Abstract
Importance
Whether mortality in type 1 diabetes mellitus is affected following intensive glycemic therapy has not been established.
Objective
To determine whether mortality differed between the original intensive and conventional treatment groups in the long-term follow-up of the Diabetes Control and Complications Trial (DCCT) cohort.
Design, Setting, and Participants
After the DCCT (1983–1993) ended, participants were followed up in a multisite (27 US and Canadian academic clinical centers) observational study (Epidemiology of Diabetes Control and Complications [EDIC]) until December 31, 2012. Participants were 1441 healthy volunteers with diabetes mellitus who, at baseline, were 13 to 39 years of age with 1 to 15 years of diabetes duration and no or early microvascular complications, and without hypertension, preexisting cardiovascular disease, or other potentially life-threatening disease.
Intervention/Exposure
During the clinical trial, participants were randomly assigned to receive intensive therapy (n = 711) aimed at achieving glycemia as close to the nondiabetic range as safely possible, or conventional therapy (n = 730) with the goal of avoiding symptomatic hypoglycemia and hyperglycemia. At the end of the DCCT, after a mean of 6.5 years, intensive therapy was taught and recommended to all participants and diabetes care was returned to personal physicians.
Main Outcomes
Total and cause-specific mortality was assessed through annual contact with family and friends and through records over 27 years mean follow-up.
Results
Vital status was ascertained for 1429 (99.2%) participants. There were 107 deaths, 64 in the conventional and 43 in the intensive group. The absolute risk difference was −109 per 100 000 patient-years (95%CI, −218 to −1), with lower all-cause mortality risk in the intensive therapy group (hazard ratio [HR] = 0.67 [95%CI, 0.46–0.99]; P = .045). Primary causes of death were cardiovascular disease (24 deaths; 22.4%), cancer (21 deaths; 19.6%), acute diabetes complications (19 deaths; 17.8%), and accidents or suicide (18 deaths; 16.8%). Higher levels of glycated hemoglobin (HbA1c) were associated with all-cause mortality (HR = 1.56 [95%CI, 1.35–1.81 per 10% relative increase in HbA1c]; P < .001), as well as the development of albuminuria (HR = 2.20 [95%CI, 1.46–3.31]; P < .001).
Conclusions and Relevance
After a mean of 27 years’ follow-up of patients with type 1 diabetes, 6.5 years of initial intensive diabetes therapy was associated with a modestly lower all-cause mortality compared with conventional therapy.
Type 1 diabetes mellitus has historically been associated with an increased risk of early mortality1,2; however, more recent epidemiologic data from Europe and the United States have suggested that this risk has been reduced.3–8 A recent US epidemiology study estimated that life expectancy for those diagnosed between 1965 and 1980 with type 1 diabetes mellitus during childhood is now 68.8 years, only 3.6 years less than the comparable general population estimate.9 Although reasons for the reduction in mortality are unclear, the Diabetes Control and Complications Trial (DCCT), conducted between 1983 and 1993, and its observational Epidemiology of Diabetes Interventions and Complications (EDIC) follow-up study have previously demonstrated that intensive therapy that lowers glycemia reduces renal10,11 and cardiovascular disease,12 the predominant causes of early mortality.13 Based on the demonstrated reductions in morbidity, intensive diabetes therapy is now the recommended standard of care14; however, it has not been established whether mortality in type 1 diabetes mellitus is affected following a period of intensive glycemic therapy. In type 2 diabetes treatment, reducing glycemia closer to the nondiabetic range has not consistently reduced mortality. Indeed, in the ACCORD (Action to Control Cardiovascular Risk in Diabetes) trial,15 all-cause and cardiovascular mortality were increased, although this has not been seen in other trials.
DCCT intensive diabetes therapy resulted in glycated hemoglobin (HbA1c) levels that were approximately 2% lower than levels in conventional therapy over an average of 6.5 years.10 However, the HbA1c levels equalized over the subsequent 19 years of EDIC. In this study, we examined the differences in all-cause and cause-specific mortality between the original treatment groups that received 6.5 years of intensive vs conventional therapy during the clinical trial. Secondary objectives were to examine the extent to which mortality was associated with glycemia or development of albuminuria.
Methods
Detailed descriptions of the DCCT clinical trial and the EDIC observational follow-up study have been published previously.10,16–18 In brief, the DCCT (1983–1993) randomized 1441 patients with type 1 diabetes mellitus between the ages of 13 and 39 years to intensive or conventional therapy, with the goal of studying the effects of near-normal glycemia on long-term diabetes complications. Approximately one-half of the participants were in the primary prevention cohort with 1 to 5 years’ diabetes duration, no retinopathy, and less than 40 mg of albuminuria per 24 hours. The secondary intervention cohort had 1 to 15 years’ diabetes duration, at least 1 microaneurysm, and less than 200 mg of albuminuria per 24 hours. Eligibility criteria excluded participants with a history of cardiovascular disease, hypertension (140/90 mm Hg or greater or use of antihypertensive medications), or hypercholesterolemia.16 During the clinical trial, the intensive and conventional treatment groups achieved mean HbA1c levels of approximately 7% and 9%, respectively, during a mean follow-up of 6.5 (range 3–9) years.10 At the end of the clinical trial, participants in the intensive therapy group were encouraged to continue intensive therapy practices, and the conventional group participants were taught intensive therapy. Thereafter, 1394 participants (representing 97% of the entire cohort) joined the EDIC observational study (1994-present) and received subsequent diabetes care from their personal physicians.17 Early in EDIC, the previously established separation in glycemia between the intensive and conventional groups diminished and was nonsignificant by year 5.18 The DCCT and EDIC protocols were approved by institutional review boards at all participating centers. All participants provided separate written consents to enroll in DCCT and later in EDIC.
Procedures
Laboratory assessments were performed centrally using standardized methods.16,17 HbA1c values, body mass index, and blood pressure were measured quarterly during the DCCT and yearly during EDIC.16,17 Fasting lipid and urine albumin excretion rate (AER), determined from a 4-hour collection, were measured annually during DCCT16 and in alternate years during EDIC.17 Serum creatinine was measured annually.
Deaths were reported to the data coordinating center through annual contact with family or friends and through search of public records. The research group pre-specified that analyses comparing mortality between the randomized treatment groups would be performed after 50 deaths in the original conventional group. As the study approached this milestone in late 2011, we attempted to ascertain by telephone, email, or mail (US Postal Service or Canada Post) the vital status of participants not known to have died. Later we commissioned a commercial vendor (OmniTrace) to attempt to determine vital status of 74 participants for whom status was uncertain. Vital status, as of December 31, 2012, was thus determined for 99.2% (1429) of all participants.
