Abstract
Oxysterols are metabolites of cholesterol that are produced in liver and other peripheral tissues as a means to eliminate cholesterol to bile acid. Recent studies have revealed that the most abundant circulating oxysterol 27-hydroxycholesterol (27HC) is the first identified endogenous selective estrogen receptor modulator. 27HC levels correlate well with that of cholesterol, and also rise progressively with age. 27HC affects estrogen receptor function by the antagonism of estrogen action and also by the direct modulation of the receptor function, and similar to estrogen/estrogen receptors, 27HC has many actions in various tissues. This review article introduces the recent progress in the understanding of the role of 27HC in breast cancer and cardiovascular dysfunction.
Keywords: 27-hydroxycholesterol, atherosclerosis, breast cancer, cholesterol metabolite, CYP27A1, CYP7B1, estrogen receptor, oxysterol, SERM
27HC, its generation & metabolism
Oxysterols are metabolites of cholesterol that are produced in liver and other peripheral tissues. Although oxysterols were originally considered as substrates for bile acid synthesis and also as a means to eliminate cholesterol, especially from peripheral tissues to the liver, there is accumulating evidence that oxysterols have unique function in various tissues [1–3]. Some oxysterols are present in foods, however, most oxysterol with the exception of 7β-hydroxycholesterol (7βHC) is only slightly absorbed in the intestinal tract and rapidly metabolized in the liver ([4,5] Umetani, Unpublished Data). The most abundant circulating oxysterol is 27-hydroxycholesterol (27HC). 27HC is a hydroxylated product in the C27 position on the lateral chain of the cholesterol structure, and serum concentrations of 27HC correlate well with that of cholesterol [1]. 27HC levels also rise progressively with age [6]. There are large differences in the ratio of esterified to unesterified 27HC among tissues, ranging from more than 90% in serum to less than 40% in kidney, with around 30% unesterified in the aorta [7–9].
The enzyme that generates 27HC, sterol 27-hydroxylase (CYP27A1), is a mitochondrial P450 enzyme and is primarily expressed in the liver, but it is also expressed in peripheral tissues, but to a lesser extent [10]. CYP27A1 catalyzes oxidation of cholesterol at C27 position to form 27HC and cholestenoic acid with NADPH, adrenodoxin and adrenodoxin reductase as co-factors [11–13]. CYP27A1 gene expression is upregulated by growth hormone and insulin-like growth hormone-1 [14], and downregulated by steroid hormones and inflammatory cytokines [14–16]. While 27HC affects cellular cholesterol homeostasis as a potent suppressor of cholesterol synthesis by regulating SREBP action in vitro [17,18], its effect on SREBP action in vivo is not clear [19]. 27HC is metabolized by another P450 enzyme, oxysterol 7α-hydroxylase (CYP7B1). CYP7B1 is expressed in the brain, particularly in the hippocampus, but is also expressed in liver and other peripheral organs [20,21]. Although the physiological concentration of these substrates should be considered, CYP7B1 has a relatively broad substrate specificity for steroids and sterols such as dehydroepiandrosterone (DHEA), 5α-androstane-3β, 17β-diol (3Adiol), 25-hydroxycholesterol, pregnenorone, 17β-estradiol (E2) and 27HC, as a 7α-hydroxylase [21–23]. Clinically, the importance of CYP7B1 has been reported in prostate cancer together with ER-β and 3Adiol because of its enzymatic function against androgen and estrogen, and CYP7B1 inhibitors can be used as chemoprevention and in the treatment of prostate cancer [23–25]. CYP7B1 shows male-predominant expression in the liver [8,26]. E2 induces transcription of CYP7B1 in culture cells through PI3K-Akt pathway [27].
There is valuable information available about the physiological roles of CYP27A1 and CYP7B1 from mutational study in human and knockout/transgenic mice. In the bile acid production, CYP27A1 acts both in the classical and alternative pathways of the conversion from cholesterol to bile acids, whereas CYP7B1 is only involved in the alternative pathway. In addition, CYP27A1 is also involved in the reverse cholesterol transport and vitamin D3 biosynthesis [28]. Therefore, it needs to be noted that in addition to not making 27HC, loss of CYP27A1 results in a number of problems associated with cholesterol and bile acid metabolism, thereby the phenotypes caused by the loss of CYP7B1 and by the loss of CYP27A1 may be due to different mechanisms of action. In addition, there are differences in the phenotypes caused by the suppression/deficiency of CYP27A1 or CYP7B1 between human and mice [29,30]. In humans, functional deficiency of CYP27A1 causes a rare disorder, CTX (cerebrotendinous xanthomatosis), connected with sterol deposition in tissue macrophages and increased risk of neuronal dysfunction and premature atherosclerosis due to diminished reverse cholesterol transport, regardless of normal circulating cholesterol levels [12,13]. In mice, loss of CYP27A1 results in a number of problems associated with cholesterol and bile acid metabolism, the combination of which contributes to the cardiovascular function. However, mice with CYP27A1 deficiency are still able to produce bile acids, and decreased bile acid synthesis caused by CYP27A1 deficiency is due to the involvement of CYP27A1 in the classical pathway, and not due to the decreased 27HC levels. Indeed, CYP27A1 overexpressed mice do not show a significant difference in cholesterol homeostasis, regardless of increased serum 27HC levels [31]. CYP7B1 mutation in humans causes spastic paraplegia 5A (SPG5A), an autosomal recessive neurologic disorder, which is due to the defect in cholesterol and neurosteroid metabolism. The phenotypes of mice with CYP7B1 deficiency are described below.
