Abstract
The effects of morphine on the morphogenesis and survival of calbindin-D28kimmunoreactive Purkinje cells was studied in organotypic explant cultures isolated from 1- or 7-day-old mouse cerebella. To reduce experimental variability, bilaterally matched pairs of organotypic cultures were used to compare the effects of opiate drug treatment. One explant within each pair was untreated, while the remaining explant was continuously treated for 7 to 10 days with morphine, morphine plus naloxone, or naloxone alone. In explants derived from 1-day-old mice, morphine treatment significantly reduced Purkinje cell dendritic length compared to symmetrically-matched untreated control explants. The concentration of morphine estimated to cause a half-maximal reduction (EC50) in dendritic length was 4.9 × 10−8 M. At higher concentrations (EC50 = 3.6 × 10−6 M), morphine also significantly decreased the number of Purkinje cells in explants from 1-day-old mice compared to untreated explants. Electron microscopy identified increased numbers of degenerating Purkinje cells in explants derived from 1-day-old mice. This showed that high concentrations (10−5 M) of morphine reduced Purkinje cell numbers by decreasing their rate of survival. In explants derived from 7-day-old mice, morphine (10−5 M) neither affected Purkinje cell dendritic length nor cell numbers compared to symmetrically-matched untreated (control) explants. Collectively, these findings suggest that morphine per se, through a direct action on the cerebellum, can affect Purkinje cell differentiation and survival. The results additionally suggest there is a critical period during development when Purkinje cells are especially vulnerable to the effects of morphine.
Keywords: Endogenous opioid systems, Calbindin-D28k, Cerebellar development, Cell death, Drug abuse, Critical period, Neurotoxicity
INTRODUCTION
Endogenous opioid systems are present during development and can modify nervous system maturation (16,21,77,78). During development, endogenous opioid neuropeptides typically act by inhibiting the growth of the nervous system (21,77,78). Opiate drugs such as morphine also affect neural development (9,12,14,16,17,42,52,54,58,62,66,70,74). Presumably opiate drugs disrupt the normal interactions between endogenous opioid peptides and their receptors.
The cerebellum is likely to provide important clues about the developmental role of opiates. Subpopulations of developing cerebellar neural cells express the opioid mRNA and/or peptide products (22,46,61,79) and opioid receptors (34,41,65,73). Cerebellar morphogenesis can be modified by manipulating endogenous opioid systems (21,23,73,77) or opiate drugs (74-76). Methadone (70) and/or morphine (23) can inhibit the outgrowth from cerebellar explants. The reduction in outgrowth results largely from the reduced growth of neurons (19) and/or glia (23).
Purkinje cells are especially important instruments for opioid action in the cerebellum. Not only is the rate of their dendritic growth affected by endogenous opioids in vivo (21), but subpopulations of developing Purkinje cells themselves express proenkephalin mRNA (46,61) and/or contain enkephalin immunoreactivity (79). Purkinje cells are important in cerebellar organization both during development (60) and in the adult (47). Purkinje cells are the first neuronal type to be generated and to migrate into the immature cerebellar cortex in rodents (28,43), and their axons provide the only efferent path from the cerebellar cortex (47).
A central question is to what extent do opiates per se affect neuronal maturation? Opiates are reported to affect neuronal development through both direct (14,15,23,70) and indirect mechanisms (23,35,36). Yet, confounding effects make it difficult to study how opiates intrinsically affect cellular growth. This is especially true when assessing the effects of opiates in the offspring of opiate dependent mothers. Psychosocial and physical problems are associated with opiate abuse, as well as concurrent multi-drug use, and opiate abstinence in newborns. These confounding effects make it difficult to assess the cellular mechanisms by which opiates by themselves affect neuronal development (7,12,32,33,71). To address this problem, opiate-dependent changes in Purkinje cell morphogenesis were examined in bilaterally matched pairs of organotypic cultures exposed to morphine for 7 to 10 days. The findings suggest that opiates can affect Purkinje cell differentiation and survival at certain critical times during cerebellar development. The results may have implications about opiate drug use during pregnancy or during early childhood.
MATERIALS AND METHODS
Organotypic Culture
Organotypic explant cultures were derived from the cerebella of a total of thirty-six, 1- or 7-day-old male ICR mice (Harlan Sprague-Dawley, Indianapolis, IN). Because there are regional differences in the rate of growth and in opioid expression by Purkinje cells within the rodent cerebellum (28,46), the precise bilateral matching of explants (i.e., the “homologous- or mirror-pair” paradigm) developed by Toran-Allerand (63,64) was used to assess the experimental effects of opiates in this culture model (23,63,64). Tissue dissection was guided by existing anatomical divisions within the cerebellum (37). Entire cerebella from 1-day-old mice were divided into rostral and caudal portions at the primary fissure. The larger caudal tissue fragment was then subdivided at the secondary fissure, except laterally where the intercrural fissure served as a guide (37). In cerebella derived from 7-day-old mice, explants were taken from the lateral portions of crus I and II, i.e., between the posterior superior fissure and the ansoparamedian fissure (37), and from the vermal portions of lobules VI, VII and VIII. Symmetric-paired tissue sections from 1- and 7-dayold mice were cut in the parasagittal plane from the right and left halves of the cerebellum using a micro-surgery scalpel (4 mm blade) and a dissecting microscope at magnifications of 8-35x. Bilaterally paired explants were trimmed until they were precisely matched. Only explant pairs that were precisely matched in size and topography were used, others were discarded (see below). Matched explant-pairs were positioned flat against the collagen substrate surface so the pial surface was at the peripheral border of the explant (19,23).