Copies of death certificates, clinical center narratives, autopsy reports, and hospital records were obtained19 and reviewed by the mortality and morbidity review committee (masked to treatment assignment, HbA1c, and glucose levels), which assigned a cause of death. The first author (T.J.O.), who was similarly masked, further classified deaths according to the Diabetes Epidemiology Research International (DERI) classification system.20 This system standardizes classification of the underlying cause of death, prioritizes secondary causes, and determines the role of diabetes. In the analyses of this study, only the underlying cause of death is considered. If death was attributed to an accident that was thought to be caused by hypoglycemia, it was classified as hypoglycemia and not as an accident. Deaths categorized as accidents included motor vehicle crashes, drowning, unintentional ingestion, and other external events, but were differentiated from suicides.
Covariates
Weighted mean HbA1c values over the study duration were computed with weights proportional to the time interval between values, quarterly during DCCT and annually during EDIC. Renal insufficiency was defined as an estimated glomerular filtration rate (eGFR) of less than 30 mL/min/1.73 m2 using the Chronic Kidney Disease Epidemiology Collaboration equation21or a history of kidney transplantation or dialysis. Albuminuria was categorized as normal (AER <40 mg/24 hours), microalbuminuria (AER from 40 to <300 mg/24 hours), macroalbuminuria (AER ≥300mg/24 hours), and as any albuminuria (AER ≥40 mg/24 hours). Nonfatal cardiovascular disease included nonfatal myocardial infarction, silent myocardial infarction (diagnosed on an annual electrocardiogram without a clinical history), revascularization (angioplasty or bypass), confirmed angina, and nonfatal cerebrovascular events, as described previously,12 and congestive heart failure (NY Heart Association functional class III or greater; ascertainment starting in 2007; EDIC year 13). Severe hypoglycemia, defined as requiring the assistance of another party, and the subset accompanied by coma, seizure, or both were ascertained at DCCT quarterly clinic visits. During EDIC, hypoglycemic events as defined previously were only recorded for the 3months preceding an annual visit and the rate annualized.
Statistical Analyses
A Cox proportional hazards model with 50 deaths in the original conventional group provided 85% power to detect a hazard ratio (HR) of 0.5 between groups with a 2-sided test at the 0.05 level. Analyses herein are based on all deaths known to have occurred up to December 31, 2012. The survival analysis follow-up time was based on the date of death (n = 107), the date last known to be alive (n = 12), or the December 31, 2012, date of data set closure (n = 1322). The patient disposition for these analyses is described in the eFigure (see Supplement).
Quantitative and categorical characteristics were compared using the Wilcoxon rank-sum test and χ2 test, respectively. Death rates per 100 000 patient-years, and 95% CIs, were computed from robust Poisson regression models.22 Inferences on the difference in absolute rates were obtained using the Δ method.22 P values and HRs comparing all-cause mortality between groups were based on Wald tests from Cox proportional hazards models and the cumulative incidence for time to death was estimated using the Kaplan-Meier method.22 The homogeneity of treatment group differences among strata was tested using a group-by-stratum interaction in the model. Estimates for HRs associated with other time-dependent covariates were calculated using separate Cox models adjusted for baseline characteristics.
Additional Cox models evaluated the association of the 2 specified major risk factors (glycemia and albuminuria/renal disease) with the risk of mortality, individually. The number of deaths was inadequate for reliable multivariable risk factor modeling, especially within each treatment group.
