Abstract
Retinal pigment epithelial (RPE) cells secrete vascular endothelial growth factor (VEGF), a cytokine known to promote angiogenesis. Results from RNase protection assays (RPAs) show that RPE from non-diabetic human donors and from adult retinal pigment epithelium-19 (ARPE-19) cells expressed significant bone morphogenetic protein-4 (BMP-4) message. In addition, ARPE-19 cells cultured in high glucose (25 mM), compared to those in physiological glucose (5.5 mM) released significantly more BMP-4 into the conditioned media (CM). However, the effect of BMP-4 on the release of VEGF by ARPE-19 cells has not been studied. Accordingly, ARPE-19 cells were treated with BMP-4 to determine VEGF secretion. BMP-4 and VEGF levels in the CM and cell lysates were measured by enzyme-linked immunosorbent assay (ELISA). Cells treated with exogenous BMP-4 had higher VEGF in the CM and this treatment effect was dose- and time-dependent, while cell lysates had low levels of VEGF. Addition of cycloheximide (CHX) or actinomycin-D (ACT) significantly reduced VEGF secretion from cells treated with BMP-4, suggesting that the BMP-4-induced secretion of VEGF requires new RNA and protein synthesis. Our results suggest that BMP-4 may play a role in the regulation of ocular angiogenesis associated with diabetic retinopathy (DR) by stimulating VEGF release from RPE cells.
Keywords: retinal pigment epithelium, ARPE-19, bone morphogenetic protein, vascular endothelial growth factor, angiogenesis, diabetic retinopathy
The retinal pigment epithelium (RPE), the outermost layer of the retina, plays an important role in the development and function of the eye. Adult retinal pigment epithelium-19 (ARPE-19) cells are from a primary cell line developed from a 19-year-old male eye donor. These cells have been shown to retain some important physiological functions of the eye, suggesting that they are appropriate for in-vitro studies of RPE functions [Dunn et al., 1996]. ARPE-19 cells have also been shown to release vascular endothelial growth factor (VEGF) in response to amino acid deprivation (metabolic stress; [Abcouwer et al., 2002]), 4-hydroxynonenal (oxidative stress; [Ayalasomayajula and Kompella, 2002]), and SA3443, a synthetic cyclic disulfide compound (anti-inflammation; [Muranaka et al., 2005]).
Pfeffer et al. [1994] showed that human RPE cells produce transforming growth factor-beta 2 (TGF-β2) and Pascal et al. [1999] showed that TGF-β2 production can be affected by different glucose concentrations. Three specific isoforms of the transforming growth factor-beta (TGF-β) were found to increase VEGF in primary cultured human retinal pigment epithelium (HRPE) cells [Nagineni et al., 2003] and VEGF is crucial for angiogenesis [Saadeh et al., 1999]. VEGF has also been identified as an initiator of proliferative diabetic retinopathy (DR) and found to be related to neovascularization [Aiello and Wong, 2000].
Bone morphogenetic proteins (BMPs) are members of TGF-β superfamily of proteins. BMPs 2–9 were identified as members of this superfamily based on similar amino acid sequences, while BMPs 10–13 were identified by lowstringency hybridization [Schmitt et al., 1999]. BMPs can trigger many cellular functions including bone formation [Mathura et al., 2000]. In a study using osteoblastic cells, BMP-7 (OP-1) induced VEGF expression by osteoblast cells [Yeh and Lee, 1999], suggesting that BMP-7, acting at least in part via VEGF, stimulates angiogenesis in bones. BMP-4, another of the many BMPs identified, has been shown to be involved in embryonic eye development and formation [Mathura et al., 2000]. Although BMP-4 diminishes after early embryonic development, it is also associated with other physiological processes [Mathura et al., 2000]. BMP-6 and -7 are known to be associated with neuro-protection from ischemia in adult rats [Martinez et al., 2001; Chang et al., 2002]. BMP-4 was found to be neuroprotective of Müller glia in the chicken retina [Fischer et al., 2004], and upregulated in malignant melanoma, promoting cell invasion and migration [Rothhammer et al., 2005]. BMP-2 and TGFb share a signaling pathway of insulin-stimulated differentiation of pancreatic β cells [Yew et al., 2005]. BMP-4 inhibits follicle-stimulating hormone in the pituitary [Faure et al., 2005] and promotes smooth muscle proliferation [Frank et al., 2005]. Furthermore, transcription of the vegf gene in zebrafish embryos was found to be regulated by BMP-4 binding to BMP-activated Smad binding elements [He and Chen, 2005]. Although there are many structural homologies between BMPs and TGF-βs [Schmitt et al., 1999], the effect of glucose on BMP-4 secretion and the possible effects of BMP-4 on VEGF secretion have not been investigated.