27HC, the first identified endogenous selective estrogen receptor modulator
Using cell-based assays and in vitro assays, we discovered that 27HC is a competitive ER antagonist in the vasculature [9]. 27HC binds directly to ER-α (Ki= 1.32 µM) and ER-β (Ki=0.42 µM). The Km of 27HC for its catabolic enzyme CYP7B1 is 24 µM [32], which is much higher than the Kd of 27HC for the ERs. Thus, unesterified 27HC achieves levels above the Kd value for the ER activity.
The generation of nitric oxide (NO) by inducible type and endothelial type of nitric oxide synthases (iNOS and eNOS, respectively) promotes endothelial cell growth and migration, and prevents leukocyte adhesion, thrombosis and vascular smooth muscle cell proliferation. Reduced vascular synthesis of NO causes several disorders, including hypercholesterolemia and diabetes mellitus [33–35]. E2 regulates vascular functions such as: vasodilation and re-endothelialization after vascular injury through its modulation of iNOS and eNOS. With the known functions of ER in the regulation of NOS in EC, the pathophysiologic implications of the findings on 27HC were determined in vivo. Increasing 27HC levels in mice by diet-induced hypercholesterolemia or pharmacologic administration decreased estrogen-dependent expression of vascular eNOS and iNOS, repressed carotid artery re-endothelialization and increased aortic tension [9]. Parallel findings were shown in mice with elevated 27HC due to deletion of CYP7B1. The binding of 27HC to nuclear receptors is highly ER specific, and 27HC did not affect other nuclear receptor activity tested [9]. Indeed, although some reports show that 27HC is a weak agonist for liver X receptor (LXR) [36] in vitro or cell culture models, 27HC does not show strong direct activity as an LXR agonist in certain cell lines [37] and in vivo [9,38]. In addition to the antiestrogenic effects of 27HC in vascular endothelial cells in the presence of estrogen, we also identified proestrogenic actions of 27HC in hepatoma and colon cancer cells, indicating that the effect of 27HC is tissue specific [9]. Thus, 27HC is the first identified endogenous selective estrogen receptor modulator, or SERM, and it has important biological actions in vivo.
Impact of 27HC on breast cancer
Breast cancer is the second most common malignancy after skin cancer in women, with approximately 1,000,000 new cases diagnosed worldwide each year. The risk of ER-positive breast cancer increases particularly in postmenopausal women despite of the decline of circulating estrogen levels. Endocrine-based therapies against ER-positive breast cancer with synthetic SERMs or aromatase inhibitors are often ineffective or resistance develops [39], suggesting that there are yet unknown, important ER-mediated mechanisms [40]. There is increasing evidence that obesity is closely related with several types of cancer development and progression [41].
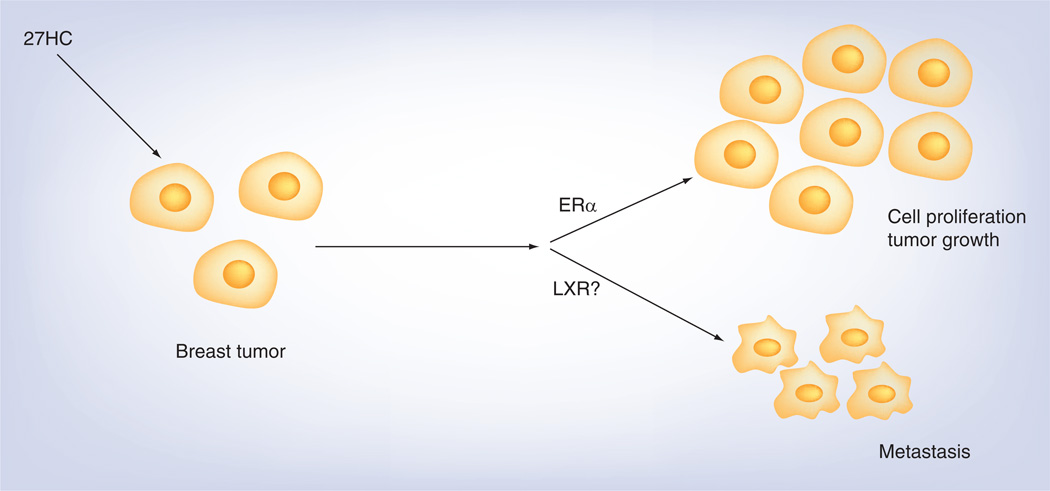
Our two recent papers by Wu et al. [42] and Nelson et al. [43], together with our previous report [44] shed light on the link between 27HC and the progression of breast tumor, in which the impact of estrogen/ER in cancer development and progression has been widely studied. In MCF-7 cells, 27HC increases cell number and has a potent impact on ER-mediated processes involved in breast cancer cell growth [44]. We further investigated the role of 27HC in breast cancer progression in vivo. First, we compared mRNA expression of cyp27a1 and cyp7b1 in ER-positive tumors versus normal breast tissue samples in the Cancer Genome Atlas (TCGA 2012). cyp27a1 expression was similar in normal breast and tumors, in contrast, cyp7b1 expression was decreased in ER-positive tumors compared with normal breast tissue. In addition, there were greater 27HC levels in tumor samples compared with controls. Furthermore, survival of cancer patients was markedly poorer for patients with low versus high tumor cyp7b1 expression. In mouse models, 27HC promoted the tumor growth and metastasis by independent mechanisms (Figure 1) [43]. Interestingly, 27HC treatment also increased macrophage infiltration and angiogenesis in the tumor, and locally produced 27HC by CYP27A1 in macrophages may determine the likelihood of a higher tumor grade. In addition, elevated levels of 27HC or cyp27a1 gene expression, or decreased levels of cyp7b1 expression correlated with tumor metastasis. Thus, 27HC, which is an endogenous SERM that does not require aromatization, has a potent impact on ER-mediated processes in breast cancer cells in vitro and in vivo.