All analyses were performed using SAS software (version 9.3; SAS Institute Inc) and R package.23 A 2-sided P value of less than .05 was considered statistically significant.
Results
During the DCCT, 1441 volunteers were randomized to receive either intensive therapy (n = 711) or conventional therapy (n = 730) for a mean of 6.5 years and subsequently observed, which resulted in 36 725 person-years of total follow-up (18 207 intensive and 18 518 conventional) during DCCT and EDIC combined. Characteristics at baseline of the 2 randomly assigned treatment groups, the primary and secondary cohorts, and of those who survived vs those who died are shown in Table 1. There were no major differences at baseline between the randomly assigned treatment groups except for a 2-mmHg higher systolic blood pressure in the conventional group. The inter-cohort differences reflect eligibility differences.
Table 1.
Clinical Characteristics at DCCT Baseline (1983 – 1989) of the DCCT/EDIC Cohort by Treatment Group Assignment, Primary Prevention versus Secondary Intervention Cohort, and Deaths versus Survivors†
| Characteristic | Intensive (N=711) |
Conventional (N=730) |
p-value | Primary (N=726) |
Secondary (N=715) |
p-value | Alive (N=1334) |
Dead (N=107) |
p-value |
|---|---|---|---|---|---|---|---|---|---|
| Age (yr) | 27 ± 7 | 27 ± 7 | 0.14 | 26 ± 7 | 27 ± 7 | 0.09 | 27 ± 7 | 29 ± 7 | <0.001 |
| Age at onset of diabetes (yr) | 22 ± 8 | 22 ± 8 | 0.64 | 24 ± 8 | 19 ± 8 | <0.001 | 22 ± 8 | 24 ± 8 | 0.003 |
| Female sex (%) | 345 (49%) | 335 (46%) | 0.32 | 348 (48%) | 332 (46%) | 0.57 | 641 (48%) | 39 (36%) | 0.02 |
| Secondary Intervention (vs. Primary Prevention) Cohort (%) | 363 (51%) | 352 (48%) | 0.28 | -- | -- | 663 (50%) | 52 (49%) | 0.83 | |
| Duration of diabetes (yr) | 6 ± 4 | 5 ± 4 | 0.14 | 3 ± 1 | 9 ± 4 | <0.001 | 6 ± 4 | 6 ± 4 | 0.96 |
| Current cigarette smoker (%) | 132(19%) | 134(18%) | 0.92 | 132(18%) | 134(19%) | 0.78 | 233(17%) | 33(31%) | <0.001 |
| Body mass index (kg/m2) | 23.3 ± 2.7 | 23.4 ± 2.9 | 0.46 | 23.2 ± 2.9 | 23.6 ± 2.7 | 0.007 | 23.4 ± 2.8 | 23.3 ± 2.9 | 0.84 |
| Obese (%) | 10 (1.4%) | 13 (1.8%) | 0.57 | 10 (1.4%) | 13 (1.8%) | 0.50 | 21 (1.6%) | 2 (1.9%) | 0.81 |
| Blood pressure (mm Hg) | |||||||||
| Systolic | 113 ± 12 | 115 ± 12 | 0.01 | 113 ± 11 | 115 ± 12 | 0.008 | 114 ± 12 | 117 ± 11 | 0.008 |
| Diastolic | 72 ± 9 | 73 ± 9 | 0.26 | 72 ± 9 | 73 ± 9 | 0.03 | 72 ± 9 | 74 ± 8 | 0.05 |
| Lipids† | |||||||||
| HDL cholesterol (mg/dl) | 51 ± 12 | 50 ± 12 | 0.50 | 52 ± 13 | 49 ± 12 | 0.002 | 50 ± 12 | 51 ± 13 | 0.61 |
| LDL cholesterol (mg/dl) | 110 ± 29 | 109 ± 29 | 0.50 | 107 ± 30 | 112 ± 28 | 0.004 | 109 ± 29 | 113 ± 26 | 0.18 |
| Total cholesterol (mg/dl) | 177 ± 33 | 176 ± 34 | 0.53 | 174 ± 34 | 179 ± 33 | 0.01 | 176 ± 33 | 182 ± 31 | 0.04 |
| Triglycerides (mg/dl) | 81 ± 43 | 82 ± 51 | 0.82 | 76 ± 50 | 87 ± 44 | <0.001 | 81 ± 47 | 90 ± 48 | 0.005 |
| Renal Function‡ | |||||||||
| Albumin excretion rate (mg/24hr) | 16.4 ± 19.6 | 15.5 ± 17.9 | 0.89 | 11.8 ± 8.3 | 20.1 ± 24.6 | <0.001 | 15.8 ± 19.0 | 17.6 ± 15.7 | 0.06 |
| Median | 11.5 | 11.5 | 10.1 | 13.0 | 10.1 | 14.4 | |||
| Interquartile Range | 10.1 | 11.5 | 8.6 | 17.3 | 10.1 | 14.4 | |||
| Albumin excretion | |||||||||
| Any albuminuria (AER ≥ 40 mg/24hr) (%) | 39 (5.5%) | 35 (4.8%) | 0.55 | 1 (0.1%) | 73 (10.2%) | <0.001 | 65 (4.9%) | 9 (8.4%) | 0.11 |
| eGFR | 126 ± 14 | 126 ± 15 | 0.78 | 127 ± 14 | 126 ± 14 | 0.43 | 126 ± 14 | 127 ± 15 | 0.94 |
| Glycated hemoglobin (%) | 9.1 ± 1.6 | 9.1 ± 1.6 | 0.55 | 9.0 ± 1.7 | 9.2 ± 1.5 | 0.002 | 9.0 ± 1.6 | 9.5 ± 1.7 | 0.005 |
| Heart rate (beats/min) | 68 ± 11 | 68 ± 11 | 0.36 | 67 ± 11 | 70 ± 11 | <0.001 | 68 ± 11 | 70 ± 11 | 0.14 |
Values are means ±SD or %. HDL denotes high-density lipoprotein, and LDL low-density lipoprotein. The body-mass index is the weight in kilograms divided by the square of the height in meters. To convert values for cholesterol to millimoles per liter, multiply by 0.02586. To convert values for trigylcerides to millimoles per liter, multiply by 0.01129.
