Abstract
Purpose.
The purpose of this study was to investigate the thiol repair systems of thioltransferase (TTase) and thioredoxin (Trx) and oxidation-damaged proteins in human cataractous lenses.
Methods.
Cataractous lenses in humans (57–85 years of age) were classified into cortical, nuclear, mixed, mature, and hypermature cataract types by using a lens opacity classification system, and were obtained by extracapsular cataract extraction (ECCE) procedure. Cortical and nuclear cataracts were grouped by decreasing order of visual acuity into optical chart reading (R), counting fingers (CF), hand motion (HM), and light perception (LP). ECCE lens homogenate was analyzed for glutathione (GSH) level and enzyme activities of TTase, glutathione reductase (GR), Trx, and thioredoxin reductase (TR). Cortical and nuclear cataractous lenses (8 of each) with visual acuity better than HM were each dissected into cortical and nuclear portions for measurement of glyceraldehyde 3-phosphate dehydrogenase (G3PD) activity. Clear lenses (in humans 49–71 years of age) were used as control.
Results.
Compared with control, all cataractous lenses lost more than 80% GSH and 70% GR; TR and Trx activity; and 40% to 70% TTase activity, corroborated with the loss in visual acuity. Among cataracts with R and CF visual acuity, cortical cataract lost more cortical G3PD activity (18% of control) than that of nuclear cataract (50% of control), whereas GSH depletion and TTase inactivation were similar in both cataracts.
Conclusions.
Thiol repair systems were damaged in all types of cataracts. Cortical and nuclear cataracts showed differential G3PD inactivation in the cortex, implying those 2 type of cataracts might be formed through different mechanisms.
Keywords: glyceraldehyde 3-phosphodehydrogenase, human cataract, oxidative stress, PSSG, PSSP, redox regulation, thiol oxidation damage repair, thioltransferase system, thioredoxin system
This study evaluated the loss of thiol oxidation repair systems in human cataractous lenses and the association with cataractogenesis.
Introduction
Human senile cataract, also known as age-related cataract, is a leading cause of blindness in the aging population and has been a topic of research for many years. The cause of age-associated human cataract development is generally believed to be multifactorial, with combined oxidative damage, truncation, and modification of lens structural proteins that may cause the proteins to unfold, aggregate, and become insoluble. Such change in lens proteins will eventually lead to light scattering and cataract formation.1–3 Of the mechanisms proposed in human cataract development, the hypothesis of oxidative stress has been studied extensively. Convincing evidence for this hypothesis has been accumulated from studies of cataractous lenses regarding oxidation-related damage found in DNA,4 proteins,5–7 and lipids.8 For instance, almost all cataractous lenses have lost more than 60% of the antioxidant glutathione (GSH), more than half of the methionine on the isolated proteins has been oxidized to methionine sulfoxide, and almost all cysteine moieties have been oxidized to cysteic acid.2,6 There are extensive protein-protein disulfide (PSSP) formations.5,6 Furthermore, it has been found that oxidized GSH is conjugated with protein thiols (glutathionylation) to form protein-glutathione mixed disulfides (PSSG).9–12 Studies using mass spectroscopy analysis have provided clear evidence that glutathionylation of the γB crystallin protein in a cultured lens exposed to H2O2 has indeed changed the protein conformation so much that it causes the buried thiol moieties to be exposed and oxidized.13
Mammalian cells and tissues, including the lens, are maintained in a reduced state for proper function. Oxidative stress can cause redox imbalance, altering or affecting cellular function. Although there are small antioxidants and enzymes to remove oxidants, the aging process can deplete or weaken such defense resources. Therefore, many of the oxidative-sensitive sulfhydryl-containing proteins can be oxidized to PSSG and PSSP and lose their respective functions. Such modification requires an efficient system to reverse or repair the damage. Recently, the class of redox-regulating enzymes in the oxidoreductases family have been found in various tissues with the specific dethiolase properties necessary to reduce and repair thiol-oxidized proteins, such as PSSG and PSSP. One such enzyme is the thioltransferase (TTase) system, also called glutaredoxin, which uses GSH as a cofactor to cleave PSSG, allowing the protein thiol to be free again.14,15 The other enzyme is the thioredoxin (Trx) system, which reduces PSSP, and the oxidized Trx is reduced by thioredoxin reductase (TR) with nicotinamide adenine dinucleotide phosphate (NADPH) as the electron donor.16 Both of these systems are found in the lens17–19 and in other ocular tissues.20 Because the lens is extremely sensitive to oxidative stress, we hypothesized that proper redox regulation to protect lens structural proteins from oxidative damage was important for lens transparency.21 We also speculated that thiol oxidation damage repair systems in cataractous lenses may have become inactivated or compromised. Thus, our current study examined such potential changes by using human cataractous lens tissues collected by extracapsular extraction (ECCE) procedure and classified by various cataractous types and severity of opacity. These tissues were examined for both the TTase and Trx systems and extensive activity loss was found in all cataract types, and such loss was corroborated with the results of examining patients' visual acuity. The finding that the loss of glyceraldehyde 3-phosphate dehydrogenase (G3PD) activity was more severe in the cortical cataract than in the nuclear cataract suggests a differential cause of these two cataract types.
Materials and Methods
Materials
NADPH, GSH, oxidized glutathione (GSSG), dithiothreitol (DTT), insulin, 5′,5′-dithiobis(2-nitrobanzoic acid) (DTNB), and β-hydroxyethyl disulfide (HEDS) were from Sigma-Aldrich Corp. (St. Louis, MO, USA). Bicinchoninic acid (BCA) protein assay reagent and SuperSignal West chemiluminescent substrate were from Pierce (Rockford, IL, USA). Antibodies specific for glutathione reductase (GR) and TR were purchased from Abcam Co. (Cambridge, MA, USA). Antibodies for TTase and TRx were custom-made by Bethyl Laboratories (Montgomery, TX, USA). Antibody for G3PD and horseradish peroxidase-conjugated secondary antibodies were from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). All other chemicals and reagents were of analytical grade.
