Abstract
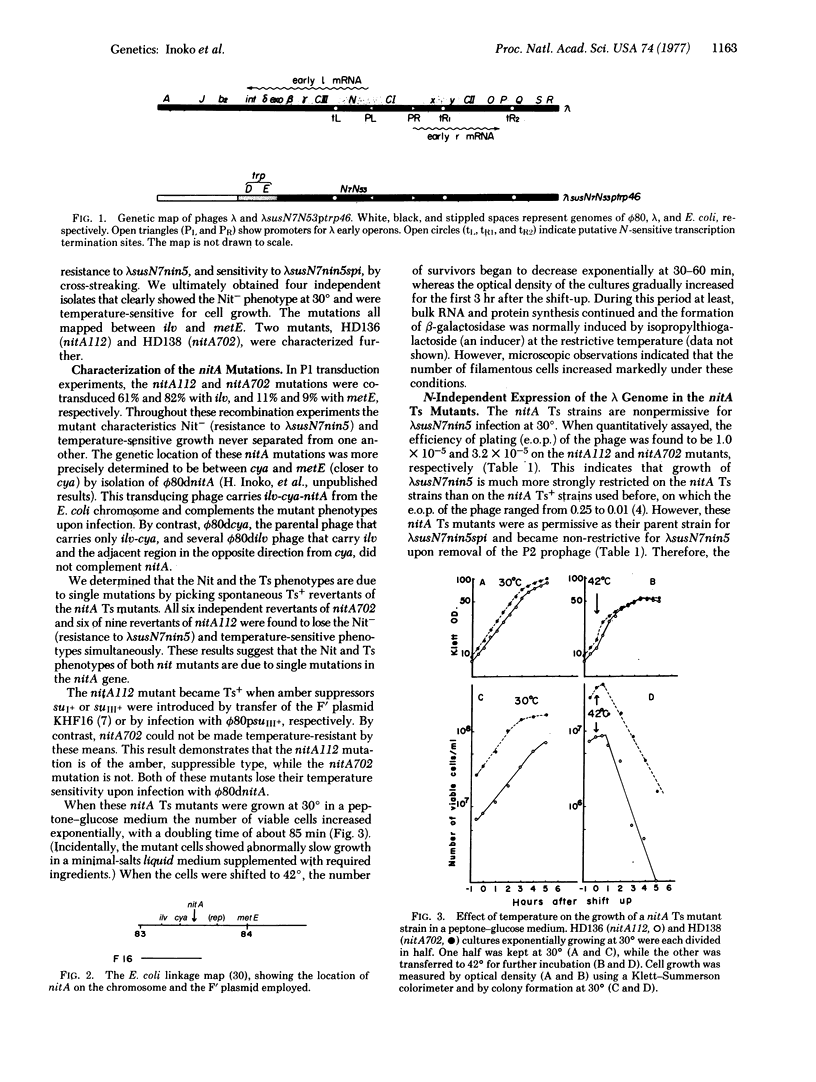
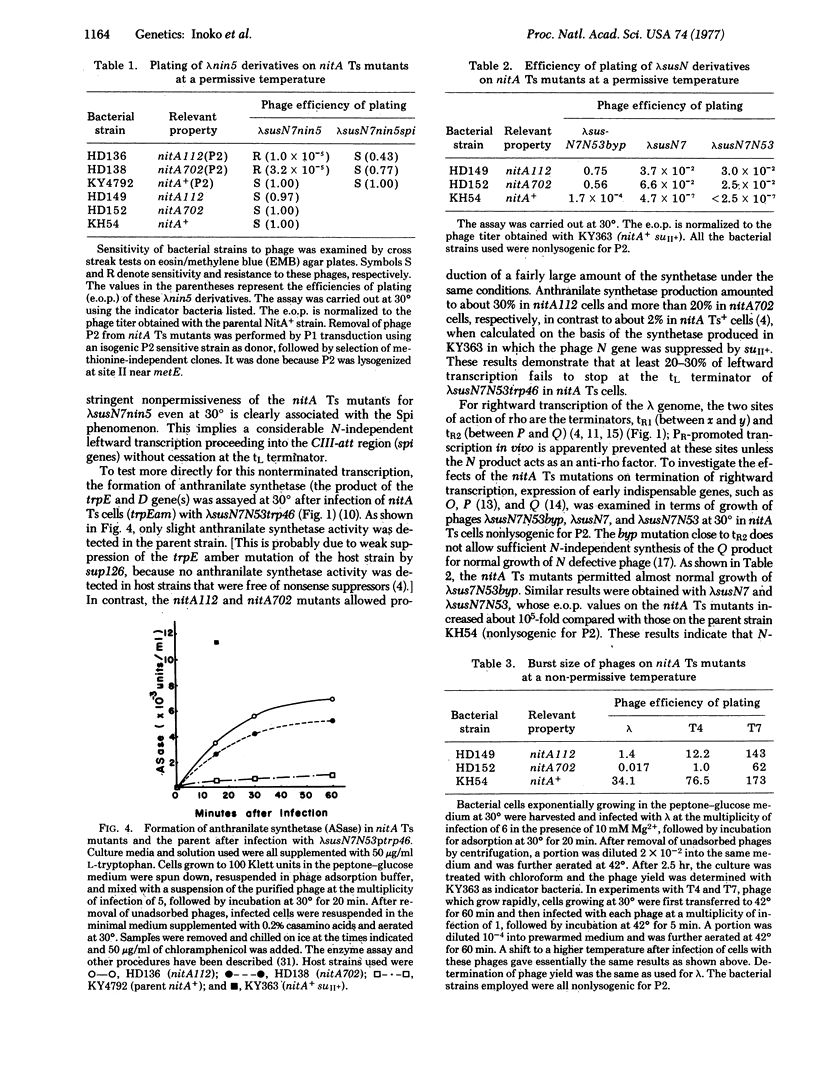
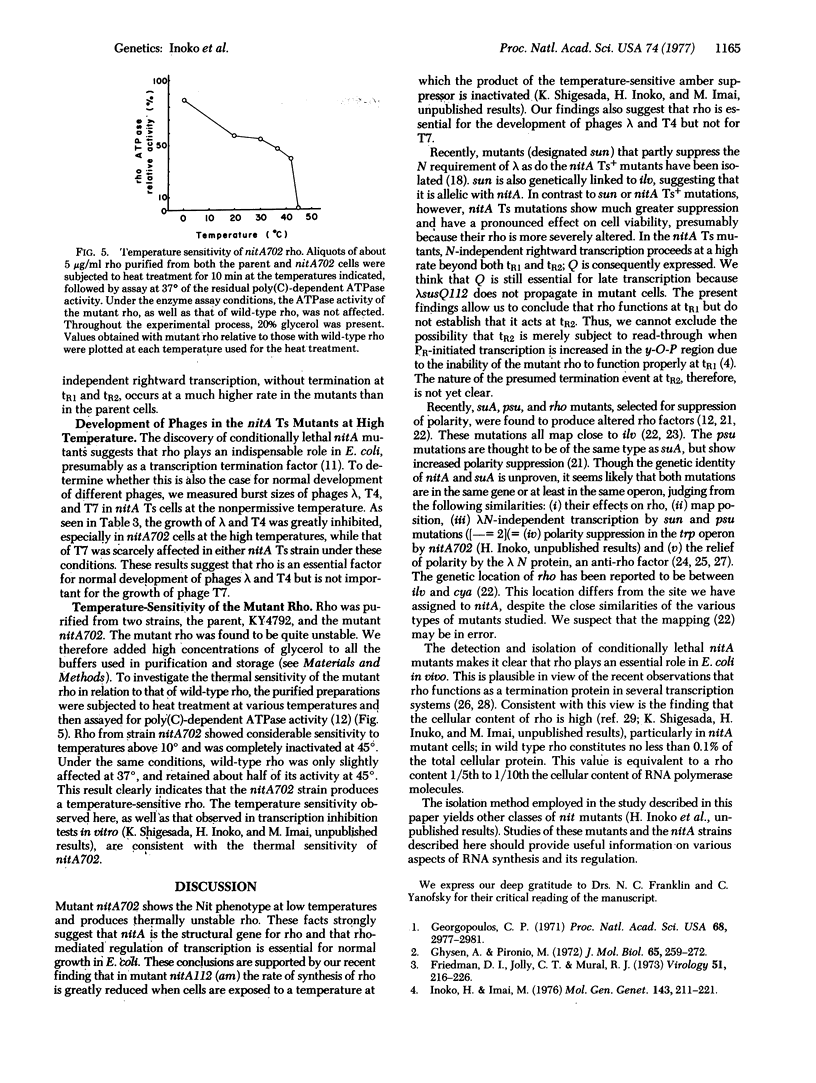
Temperature-sensitive nitA (rho) mutants of E. coli were isolated; one of them was characterized as an amber mutant. These strains show the Nit phenotype (transcription of phage lambda DNA independent of the N gene) at low temperatures and are inviable at high temperatures. The mutated sites appear to be between cya and metE on the chromosome. Temperature-sensitive nitA bacteria not only permit leftward transcription of the lambda genome at a high rate in the absence of the lambda N protein, but also allow lambda growth at low temperatures. At high temperatures, phages lambda and T4 are incapable of normal development in these cells, while growth of T7 is not affected. The production of thermally unstable rho by the nitA temperature-sensitive mutant suggests that nitA is the structural gene for rho.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adhya S., Gottesman M., De Crombrugghe B. Release of polarity in Escherichia coli by gene N of phage lambda: termination and antitermination of transcription. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2534–2538. doi: 10.1073/pnas.71.6.2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERTANI G. Lysogeny. Adv Virus Res. 1958;5:151–193. doi: 10.1016/s0065-3527(08)60673-9. [DOI] [PubMed] [Google Scholar]
- Bachmann B. J., Low K. B., Taylor A. L. Recalibrated linkage map of Escherichia coli K-12. Bacteriol Rev. 1976 Mar;40(1):116–167. doi: 10.1128/br.40.1.116-167.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunel F., Davison J. Bacterial mutants able to partly suppress the effect of N mutations in bacteriophage lambda. Mol Gen Genet. 1975;136(2):167–180. doi: 10.1007/BF00272037. [DOI] [PubMed] [Google Scholar]