Figure 1. 27-hydroxycholesterol promotes breast tumor progression and metastasis.
27HC promotes cell proliferation and tumor growth of breast cancer cells via ER-α. It also increases tumor metastasis through actions independent from ER function, probably via LXR.
27HC: 27-Hydroxycholesterol; ER: estrogen receptor; LXR: Liver X receptor.
This discovery will be of great interest, because similar mechanisms are potentially operative in a number of other steroid hormone-responsive cancers, and also 27HC and its regulatory enzymes in cancer may explain treatment failures with aromatase inhibition. Therefore, assessments of 27HC or the enzyme abundance in tumors may aid in personalizing hormone-based therapy. However, the limitation of these studies should be noted. For example, these studies were performed in postmenopausal women, who have reduced estrogen levels. In addition, estrogen is also produced from stromal cells of adipose tissue [45], and increased fat mass in women with obesity can enhance the aromatization of adrenal androgens and consequently increase estrogens [46]. From cell culture assays, 27HC has proestrogenic effects in breast cancer cells in the absence of estrogen, in contrast, it shows suppressive effects on the ER activity in the presence of estrogen [9]. Sometimes, the effects of 27HC in cell culture and in vivo are different, therefore, how 27HC acts against breast tumors in premenopausal women should be investigated. In this regard, it is interesting that 27HC promotes tamoxifen-resistant tumors in the similar fashion as E2 [43], suggesting that 27HC promotes breast tumors that have acquired resistance to chemical therapy such as tamoxifen and other SERMs.
Impact of 27HC on atherosclerosis & metabolism
Atheroscrelosis is a complex disease, and inflammation is a major component that is involved in the pathological process [47,48]. Oxidized lipids modulate inflammation-related gene expression, which induces proatherogenic responses and plaque instability [49]. Oxysterols, such as 7βHC and 7keto-cholesterol but not cholesterol with the same levels, have cytotoxic effects and cause superoxide anion production [50]. In contrast, 27HC is also important for cholesterol elimination from macrophages, and this works as an atheroprotective effect [13]. Furthermore, in mice CYP27A1 shows gene dose-dependent effect which is likely due to affected reverse cholesterol transport, and atherosclerosis is accelerated in cyp27a1+/−, but reduced in cyp27a1−/− mice [51]. As described above, plasma 27HC concentration is strongly correlated with the concentration of cholesterol. Compared with plasma 27HC levels, 27HC levels in atherosclerotic lesions are much higher and approach millimolar concentrations [1]. The amount of 27HC in atherosclerotic lesions increases with the severity of the lesion and with the abundance of macrophages [10,52]. There is one report showing no correlation between plasma 27HC levels and CHD risk in the WHI study [53], however, the plasma levels of 27HC in that paper are very low compared with previously published data from human serum [1]. In addition to the abundance of cholesterol, CYP27A1 is also upregulated in atherosclerotic lesions [10,54], and its expression increases during monocyte to macrophage differentiation [55]. High cholesterol/fat diet increases plasma, hepatic and adipose levels of 27HC in mice [56]. 27HC is also locally produced as one of the major de novo cholesterol products in adipocytes and suppresses adipocyte differentiation, suggesting the prevention of the formation of new fat cells upon overfeeding with dietary cholesterol [57].
The role of estrogen in atherosclerosis and cardiovascular function is also complex. At the early stages of atherosclerotic lesion development, estrogen prevents atheroma formation, and it also protects vasculature from ischemia [58]. In contrast, estrogen has deleterious effects such as promoting inflammation, unstabilizing plaque and increasing coagulation and thrombosis, especially at the late stages when the fatty streak is established [59]. Work in multiple animal models and observational clinical studies suggests that estrogen has the potential to provide potent cardiovascular protection. However, following more recent clinical trials, it is now apparent that there are mechanisms that modify the vascular actions of estrogen, particularly in the setting of hypercholesterolemia. This raises the critically important and novel possibility that there are endogenous counter-regulatory mechanisms.