Human Lens Sample Collection
Normal donor lenses of humans 49 to 71 years of age (n = 9) were received from Omaha Lions Eye Bank (Omaha, NE, USA) and served as controls. The intact lenses were enucleated and immediately put into individual containers and placed on dry ice for overnight delivery. Human cataractous lenses of patients 57 to 85 years of age were collected in Sichuan, China, through ECCE procedure and stored in liquid nitrogen until delivery in a dry-ice container. The ECCE lenses were classified into cortical (n = 28), nuclear (n = 26), mixed cortical and nuclear (n = 17), mature (n = 17), and hypermature (n = 9) cataract types, according to the Lens Opacities Classification System III (LOCS III; Chylack, Inc., Duxbury, MA, USA). Patients with either cortical or nuclear cataract only were screened for visual acuity in the clinic prior to surgery. Visual acuity test results in decreasing acuity order included optical chart reading (R), counting fingers (CF), hand motion (HM), and light perception (LP). There were 13 cortical cataracts with visual acuities of R and CF, 4 cortical cataracts with HM and LP, 11 nuclear cataracts with R and CF, and 4 nuclear cataracts with HM and LP. Lens samples were stored in individual vials at −80°C until use.
Human Lens Dissection and Homogenization
Individual normal (clear) human lenses were dissected into cortex (with epithelium) and nucleus, using a cork borer. Briefly, a center cone of the lens (anterior-to-posterior) was removed using a 5-mm stainless steel cork borer. After 1 mm was cut off at both ends, the remaining section of the center cone was considered the nucleus (25%–30% of total lens weight). The remaining portions were considered the cortex (70%–75% of the lens weight). For ECCE lenses, selected cortical or nuclear cataract lenses were each dissected using the method described above. The ECCE method of cataract extraction typically collected tissues containing mostly nuclear and inner cortical layers of the whole lens, whereas the cortical region dissected by cork borer was considerably less than that of the control whole lens. Each lens sample was homogenized in 1.5 mL ice-cold buffer containing 50 mM Tris-HCl (pH 7.5), 100 mM NaCl, and 10 mM EDTA. The homogenate was centrifuged at 13,000g for 15 minutes at 4°C, and the supernatant was stored at −80°C pending analysis. For studies of enzyme regional distribution, seven cortical and seven nuclear cataracts with the least amount of opacity were selected from the ECCE samples along with four clear control lenses. These lenses were dissected into cortical and nuclear portions, following the method described by Huang et al.22
Quantification of GSH
An aliquot of ice-cold lens homogenate was mixed with an equal volume of 20% trichloroacetic acid to remove proteins. The mixture was centrifuged at 13,000g for 10 minutes at 4°C, and the supernatant were measured immediately for free GSH, using a micro-assay with Ellman's reagent.23
Enzyme Assays of Lens Homogenate
In each enzyme assay, the reaction mixture containing lysis buffer was used as the blank without lens homogenate. The activity of each enzyme was expressed as either milliunits or units of homogenate per gram of lens wet weight.
TTase was assayed using HEDS as the substrate following the method described in the report by Raghavachari and Lou.17 Briefly, lens homogenate was mixed with potassium phosphate buffer (200 mM, pH 7.5) containing 0.5 mM GSH, 0.4 unit/mL GR, 0.2 mM NADPH, and 2 mM HEDS. The reaction was carried out at 30°C, and the decrease in absorbance at 340 nm was monitored for 5 minutes and used to determine the TTase activity. Glutathione reductase assay was performed following the method of Straatsma et al.24 Briefly, lens homogenate was mixed with potassium phosphate buffer (100 mM, pH 7.0, with 2 mM EDTA) containing 0.2 mM NADPH and 2 mM GSSG. The reaction was carried out at 30°C, and the decrease in absorbance at 340 nm was monitored for 5 minutes and used to determine the GR activity.
Activity levels of both Trx and TR were determined based on the methods of Holmgren and Bjornstedt.25 For TRx activity assay, lens homogenate was mixed with potassium phosphate buffer (100 mM, pH 7.0, with 2 mM EDTA) containing 0.05 U/mL TR, 0.2 mM NADPH, and 0.14 mM insulin. The reaction was carried out at 30°C, and the decrease in absorbance at 340 nm was monitored for 5 minutes and used to determine Trx activity. For TR activity assay, lens homogenate was mixed with potassium phosphate buffer (100 mM, pH 7.0, with 2 mM EDTA) containing 0.2 mM NADPH and 5 mM DTNB. The reaction was carried out at 30°C, and the increase in absorbance at 412 nm was monitored for 5 minutes and used to quantify TR activity.
G3PD activity was analyzed according to the method described by Bergmeyer et al.26 Briefly, lens homogenate was mixed with assay buffer (200 mM triethanolamine, pH 7.6) containing 1 mM ATP, 1 mM EDTA, 2 mM MgSO4, 0.2 mM NADPH, 15 U/mL 3-phosphoglyceric phosphokinase, and 6 mM 3-phosphoglyceric acid, and the reaction was carried out at 25°C. The decrease in absorbance at 340 nm was monitored for 5 minutes and used to determine G3PD activity.
Immunoblot Analysis
Lens homogenate containing equal amount of proteins was applied on 10% SDS-PAGE, and resolved protein bands were transferred to enhanced chemiluminescence membrane (Hybond; GE Healthcare, Piscataway, NJ, USA) and probed with specific antibodies for GR, TRx, TR, and G3PD. Corresponding protein bands were detected and visualized using a SuperSignal West Pico chemiluminescent substrate (Thermoscientific, Rockford, IL, USA).