In the present study, we have confirmed BMP-4 expression in ARPE-19 cells and in the RPE cells from a non-diabetic human donor. BMP-4 secretion from ARPE-19 cells cultured in high glucose concentration (25 mM), in comparison to physiological level (5.5 mM), was elevated. Treatments of ARPE-19 cells with BMP-4 resulted in an upregulation of VEGF synthesis and secretion. Therefore, we propose that BMP-4, similar to TGF-β, may play a regulatory role in angiogenesis via VEGF synthesis and secretion.
MATERIALS AND METHODS
ARPE-19 Cell Culture
The adult retinal pigmented epithelium cell line, ARPE-19, was purchased from American Type Culture Collection (ATCC, Rockville, MD) and cultured as described by Dunn et al. [1996] except they were revived in 5.5 mM instead of 18 mM glucose and were grown in 5% CO2 instead of 10%. ARPE-19 cells were grown to confluence in T-75 flasks (~3–5 days) in Dulbecco's Modified Eagle Medium/F12-Nutrient Mixture (DMEM, Gibco; F-12 Nutrient Mixture, Gibco; obtained without glucose and then supplemented by adding glucose to the appropriate concentrations) with 15 mM HEPES buffer (Gibco), 10% fetal bovine serum (Gibco), 5.5 mM glucose, 0.35% additional sodium bicarbonate, 2.5 mM l-glutamine, and 1% penicillin/streptomycin at 37°C. Media was changed every other day. At confluence, these cells are undifferentiated (i.e., without melanin) but express several visual proteins [Dunn et al., 1996] including the LRAT enzyme [Trevino et al., 2005].
RPE Tissue From Human Donor Eyes
Human donor eyes were from National Disease Research Interchange. NDRI # 47700 was a non-diabetic Caucasian male, 39 years old. The eyes were frozen within 9 h post-enucleation before being transported to the laboratory. RPE tissues were dissected from donor eyes immediately upon arrival.
Experiments on the Effect of Glucose Concentration on BMP-4 Secretion
Cells at confluence were treated with 0.25% trypsin in 0.02% EDTA and subcultured into 24-well plates with serum-free (SF) media containing 5.5 mM glucose (day 0, passage 25). On day 5, media was changed to 5.5 mM glucose, 25 mM glucose, or 5.5 mM glucose +19.5 mM mannitol. Experimental media was changed every other day two more times (until day 10). Conditioned media (CM) containing proteins secreted during a 24-h treatment period after having been treated for five days was collected and assayed for BMP-4 using enzyme-linked immunosorbent assay (ELISA).
Physiological serum glucose level has been reported in the literature as 4.4–6.0 mM [Guyton, 1982; Norman and Litwack, 1987; Stryer, 1995], therefore, 5.5 mM glucose is an appropriate normal, physiological concentration. Serum glucose levels in type I and II diabetics have been reported to be 17.5–66.0 mM glucose [Guyton, 1982; Norman and Litwack, 1987; Stryer, 1995], therefore, 25 mM glucose is a representative hyperglycemic level.
Experiments on the Effect of Concentration of BMP-4 on VEGF Secretion From ARPE-19 Cells
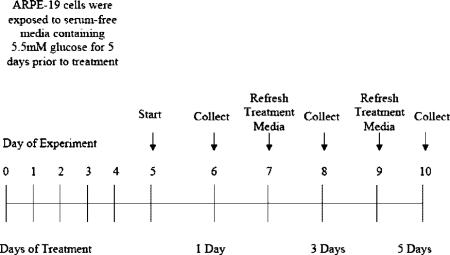
Cells at confluence were treated with 0.25% trypsin in 0.02% EDTA and subcultured in 24-well plates into SF media containing 5.5 mM glucose (day 0, passage 25). On day 5, media containing exogenous BMP-4 at a final concentration of 0.1, 1.0, and 10 ng/ml was added to the cells. Experimental media was changed every other day two more times (until day 10). Cells and CM containing proteins secreted during a 24-h period of treatment were collected on day 10, after 5 days of treatment, and assayed for VEGF using ELISA according to the following experimental paradigm:
To determine VEGF levels in the cell lysate, cells pre-washed with Hank's balanced salt solution (HBSS) were first recovered in 250 μl 0.1% Triton X-100 solution in phosphate buffered saline (PBS) by scraping them from the culture plate. An additional 250 μl of 0.1% Triton X-100 solution in PBS was added to each well to ensure complete cell collection. The total cell suspension recovered from each well (in 500 μl, 0.57 mg protein) was then sonicated for 2 min before ELISA assays.