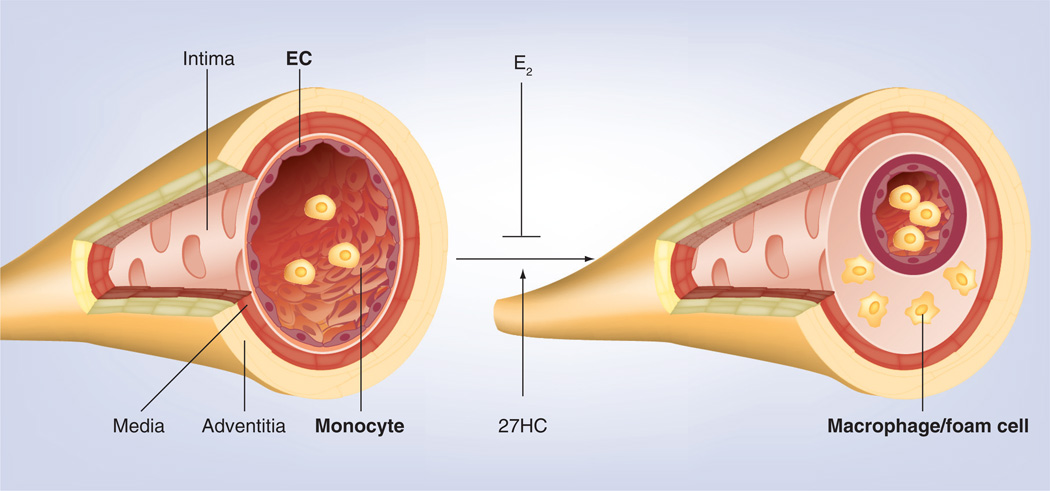
Based on the results showing that 27HC as a SERM inhibits both transcriptional and nontranscriptional estrogen-dependent production of NO in vascular endothelial cells, we tested the impact of 27HC on atherosclerosis by crossing apoE-deficient mice, commonly used as an atherosclerosis model, with cyp7b1−/− mice and studied the resulting littermates [60]. This animal model enabled us to compare the impact of 27HC with or without hypercholesterolemia. We found that elevations in 27HC via the deletion of cyp7b1 caused exaggerated atherosclerosis without altering lipid status in the setting of normo- and hypercholesterolemia; in addition, estrogen-related atheroprotection is markedly attenuated. Furthermore, dose-related capacity of E2 is observed to reverse the exaggerated atherosclerosis with elevated endogenous 27HC. The effects of 27HC on atherosclerosis are ER-α-dependent. Taken together, these results show that 27HC is an important contributing factor in the loss of estrogen protection from vascular disease, and has a potent impact on atherosclerotic lesion development regardless of the blood cholesterol levels (Figure 2). Elevated 27HC also caused increased inflammatory cytokine production in aorta. This suggests that 27HC also induces inflammatory responses in vasculature. Indeed, in monocytes/macrophages 27HC upregulates proinflammatory genes via ER-α, and also activates NFκB in EC via the activation of JNK and ERK. Subsequently, 27HC increased monocyte adhesion to EC in cell culture and in vivo. Since estrogen blunts NFκB activation in EC [61], it is indicated that 27HC promotes atherosclerosis via unique proinflammatory processes mediated by ER-α, the impact of elevated 27HC occurs through the direct modulation of ER function rather than through the antagonism of estrogen action, and it potently impairs the beneficial effects of ER on vascular function. Although the results of hormone replacement therapy to date are surprisingly negative in clinical trials [62,63], this study will lead to a greater understanding of how estrogen replacement therapy is ineffective or even harmful in postmenopausal women after a certain period without estrogen replacement after menopause has passed. Strategies to lower 27HC may complement existing approaches targeting cholesterol to prevent vascular disease.
Figure 2. 27-hydroxycholesterol develops atherosclerosis in an estrogen receptor-dependent manner.
Estrogen suppresses atherosclerotic lesion development, while 27HC stimulates inflammatory responses via estrogen receptor-α in macrophages and vascular endothelial cells. 27HC also promotes monocyte/macrophage infiltration into the intimal layer, leading to atherosclerosis development.
27HC: 27-Hydroxycholesterol; EC: Endothelial cells.
Future perspective
Considering that estrogen/ER play many important roles in various tissues in men and women, 27HC may have more actions than have been investigated so far. One area of great interest is the impact of 27HC in brain function, and there is growing evidence to show the importance [64]. Hypercholesterolemia is a risk factor for Alzheimer’s disease, and cholesterol does not pass through the blood–brain barrier, in contrast 27HC does. This implies that 27HC has an important role in the cholesterol metabolism in the brain. Indeed, elevated 27HC is linked with Alzheimer’s disease progression, although the accumulation in brains of patients is likely secondary to neurodegeneration caused by decreased CYP7B1 activity. The sequence variations in the cyp7b1 gene have been reported in the patients of spastic paraplegia type 5 with possibly decreased CYP7B1 activity that causes high cholesterol and 27HC levels, especially in the brain [65–69]. Mutations in cyp27a1 gene cause CTX, which results in the accumulation of cholesterol in brain and progression of neurological dysfunction. In addition, 24(S)-hydroxycholesterol, one of the major oxysterol in brain, is also metabolized by CYP27A1 [70]. Therefore, it is intriguing to investigate the roles of 27HC/CYP27A1/CYP7B1 in the brain in the relationship with ER function.
Another area of great interest is the impact of 27HC through GPR30, recently identified membrane estrogen receptor [71,72]. Although it is still controversial whether GPR30 has physiological function as an estrogen receptor [73], this receptor may have an important role in a certain context. It is still unknown whether 27HC is a ligand to this receptor, however, it is possible that 27HC functions via this receptor in certain tissues.
There are still unknown physiological functions for the role of 27HC even in metabolism, cancer and cardiovascular system. In addition, our discovery of 27HC as an endogenous SERM suggests the existence of other endogenous ligands for ER or other steroid receptors. Further work will be warranted to explore the role of 27HC as an important regulator of the crosstalk between metabolism, cancer and cardiovascular diseases.
Executive summary.
27-hydroxycholesterol (27HC) is a cholesterol metabolite that is produced and catabolyzed by P450 enzymes, sterol 27-hydroxylase (CYP27A1) and oxysterol 7α-hydroxylase (CYP7B1), respectively, and its concentration correlates well with that of cholesterol.
27HC is the first identified endogenous selective estrogen receptor modulator, and suppresses estrogen-induced genomic and nonnuclear action of estrogen receptor (ER) in vascular endothelial cells.