Protein Concentration Determination and Statistical Analysis
Protein concentration was determined by microanalysis using the BCA method.27 Statistical data were analyzed using the Student's t-test. For all tests, a P value of <0.05 was considered significant. Error bars in all pixel intensity analyses of Western blots are standard deviation values.
Results
Comparison of Expression Levels and Activities of TTase and TRx Systems in Normal and Cataractous Human Lenses
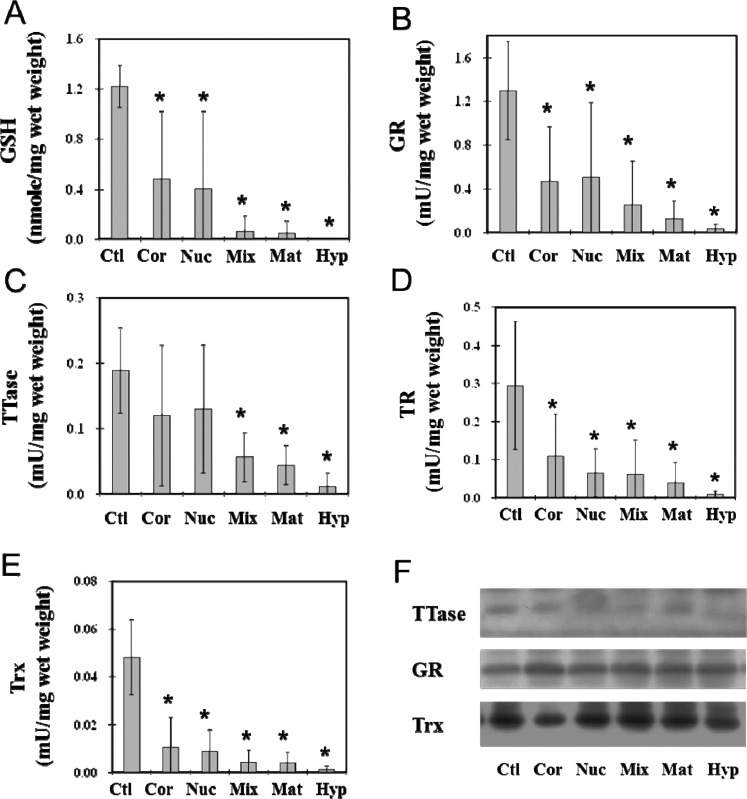
To understand the effects of opacity and types of cataracts on TTase and Trx thiol repair systems in the lens, we analyzed the enzyme activities of GR and TTase in the TTase system and the TR and Trx enzymes in the Trx system, using whole ECCE lenses from cortical, nuclear, mixed, mature, or hypermature cataracts. The nuclear portion of the normal, clear lens covering a region similar to that of the ECCE lens was used as the control. As shown in Figure 1A, GSH levels in both cortical and nuclear cataractous samples suffered extensive loss (nearly 60%) in comparison to that of controls. The mixed, mature, and hypermature cataractous lenses lost almost all their respective GSH pools. The GR, TTase, TR, and Trx enzymes in the TTase and Trx repair systems were all inactivated in each cataract type (Figs. 1B–E). The less severe opacity in cortical and nuclear cataracts retained nearly 40% of the enzyme activity in GR (Fig. 1B), less than 20% to 30% activity for TR (Fig. 1D) and Trx (Fig. 1E), and nearly 60% of the TTase activity (Fig. 1C). In the cataracts with more severe opacity, such as the mixed, mature, and hypermature types, the activities of all four enzymes were depleted to less than 10% of that of the controls, and the loss was proportional to the cataract severity, from the relatively less severe mixed cataract type to the most severe hypermature type. In hypermature cataracts, essentially no enzyme activity could be found for all four enzymes analyzed.
Figure 1.
Comparison between thiol repair enzyme activities in normal nucleus and those in ECCE cataractous lenses. Nuclear portions of nine normal, clear human lenses (Ctl) and whole tissues of human ECCE cataractous lenses of different types of cataracts were used. The cataractous lens samples included 28 cortical (Cor), 26 nuclear (Nuc), 17 mixed cortical and nuclear (Mix), 17 mature cataracts (Mat), and 9 hypermature cataracts (Hyp). Each lens preparation was assayed for GR, TR, TTase, and TRx activities. An aliquot of deproteinized lens homogenate was used for GSH analysis. Data are expressed as means ± SD. *P < 0.05 by comparison with the control. Equal amounts of proteins from the above sample preparation were used for Western blot analysis for the protein content of TTase, GR, and Trx, using specific antibodies for TTase, GR, and Trx. (A) GSH level; (B) GR activity; (C) TTase activity; (D) TR activity; (E) Trx activity; (F) Western blot analysis of TTase, GR, and TRx in human cataractous lenses.
Figure 1F shows the results of Western blot analysis of TTase, GR, and Trx, in which the protein content of each enzyme remained at similar levels between the clear control lens and the ECCE lenses from five cataract types, regardless of the severity of opacity. This indicates that the loss of enzyme activity found in Figure 1 is not related to the protein contents of these enzymes in the cataractous lenses.