Renal function and lipid levels (HDL and LDL cholesterol) were determined from the biennial evaluation conducted at year 17 or 18 of the EDIC study on the basis of the year of entry into the DCCT.
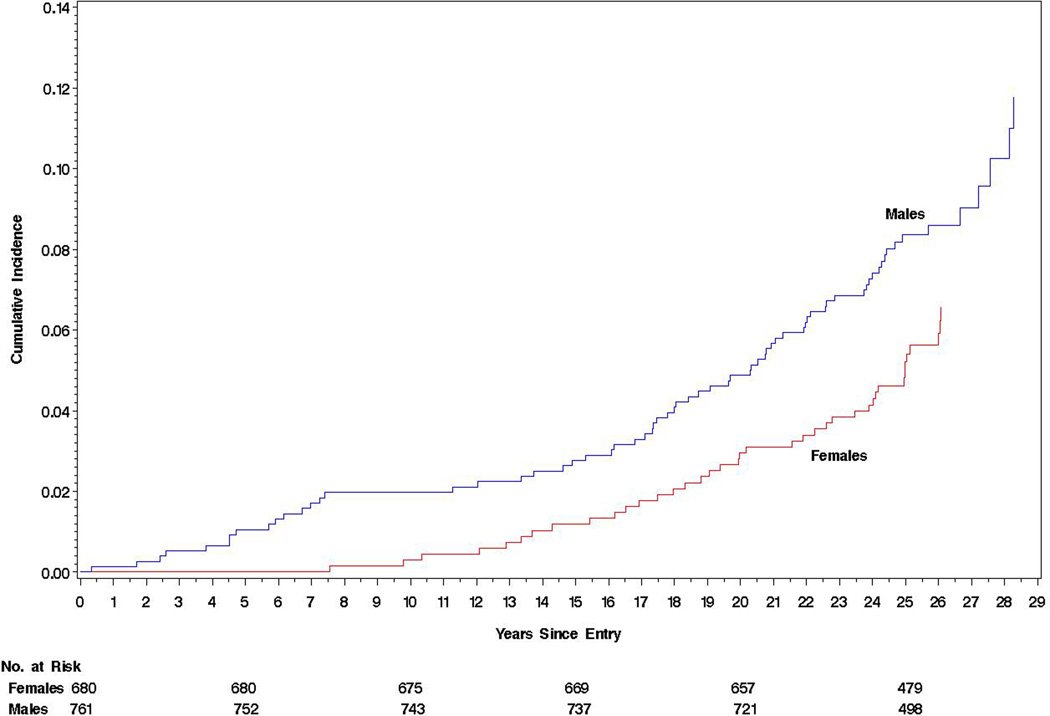
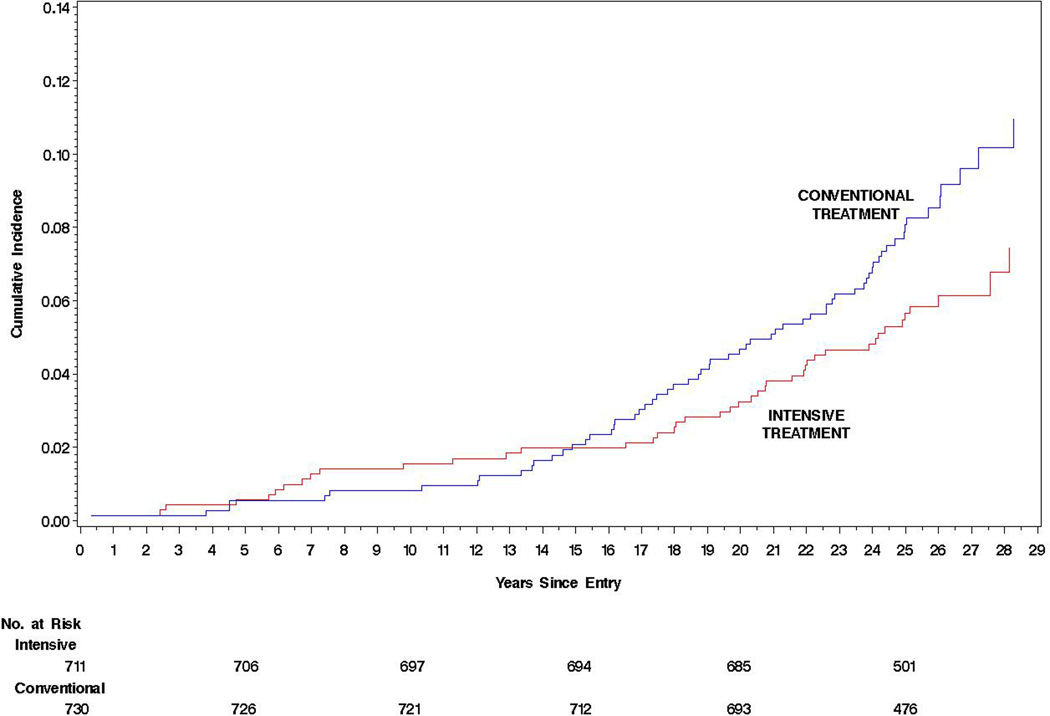
The all-cause mortality rate was low, only 291/100 000 patient-years (95%CI, 242–351) or 0.29% per year, and was greater in men than women (hazard ratio [HR], 1.61 [95% CI, 1.09–2.39; P = .02]), absolute risk difference (ARD), 132/100 000 patient-years (95%CI, 24– 240; Table 2 and Figure). In addition, those who died were older, had an older age at diabetes onset, were more likely to be smokers, and had higher baseline systolic blood pressure, cholesterol, and HbA1c levels (Table 1). Of the 107 (7.4%) deaths, 43 (6.0%) were in the intensive treatment group and 64 (8.8%) were in the conventional treatment group. Mortality risk per 100 000 patient-years was lower in the intensive group (HR = 0.67 [95%CI, 0.46–0.99];P = .045; ARD = −109/100 000 patient-years [95% CI, −218 to −1]; P = .048; Table 2 and Figure), with a similar HR for both sexes (Table 2). Mortality between treatment groups did not differ until after the first 15 years of follow-up (Figure).
Table 2.
Deaths and Death Rates by Treatment Group, Primary versus Secondary Cohort, and Gender
| A. Total | Comparator Groups | HR (95% CI)* | p-value | ARD (95% CI)* | p-value | |
| Intensive | Conventional | |||||
| No. of deaths/total | 43/711 | 64/730 | 0.67 (0.46, 0.99) | 0.045 | ||
|
Rate/100,000 patient-years (95%CI) |
236 (176, 318) | 346 (272, 440) | −109 (−218, −1) | 0.048 | ||
| Females | Males | |||||
| No. of deaths/total | 39/680 | 68/761 | 1.61 (1.09, 2.39) | 0.02 | ||
|
Rate/100,000 patient-years (95%CI) |
222 (163, 303) | 354 (280, 448) | 132 (24, 240) | 0.02 | ||
| Primary | Secondary | |||||
| No. of deaths/total | 55/726 | 52/715 | 0.87 (0.59, 1.27) | 0.47 | ||
|
Rate/100,000 patient-years (95%CI) |
303 (234, 393) | 280 (214, 366) | −23 (−132, 86) | 0.68 | ||
| B. Treatment Group by Gender§ | ||||||
| Males | Intensive | Conventional | ||||
| No. of deaths/total | 27/366 | 41/395 | 0.69 (0.42, 1.12) | 0.13 | ||
| Rate/100,000 patient-years (95%CI) |
291 (200, 424) | 413 (307, 558) | −122 (−287, 43) | 0.15 | ||
| Females | ||||||
| No. of deaths/total | 16/345 | 23/335 | 0.66 (0.35, 1.26) | 0.21 | ||
| Rate/100,000 patient-years (95%CI) |
179 (110, 291) | 267 (179, 400) | −88 (−226, 50) | 0.21 | ||
| C. Group by Cohort‖ | ||||||
| Primary | Intensive | Conventional | ||||
| No. of deaths/total | 24/348 | 31/378 | 0.83 (0.49, 1.42) | 0.50 | ||
| Rate/100,000 patient-years (95%CI) |
276 (186,409) | 328 (232,463) | −52 (−209, 105), | 0.52 | ||
| Secondary | ||||||
| No. of deaths/total | 19/363 | 33/352 | 0.54 (0.31, 0.95) | 0.03 | ||
| Rate/100,000 patient-years (95%CI) |
200 (128,313) | 364 (260,509) | −164 (−315, −13) | 0.03 | ||
| D. Gender by Cohort¶ | ||||||
| Primary | Female | Male | ||||
| No. of deaths/total | 24/348 | 31/378 | 1.22 (0.71, 2.07) | 0.47 | ||
| Rate/100,000 patient-years (95%CI) |
274 (185, 406) | 330 (234, 467) | 56 (−101, 213) | 0.48 | ||
| Secondary | ||||||
| No. of deaths/total | 15/332 | 37/383 | 2.25 (1.23, 4.10) | < 0.01 | ||
| Rate/100,000 patient-years (95%CI) |
171 (104, 282) | 378 (275, 519) | 207 (59, 354) | < 0.01 | ||
Abbreviations: ARD, adjusted risk difference; HR, hazard ratio.
HRs from an unadjusted Cox proportional hazard model and ARDs from a Poisson model
P = 0.92 for the test of group by gender interaction, or that the Int:Conv hazard ratio among males equals that among females, based on the Wald statistic from Cox proportional model.
P = 0.27 for the test of group by study cohort interaction based on Wald statistic from Cox proportional model.
P = 0.13 for the test of gender by study cohort interaction based on Wald statistic from Cox proportional model (data not shown).
Figure 1.