Experiments on the Effect of Duration of BMP-4 Treatment on VEGF Secretion From ARPE-19 Cells
Cells at confluence were treated with 0.25% trypsin in 0.02% EDTA and subcultured in 24-well plates into SF media containing 5.5 mM glucose (day 0, passage 25). On day 5, media containing exogenous BMP-4 at a final concentration of 10 ng/ml was added to the cells. Experimental media was changed every other day two more times (until day 10). Cells and CM containing proteins secreted during a 24-h period of treatment were collected on days 8 and 10, after 3 and 5 days of treatment, and assayed for VEGF using ELISA according to the experimental paradigm illustrated below.
Experiments on the Effect of Cycloheximide (CHX) and Actinomycin-D (ACT) on VEGF Secretion
Cells at confluence were treated with 0.25% trypsin in 0.02% EDTA and subcultured in 24-well plates into SF media containing 5.5 mM glucose (day 0, passage 25). On day 5, media containing exogenous BMP-4 at a final concentration of 10 ng/ml was added to the cells along with either 3.6 μM CHX or 0.5 μM ACT. Experimental media was changed every other day two more times (until day 10). Cells and CM-containing proteins secreted during a 24-h period of treatment was collected on day 10, after 5 days of treatment, and assayed for VEGF using ELISA.
Enzyme-Linked Immunosorbent Assay
BMP-4 and VEGF were measured according to manufacturer's instructions (R&D Systems). To determine BMP-4 and VEGF levels in CM, triplicate samples of 300-μl aliquots were removed for ELISA assays. The Quantikine human VEGF ELISA is designed to measure total VEGF165 and VEGF121 levels. There are four VEGF isoforms and only VEGF165 and VEGF121 are diffusible proteins that are secreted into the medium.
VEGF165 is the most predominant form of VEGF and is associated with pathologic intravitreous neovascularization [Treins et al., 2001; Ishida et al., 2003; McColm et al., 2004]. The minimum detectable dose of VEGF in the ELISA is typically less than 5 pg/ml. The minimum detectable dose of BMP-4 in the ELISA is typically less than 0.43 pg/ml.
RNase Protection Assay (RPA)
Total RNA was extracted using the TRI reagent following the manufacturer's recommendation (Sigma). RPE cells dissected from donor eyes were homogenized in 1 ml of TRI reagent. Total RNA was used to determine mRNA levels of BMP-4 by RPA. An mBMP RiboQuant RPA kit with multi-probe template sets from BD Phar-Mingen (San Diego, CA) was used according to manufacturer's instructions. L32, a ribosomal protein, was used as an internal control and to evaluate sample loading.
Statistical Analysis
Experimental data were collected in triplicate except where noted. The results are expressed as the mean ± SE. The statistical analysis was performed using Student's paired t-test. A confidence level of P ≤ 0.05 was considered statistically significant.
RESULTS
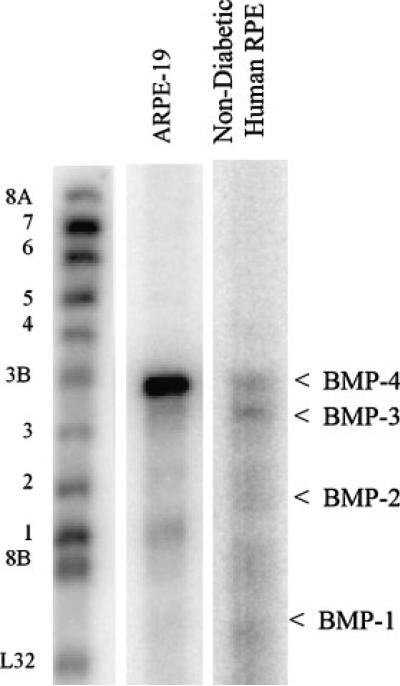
RNase protection assay was used to assess the expression of the members of the BMP family in ARPE-19 cells and in RPE cells from a non-diabetic human donor. Figure 1 shows that BMP-1, -2, -3, and -4 mRNAs were detected in ARPE-19 cells, with BMP-4 being the predominant isoform. Human RPE cells also expressed BMP-4 with a similar level of BMP-3 but with much lower levels of BMP-2 and BMP-1.