Relationship of Thiol Repair System and Loss of Vision Acuity in Human Cataractous Lenses
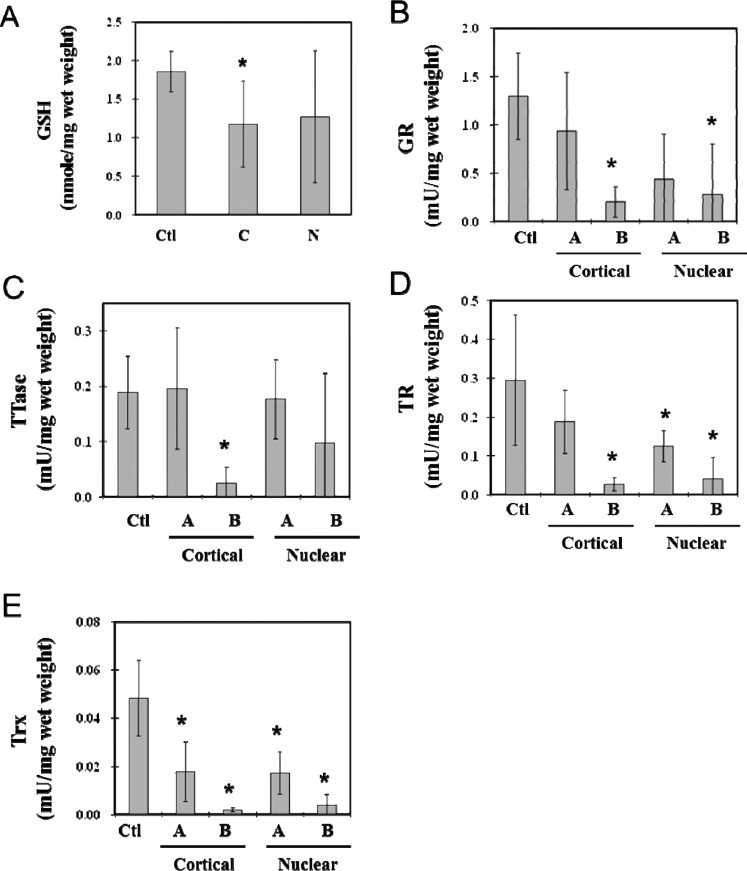
It is well accepted clinically that the severity of cataract in a patient can affect visual acuity. Therefore, before cataract surgery, selected patients with cortical or nuclear cataracts were screened for four categories of visual acuity: optical chart reading, counting fingers, hand motion, and light perception. The first two categories, group A, are considered to have better visual acuity, and the next two, group B, have poor vision, thus, patients have a more severe lens opacity. We examined the biochemical changes in redox-regulating parameters of GSH level and activities of the TTase and Trx enzyme systems in these lenses for any potential relationship to patients' visual acuity. Cortical and nuclear cataract samples were each divided into the subgroups A and B and analyzed in comparison with the clear lens control group.
As shown in Figure 2A, the GSH level was depleted nearly 40% in each of the A groups of cortical and nuclear cataracts, but almost no GSH was detected in the B groups. A similar trend was found in the loss of enzyme activities of the four enzymes (GR, TTase, TR, and Trx) examined. Both of the cataract types retained more respective enzyme activity in the A group than in the B group (Figs. 2B–E), with the exception of TTase, which showed no change in the A group, but an extensive loss was seen in the B group (Fig. 2C).
Figure 2.
Relationship between the status of thiol repair systems and the loss of vision acuity in human lenses with cortical or nuclear opacity. Cortical (n = 17) and nuclear (n = 15) cataractous lenses were selected and divided into two groups based on visual acuity: group A: optical chart reading (R), counting fingers (CF); group B: hand motion (HM), light perception (LP). Samples included cortical A (n = 13), cortical B (n = 4), nuclear A (n = 11), nuclear B (n = 4), and control (n = 8) cataract samples. Each sample homogenate was assayed for GR, TR, TTase, and TRx activities and GSH level. Data are means ± SD. *P < 0.05 by comparison with control. (A) GSH level; (B) GR activity; (C) TTase activity; (D) TR activity; (E) Trx activity.
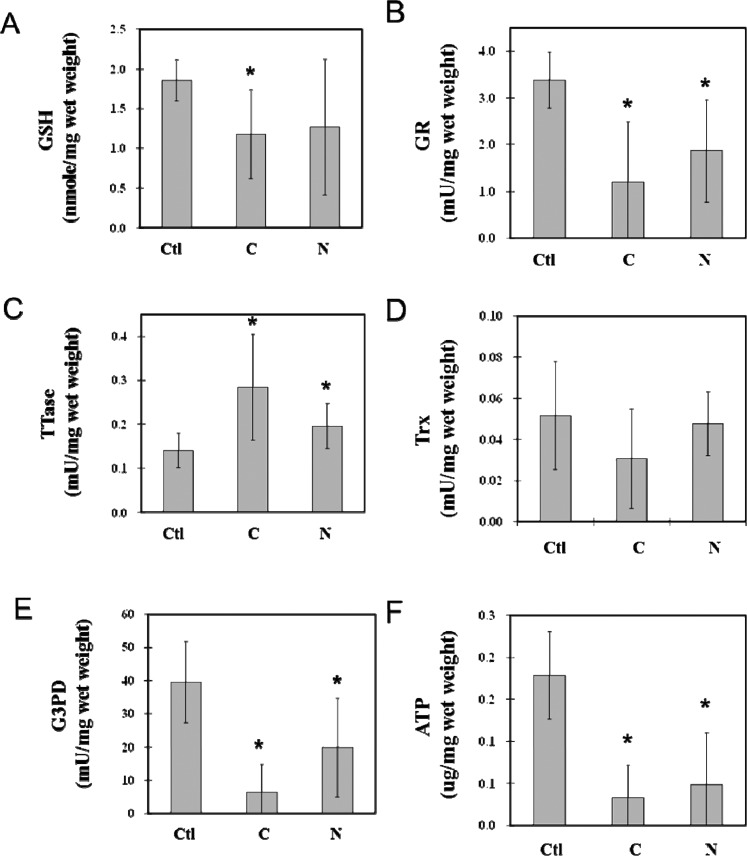
Comparative Studies of G3PD Activity, ATP Level, and Thiol Repair Systems in the Cortical Region of Cataractous Lenses
To determine whether the regional activities of redox-regulating enzymes (TTase and Trx) and the ATP-generating enzyme (G3PD) in the lens are associated with the cause of cataract formation, we selected seven cortical and seven nuclear cataractous lenses with the least opacity and compared their biochemical alterations with those of four normal, clear lenses. The results of the assays of cortical regions are summarized in Figure 3, in which the GSH level was depleted almost to the same degree (~40%) in both the cortical and nuclear cataracts (Fig. 3A). However, more than 80% of the ATP pool was lost in both cataract types (Fig. 3F). Enzyme activity levels for GR, G3PD, and Trx were each depleted more in the cortical cataract than in the nuclear cataract. GR activity was only 30% in the cortical cataract but 40% in the nuclear cataract (Fig. 3B), whereas 55% of Trx activity was retained in the cortical cataract, but no loss was detected in the nuclear cataract (Fig. 3D). G3PD suffered extensive activity loss in the cortical cataract, with only 10% retained, but the nuclear cataract still preserved nearly 40% of G3PD activity (Fig. 3E). Interestingly, TTase activity was actually elevated in both cataract types, with nearly doubled activity found in the cortical cataract (Fig. 3C).