Cumulative incidence of mortality from the date of randomization in the DCCT (starting in 1983) to December 31, 2012 with estimates of the Hazard Ratio, 95% confidence limits and p-value obtained from a Cox Proportional Hazards model. A) For males versus females, HR = 1.61 (1.09, 2.39), p = 0.02. B) For the intensive versus conventional treatment groups (intent-to-treat analysis), HR = 0.67 (0.46, 0.99), p = 0.045.
There were no significant differences in mortality between the primary prevention and secondary intervention cohorts (Table 2). The intensive to conventional group HR within the secondary intervention cohort was nominally significant (HR = 0.54 [95% CI, 0.31–0.95]; P = .03; ARD = −164/100 000 patient-years [95% CI, −315 to −13]; P = .03), whereas intensive to conventional group HR in the primary prevention cohort was not (HR = 0.83 [95% CI, 0.49–1.42]; P = .50; ARD = −52/100 000 patient-years [95% CI, −209 to 105]; P = .52). However, a test of homogeneity of the difference between the intensive vs the conventional treatment group HRs in the 2 cohorts (0.83 in the primary vs 0.54 in the secondary) was not significant (ie, within the realm of chance variation).
The most common causes of death (Table 3) were cardiovascular events (24 [22.4%]), cancer (21 [19.6%]), acute diabetes complications (19 [17.8%]), and accidents or suicide (18 [16.8%]). In the intensive vs the conventional group, there were fewer deaths from diabetic renal (1 vs 6), cardiovascular (9 vs 15), and cancer causes (7 vs 14). The most frequently occurring fatal cancer was lung cancer (n = 5; 2 in the intensive vs 3 in the conventional group). There were nominally more deaths due to accident or suicide in the intensive vs the conventional group (13 of 711 vs 5 of 730). None of the accidents were directly attributable to hypoglycemia. The proportion of deaths attributed to accident or suicide was significantly greater among men (23.5% [95% CI, 14.1%–35.4%) than women (5.1% [95% CI, 0.6%–17.3%]; P < .001).
Table 3.
Distribution of the DERI (Diabetes Epidemiology Research International Mortality Study) Causes of Death and the Classification by the Role of Diabetes
| Total | Intensive | Conventional | Females | Males | Primary | Secondary | |
|---|---|---|---|---|---|---|---|
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | |
| Overall | 107 (100) | 43 (40.2) | 64 (59.8) | 39 (36.5) | 68 (63.5) | 55 (51.4) | 52 (48.6) |
| DERI Causes of Death | |||||||
| Diabetic renal | 7 (6.5) | 1 (2.3) | 6 (9.4) | 1 (2.6) | 6 (8.8) | 3 (5.5) | 4 (7.7) |
| Acute complications | 19 (17.8) | 9 (20.9) | 10 (15.6) | 10 (25.6) | 9 (13.2) | 9 (16.4) | 10 (19.2) |
| DKA | 8 (7.5) | 3 (7.0) | 5 (7.8) | 3 (7.7) | 5 (7.4) | 4 (7.3) | 4 (7.7) |
| Diabetic Coma Unspecified | 2 (1.9) | 1 (2.3) | 1 (1.6) | 1 (2.6) | 1 (1.5) | 1 (1.8) | 1 (1.9) |
| Hypoglycemia | 9 (8.4) | 5 (11.6) | 4 (6.3) | 6 (15.4) | 3 (4.4) | 4 (7.3) | 5 (9.6) |
| Accident / Suicide | 18 (16.8) | 13 (30.2) | 5 (7.8) | 2 (5.1) | 16 (23.5) | 12 (21.8) | 6 (11.5) |
| Accident | 11 (10.3) | 8 (18.6) | 3 (4.7) | 0 (0) | 11 (16.2) | 6 (10.9) | 5 (9.6) |
| Suicide | 7 (6.5) | 5 (11.6) | 2 (3.1) | 2 (5.1) | 5 (7.4) | 6 (10.9) | 1 (1.9) |
| Cardiovascular | 24 (22.4) | 9 (20.9) | 15 (23.4) | 10 (25.6) | 14 (20.6) | 12 (21.8) | 12 (23.1) |
| Infections | 4 (3.7) | 1 (2.3) | 3 (4.7) | 0 (0) | 4 (5.9) | 1 (1.8) | 3 (5.8) |
| Cancer | 21 (19.6) | 7 (16.3) | 14 (21.9) | 11 (28.2) | 10 (14.7) | 11 (20.0) | 10 (19.2) |
| Other, non-diabetes | 5 (4.7) | 1 (2.3) | 4 (6.3) | 1 (2.6) | 4 (5.9) | 2 (3.6) | 3 (5.8) |
| Other, diabetes | 1 (0.9) | 0 (0) | 1 (1.6) | 1 (2.6) | 0 (0) | 1 (1.8) | 0 (0) |
| Unknown | 8 (7.5) | 2 (4.7) | 6 (9.4) | 3 (7.7) | 5 (7.4) | 4 (7.3) | 4 (7.7) |
| Death by the Role of Diabetes | |||||||
| Diabetes had a role* | 62 (57.9) | 26 (60.5) | 36 (56.3) | 22 (56.4) | 40 (58.8) | 29 (52.7) | 33 (63.5) |
| Diabetes caused death | 29 (27.1) | 13 (30.2) | 16 (25.0) | 12 (30.8) | 17 (25.0) | 15 (27.3) | 14 (26.9) |
| Diabetes was necessary for the death but another process was the underlying cause | 23 (21.5) | 7 (16.3) | 16 (25.0) | 8 (20.5) | 15 (22.1) | 10 (18.2) | 13 (25.0) |
| Diabetes made a marginal contribution to the death but was not necessary for death | 10 (9.4) | 6 (14.0) | 4 (6.3) | 2 (5.1) | 8 (11.8) | 4 (7.3) | 6 (11.5) |
| Diabetes had no role† | 37 (34.6) | 15 (34.9) | 22 (34.4) | 14 (35.9) | 23 (33.8) | 22 (40.0) | 15 (28.9) |
| Unknown | 8 (7.5) | 2 (4.7) | 6 (9.4) | 3 (7.7) | 5 (7.4) | 4 (7.3) | 4 (7.7) |
Death rate related to “diabetes had a role” is 62/36725=169/100000 patient year.
Death rate related to “diabetes had no role” is 37/36725=101/100000 patient year.
Primary- primary prevention cohort; Secondary- secondary intervention cohort.