Fig. 1.
BMP mRNA expression in ARPE-19 cells and in RPE cells from a non-diabetic human donor. Total RNA was isolated using the TRI reagent. Twenty micrograms of total RNA was used for the measurement of BMP mRNA expression by the RPA. The protected RNA fragments were fractionated on 5% polyacrylamide gels containing 8 M urea and detected by PhosphorImaging. Positions of labeled probes for the different BMPs and a housekeeping gene control (ribosomal protein L32) are marked on the left of the image. The protected fragments are indicated on the right with arrows.
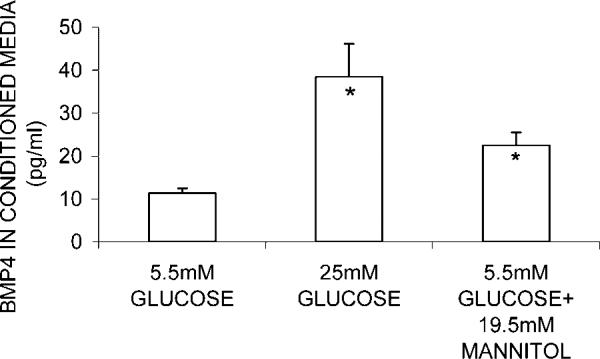
ARPE-19 cells were cultured in 5.5 mM glucose, 25 mM glucose, and 5.5 mM glucose + 19.5 mM mannitol. Figure 2 shows that CM from cells in 25 mM glucose had a significantly higher (237%, P = 0.0035) level of BMP-4 in comparison to those in 5.5 mM glucose. However, CM from cells cultured in 5.5 mM glucose +19.5 mM mannitol (osmotic control) also showed a significant (97.4%, P = 0.016) but somewhat smaller increase of BMP-4 level from the control (5.5 mM glucose). Therefore, the overall increase of BMP-4 protein secretion in cells grown in high glucose (25 mM) may include this increase of BMP-4 due to osmolarity.
Fig. 2.
Effect of glucose treatment on BMP-4 secretion by ARPE-19 cells. Cells were grown to confluence in media containing physiological glucose (5.5 mM) before being harvested and re-seeded into different media for 24 h. After cells were treated for 5 days, CM was collected for ELISA measurement of BMP-4. No change in total cell protein (ranged from 0.48 to 0.66 mg/well) was observed in glucose treatment. Mean and standard error from results of three experiments are indicated in the diagram. In each experiment, five observations were made in each treatment group. *Indicates statistically significant difference between BMP-4 concentration in CM from cells grown in 25 mM glucose and those from cells in 5.5 mM glucose+ 19.5 mM mannitol as compared to those from cells grown in 5 mM glucose, P ≤ 0.05.
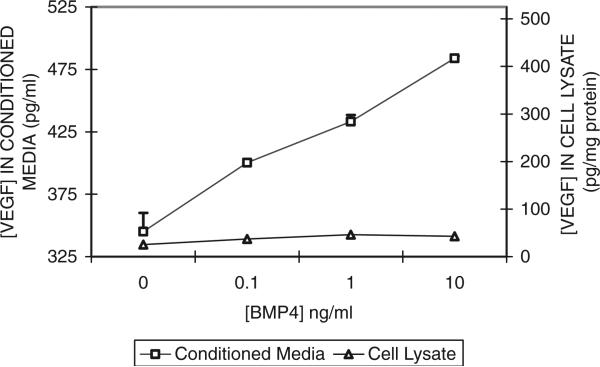
In order to study how exogenous BMP-4 induced VEGF secretion by ARPE-19 cells, different concentrations of exogenous BMP-4 were added to the media of ARPE-19 cells using the schedule described in Materials and Methods. Figure 3 shows the relationship between VEGF levels in the CM from cells cultured in media containing 5.5 mM glucose plus vehicle, 0.1, 1.0, or 10 ng/ml exogenous BMP-4. The level of VEGF increased in proportion to the (log) increase of BMP-4 concentration, suggesting a strong dose-response relationship between exogenous BMP-4 and VEGF secretion by ARPE-19 cells.