Figure 3.
Comparison between the cortical regional loss in thiol repair enzyme activity and the ATP production of cortical and nuclear cataract lenses. Four normal lenses (Ctl), seven cortical (C), and seven nuclear (N) cataractous lenses with the least opacity were dissected into cortical and nuclear portions. Cortical and nuclear portions of each sample were homogenized and assayed for the levels of ATP and GSH pool, as well as enzyme activities for G3PD, GR, TTase, and Trx. Data are means ± SD. *P < 0.05 by comparison with control. Analysis of the cortical region of the cortical (C) and nuclear (N) cataract lenses was carried out with normal, clear lenses as the control (Ctl). Data include (A) GSH level; (B) GR activity; (C) TTase activity; (D) Trx activity; (E) G3PD activity; and (F) ATP level.
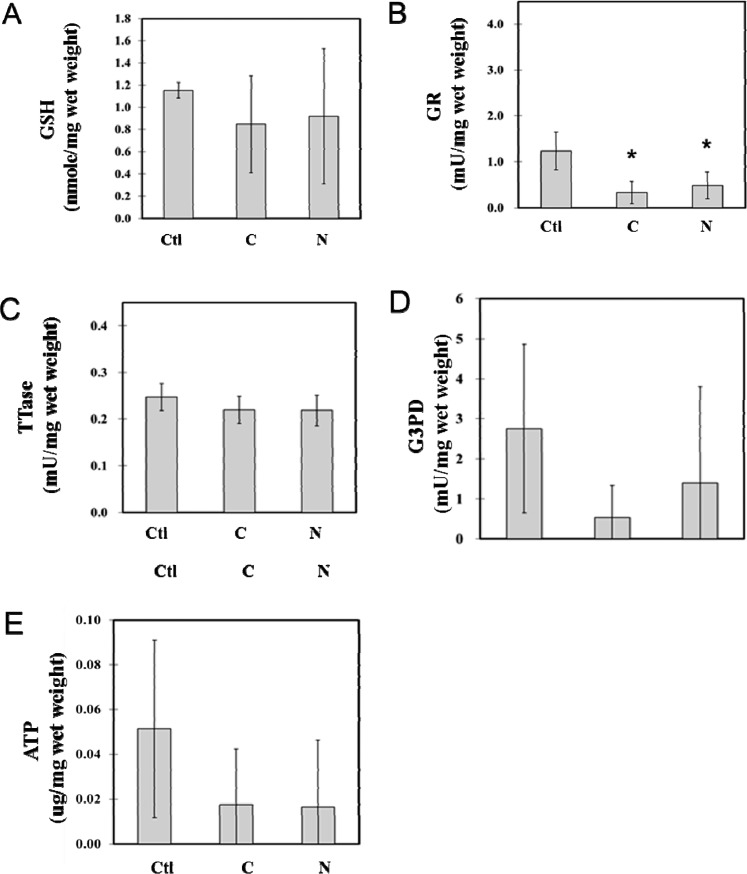
Comparative Studies of G3PD Activity, ATP Level, and Thiol Repair Systems in the Nuclear Region of Cataractous Lenses
A similar trend was found in the nuclear portions of the same cataract samples used in Figure 3. As shown in Figure 4, the GSH level in the nuclear region was slightly lower than that of the cortical region of the same lens (Fig. 3A), but the loss in each of the cataract type in the nucleus was relatively minor (20%) in comparison with that of the cortical region (Fig. 4A). ATP level in the nuclear region was much lower (10%) than that of the cortical region of the same tissue (Fig. 4E), but again, the extent of loss in ATP level in both cataract types was also much less in comparison with that of the cortical samples (70%). The enzymatic activities of GR, TTase in the nuclear region (Figs. 4B, 4C) were in the same range as that in the cortical region (Figs. 3B, 3C) from the same lens tissue, except for G3PD (Fig. 4D), which was only 10% of the cortical region (Fig. 3E). Similar to the results for G3PD and GR activity changes found in the cortical region, activity levels in nuclear tissue showed more loss in cortical cataract type than in the nuclear cataract type, and the damage appeared to be more severe for G3PD (Figs. 4B, 4D). There was no apparent change in TTase activity in this region for both of the cataract types (Fig. 4C).
Figure 4.