In breast cancer patients, the tissue expression of cyp7b1 reversely correlates with overall outcome, and 27HC promotes breast tumor cell proliferation and metastasis.
27HC promotes atherosclerosis via unique proinflammatory processes mediated by ER-α, the impact of elevated 27HC occurs through the direct modulation of ER function rather than through the antagonism of estrogen action, and potently impairs the beneficial effects of ER on vascular function.
Further investigation will be warranted to explore the role of 27HC as an important regulator of the crosstalk between metabolism, cancer and cardiovascular diseases.
Acknowledgments
We thank many colleagues and collaborators who have contributed to our understanding of the biology of 27HC.
This work was supported by National Institutes of Health grant DK079328 and American Diabetes Association Grant 7–11-JF-46.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- 1. Brown AJ, Jessup W. Oxysterols and atherosclerosis. Atherosclerosis. 1999;142(1):1–28. doi: 10.1016/s0021-9150(98)00196-8. •• This review article describes the sources of oxysterols as well as their levels in plasma and atherosclerotic lesions.
- 2.Russell DW. Oxysterol biosynthetic enzymes. Biochim. Biophys. Acta. 2000;1529(1–3):126–135. doi: 10.1016/s1388-1981(00)00142-6. [DOI] [PubMed] [Google Scholar]
- 3.Schroepfer GJ., Jr Oxysterols: modulators of cholesterol metabolism and other processes. Physiol. Rev. 2000;80(1):361–554. doi: 10.1152/physrev.2000.80.1.361. [DOI] [PubMed] [Google Scholar]
- 4.Linseisen J, Wolfram G. Absorption of cholesterol oxidation products from ordinary foodstuff in humans. Ann. Nutr. Metab. 1998;42(4):221–230. doi: 10.1159/000012737. [DOI] [PubMed] [Google Scholar]
- 5.Lyons MA, Samman S, Gatto L, Brown AJ. Rapid hepatic metabolism of 7-ketocholesterol in vivo: implications for dietary oxysterols. J. Lipid Res. 1999;40(10):1846–1857. [PubMed] [Google Scholar]
- 6.Burkard I, Von Eckardstein A, Waeber G, Vollenweider P, Rentsch KM. Lipoprotein distribution and biological variation of 24S- and 27-hydroxycholesterol in healthy volunteers. Atherosclerosis. 2007;194(1):71–78. doi: 10.1016/j.atherosclerosis.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 7.Dzeletovic S, Breuer O, Lund E, Diczfalusy U. Determination of cholesterol oxidation products in human plasma by isotope dilution-mass spectrometry. Anal. Biochem. 1995;225(1):73–80. doi: 10.1006/abio.1995.1110. [DOI] [PubMed] [Google Scholar]
- 8. Li-Hawkins J, Lund EG, Turley SD, Russell DW. Disruption of the oxysterol 7alpha-hydroxylase gene in mice. J. Biol Chem. 2000;275(22):16536–16542. doi: 10.1074/jbc.M001811200. •• The authors created the cyp7b1-deficient mice and characterized the phenotypes including elevated 27-hydroxycholesterol (27HC) levels.
- 9. Umetani M, Domoto H, Gormley AK, et al. 27-Hydroxycholesterol is an endogenous SERM that inhibits the cardiovascular effects of estrogen. Nat. Med. 2007;13(10):1185–1192. doi: 10.1038/nm1641. •• We found that 27HC is the first identified endogenous selective estrogen receptor modulator.
- 10.Crisby M, Nilsson J, Kostulas V, Bjorkhem I, Diczfalusy U. Localization of sterol 27-hydroxylase immuno-reactivity in human atherosclerotic plaques. Biochim. Biophys. Acta. 1997;1344(3):278–285. doi: 10.1016/s0005-2760(96)00152-x. [DOI] [PubMed] [Google Scholar]
- 11.Andersson S, Davis DL, Dahlback H, Jornvall H, Russell DW. Cloning, structure, and expression of the mitochondrial cytochrome P-450 sterol 26-hydroxylase, a bile acid biosynthetic enzyme. J. Biol. Chem. 1989;264(14):8222–8229. [PubMed] [Google Scholar]
- 12.Cali JJ, Hsieh CL, Francke U, Russell DW. Mutations in the bile acid biosynthetic enzyme sterol 27-hydroxylase underlie cerebrotendinous xanthomatosis. J. Biol. Chem. 1991;266(12):7779–7783. [PMC free article] [PubMed] [Google Scholar]
- 13.Bjorkhem I, Andersson O, Diczfalusy U, et al. Atherosclerosis and sterol 27-hydroxylase: evidence for a role of this enzyme in elimination of cholesterol from human macrophages. Proc. Natl Acad. Sci. USA. 1994;91(18):8592–8596. doi: 10.1073/pnas.91.18.8592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Araya Z, Tang W, Wikvall K. Hormonal regulation of the human sterol 27-hydroxylase gene CYP27A1. Biochem. J. 2003;372(Pt 2):529–534. doi: 10.1042/BJ20021651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Segev H, Honigman A, Rosen H, Leitersdorf E. Transcriptional regulation of the human sterol 27-hydroxylase gene (CYP27) and promoter mapping. Atherosclerosis. 2001;156(2):339–347. doi: 10.1016/s0021-9150(00)00654-7. [DOI] [PubMed] [Google Scholar]
- 16.Memon RA, Moser AH, Shigenaga JK, Grunfeld C, Feingold KR. In vivo and in vitro regulation of sterol 27-hydroxylase in the liver during the acute phase response. potential role of hepatocyte nuclear factor-1. J. Biol. Chem. 2001;276(32):30118–30126. doi: 10.1074/jbc.M102516200. [DOI] [PubMed] [Google Scholar]