DKA- diabetic ketoacidosis
Albuminuria and end-stage renal disease during the study were associated with the risk of mortality (Table 4). Among the 635 patients with any albuminuria (AER ≥40 mg/24 hours), mortality risk was higher compared with those with a history of normal albuminuria (HR = 2.20 [95%CI, 1.46–3.31]; P < .01; model A). The HR for mortality in the 464 patients who developed microalbuminuria (AER ranging from40mg/24 hours to <300 mg/24 hours) vs those with normal albuminuria was 1.52 (95%CI, 0.97–2.37; P = .07), and the HR for mortality in the 171 patients who developed macroalbuminuria (AER ≥300 mg/24 hours) vs those with normal albuminuria was 3.00 (95%CI, 1.82–4.93; P < .001; model B). Risk was also higher in the 44 patients with renal insufficiency vs those without (HR = 8.51[95%CI, 4.45–16.27]; P < .001; model C). Although a history of severe hypoglycemia requiring assistance was not significantly associated with mortality (model E), the subset of participants who had experienced severe hypoglycemia with coma, seizure, or both (model D) had a HR of 1.63 (95%CI, 1.10–2.40; P = .02), and a nonsignificantly higher risk of death due to accident or suicide (HR = 2.64 [95%CI, 0.99–7.04]; P = .06). Mortality risk was also associated with higher mean HbA1c levels over the full follow-up period (HR = 1.56 [95%CI, 1.35–1.81 per 10% relative increase in HbA1c]; P < .001). The eTable in the Supplement presents the treatment group differences in these covariates.
Table 4.
Separate Models of the Association of Each Time-Dependent Covariate with the Risk of Death and the Corresponding Death Rate Within Categories of each Covariate.
| Time-Dependent Covariate | Effect of Time-Dependent Covariate* |
Number of Deaths |
N | Death Rate† (per 100,000 pt yrs) |
||
|---|---|---|---|---|---|---|
| Hazard Ratio (95% CI) | P-value | |||||
| Renal disease‡ | ||||||
| A. | AER# < 40 mg/24 h | Reference | 46 | 806 | 225 | |
| AER# ≥ 40 mg/24 h | 2.20 (1.46, 3.31) | < 0.01 | 61 | 635 | 1165 | |
| B. | AER# < 40 mg/24 h | Reference | 46 | 806 | 225 | |
| 40 ≤ AER# < 300 mg/24 h | 1.52 (0.97, 2.37) | 0.07 | 31 | 464 | 746 | |
| AER# ≥ 300 mg/24 h | 3.00 (1.82, 4.93) | < 0.01 | 30 | 171 | 1482 | |
| C. | No Renal Insufficiency | Reference | 95 | 1397 | 267 | |
| Renal Insufficiency | 8.51 (4.45, 16.27) | < 0.01 | 12 | 44 | 1527 | |
| Hypoglycemia | ||||||
| D. | No Coma/Seizure | Reference | 52 | 773 | 267 | |
| Coma/Seizure | 1.63 (1.10, 2.40) | 0.02 | 55 | 668 | 1153 | |
| E. | No Severe Hypoglycemia § | Reference | 34 | 444 | 306 | |
| Severe Hypoglycemia § | 1.36 (0.90, 2.07) | 0.15 | 73 | 997 | 1361 | |
| HbA1c | ||||||
| F. | Mean During DCCT/EDIC‖ | |||||
| Per 10% higher HbA1c** | 1.56 (1.35, 1.81) | < 0.01 | ||||
All models were adjusted for the glycated hemoglobin value, age, sex, systolic blood pressure, and smoking status at baseline in the DCCT.
Death rate among those who had renal disease, microalbuminuria, albuminuria, or renal insufficiency or hypoglycemia and those who did not.
Microalbuminuria/albuminuria included participants who progressed to end stage renal staage that was not preceded by a timed renal collection to measure the albumin excretion rate. Renal insufficiency was defined by a history of an eGFR < 30 ml/min/1.73m2 using the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) equation [22], of kidney transplantation, or the implementation of dialysis.
Albumin Excretion Rate
Any hypoglycemic episode requiring assistance from any party to treat
The log mean glycated hemoglobin value was used so that the hazard ratio per c-fold change in risk is c4.69593, where 4.69593 is the estimated regression coefficient; a c of 1.1 corresponds to a 10 percent increase in the mean glycated hemoglobin value, such as comparing the risk with an HbA1c of 8% versus an HbA1c of 8.8% (10% higher). Likewise, a c of 0.9 corresponds to a 10 percent decrease.
Glycated hemoglobin
The risk of mortality was higher among those with a history of hypoglycemia (Table 4), and hypoglycemia occurred more frequently in the intensive group (eTable in the Supplement). When adjusting for hypoglycemia with coma, seizure, or both, the model estimates the intensive-to-conventional HR as if there were no difference in hypoglycemia between the 2 treatment groups so that the reduced risk associated with intensive treatment is greater (adjusted HR = 0.61 [95%CI, 0.41-0.9]; P = .01).
Discussion
Over an average of 27 years of follow-up in the DCCT/EDIC cohort with type 1 diabetes mellitus, overall mortality risk in the intensive group was lower than that in the conventional group (P = .045), although the absolute risk reduction was small at approximately 1/1000 patient-years. The DCCT was designed to examine the effects of intensive therapy on microvascular complications and, on the basis of the large demonstrated benefits,10–12 intensive therapy has become the recommended therapy for type 1 diabetes.14 However, sustaining intensive therapy is difficult for many patients, as demonstrated by the rise in mean HbA1c levels after completion of the DCCT, which became nonsignificantly different from the control group within 5 years. Furthermore, intensive therapy is associated with increased hypoglycemic risk, which in turn has been associated with increased mortality.24 The current data suggest net mortality benefit from intensive therapy, even when the controlled clinical trial comparing intensive with conventional therapy was implemented over less than 25% of the 27-year follow-up time.
These results provide reassurance that adoption of 6.5 years of intensive therapy in type 1 diabetes does not incur increased risk of overall mortality, and are in contrast to findings from the ACCORD trial in type 2 diabetes.15 The UKPDS (UK Prospective Diabetes Study), a controlled clinical trial with a population of newly diagnosed patients with type 2 diabetes and a study duration that was more analogous to the DCCT/EDIC than ACCORD, also showed a reduced mortality with intensive therapy.25 The lower mortality in the intensive group in our study was consistent across all causes of death except for accident and suicide; however, possibly owing to the small number of deaths, the reductions in specific causes did not achieve statistical significance. The intensive-to-conventional differences in mortality parallel the previously demonstrated reductions in cardiovascular and renal disease in DCCT/EDIC,12–14 and HbA1c levels and albuminuria (to a lesser degree) were associated with mortality.