Fig. 3.
Effect of exogenous BMP-4 concentration on VEGF secretion by ARPE-19 cells. ARPE-19 cells were grown to confluence in SF media containing 5.5 mM glucose before the addition of exogenous BMP-4 at 0.1, 1.0, or 10 ng/ml. For detailed treatment paradigm, see Materials and Methods. CM and cells were collected after a 24-h period of BMP-4 treatments. VEGF levels in the CM and cell lysate were measured using ELISA. Results of VEGF in the CM are mean and standard error from two representative experiments (with triplicate observations per concentration per experiment). Results of VEGF in the cell lysates were derived from one experiment (with triplicate measurements for each concentration).
VEGF in the cell lysate was also measured using ELISA in order to determine whether BMP-4 caused an increase in the release of previously accumulated VEGF from within the cells. Results in Figure 3 indicate that the cell lysates consistently displayed lower concentrations of VEGF, suggesting that the increased VEGF secretion may not be due to previously accumulated VEGF.
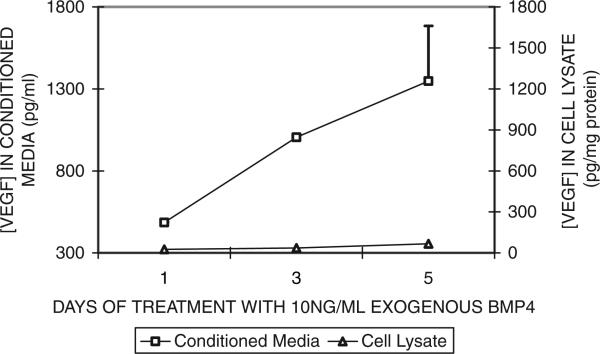
The amount of VEGF in CM of cells treated with BMP-4 also increased with the duration of BMP-4 treatment. Figure 4 shows that at 10 ng/ ml of exogenous BMP-4, levels of VEGF protein in the CM of ARPE-19 increased proportionately with the increased treatment period, indicating a strong time-dependency in the BMP-4 treatment response.
Fig. 4.
Effect of exogenous BMP-4 treatment duration on VEGF secretion by ARPE-19 cells. ARPE-19 cells were grown to confluence in SF media containing 5.5 mM glucose before the addition of exogenous BMP-4 (10 ng/ml). For detailed treatment paradigm, see Materials and Methods. CM was collected from cells after the specified period of BMP-4 treatments. VEGF levels in the CM and cell lysate were measured using ELISA. Results of VEGF in the CM are mean and standard error from two representative experiments (with triplicate observations per time point per experiment). Results of VEGF in the cell lysates were derived from one experiment (with triplicate measurements for each time point).
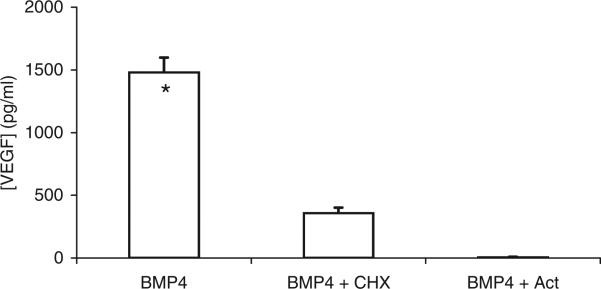
To examine whether BMP-4 induced synthesis of VEGF in ARPE-19 cells, 3.6 μM CHX or 0.5 μM ACT was added to the media of cells treated with 10 ng/ml of BMP-4. Treatment with ACT decreased the amount of VEGF in the CM by 94.6% and the addition of CHX reduced the amount of VEGF in the CM by 58.2% (Fig. 5), and these changes are statistically significant.These results support the suggestion that increased amounts of VEGF released by ARPE-19 cells in response to BMP-4 treatment are due to increased RNA and protein synthesis rather than an increased release of VEGF from cell storage.
Fig. 5.