- 17.Westman J, Kallin B, Bjorkhem I, Nilsson J, Diczfalusy U. Sterol 27-hydroxylase- and apoAI/phospholipid-mediated efflux of cholesterol from cholesterol-laden macrophages: evidence for an inverse relation between the two mechanisms. Arterioscler. Thromb. Vasc. Biol. 1998;18(4):554–561. doi: 10.1161/01.atv.18.4.554. [DOI] [PubMed] [Google Scholar]
- 18.Sun LP, Seemann J, Goldstein JL, Brown MS. Sterol-regulated transport of SREBPs from endoplasmic reticulum to Golgi: Insig renders sorting signal in Scap inaccessible to COPII proteins. Proc. Natl Acad. Sci. USA. 2007;104(16):6519–6526. doi: 10.1073/pnas.0700907104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ali Z, Heverin M, Olin M, et al. On the regulatory role of side-chain hydroxylated oxysterols in the brain. Lessons from CYP27A1 transgenic and Cyp27a1(−/−) mice. J. Lipid Res. 2013;54(4):1033–1043. doi: 10.1194/jlr.M034124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stapleton G, Steel M, Richardson M, et al. A novel cytochrome P450 expressed primarily in brain. J. Biol. Chem. 1995;270(50):29739–29745. doi: 10.1074/jbc.270.50.29739. [DOI] [PubMed] [Google Scholar]
- 21.Rose KA, Stapleton G, Dott K, et al. Cyp7b, a novel brain cytochrome P450, catalyzes the synthesis of neurosteroids 7alpha-hydroxy dehydroepiandrosterone and 7alpha-hydroxy pregnenolone. Proc. Natl Acad. Sci. USA. 1997;94(10):4925–4930. doi: 10.1073/pnas.94.10.4925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yantsevich AV, Dichenko YV, Mackenzie F, et al. Human steroid and oxysterol 7alpha-hydroxylase CYP7B1: substrate specificity, azole binding and misfolding of clinically relevant mutants. FEBS J. 2014;281(6):1700–1713. doi: 10.1111/febs.12733. [DOI] [PubMed] [Google Scholar]
- 23.Martin C, Bean R, Rose K, Habib F, Seckl J. cyp7b1 catalyses the 7alpha-hydroxylation of dehydroepiandrosterone and 25-hydroxycholesterol in rat prostate. Biochem. J. 2001;355(Pt 2):509–515. doi: 10.1042/0264-6021:3550509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weihua Z, Lathe R, Warner M, Gustafsson JA. An endocrine pathway in the prostate, ERbeta, AR, 5alpha-androstane-3beta,17beta-diol, and CYP7B1, regulates prostate growth. Proc. Natl Acad. Sci. USA. 2002;99(21):13589–13594. doi: 10.1073/pnas.162477299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lundqvist J, Norlin M. Effects of CYP7B1-related steroids on androgen receptor activation in different cell lines. Biochim. Biophys. Acta. 2012;1821(7):973–979. doi: 10.1016/j.bbalip.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 26.Uppal H, Saini SP, Moschetta A, et al. Activation of LXRs prevents bile acid toxicity and cholestasis in female mice. Hepatology. 2007;45(2):422–432. doi: 10.1002/hep.21494. [DOI] [PubMed] [Google Scholar]
- 27.Tang W, Pettersson H, Norlin M. Involvement of the PI3K/Akt pathway in estrogen-mediated regulation of human CYP7B1: identification of CYP7B1 as a novel target for PI3K/Akt and MAPK signalling. J. Steroid Biochem. Mol. Biol. 2008;112(1–3):63–73. doi: 10.1016/j.jsbmb.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 28.Gupta RP, Patrick K, Bell NH. Mutational analysis of CYP27A1: assessment of 27-hydroxylation of cholesterol and 25-hydroxylation of vitamin D. Metabolism. 2007;56(9):1248–1255. doi: 10.1016/j.metabol.2007.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goodwin B, Gauthier KC, Umetani M, et al. Identification of bile acid precursors as endogenous ligands for the nuclear xenobiotic pregnane X receptor. Proc. Natl Acad. Sci. USA. 2003;100(1):223–228. doi: 10.1073/pnas.0237082100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lorbek G, Lewinska M, Rozman D. Cytochrome P450s in the synthesis of cholesterol and bile acids--from mouse models to human diseases. FEBS J. 2012;279(9):1516–1533. doi: 10.1111/j.1742-4658.2011.08432.x. [DOI] [PubMed] [Google Scholar]
- 31.Meir K, Kitsberg D, Alkalay I, et al. Human sterol 27-hydroxylase (CYP27) overexpressor transgenic mouse model. Evidence against 27-hydroxycholesterol as a critical regulator of cholesterol homeostasis. J. Biol. Chem. 2002;277(37):34036–34041. doi: 10.1074/jbc.M201122200. [DOI] [PubMed] [Google Scholar]
- 32.Martin KO, Budai K, Javitt NB. Cholesterol and 27-hydroxycholesterol 7 alpha-hydroxylation: evidence for two different enzymes. J. Lipid Res. 1993;34(4):581–588. [PubMed] [Google Scholar]
- 33.Christopherson KS, Bredt DS. Nitric oxide in excitable tissues: physiological roles and disease. J. Clin. Invest. 1997;100(10):2424–2429. doi: 10.1172/JCI119783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Papapetropoulos A, Rudic RD, Sessa WC. Molecular control of nitric oxide synthases in the cardiovascular system. Cardiovasc. Res. 1999;43(3):509–520. doi: 10.1016/s0008-6363(99)00161-3. [DOI] [PubMed] [Google Scholar]