- Butler B., Echols H. Regulation of bacteriophage lambda development by gene N: properties of a mutation that bypasses N control of late protein synthesis. Virology. 1970 Feb;40(2):212–222. doi: 10.1016/0042-6822(70)90396-x. [DOI] [PubMed] [Google Scholar]
- Das A., Court D., Adhya S. Isolation and characterization of conditional lethal mutants of Escherichia coli defective in transcription termination factor rho. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1959–1963. doi: 10.1073/pnas.73.6.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Crombrugghe B., Adhya S., Gottesman M., Pastan I. Effect of Rho on transcription of bacterial operons. Nat New Biol. 1973 Feb 28;241(113):260–264. doi: 10.1038/newbio241260a0. [DOI] [PubMed] [Google Scholar]
- Franklin N. C. Altered reading of genetic signals fused to the N operon of bacteriophage lambda: genetic evidence for modification of polymerase by the protein product of the N gene. J Mol Biol. 1974 Oct 15;89(1):33–48. doi: 10.1016/0022-2836(74)90161-2. [DOI] [PubMed] [Google Scholar]
- Friedman D. I., Jolly C. T., Mural R. J. Interference with the expression of the N gene function of phage lambda in a mutant of Escherichia coli. Virology. 1973 Jan;51(1):216–226. doi: 10.1016/0042-6822(73)90381-4. [DOI] [PubMed] [Google Scholar]
- Georgopoulos C. P. Bacterial mutants in which the gene N function of bacteriophage lambda is blocked have an altered RNA polymerase. Proc Natl Acad Sci U S A. 1971 Dec;68(12):2977–2981. doi: 10.1073/pnas.68.12.2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghysen A., Pironio M. Relationship between the N function of bacteriophage lambda and host RNA polymerase. J Mol Biol. 1972 Mar 28;65(2):259–272. doi: 10.1016/0022-2836(72)90281-1. [DOI] [PubMed] [Google Scholar]