Effect of CHX and ACT on VEGF secretion by ARPE-19 cells. ARPE-19 cells were grown to confluence in SF media containing 5 mM glucose before the addition of exogenous BMP-4 (10 ng/ml) and CHX (3.6 μM) or ACT (0.5 μM). CM was collected from cells at the 5th day of treatment with BMP-4. VEGF in the CM was assayed using ELISA. One experiment was performed and four observations were made per treatment group. The mean ± SE are indicated. *Indicates significant difference between VEGF concentration in CM without inhibitors compared to those with CHX and with ACT.
DISCUSSION
Diabetic retinopathy is one of the leading causes of blindness in the United States [Burgos et al., 1997]. DR may be caused by increased retinal vascular permeability, new retinal vessel development, and/or loss of retinal capillaries [Aiello and Wong, 2000]. VEGF is considered to be a key angiogenic factor in the neovascularization of proliferative DR [Castellon et al., 2002]. The present study is the first to examine whether BMP-4, a cytokine promoting bone and eye development [Mathura et al., 2000], may enhance VEGF secretion and thus play a role in VEGF-induced angiogenesis.
Our results show that ARPE-19 cells grown in media containing a hyperglycemic level (25 mM) of glucose secrete more BMP-4 protein than those grown in media with a physiological level (5.5 mM) of glucose (Fig. 2). As BMP-4 mRNA is also expressed in both ARPE-19 and human RPE cells (Fig. 1) and it plays a strong role in cell differentiation, it would be of great interest to study whether this elevated BMP-4 level may be involved in the development of DR via VEGF secretion by ocular cells.
Because BMP-4 is a member of the TGF-β family of proteins which affect VEGF expression, we hypothesized that BMP-4 may also affect VEGF synthesis and release. Therefore, we investigated the effects of exogenous BMP-4 on VEGF secretion by ARPE-19 cells. Based on our results shown in Figures 3 and 4, it is clear that BMP-4 increased VEGF secretion by ARPE-19 cells in a dose- and time-dependent manner. Our results on cell lysate suggest that VEGF may be stored at low levels intracellularly, and that the observed BMP-4-induced VEGF secretion may not be due to release of previously stored VEGF in these cells. This conclusion was further substantiated by the results of ARPE-19 treated with BMP-4 and CHX or ACT, which inhibited VEGF synthesis leading to a significantly lower secretion of VEGF (Fig. 5). At present, the transduction pathways of BMP-4-induced VEGF production remain to be established [Nagineni et al., 2003; He and Chen, 2005].
Our experimental results seem different from a recent study, using HRPE cells, in which BMP-4 did not affect VEGF secretion [Nagineni et al., 2003]. It is possible that the ARPE-19 cells used in our study have different gene expression than the HRPE cells in that study. In addition, 10 ng/ml of BMP-4 was used in our study, as compared with a higher concentration of 40 ng/ ml used in the reported study [Nagineni et al., 2003]. Furthermore, in our study, BMP-4 treatment of ARPE-19 cells shows significant time- and dose-dependency of VEGF secretion (Figs. 4, 5), suggesting a strong effect of BMP-4 on these cells. Additional studies will be needed in order to examine why HRPE cells did not respond to BMP-4 treatments.
ACKNOWLEDGMENTS
The authors thank National Disease Research Interchange (NDRI) for supplying human eyes.
Grant sponsor: NIH; Grant numbers: GM08194, GM07761.