EDIC has established that glycemic control plays a central role in determining the risks of microvascular and macrovascular complications,12,18 and we now show its association with subsequent mortality. Although the numbers are small, there were fewer diabetic renal deaths (1 vs 6) and cardiovascular deaths (9 vs 15) with intensive vs conventional therapy, causes for which glycemia might be expected to play a major role.
These results also support the emerging concept that most of the excess mortality in type 1 diabetes is mediated by the development of albuminuria (whether microalbuminuria or greater).26,27 In EDIC, the persistent effect of early metabolic control (achieved with intensive therapy during the average 6.5 years of DCCT) on subsequent risk of albuminuria and other longer-term complications (so-called metabolic memory)18,28,29 appears to be related to a reduction in mortality over the subsequent 20-year follow-up period.
The small numbers of accidents and suicides (13 in the intensive group and 5 in the conventional group), none of which were clearly related to acute hypoglycemia, preclude any definitive conclusions regarding the relative risk of former intensive therapy. However, acute hypoglycemia has been recognized as an important cause of mortality in type 1 diabetes mellitus24 and a history of severe hypoglycemia accompanied by coma, seizure, or both in DCCT/EDIC was associated with greater overall mortality (HR = 1.63 [95% CI, 1.10–2.40]; P = .02), supporting the concept that a history of severe hypoglycemia increases or is a marker for mortality risk. Despite the putative adverse effect of severe hypoglycemia on mortality risk, mortality was lower in the intensive therapy group. Studies in type 2 diabetes have been conflicting and inconclusive as to the chronic role of severe hypoglycemia and mortality.30,31
There are a number of limitations to this study that include the small number of deaths, the initial selection of a lower cardiovascular disease risk population, and underrepresentation of mortality experience in the childhood years of diabetes. The lack of racial diversity may also be considered a limitation. Another limitation is that our current analyses do not account for any potential treatment group differences in cardio-protective medication use (renin-angiotensin-aldosterone system inhibitor, statin, or β-blocker). However, the fraction of participants using such medications up to December 31, 2012, was 84% in the conventional group vs 83% in the intensive group, and the benefit of intensive therapy was unchanged after adjusting for medication use. Additionally, the numbers of deaths are inadequate to permit reliable multivariable risk factor modeling to assess the joint association of various risk factors with the risk of mortality overall and within each treatment group.
Conclusion
After a mean of 27 years’ follow-up of patients with type 1 diabetes, 6.5 years of initial intensive diabetes therapy was associated with a modestly lower all-cause mortality compared with conventional therapy.
Supplementary Material
ACKNOWLEDGEMENTS
Funding/Support: The DCCT/EDIC has been supported by U01 cooperative agreement grants (1982–1993 and 2011–2016) and contracts (1982–2011) with the Division of Diabetes Endocrinology and Metabolic Diseases of the National Institute of Diabetes and Digestive and Kidney Disease (grants U01 DK094176 and U01 DK094157), and through support by the National Eye Institute, the National Institute of Neurological Disorders and Stroke, the General Clinical Research Centers Program (1993–2007), and the Clinical Translational Science Center Program (2006-present), Bethesda, Maryland. The following industry contributors provided free or discounted supplies or equipment to support participants’ adherence to the study: Abbott Diabetes Care (Alameda, CA), Animas (Westchester, PA), Bayer Diabetes Care (North America Headquarters, Tarrytown, NY), Becton Dickinson (Franklin Lakes, NJ), Eli Lilly (Indianapolis, IN), Extend Nutrition (St. Louis, MO), Lifescan (Milpitas, CA), Medtronic Diabetes (Minneapolis, MN), Nipro Home Diagnostics (Ft. Lauderdale, FL), Nova Diabetes Care (Billerica, MA), Omron (Shelton, CT), OmniPod Insulin Management System (Bedford, MA), Perrigo Diabetes Care (Allegan, MI), Roche Diabetes Care (Indianapolis, IN), and sanofi-aventis (Bridgewater NJ).
Role of the Sponsor: Representatives of the National Institutes of Health (NIH) and other federal agencies participated in the design and conduct of the study but had no role in the collection, management, analysis, or interpretation of the data; in the preparation, review, or approval of the manuscript; or in the decision to submit the manuscript for publication. Industry contributors had no role in the DCCT/EDIC study.
Appendix
Authors/Writing Group for the DCCT/EDIC Research Group take authorship responsibility for the study results. Trevor J. Orchard, MD; DavidM. Nathan, MD; Bernard Zinman, MD; Patricia Cleary, MS; David Brillon, MD; Jye-Yu C. Backlund, MPH; John M. Lachin, ScD.
Affiliations of Group for the DCCT/EDIC Research Group: University of Pittsburgh, Pittsburgh, Pennsylvania (Orchard); Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts (Nathan); Lunefeld Tanenbaum Research Institute, Mount Sinai Hospital, University of Toronto, Toronto, Ontario, Canada (Zinman); The George Washington University Biostatistics Center, Rockville, Maryland (Cleary, Backlund, Lachin); Cornell University Medical Center, New York, New York (Brillon).
Footnotes
Author Contributions: Dr Lachin had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Orchard, Nathan, Zinman, Brillon, Lachin.
Acquisition, analysis, or interpretation of data: Orchard, Nathan, Zinman, Cleary, Brillon, Backlund, Lachin.
Drafting of the manuscript: Orchard, Nathan, Cleary, Brillon, Backlund, Lachin.
Critical revision of the manuscript for important intellectual content: Orchard, Nathan, Zinman, Brillon, Lachin.
Statistical analysis: Orchard, Cleary, Backlund,Lachin.
Obtained funding: Orchard, Nathan, Zinman, Lachin.
Administrative, technical, or material support: Orchard, Zinman, Cleary, Backlund.
Study supervision: Orchard, Nathan, Zinman, Cleary.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest and none were reported.