- 35.Li H, Forstermann U. Nitric oxide in the pathogenesis of vascular disease. J. Pathol. 2000;190(3):244–254. doi: 10.1002/(SICI)1096-9896(200002)190:3<244::AID-PATH575>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 36.Janowski BA, Willy PJ, Devi TR, Falck JR, Mangelsdorf DJ. An oxysterol signalling pathway mediated by the nuclear receptor LXR alpha. Nature. 1996;383(6602):728–731. doi: 10.1038/383728a0. [DOI] [PubMed] [Google Scholar]
- 37.Lehmann JM, Kliewer SA, Moore LB, et al. Activation of the nuclear receptor LXR by oxysterols defines a new hormone response pathway. J. Biol. Chem. 1997;272(6):3137–3140. doi: 10.1074/jbc.272.6.3137. [DOI] [PubMed] [Google Scholar]
- 38.Escher G, Krozowski Z, Croft KD, Sviridov D. Expression of sterol 27-hydroxylase (CYP27A1) enhances cholesterol efflux. J. Biol. Chem. 2003;278(13):11015–11019. doi: 10.1074/jbc.M212780200. [DOI] [PubMed] [Google Scholar]
- 39.Patel RR, Sharma CG, Jordan VC. Optimizing the antihormonal treatment and prevention of breast cancer. Breast Cancer. 2007;14(2):113–122. doi: 10.2325/jbcs.966. [DOI] [PubMed] [Google Scholar]
- 40.Chen S, Masri S, Wang X, Phung S, Yuan YC, Wu X. What do we know about the mechanisms of aromatase inhibitor resistance? J. Steroid Biochem. Mol. Biol. 2006;102(1–5):232–240. doi: 10.1016/j.jsbmb.2006.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Al-Zhoughbi W, Huang J, Paramasivan GS, et al. Tumor macroenvironment and metabolism. Semin. Oncol. 2014;41(2):281–295. doi: 10.1053/j.seminoncol.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wu Q, Ishikawa T, Sirianni R, et al. 27-Hydroxycholesterol promotes cell-autonomous, ER-positive breast cancer growth. Cell Rep. 2013;5(3):637–645. doi: 10.1016/j.celrep.2013.10.006. •• Shows the role of 27HC in breast tumor progression in human and mouse.
- 43. Nelson ER, Wardell SE, Jasper JS, et al. 27-Hydroxycholesterol links hypercholesterolemia and breast cancer pathophysiology. Science. 2013;342(6162):1094–1098. doi: 10.1126/science.1241908. •• Shows the role of 27HC in breast tumor progression and metastasis.
- 44.Dusell CD, Umetani M, Shaul PW, Mangelsdorf DJ, Mcdonnell DP. 27-hydroxycholesterol is an endogenous selective estrogen receptor modulator. Mol. Endocrinol. 2008;22(1):65–77. doi: 10.1210/me.2007-0383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Simpson ER, Ackerman GE, Smith ME, Mendelson CR. Estrogen formation in stromal cells of adipose tissue of women: induction by glucocorticosteroids. Proc. Natl Acad. Sci. USA. 1981;78(9):5690–5694. doi: 10.1073/pnas.78.9.5690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaaks R, Lukanova A, Kurzer MS. Obesity, endogenous hormones, and endometrial cancer risk: a synthetic review. Cancer Epidemiol. Biomarkers Prev. 2002;11(12):1531–1543. [PubMed] [Google Scholar]
- 47.Galkina E, Ley K. Immune and inflammatory mechanisms of atherosclerosis. Annu. Rev. Immunol. 2009;27:165–197. doi: 10.1146/annurev.immunol.021908.132620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Libby P. Inflammation in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2012;32(9):2045–2051. doi: 10.1161/ATVBAHA.108.179705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leonarduzzi G, Gamba P, Gargiulo S, Biasi F, Poli G. Inflammation-related gene expression by lipid oxidation-derived products in the progression of atherosclerosis. Free Radic. Biol. Med. 2012;52(1):19–34. doi: 10.1016/j.freeradbiomed.2011.09.031. [DOI] [PubMed] [Google Scholar]
- 50.Vejux A, Lizard G. Cytotoxic effects of oxysterols associated with human diseases: induction of cell death (apoptosis and/or oncosis), oxidative and inflammatory activities, and phospholipidosis. Mol. Aspects Med. 2009;30(3):153–170. doi: 10.1016/j.mam.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 51.Zurkinden L, Solca C, Vogeli IA, et al. Effect of Cyp27A1 gene dosage on atherosclerosis development in ApoE-knockout mice. FASEB. J. 2014;28(3):1198–1209. doi: 10.1096/fj.13-233791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Upston JM, Niu X, Brown AJ, et al. Disease stage-dependent accumulation of lipid and protein oxidation products in human atherosclerosis. Am. J. Pathol. 2002;160(2):701–710. doi: 10.1016/S0002-9440(10)64890-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rossouw JE, Prentice RL, Manson JE, et al. Relationships of coronary heart disease with 27-hydroxycholesterol, low-density lipoprotein cholesterol, and menopausal hormone therapy. Circulation. 2012;126(13):1577–1586. doi: 10.1161/CIRCULATIONAHA.112.103218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shanahan CM, Carpenter KL, Cary NR. A potential role for sterol 27-hydroxylase in atherogenesis. Atherosclerosis. 2001;154(2):269–276. doi: 10.1016/s0021-9150(00)00473-1. [DOI] [PubMed] [Google Scholar]