Abbreviations used
- BMP
bone morphogenetic protein
- ARPE-19
adult retinal pigment epithelium
- RPA
RNAse protection assay
- CM
conditioned media
- ELISA
enzyme-linked immunosorbent assay
- SF
serum-free
- VEGF
vascular endothelial growth factor
- CHX
cycloheximide
- ACT
actinomycin-D
REFERENCES
- Abcouwer SF, Marjon PL, Loper RK, Vander Jagt DL. Response of VEGF expression to amino acid deprivation and inducers of endoplasmic reticulum stress. Invest Ophthalmol Vis Sci. 2002;43:2791–2798. [PubMed] [Google Scholar]
- Aiello LP, Wong JS. Role of vascular endothelial growth factor in diabetic vascular complications. Kidney Int Suppl. 2000;77:S113–119. doi: 10.1046/j.1523-1755.2000.07718.x. [DOI] [PubMed] [Google Scholar]
- Ayalasomayajula SP, Kompella UB. Induction of vascular endothelial growth factor by 4-hydroxynonenal and its prevention by glutathione precursors in retinal pigment epithelial cells. Eur J Pharmacol. 2002;449:213–220. doi: 10.1016/s0014-2999(02)02043-5. [DOI] [PubMed] [Google Scholar]
- Burgos R, Simo R, Audi L, Mateo C, Mesa J, Garcia-Ramirez M, Carrascosa A. Vitreous levels of vascular endothelial growth factor are not influenced by its serum concentrations in diabetic retinopathy. Diabetologia. 1997;40:1107–1109. doi: 10.1007/s001250050794. [DOI] [PubMed] [Google Scholar]
- Castellon R, Caballero S, Hamdi HK, Atilano SR, Aoki AM, Tarnuzzer RW, Kenney MC, Grant MB, Ljubimov AV. Effects of tenascin-C on normal and diabetic retinal endothelial cells in culture. Invest Ophthalmol Vis Sci. 2002;43:2758–2766. [PubMed] [Google Scholar]
- Chang CF, Morales M, Chou J, Chen HL, Hoffer B, Wang Y. Bone morphogenetic proteins are involved in fetal kidney tissue transplantation-induced neuroprotection in stroke rats. Neuropharmacology. 2002;43:418–426. doi: 10.1016/s0028-3908(02)00092-8. [DOI] [PubMed] [Google Scholar]
- Dunn KC, Aotaki-Keen AE, Putkey FR, Hjelmeland LM. ARPE-19, a human retinal pigment epithelial cell line with differentiated properties. Exp Eye Res. 1996;62:155–169. doi: 10.1006/exer.1996.0020. [DOI] [PubMed] [Google Scholar]
- Faure MO, Nicol L, Fabre S, Fontaine J, Mohoric N, McNeilly A, Taragnat C. BMP-4 inhibits follicle-stimulating hormone secretion in ewe pituitary. J Endocrinol. 2005;186:109–121. doi: 10.1677/joe.1.05988. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Schmidt M, Omar G, Reh TA. BMP4 and CNTF are neuroprotective and suppress damage-induced proliferation of Muller glia in the retina. Mol Cell Neurosci. 2004;27:531–542. doi: 10.1016/j.mcn.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Frank DB, Abtahi A, Yamaguchi DJ, Manning S, Shyr Y, Pozzi A, Baldwin HS, Johnson JE, de Caestecker MP. Bone morphogenetic protein 4 promotes pulmonary vascular remodeling in hypoxic pulmonary hypertension. Circ Res. 2005;97:496–504. doi: 10.1161/01.RES.0000181152.65534.07. [DOI] [PubMed] [Google Scholar]
- Guyton AC. Human physiology and mechanisms of disease. Saunders; Philadelphia: 1982. p. 607. [Google Scholar]
- He C, Chen X. Transcription regulation of the vegf gene by the BMP/Smad pathway in the angioblast of zebrafish embryos. Biochem Biophys Res Commun. 2005;329:324–330. doi: 10.1016/j.bbrc.2005.01.133. [DOI] [PubMed] [Google Scholar]
- Ishida S, Usui T, Yamashiro K, Kaji Y, Amano S, Ogura Y, Hida T, Oguchi Y, Ambati J, Miller JW, Gragoudas ES, Ng YS, D'Amore PA, Shima DT, Adamis AP. VEGF164-mediated inflammation is required for pathological, but not physiological, ischemia-induced retinal neovascularization. J Exp Med. 2003;198:483–489. doi: 10.1084/jem.20022027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez G, Carnazza ML, Di Giacomo C, Sorrenti V, Vanella A. Expression of bone morphogenetic protein-6 and transforming growth factor-beta1 in the rat brain after a mild and reversible ischemic damage. Brain Res. 2001;894:1–11. doi: 10.1016/s0006-8993(00)03140-1. [DOI] [PubMed] [Google Scholar]