- Herskowitz I. Control of gene expression in bacteriophage lambda. Annu Rev Genet. 1973;7:289–324. doi: 10.1146/annurev.ge.07.120173.001445. [DOI] [PubMed] [Google Scholar]
- Horiuchi T., Nagata T. Mutations affecting growth of the Escherichia coli cell under a condition of DNA polymerase I-deficiency. Mol Gen Genet. 1973;123(1):89–110. doi: 10.1007/BF00282992. [DOI] [PubMed] [Google Scholar]
- Inoko H., Imai M. Isolation and genetic characterization of the nitA mutants of Escherichia coli affecting the termination factor rho. Mol Gen Genet. 1976 Jan 16;143(2):211–221. doi: 10.1007/BF00266924. [DOI] [PubMed] [Google Scholar]
- Inoko H., Naito S., Ito K., Imai M. Regulation of the tryptophan operon of Escherichia coli integrated into the phage phi 80 genome. Mol Gen Genet. 1974 Mar 6;129(1):37–48. doi: 10.1007/BF00269264. [DOI] [PubMed] [Google Scholar]
- KELLY B. Localization of P2 prophage in two strains of Escherichia coli. Virology. 1963 Jan;19:32–39. doi: 10.1016/0042-6822(63)90021-7. [DOI] [PubMed] [Google Scholar]
- Korn L. J., Yanofsky C. Polarity suppressors defective in transcription termination at the attenuator of the tryptophan operon of Escherichia coli have altered rho factor. J Mol Biol. 1976 Sep 15;106(2):231–241. doi: 10.1016/0022-2836(76)90082-6. [DOI] [PubMed] [Google Scholar]
- Korn L. J., Yanofsky C. Polarity suppressors increase expression of the wild-type tryptophan operon of Escherichia coli. J Mol Biol. 1976 May 15;103(2):395–409. doi: 10.1016/0022-2836(76)90319-3. [DOI] [PubMed] [Google Scholar]