Comparison between the nuclear regional loss in TTase thiol repair enzyme activity and the ATP production of cortical and nuclear cataract lenses. The same samples described in Figure 3 were used for analysis of the nuclear portion of the cortical (C) and nuclear (N) cataracts, with normal lens as the control (Ctl). Data include (A) GSH level; (B) GR activity; (C) TTase activity; (D) G3PD activity; and (E) ATP level.
Comparison of TTase, Trx, GR, and G3PD Levels in the Cortex of Cortical and Nuclear Cataracts
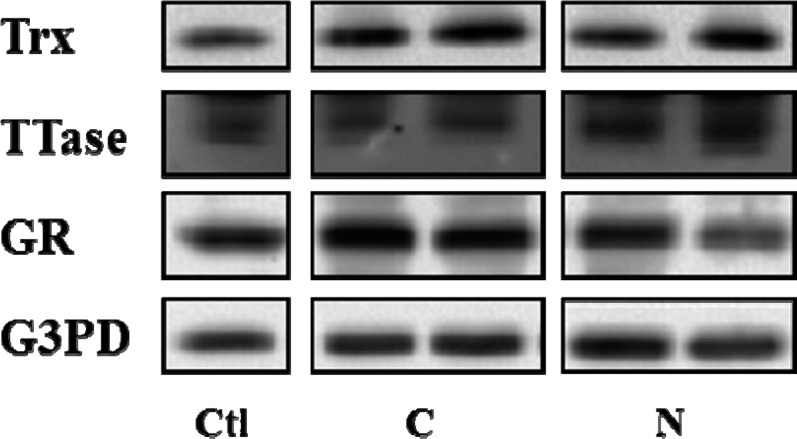
When the enzyme protein level for Trx, TTase, GR, and G3PD were examined using the cortical region of these ECCE lenses, we did not find any apparent difference in either cataract types in comparison with the protein levels in the age-matched clear lenses (Fig. 5). This indicates that the activity loss in the redox-regulating enzymes is unrelated to the protein level found in the tissue.
Figure 5.
Comparison of protein levels of TTase, Trx, GR, and G3PD in the cortex of cortical and nuclear cataracts. Equal amount of proteins from the cortical portion was used for Western blot analysis for the protein levels of Trx, TTase, GR, and G3PD, with respective specific antibodies. C, cortical cataract; N, nuclear cataract; Ctl, control.
Discussion
Status of the redox-regulating systems in the cataract tissues has never been explored before; this study is the first of its kind. It is clearly shown in the results (Fig. 1) that both the TTase and Trx systems were inactivated in proportion to the severity of lens opacity. In fact, the most advanced categories of mature and hypermature cataracts were essentially depleted of all enzymatic activities in these redox systems. GSH, a well-known indicator of the reduced status of a lens, was also severely suppressed in all cataract types, with total loss found in the mixed, mature, and hypermature cataracts. These results confirm the potential role of oxidative stress in the cause of cataract development, as we and others have hypothesized.5,6,21 Previously, we observed that these redox-regulating systems became slowly inactivated as the lens aged.28 It was reported that in human cataract lenses, enzymes for ATP production (G3PD) and GSH recycling (glutathione reductase, or GR) had very low activity levels; however, activities could be restored in vitro when treated with recombinant TTase or Trx in a reaction mixture.29,30 Bhuyan et al.19 reported that expression of Trx was suppressed in cataract lenses, but in our current study, the protein levels of Trx as well as GR and TTase all remained at relatively the same level as that of the age-matched, clear control lenses (Fig. 3C). Due to insufficient tissue homogenate, TR assay was not performed. This indicates that the activity loss in the cataractous lenses was due to posttranslational modification of the enzyme that led to activity loss but not from the capacity of gene expression. This conclusion is very much in agreement with those in published reports that formation of age-related cataracts may be initiated in part from the oxidation-induced protein modification in their thiol groups that progressively advance to protein crosslinking with each other and/or with lens membranes.21,31 Such alteration can lead to formation of large-molecular-weight molecules to a size that would cause light deflection or opacification in the lens.
We speculate that the diminished activity of these thiol-regulating enzymes in the cataractous lenses is not due to insufficient extraction of the soluble proteins from the ECCE tissues. In all, the cataract samples we analyzed all had amounts of soluble proteins that were similar to the amounts in age-matched normal, clear lenses, except for the cortical cataract, which was approximately 20% less (data not shown). In the cortical cataract, the cortex region is known to have more aggregated, high-molecular-weight proteins, thus less soluble proteins can be extracted. However, the aggregated proteins are likely made of lens structural proteins with a very small portion of enzyme molecules. Even if enzymes in the lens were forming aggregates, the amount of thiol-regulating enzymes being conjugated in the large-protein aggregates would likely be negligible. Furthermore, in comparison to the total protein levels of the thiol-regulating enzymes in the cortical region, cortical cataract showed amounts similar to those of nuclear cataracts and normal, clear lenses (Fig. 5). Therefore, we speculate that the loss of thiol-regulating enzyme activity in the cataract lenses, including the cortical cataract, is not an artifact but an alteration closely related to the pathological condition of the lens.
Most interestingly, our studies provide new evidence showing that the oxidative damage protection system can be linked to the status of visual acuity. As shown in Figure 2, GSH and the thiol damage repair enzyme systems are only partially depleted or inactivated when the patients have either cortical or nuclear opacity but still have some vision (group A), but not in the patients who have very poor vision (group B). This suggests that, when the redox-protecting systems are compromised or degenerated, opacity may develop more easily and more quickly so that vision loss becomes a direct consequence. The corroborated positive findings in clinical observation and biochemical results are encouraging and need to be further developed and adopted in the future studies of research of cataract or other degenerative diseases of the eye.
