Abstract
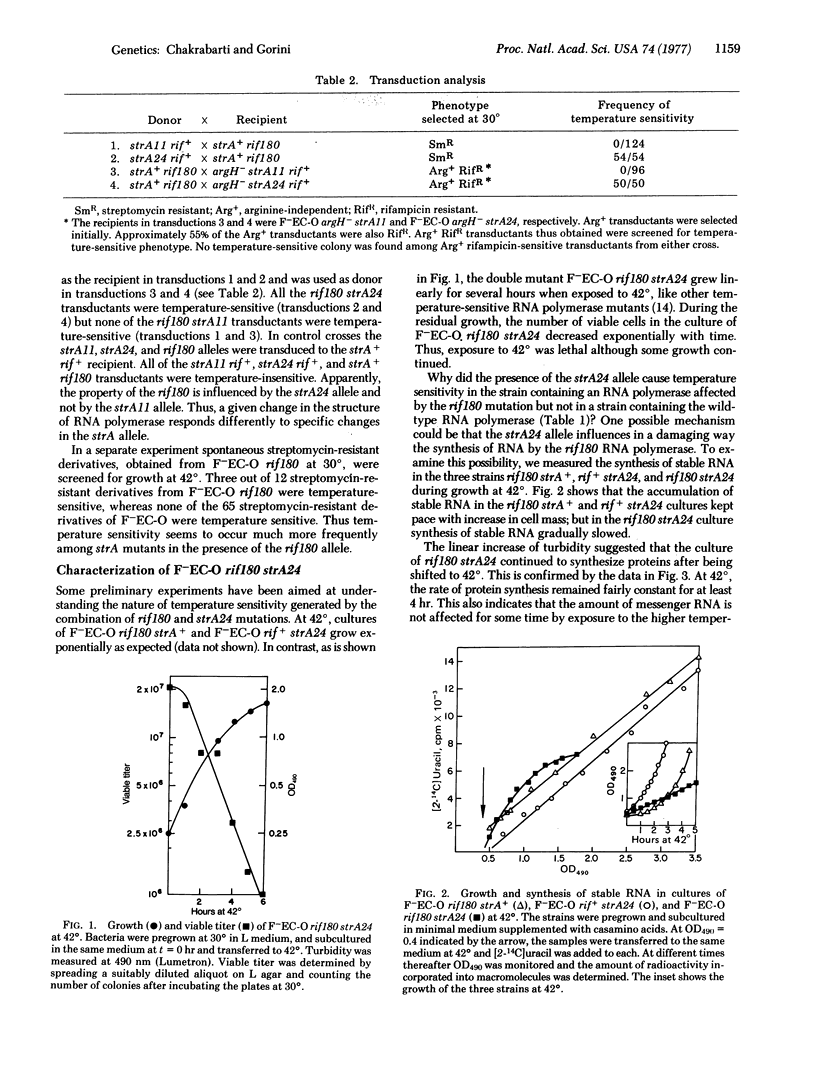
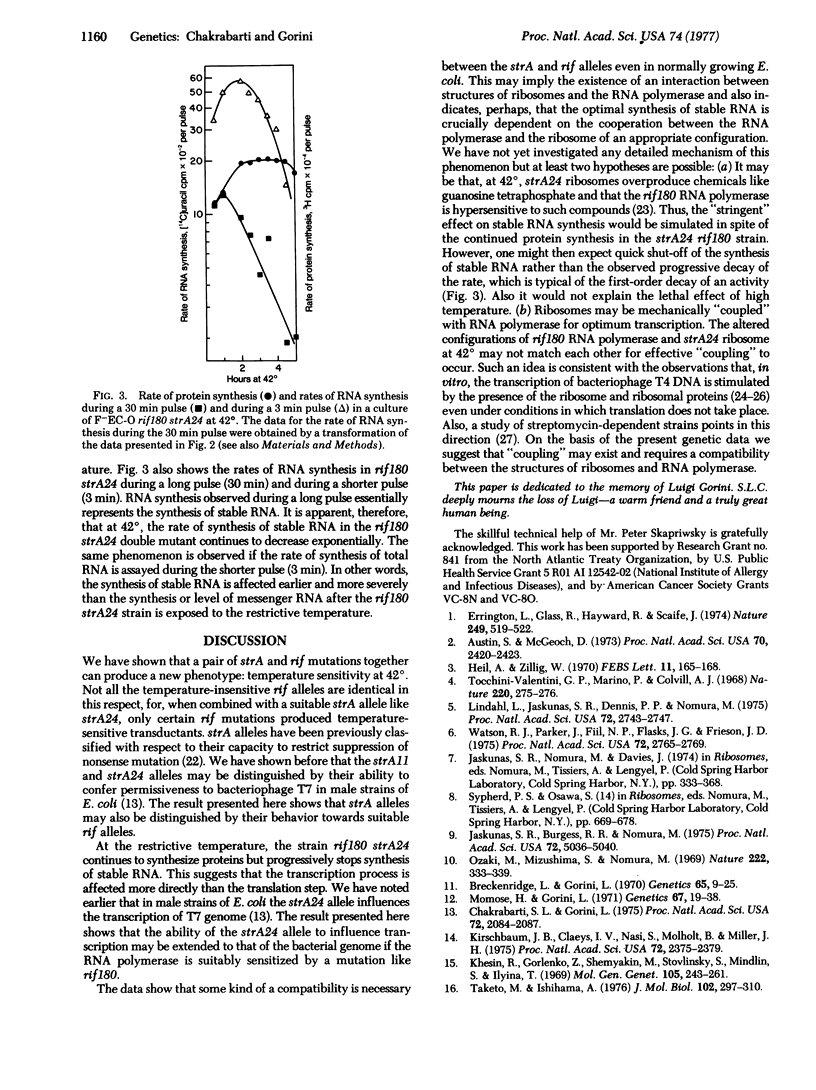
A temperature-sensitive lethal mutant of Escherichia coli has been constructed by combining two temperature-insensitive mutations: a rif180 mutation that modifies RNA polymerase (RNA nucleotidyltransferase; nucleosidetriphosphate:RNA nucleotidyltransferase, EC 2.7.7.6) and a strA24 mutation that modifies the ribosomal protein S12. The temperature sensitivity is a property of the combination of these two particular alleles; replacement of either of the alleles relieves the temperature sensitivity. An isogenic strain containing a different strA mutation (i.e., rif180 strA11) is not temperature sensitive. Evidently ribosomes modified by the particular strA24 polymerase altered by the rif180 mutation, which suggests that in vivo there may exist some interaction between structures of ribosomes and the RNA polymerase.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austin S., McGeoch D. The synthesis in vitro of RNA polymerase subunits of Escherichia coli. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2420–2423. doi: 10.1073/pnas.70.8.2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breckenridge L., Gorini L. Genetic analysis of streptomycin resistance in Escherichia coli. Genetics. 1970 May;65(1):9–25. doi: 10.1093/genetics/65.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess R. R. RNA polymerase. Annu Rev Biochem. 1971;40:711–740. doi: 10.1146/annurev.bi.40.070171.003431. [DOI] [PubMed] [Google Scholar]
- Cashel M., Gallant J. Two compounds implicated in the function of the RC gene of Escherichia coli. Nature. 1969 Mar 1;221(5183):838–841. doi: 10.1038/221838a0. [DOI] [PubMed] [Google Scholar]
- Chakrabarti S. L., Gorini L. A link between streptomycin and rifampicin mutation. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2084–2087. doi: 10.1073/pnas.72.6.2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti S., Gorini L. Growth of bacteriophages MS2 and T7 on streptomycin-resistant mutants of Escherichia coli. J Bacteriol. 1975 Feb;121(2):670–674. doi: 10.1128/jb.121.2.670-674.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Errington L., Glass R. E., Hayward R. S., Scaife J. G. Structure and orientation of an RNA polymerase operon in Escherichia coli. Nature. 1974 Jun 7;249(457):519–522. doi: 10.1038/249519a0. [DOI] [PubMed] [Google Scholar]