- Lowery-Goldhammer C., Richardson J. P. An RNA-dependent nucleoside triphosphate phosphohydrolase (ATPase) associated with rho termination factor. Proc Natl Acad Sci U S A. 1974 May;71(5):2003–2007. doi: 10.1073/pnas.71.5.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse D. E., Primakoff P. Relief of polarity in E. coli by "suA". Nature. 1970 Apr 4;226(5240):28–31. doi: 10.1038/226028a0. [DOI] [PubMed] [Google Scholar]
- Nagata T., Horiuchi T. Isolation and characterization of a temperature-sensitive amber suppressor mutant of Escherichia coli K12. Mol Gen Genet. 1973;123(1):77–88. doi: 10.1007/BF00282991. [DOI] [PubMed] [Google Scholar]
- Oeschger M. P., Berlyn M. K. A simple procedure for localized mutagenesis using nitrosoguanidine. Mol Gen Genet. 1974;134(1):77–83. doi: 10.1007/BF00332814. [DOI] [PubMed] [Google Scholar]
- Ratner D. Evidence that mutations in the suA polarity suppressing gene directly affect termination factor rho. Nature. 1976 Jan 15;259(5539):151–153. doi: 10.1038/259151a0. [DOI] [PubMed] [Google Scholar]
- Richardson J. P., Grimley C., Lowery C. Transcription termination factor rho activity is altered in Escherichia coli with suA gene mutations. Proc Natl Acad Sci U S A. 1975 May;72(5):1725–1728. doi: 10.1073/pnas.72.5.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. W. Termination factor for RNA synthesis. Nature. 1969 Dec 20;224(5225):1168–1174. doi: 10.1038/2241168a0. [DOI] [PubMed] [Google Scholar]
- Segawa T., Imamoto F. Diversity of regulation of genetic transcription. II. Specific relaxation of polarity in read-through transcription of the translocated trp operon in bacteriophage lambda trp. J Mol Biol. 1974 Aug 25;87(4):741–754. doi: 10.1016/0022-2836(74)90082-5. [DOI] [PubMed] [Google Scholar]
- Shimizu N., Hayashi M. In vitro transcription of the tryptophan operon integrated into a transducing phage genome. J Mol Biol. 1974 Apr 5;84(2):315–335. doi: 10.1016/0022-2836(74)90587-7. [DOI] [PubMed] [Google Scholar]