References
- 1.Borch-Johnsen K, Kreiner S, Deckert T. Mortality of type 1 (insulin-dependent) diabetes mellitus in Denmark: a study of relative mortality in 2930 Danish type 1 diabetic patients diagnosed from 1933 to 1972. Diabetologia. 1986;29(11):767–772. doi: 10.1007/BF00873214. [DOI] [PubMed] [Google Scholar]
- 2.Harjutsalo V, Forsblom C, Groop PH. Time trends in mortality in patients with type 1 diabetes: nationwide population based cohort study. BMJ. 2011 Sep 8;343:d5364. doi: 10.1136/bmj.d5364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Skrivarhaug T, Bangstad HJ, Stene LC, Sandvik L, Hanssen KF, Joner G. Long-term mortality in a nationwide cohort of childhood-onset type 1 diabetic patients in Norway. Diabetologia. 2006;49(2):298–305. doi: 10.1007/s00125-005-0082-6. [DOI] [PubMed] [Google Scholar]
- 4.Soedamah-Muthu SS, Fuller JH, Mulnier HE, Raleigh VS, Lawrenson RA, Colhoun HM. All-cause mortality rates in patients with type 1 diabetes mellitus compared with a non-diabetic population from the UK general practice research database, 1992–1999. Diabetologia. 2006;49:660–666. doi: 10.1007/s00125-005-0120-4. [DOI] [PubMed] [Google Scholar]
- 5.Shankar A, Klein R, Klein BEK, Moss SE. Association between glycosylated hemoglobin level and cardiovascular and all-cause mortality in type 1 diabetes. Am J Epidemiol. 2007;166(4):393–402. doi: 10.1093/aje/kwm096. [DOI] [PubMed] [Google Scholar]
- 6.Pambianco G, Costacou T, Ellis D, Becker DJ, Klein R, Orchard TJ. The 30-year natural history of type 1 diabetes complications: the Pittsburgh Epidemiology of Diabetes Complications Study experience. Diabetes. 2006;55(5):1463–1469. doi: 10.2337/db05-1423. [DOI] [PubMed] [Google Scholar]
- 7.Secrest AM, Becker DJ, Kelsey SF, Laporte RE, Orchard TJ. All-cause mortality trends in a large population-based cohort with long-standing childhood-onset type 1 diabetes. Diabetes Care. 2010;33(12):2573–2579. doi: 10.2337/dc10-1170. PMCID: PMC2992193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McNally PG, Raymond NT, Burden ML, et al. Trends in mortality of childhood-onset insulin-dependent diabetes mellitus in Leicestershire: 1940–1991. Diabetic Medicine. 1995;12:961–966. doi: 10.1111/j.1464-5491.1995.tb00406.x. [DOI] [PubMed] [Google Scholar]
- 9.Miller R, Secrest A, Sharma R, Songer T, Orchard T. Improvements in the life expectancy of type 1 diabetes: the Pittsburgh Epidemiology of Diabetes Complications Study cohort. Diabetes. 2012;61(11):2987–2992. doi: 10.2337/db11-1625. PMCID: PMC3478551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 11.The DCCT/EDIC Research Group. Intensive diabetes therapy and glomerular filtration rate in type 1 diabetes. N Engl J Med. 2011;365:2366–2376. doi: 10.1056/NEJMoa1111732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nathan DM, Cleary PA, Backlund JY, et al. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353(25):2643–2653. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dorman JS, LaPorte RE, Kuller LH, et al. The Pittsburgh Insulin-Dependent Diabetes Mellitus (IDDM) Morbidity and Mortality Study: mortality results. Diabetes. 1984;33:271–276. doi: 10.2337/diab.33.3.271. [DOI] [PubMed] [Google Scholar]
- 14.Standards of Medical Care in Diabetes—2014. Diabetes Care. 2014;37(Supplement 1):S14–S80. doi: 10.2337/dc14-S014. [DOI] [PubMed] [Google Scholar]
- 15.Action to Control Cardiovascular Risk in Diabetes Study Group. Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.The DCCT Research Group. The Diabetes Control and Complications Trial (DCCT). Design and methodologic considerations for the feasibility phase. Diabetes. 1986;35(5):530–545. [PubMed] [Google Scholar]
- 17.Epidemiology of Diabetes Interventions and Complications (EDIC) Research Group. Design, implementation, and preliminary results of a long-term follow-up of the Diabetes Control and Complications Trial cohort. Diabetes Care. 1999;22(1):99–111. doi: 10.2337/diacare.22.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nathan DM, Bayless M, Cleary P, et al. for the DCCT/EDIC Research Group. The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study at 30 Years: Advances and Contributions. Diabetes. 2013;62:3976–3986. doi: 10.2337/db13-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Epidemiology of Diabetes Interventions and Complications Research Group. Protocol. 10/24/2013. ( https://edic.bsc.gwu.edu/.) [Google Scholar]
- 20.Diabetes Epidemiology Research International Mortality Study Group. International evaluation of cause-specific mortality and IDDM. Diabetes Care. 1991;14:55–60. doi: 10.2337/diacare.14.1.55. [DOI] [PubMed] [Google Scholar]
- 21.Levey AS, Stevens LA, Schmid CH, et al. CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lachin JM. Biostatistical Methods: The Assessment of Relative Risks. Second Edition. John Wiley and Sons; 2011. [Google Scholar]
- 23.R Core Team. R Foundation for Statistical Computing. Vienna, Austria: 2013. R: A language and environment for statistical computing. [Google Scholar]
- 24.Alsahli M, Gerich JE. Hypoglycemia. Endocrinol Metab Clin North Am. 2013;42(4):657–676. doi: 10.1016/j.ecl.2013.07.002. PMID: 24286945. [DOI] [PubMed] [Google Scholar]
- 25.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 26.Groop PH, Thomas MC, Moran JL, et al. FinnDiane Study Group. The presence and severity of chronic kidney disease predicts all-cause mortality in type 1 diabetes. Diabetes. 2009;58(7):1651–1658. doi: 10.2337/db08-1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Orchard TJ, Secrest AM, Miller RG, Costacou T. In the absence of renal disease, 20-year mortality risk in type 1 diabetes is comparable to that of the general population: a report from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetologia. 2010;53(11):2312–2319. doi: 10.1007/s00125-010-1860-3. PMCID: PMC3057031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lachin JM, Genuth S, Cleary P, Nathan DM for the EDIC Research Group. Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. N Engl J Med. 2000;342:381–389. doi: 10.1056/NEJM200002103420603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.EDIC Research Group. Sustained effect of intensive treatment of type 1 diabetes mellitus on development and progression of diabetic nephropathy: the Epidemiology of Diabetes Interventions and Complications (EDIC) study. JAMA. 2003;290:2159–2167. doi: 10.1001/jama.290.16.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seaquist ER, Miller ME, Bonds DE, Feinglos M, Goff DC, Jr, Peterson K ACCORD Investigators. The impact of frequent and unrecognized hypoglycemia on mortality in the ACCORD study. Diabetes Care. 2012;35(2):409–414. doi: 10.2337/dc11-0996. PMICD:PMC3263892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bloomgarden ZT, Einhorn D. Hypoglycemia in type 2 diabetes: current controversies and changing practices. Frontiers in Endocrinology. 2012;3:66. doi: 10.3389/fendo.2012.00066. PMID: 22661969. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.