- Gorini L. Ribosomal discrimination of tRNAs. Nat New Biol. 1971 Dec 29;234(52):261–264. doi: 10.1038/newbio234261a0. [DOI] [PubMed] [Google Scholar]
- Gupta R. S., Schlessinger D. Coupling of rates of transcription, translation, and messenger ribonucleic acid degradation in streptomycin-dependent mutants of Escherichia coli. J Bacteriol. 1976 Jan;125(1):84–93. doi: 10.1128/jb.125.1.84-93.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heil A., Zillig W. Reconstitution of bacterial DNA-dependent RNA-polymerase from isolated subunits as a tool for the elucidation of the role of the subunits in transcription. FEBS Lett. 1970 Dec;11(3):165–168. doi: 10.1016/0014-5793(70)80519-1. [DOI] [PubMed] [Google Scholar]
- Jaskunas S. R., Burgess R. R., Nomura M. Identification of a gene for the alpha-subunit of RNA polymerase at the str-spc region of the Escherichia coli chromosome. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5036–5040. doi: 10.1073/pnas.72.12.5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khesin R. B., Gorlenko Z. M., Shemyakin M. F., Stvolinsky S. L., Mindlin S. Z., Ilyina T. S. Studies on the functions of the RNA polymerase components by means of mutations. Mol Gen Genet. 1969;105(3):243–261. doi: 10.1007/BF00337475. [DOI] [PubMed] [Google Scholar]
- Kirschbaum J. B., Claeys I. V., Nasi S., Molholt B., Miller J. H. Temperature-sensitive RNA polymerase mutants with altered subunit synthesis and degradation. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2375–2379. doi: 10.1073/pnas.72.6.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Leavitt J. C., Moldave K., Nakada D. Stimulation of in vitro RNA synthesis by ribosomes and ribosomal proteins. J Mol Biol. 1972 Sep 14;70(1):15–40. doi: 10.1016/0022-2836(72)90161-1. [DOI] [PubMed] [Google Scholar]