How the redox-regulating system preserves lens transparency is an intriguing question. Previously, we have observed that TTase and Trx are each distributed throughout the lens, with activity in the epithelium that becomes progressively decreased in the cortical and nuclear regions. Bhuyan et al.19 has reported that TR activity is higher in epithelial cells than in fiber cells and both decrease with age. They also reported that the TR activity is quite low in cataractous lenses. We also found that both TR and Trx extensively lose activity in all cataract types. Our findings of lower GR activity in cortical cataract than in nuclear cataract (Figs. 3, 4) are in agreement with that of Ohrloff et al.32 Those authors found that GR activity loss was specifically associated with cortical opacity. Generally, the activity of each of these enzymes we studied was lower in the nucleus than in the cortex. This is to be expected, as the nuclear region is much less active metabolically than that of the cortex region within a lens.
Distribution of both thiol repair systems in the cortical and nuclear regions has never been examined in an opaque lens before. We purposely chose cortical and nuclear cataracts with less opacity for this study because they each may still have some active tissues remaining in the respective regions. It is not surprising to observe that GSH and ATP were lowered in both cortical and nuclear cataracts equally. It is also reasonable to find that most of the activities of the redox-regulating enzymes were suppressed more in a lens with opacity in the cortex than in the nucleus. Because the latter tissue is less active, the oxidation-related damage may not be as obvious as that of the cortical region. However, it is surprising to find such extensively diminished G3PD activity (down to only 10% of the control) and ATP pool (only 17% of the control) in both the cortical and nuclear regions of the same cataract with cortical involvement than that with nuclear involvement. The slow or weakened glycolytic metabolic pathway can be detrimental to the well being of a lens as the lens is mostly an anaerobic organ, which depends on G3PD to generate ATP from glucose metabolism. Therefore, the low ATP produced would affect many important ATP-dependent biological functions, including ion transport, GSH synthesis, and others. With such disparity of G3PD activity and ATP pool in the cortical cataract, it is likely that the cause and biochemical mechanism for cataract formation may differ between cortical and nuclear cataracts.
This study also revealed that loss of TTase activity was much less than that of the other enzymes examined (Fig. 1). In fact, TTase activity was elevated in the cortex of a cortical cataract (Fig. 3). TTase is known to be one of the redox-regulating enzymes whose expression can be upregulated during oxidative stress. We have observed a transient upregulation of TTase and Trx in lens epithelial cells,33–35 as well as in lens organ culture,36 when exposed to H2O2 stress. It is unclear from our current study, why only TTase showed such properties in the cortical region of a lens with cortical cataract.
In summary, as far as we know, this is the first study of the status of redox-regulating enzymes in human ECCE cataractous lenses. We found a correlation between loss of activity and severity of cataract type. Most importantly, the activity loss is closely corroborated with visual acuity. Finally, the extensive inactivation of G3PD activity and loss in its ATP production in cortical cataracts but not in the nuclear cataract suggest a difference in biochemical mechanism that may lead to cataract progression, either in the cortical region or in the nuclear region.
Acknowledgments
Presented in part at the annual meetings of the Association for Research in Vision and Ophthalmology, Fort Lauderdale, Florida, United States, May 2009 and May 2011.
Supported by National Institutes of Health Grant RO1 EY10595 (MFL).
Disclosure: M. Wei, None; K-Y. Xing, None; Y-C. Fan, None; T. Libondi, None; M.F. Lou, None
References
- 1. Jedziniak JA, Kinoshita JH, Yates EM, Benedek GB. On the presence and formation of heavy molecular weight aggregates in human normal and cataractous lenses. Exp Eye Res. 1973; 15: 185–192. [DOI] [PubMed] [Google Scholar]
- 2. Harding JJ, Crabbe MJ. The lens: development, proteins, metabolism and cataract. In: Davson H, ed. The Eye. Vol 1B Orlando, FL: Academic Press; 1984: 207–492. [Google Scholar]
- 3. Andley UP, Liang JJ-N, Lou MF. Biochemical mechanisms of age-related cataract. In: Albert DM, Jakobiec FA, eds. Principle Practices in Ophthalmology. Philadelphia: WB Saunders; 2002: 1428–1449. [Google Scholar]
- 4. Andley UP, Song Z, Mitchell DL. DNA repair and survival in human lens epithelial cells with extended lifespan. Curr Eye Res. 1999; 18: 224–230. [DOI] [PubMed] [Google Scholar]
- 5. Augusteyn RC. Protein modification in cataract: possible oxidative mechanisms. In: Duncan G, ed. Mechanisms of Cataract Formation in the Human Lens. New York: Academic Press; 1981: 72–115. [Google Scholar]
- 6. Spector A. Oxidative stress-induced cataract: mechanism of action. FASEB J. 1995; 9: 1173–1182. [PubMed] [Google Scholar]
- 7. Takemoto L, Boyle D. Increased deamidation of asparagine during human senile cataractogenesis. Mol Vis. 2000; 6: 164–168. [PubMed] [Google Scholar]