- 55.Hansson M, Ellis E, Hunt MC, Schmitz G, Babiker A. Marked induction of sterol 27-hydroxylase activity and mRNA levels during differentiation of human cultured monocytes into macrophages. Biochim. Biophys. Acta. 2003;1593(2–3):283–289. doi: 10.1016/s0167-4889(02)00398-1. [DOI] [PubMed] [Google Scholar]
- 56.Wooten JS, Wu H, Raya J, Perrard XD, Gaubatz J, Hoogeveen RC. The influence of an obesogenic diet on oxysterol metabolism in C57BL/6J mice. Cholesterol. 2014;2014:843468. doi: 10.1155/2014/843468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li J, Daly E, Campioli E, Wabitsch M, Papadopoulos V. De novo synthesis of steroids and oxysterols in adipocytes. J. Biol. Chem. 2014;289(2):747–764. doi: 10.1074/jbc.M113.534172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nofer JR. Estrogens and atherosclerosis: insights from animal models and cell systems. J. Mol. Endocrinol. 2012;48(2):R13–R29. doi: 10.1530/JME-11-0145. [DOI] [PubMed] [Google Scholar]
- 59. Lenfant F, Tremollieres F, Gourdy P, Arnal JF. Timing of the vascular actions of estrogens in experimental and human studies: why protective early, and not when delayed? Maturitas. 2011;68(2):165–173. doi: 10.1016/j.maturitas.2010.11.016. •• Reviews the impacts of estrogen on the vascular function in mouse and human, and also discusses about the discrepancy between the results in human and animal models.
- 60. Umetani M, Ghosh P, Ishikawa T, et al. The cholesterol metabolite 27-hydroxycholesterol promotes atherosclerosis via proinflammatory processes mediated by estrogen receptor alpha. Cell Metab. 2014;20:1–11. doi: 10.1016/j.cmet.2014.05.013. •• We found that 27HC promotes atherosclerosis, and that its ER-α-dependent proinflammatory effects on macrophages and endothelial cells affect the atherosclerotic lesion development.
- 61.Simoncini T, Maffei S, Basta G, et al. Estrogens and glucocorticoids inhibit endothelial vascular cell adhesion molecule-1 expression by different transcriptional mechanisms. Circ. Res. 2000;87(1):19–25. doi: 10.1161/01.res.87.1.19. [DOI] [PubMed] [Google Scholar]
- 62.Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA. 1998;280(7):605–613. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- 63.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 64.Gamba P, Testa G, Sottero B, Gargiulo S, Poli G, Leonarduzzi G. The link between altered cholesterol metabolism and Alzheimer’s disease. Ann. NY Acad. Sci. 2012;1259:54–64. doi: 10.1111/j.1749-6632.2012.06513.x. [DOI] [PubMed] [Google Scholar]
- 65.Setchell KD, Schwarz M, O’Connell NC, et al. Identification of a new inborn error in bile acid synthesis: mutation of the oxysterol 7alpha-hydroxylase gene causes severe neonatal liver disease. J. Clin. Invest. 1998;102(9):1690–1703. doi: 10.1172/JCI2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jakobsson J, Karypidis H, Johansson JE, Roh HK, Rane A, Ekstrom L. A functional C-G polymorphism in the CYP7B1 promoter region and its different distribution in Orientals and Caucasians. Pharmacogenomics J. 2004;4(4):245–250. doi: 10.1038/sj.tpj.6500236. [DOI] [PubMed] [Google Scholar]
- 67.Tsaousidou MK, Ouahchi K, Warner TT, et al. Sequence alterations within CYP7B1 implicate defective cholesterol homeostasis in motor-neuron degeneration. Am. J. Hum. Genet. 2008;82(2):510–515. doi: 10.1016/j.ajhg.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schule R, Brandt E, Karle KN, et al. Analysis of CYP7B1 in non-consanguineous cases of hereditary spastic paraplegia. Neurogenetics. 2009;10(2):97–104. doi: 10.1007/s10048-008-0158-9. [DOI] [PubMed] [Google Scholar]
- 69.Goizet C, Boukhris A, Durr A, et al. CYP7B1 mutations in pure and complex forms of hereditary spastic paraplegia type 5. Brain. 2009;132(Pt 6):1589–1600. doi: 10.1093/brain/awp073. [DOI] [PubMed] [Google Scholar]
- 70.Bjorkhem I, Andersson U, Ellis E, et al. From brain to bile. Evidence that conjugation and omega-hydroxylation are important for elimination of 24S-hydroxycholesterol (cerebrosterol) in humans. J. Biol. Chem. 2001;276(40):37004–37010. doi: 10.1074/jbc.M103828200. [DOI] [PubMed] [Google Scholar]
- 71.Filardo EJ, Quinn JA, Bland KI, Frackelton AR., Jr Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol. Endocrinol. 2000;14(10):1649–1660. doi: 10.1210/mend.14.10.0532. [DOI] [PubMed] [Google Scholar]
- 72.Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307(5715):1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- 73.Pedram A, Razandi M, Levin ER. Nature of functional estrogen receptors at the plasma membrane. Mol. Endocrinol. 2006;20(9):1996–2009. doi: 10.1210/me.2005-0525. [DOI] [PubMed] [Google Scholar]