- Lindahl L., Jaskunas S. R., Dennis P. P., Nomura M. Cluster of genes in Escherichia coli for ribosomal proteins, ribosomal RNA, and RNA polymerase subunits. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2743–2747. doi: 10.1073/pnas.72.7.2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momose H., Gorini L. Genetic analysis of streptomycin dependence in Escherichia coli. Genetics. 1971 Jan;67(1):19–38. doi: 10.1093/genetics/67.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki M., Mizushima S., Nomura M. Identification and functional characterization of the protein controlled by the streptomycin-resistant locus in E. coli. Nature. 1969 Apr 26;222(5191):333–339. doi: 10.1038/222333a0. [DOI] [PubMed] [Google Scholar]
- Revel M., Herzberg M., Becarevic A., Gros F. Role of protein factor in the functional binding of ribosomes to natural messenger RNA. J Mol Biol. 1968 Apr 14;33(1):231–249. doi: 10.1016/0022-2836(68)90291-x. [DOI] [PubMed] [Google Scholar]
- Shin D. H., Moldave K. Effect of ribosomes on the biosynthesis of ribonucleic acid in vitro. J Mol Biol. 1966 Nov 14;21(2):231–245. doi: 10.1016/0022-2836(66)90094-5. [DOI] [PubMed] [Google Scholar]
- Taketo M., Ishihama A. Biosynthesis of RNA polymerase in Escherichia coli. IV. Accumulation of intermediates in mutants defective in the subunit assembly. J Mol Biol. 1976 Apr 5;102(2):297–310. doi: 10.1016/s0022-2836(76)80055-1. [DOI] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Linkage map of Escherichia coli strain K-12. Bacteriol Rev. 1972 Dec;36(4):504–524. doi: 10.1128/br.36.4.504-524.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tocchini-Valentini G. P., Marino P., Colvill A. J. Mutant of E. coli containing an altered DNA-dependent RNA polymerase. Nature. 1968 Oct 19;220(5164):275–276. doi: 10.1038/220275a0. [DOI] [PubMed] [Google Scholar]
- Watson R. J., Parker J., Fiil N. P., Flaks J. G., Friesen J. D. New chromosomal location for structural genes of ribosomal proteins. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2765–2769. doi: 10.1073/pnas.72.7.2765. [DOI] [PMC free article] [PubMed] [Google Scholar]