- 8. Bhuyan KC, Bhuyan DK, Podos SM. Lipid peroxidation in cataract of the human. Life Sci. 1986; 38: 1463–1471. [DOI] [PubMed] [Google Scholar]
- 9. Harding JJ. Free and protein-bound glutathione in normal and cataractous human lenses. Biochem J. 1970; 117: 957–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Truscott RJ, Augusteyn RC. The state of sulphydryl groups in normal and cataractous human lenses. Exp Eye Res. 1977; 25: 139–148. [DOI] [PubMed] [Google Scholar]
- 11. Anderson EI, Spector A. The state of sulfhydryl groups in normal and cataractous human lens proteins. I. Nuclear region. Exp Eye Res. 1978; 26: 407–417. [DOI] [PubMed] [Google Scholar]
- 12. Lou MF, Dickerson JE Jr, Wolfe JK, Tung W, Chylack L Jr. Correlation of protein-thiol mixed disulfide level with the opacity and brunescence in human lens. Exp Eye Res. 1999; 68: 547–552. [DOI] [PubMed] [Google Scholar]
- 13. Hanson SR, Chen AA, Smith JB, Lou MF. Thiolation of the gammaB-crystallins in intact bovine lens exposed to hydrogen peroxide. J Biol Chem. 1999; 274: 4735–4742. [DOI] [PubMed] [Google Scholar]
- 14. Holmgren A. Glutathione-dependent synthesis of deoxyribonucleotides. Characterization of the enzymatic mechanism of Escherichia coli glutaredoxin. J Biol Chem. 1979; 254: 3672–3678. [PubMed] [Google Scholar]
- 15. Lillig CH, Berndt C, Holmgren A. Glutaredoxin systems. Biochim Biophys Acta. 2008; 1780: 1304–1317. [DOI] [PubMed] [Google Scholar]
- 16. Holmgren A. Enzymatic reduction-oxidation of protein disulfides by thioredoxin. Methods Enzymol. 1984; 107: 295–300. [DOI] [PubMed] [Google Scholar]
- 17. Raghavachari N, Lou MF. Evidence for the presence of thioltransferase in the lens. Exp Eye Res. 1996; 63: 433–441. [DOI] [PubMed] [Google Scholar]
- 18. Yegorova S, Liu A, Lou MF. Human lens thioredoxin: molecular cloning and functional characterization. Invest Ophthalmol Vis Sci. 2003; 44: 3262–3271. [DOI] [PubMed] [Google Scholar]
- 19. Bhuyan KC, Reddy PG, Bhuyan DK. Thioredoxin genes in lens: regulation by oxidative stress. Methods Enzymol. 2002; 347: 421–435. [DOI] [PubMed] [Google Scholar]
- 20. Wu F, Raghavachari N, Wang G-M, Lou MF. Distribution of thioltransferase in ocular tissues. Invest Ophthalmol Vis Sci. 1998; 39: 476–480. [PubMed] [Google Scholar]
- 21. Lou MF. Redox regulation in the lens. Prog Retin Eye Res. 2003; 22: 657–682. [DOI] [PubMed] [Google Scholar]
- 22. Huang QL, Lou MF, Straatsma BR, Horwitz J. Distribution and activity of glutathione-S-transferase in normal human lens and in cataractous human epithelia. Curr Eye Res. 1993; 12: 433–437. [DOI] [PubMed] [Google Scholar]
- 23. Lou MF, Dickerson JE Jr, Garadi R, York BM Jr. Glutathione depletion in the lens of galactosemic and diabetic rats. Exp Eye Res. 1988; 46: 517–530. [DOI] [PubMed] [Google Scholar]
- 24. Straatsma BR, Lightfoot DO, Barke R, Horwitz J. Lens capsule and epithelium in age-related cataract. Am J Ophthalmol. 1991; 112: 183–196. [DOI] [PubMed] [Google Scholar]
- 25. Holmgren A, Bjornstedt M. Thioredoxin and thioredoxin reductase. Methods Enzymol. 1995; 252: 199–208. [DOI] [PubMed] [Google Scholar]
- 26. Bergmeyer HU, Gawehn K, Grassl M. Methods of Enzymatic Analysis. 2nd ed. Weinheim, Germany: Verlag Chenie; 1974; 466–467. [Google Scholar]
- 27. Smith PK, Krohn RI, Hermanson GT, et al. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985; 150: 76–85. [DOI] [PubMed] [Google Scholar]
- 28. Xing K-Y, Lou MF. Effect of age on thioltransferase (glutaredoxin) and thioredoxin systems in the human lens. Invest Ophthalmol Vis Sci. 2010; 51: 6598–6 604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yan H, Harding JJ, Xing K, Lou MF. Revival of glutathione reductase in human cataractous and clear lens extracts by thioredoxin and thioredoxin reductase, in conjunction with alpha-crystallin or thioltransferase. Curr Eye Res. 2007; 32: 455–463. [DOI] [PubMed] [Google Scholar]
- 30. Yan H, Lou MF, Fernando MR, Harding JJ. Thioredoxin, thioredoxin reductase, and alpha-crystallin revive inactivated glyceraldehyde 3-phosphate dehydrogenase in human aged and cataract lens extracts. Mol Vis. 2006; 12: 1153–1159. [PubMed] [Google Scholar]
- 31. Spector A, Roy D. Disulfide-linked high molecular weight protein associated with human cataract. Proc Natl Acad Sci U S A. 1978; 75: 3244–3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ohrloff C, Hockwin O, Olson R, Dickman S. Glutathione peroxidase, glutathione reductase and superoxide dismutase in the aging lens. Curr Eye Res. 1984; 3: 109–115. [DOI] [PubMed] [Google Scholar]
- 33. Krysan K, Lou MF. Human thioltransferase (TTase) gene is controlled by AP-1 and mediated through redox signaling. Invest Ophthalmol Vis Sci. 2003; 43: 1876–1883. [PubMed] [Google Scholar]
- 34. Xing K, Lou MF. The possible physiological function of thioltransferase in cells. FASEB J. 2003; 17: 2088–2090. [DOI] [PubMed] [Google Scholar]
- 35. Yegorova S, Liu A-M, Lou MF. Cloning, expression and characterization of human lens thioredoxin. Invest Ophthalmol Vis Sci. 2003; 44: 3263–3271. [DOI] [PubMed] [Google Scholar]
- 36. Moon S, Fernando MR, Lou MF. Induction of thioltransferase and thioredoxin/thioredoxin reductase systems in cultured pig lenses under oxidative stress. Invest Ophthalmol Vis Sci. 2005; 46: 3783–3789. [DOI] [PubMed] [Google Scholar]