- Mathura JR, Jr., Jafari N, Chang JT, Hackett SF, Wahlin KJ, Della NG, Okamoto N, Zack DJ, Campochiaro PA. Bone morphogenetic proteins-2 and -4: Negative growth regulators in adult retinal pigmented epithelium. Invest Ophthalmol Vis Sci. 2000;41:592–600. [PubMed] [Google Scholar]
- McColm JR, Geisen P, Hartnett ME. VEGF isoforms and their expression after a single episode of hypoxia or repeated fluctuations between hyperoxia and hypoxia: Relevance to clinical ROP. Mol Vis. 2004;10:512–520. [PMC free article] [PubMed] [Google Scholar]
- Muranaka K, Yanagi Y, Tamaki Y, Takahashi H, Usui T, Ohashi K, Matsuoka H, Senda T. Suppression of laser-induced choroidal neovascularization by oral administration of SA3443 in mice. FEBS Lett. 2005;579:6084–6088. doi: 10.1016/j.febslet.2005.09.079. [DOI] [PubMed] [Google Scholar]
- Nagineni CN, Samuel W, Nagineni S, Pardhasaradhi K, Wiggert B, Detrick B, Hooks JJ. Transforming growth factor-beta induces expression of vascular endothelial growth factor in human retinal pigment epithelial cells: Involvement of mitogen-activated protein kinases. J Cell Physiol. 2003;197:453–462. doi: 10.1002/jcp.10378. [DOI] [PubMed] [Google Scholar]
- Norman AW, Litwack G. Hormones. Academic Press; Orlando: 1987. p. 264. [Google Scholar]
- Pascal MM, Forrester JV, Knott PM. Glucose-mediated regulation of transforming growth factor-beta (TGF-beta) and TGF-beta receptors in human retinal endothelial cells. Curr Eye Res. 1999;19:162–170. doi: 10.1076/ceyr.19.2.162.5332. [DOI] [PubMed] [Google Scholar]
- Pfeffer BA, Flanders KC, Guerin CJ, Danielpour D, Anderson DH. Transforming growth factor beta 2 is the predominant isoform in the neural retina, retinal pigment epithelium-choroid and vitreous of the monkey eye. Exp Eye Res. 1994;59:323–333. doi: 10.1006/exer.1994.1114. [DOI] [PubMed] [Google Scholar]
- Rothhammer T, Poser I, Soncin F, Bataille F, Moser M, Bosserhoff AK. Bone morphogenic proteins are overexpressed in malignant melanoma and promote cell invasion and migration. Cancer Res. 2005;65:448–456. [PubMed] [Google Scholar]
- Saadeh PB, Mehrara BJ, Steinbrech DS, Dudziak ME, Greenwald JA, Luchs JS, Spector JA, Ueno H, Gittes GK, Longaker MT. Transforming growth factor-beta1 modulates the expression of vascular endothelial growth factor by osteoblasts. Am J Physiol. 1999;277:C628–637. doi: 10.1152/ajpcell.1999.277.4.C628. [DOI] [PubMed] [Google Scholar]
- Schmitt JM, Hwang K, Winn SR, Hollinger JO. Bone morphogenetic proteins: An update on basic biology and clinical relevance. J Orthop Res. 1999;17:269–278. doi: 10.1002/jor.1100170217. [DOI] [PubMed] [Google Scholar]
- Stryer L. Biochemistry. W.H. Freeman; New York: 1995. p. 774. [Google Scholar]
- Treins C, Giorgetti-Peraldi S, Murdaca J, Van Obberghen E. Regulation of vascular endothelial growth factor expression by advanced glycation end products. J Biol Chem. 2001;276:43836–43841. doi: 10.1074/jbc.M106534200. [DOI] [PubMed] [Google Scholar]
- Trevino SG, Schuschereba ST, Bowman PD, Tsin A. Lecithin:retinol acyltransferase in ARPE-19. Exp Eye Res. 2005;80:897–900. doi: 10.1016/j.exer.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Yeh LC, Lee JC. Osteogenic protein-1 increases gene expression of vascular endothelial growth factor in primary cultures of fetal rat calvaria cells. Mol Cell Endocrinol. 1999;153:113–124. doi: 10.1016/s0303-7207(99)00076-3. [DOI] [PubMed] [Google Scholar]
- Yew KH, Hembree M, Prasadan K, Preuett B, McFall C, Benjes C, Crowley A, Sharp S, Tulachan S, Mehta S, Tei E, Gittes G. Cross-talk between bone morphogenetic protein and transforming growth factor-beta signaling is essential for exendin-4-induced insulin-positive differentiation of AR42J cells. J Biol Chem. 2005;280:32209–32217. doi: 10.1074/jbc.M505465200. [DOI] [PubMed] [Google Scholar]