H., Burns J. J. Metabolic interactions among environmental chemicals and drugs. Science. 1972 Nov 10;178(4061):576–586. doi: 10.1126/science.178.4061.576. [DOI] [PubMed] [Google Scholar]
- Goldberg M. L. The glucose effect: carbohydrate repression of enzyme induction, RNA synthesis, and glucocorticoid activity -- a role for cyclic AMP and cyclic GMP. Life Sci. 1975 Dec 15;17(12):1747–1754. doi: 10.1016/0024-3205(75)90456-7. [DOI] [PubMed] [Google Scholar]
- Jenne H., Nagasawa H., McHugh R., MacDonald F., Wyse E. Decreased theophylline half-life in cigarette smokers. Life Sci. 1975 Jul 15;17(2):195–198. doi: 10.1016/0024-3205(75)90503-2. [DOI] [PubMed] [Google Scholar]
- Kato R., Oshima T., Tomizawa S. Toxicity and metabolism of drugs in relation to dietary protein. Jpn J Pharmacol. 1968 Sep;18(3):356–366. doi: 10.1254/jjp.18.356. [DOI] [PubMed] [Google Scholar]
- Kato R., Takanaka A. Effect of starvation on the in vivo metabolism and effect of drugs in female and male rats. Jpn J Pharmacol. 1967 Jun;17(2):208–217. doi: 10.1254/jjp.17.208. [DOI] [PubMed] [Google Scholar]
- Koysooko R., Ellis E. F., Levy G. Relationship between theophylline concentration in plasma and saliva of man. Clin Pharmacol Ther. 1974 May;15(5):454–460. doi: 10.1002/cpt1974155454. [DOI] [PubMed] [Google Scholar]
- Lohmann S. M., Miech R. P. Theophylline metabolism by the rat liver microsomal system. J Pharmacol Exp Ther. 1976 Jan;196(1):213–225. [PubMed] [Google Scholar]
- Norred W. P., Wade A. E. Dietary fatty acid-induced alterations of hepatic microsomal drug metabolism. Biochem Pharmacol. 1972 Nov 1;21(21):2887–2897. doi: 10.1016/0006-2952(72)90213-4. [DOI] [PubMed] [Google Scholar]
- Peraino C., Lamar C., Jr, Pitot H. C. Studies on the mechanism of carbohydrate repression in rat liver. Adv Enzyme Regul. 1966;4:199–217. doi: 10.1016/0065-2571(66)90015-x. [DOI] [PubMed] [Google Scholar]
- Pitot H. C., Peraino C., Pries N., Kennan A. L. Glucose repression and induction of enzyme synthesis in rat liver. Adv Enzyme Regul. 1964;2:237–247. doi: 10.1016/s0065-2571(64)80016-9. [DOI] [PubMed] [Google Scholar]
- ROSE J. A., HELLMAN E. S., TSCHUDY D. P. Effect of diet on induction of experimental porphyria. Metabolism. 1961 Jul;10:514–521. [PubMed] [Google Scholar]
- Reidenberg M. M., Vesell E. S. Unaltered metabolism of antipyrine and tolbutamide in fasting man. Clin Pharmacol Ther. 1975 Jun;17(6):650–656. doi: 10.1002/cpt1975176650. [DOI] [PubMed] [Google Scholar]
- SCHACK J. A., WAXLER S. H. An ultraviolet spectrophotometric method for the determination of theophylline and theobromine in blood and tissues. J Pharmacol Exp Ther. 1949 Nov;97(3):283–291. [PubMed] [Google Scholar]
- SCHLUGER J., MCGINN J. T., HENNESSY D. J. Comparative theophylline blood levels following the oral administration of three different theophylline preparations. Am J Med Sci. 1957 Mar;233(3):296–302. doi: 10.1097/00000441-195703000-00009. [DOI] [PubMed] [Google Scholar]
- Short J., Armstrong N. B., Zemel R., Lieberman I. A role for amino acids in the induction of deoxyribonucleic acid synthesis in liver. Biochem Biophys Res Commun. 1973 Jan 23;50(2):430–437. doi: 10.1016/0006-291x(73)90858-9. [DOI] [PubMed] [Google Scholar]
- Strobel H. W., Lu A. Y., Heidema J., Coon M. J. Phosphatidylcholine requirement in the enzymatic reduction of hemoprotein P-450 and in fatty acid, hydrocarbon, and drug hydroxylation. J Biol Chem. 1970 Sep 25;245(18):4851–4854. [PubMed] [Google Scholar]
- Strother A., Throckmorton J. K., Herzer C. The influence of high sugar consumption by mice on the duration of action of barbiturates and in vitro metabolism of barbiturates, aniline and p-nitroanisole. J Pharmacol Exp Ther. 1971 Dec;179(3):490–498. [PubMed] [Google Scholar]
- Vesell E. S., Page J. G. Genetic control of the phenobarbital-induced shortening of plasma antipyrine half-lives in man. J Clin Invest. 1969 Dec;48(12):2202–2209. doi: 10.1172/JCI106186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesell E. S., Passananti G. T., Greene F. E., Page J. G. Genetic control of drug levels and of the induction of drug-metabolizing enzymes in man: individual variability in the extent of allopurinol and nortriptyline inhibition of drug metabolism. Ann N Y Acad Sci. 1971 Jul 6;179:752–773. doi: 10.1111/j.1749-6632.1971.tb46950.x. [DOI] [PubMed] [Google Scholar]
- WELLAND F. H., HELLMAN E. S., GADDIS E. M., COLLINS G., HUNTER G. W., Jr, TSCHUDY D. P. FACTORS AFFECTING THE EXCRETION OF PORPHYRIN PRECURSORS BY PATIENTS WITH ACUTE INTERMITTENT PORPHYRIA. I. THE EFFECT OF DIET. Metabolism. 1964 Mar;13:232–250. doi: 10.1016/0026-0495(64)90103-9. [DOI] [PubMed] [Google Scholar]