Explants were maintained in organotypic culture in 18.6 % donor horse serum-containing nutrient media in a rigorously controlled, sterile-environment as described before (22,23). Explants were grown on 22-mm diameter glass or ACLAR plastic coverslips inserted into 12-well (22 mm diameter) plastic tissue culture chambers (22,23). Explants derived from 1-day-old mice were treated with opiates for 7-10 days, because prior studies showed that Purkinje cell development in 10-day-old rats are affected by opioid manipulation during the first 10 postnatal days (21). Explants were treated continuously for 7 to 10 days with media alone (controls), media containing morphine sulfate (Sigma), morphine sulfate plus 3-fold greater concentrations of naloxone (DuPont, Wilmington, DE), or naloxone alone. The medium was changed twice weekly.
Calbindin-D28k Immunocytochemistry
After 7-10 days in vitro, explants were fixed for 30-60 min. in ice-cold Zamboni's fixative containing 3% paraformaldehyde. To allow penetration of the immunocytochemical reagents, explants were permeabilized by sequential treatment in 50% ethanol in 0.1 M PBS (pH 7.4) (30 min.), 70% ethanol in 0.1 M PBS (30 min.), and 50% ethanol in 0.1 M PBS (30 min.) followed by 3 x 5 min. rinses in 0.1 M PBS before addition of the primary antibody (23) as modified from Jaeger et al. (29). Organotypic explant cultures were immunostained for calbindin-D28k, a marker for Purkinje cells in adult (11) and developing (39,59) rodents. Primary monoclonal antibodies against calbindin-D28k (Sigma, St. Louis, MO) were diluted 1:2000 (w/v) in pH 7.4 phosphate buffered saline (PBS) containing 0.1% Triton-X 100 and 1% crystalline grade bovine serum albumin (BSA; Calbiochem, CA). Permeabilized tissue was incubated with primary antibodies for 48 h at 4°C on an orbital shaker at 40-60 rpm. Anti-calbindin-D28k monoclonal antibodies were detected using a biotin-avidin-peroxidase detection system (Vectastain ABC kit, Vector Laboratories, Burlingame, CA). Tissue was incubated overnight with the biotinylated anti-mouse second antibody at 4°C (Vectastain ABC kit) followed by incubation in avidin-conjugated peroxidase for 1.5 h at room temperature. Explants were reacted in diaminobenzidine (DAB) plus H2O2 as directed (Vectastain ABC kit), except that the duration of the DAB reaction was adjusted to optimize the signal-to-noise ratio based on direct observation of the reaction using an inverted microscope. Incubations and rinses were performed on an orbital shaker at 40-60 rpm. Calbindin-D28k immunoreactivity was not seen when primary antibodies were excluded from the reaction.
Dendritic Length and Cell Numbers
Multiple explants from individual mice were randomly distributed across experimental groups. Each value for dendritic length or cell numbers is the mean determination of multiple cells and explants from at least n = 6 mice. To determine dendritic length, an equal numbers (typically 6-12) of randomly chosen Purkinje cells were sampled from each explant within a matched explant pair (at least 6 explant pairs per group). Total dendritic length was determined using a calibrated eyepiece reticle with concentric circles as previously described (21), except that measurements were made using a long working-distance 100x oil immersion objective and differential interference contrast optics. To determine cell numbers, all Purkinje cells in each explant (at least 6 explant pairs per group) were counted using an Olympus Vanox microscope at a magnification of 40x aided with a 10 × 10 square-lattice eyepiece reticle. In studies demonstrating antagonist (naloxone) reversibility of morphine (10−5 M morphine and/or 3 × 10−5 M naloxone) effects on the length of dendrites and the number of calbindin-D28k-immunoreactive Purkinje cells, individual explants were randomly sampled without knowledge of the treatment group, or matched pair, an explant belonged (blind study). The concentration-dependent effects of morphine were assessed using sampling procedures and statistical analyses as previously described (19).
Statistical Analysis
The two-tailed, paired Student's t test (Statistica; StatSoft, Tulsa, OK) was used to compare dendritic length and cell numbers within homologous untreated (control) and opiate-treated explant pairs. Values (mean ± SEM) for treated explants are reported as a percentage of their matched pair controls. Additional determinations were made in explants derived from 1-day-old mice where opiates affected Purkinje cell numbers and dendritic length. Non-linear, least squares regression analysis (GraphPad Inplot, GraphPad Software) was performed to estimate the concentration of morphine that would cause a half-maximal reduction in cell numbers and dendritic length. Explant pairs from single mice were distributed across experimental groups (for example, control versus morphine, control versus morphine plus naloxone, control versus naloxone). For dendritic length, 8-12 Purkinje cells were randomly sampled from each control and treated explant within a matched explant pair. Purkinje cell dendritic lengths and numbers were determined for each experimental group from matched explant pairs taken from n = 6 to 12 mice.
Ultrastructural Observations
Cerebellar explants were grown on collagen-coated ACLAR plastic coverslips. After 7-10 days in culture, explants were fixed in situ (on their coverslips) for 8 h at room temperature in 4% paraformaldehyde (v/v), 2% glutaraldehyde (v/v) in Sorenson's phosphate buffer, pH 7.2 and transferred into ice-cold fixative for an additional 3.5 h. After fixation, coverslips with explants were rinsed 3 times (20 min per rinse) in ice-cold, phosphate buffered saline, postfixed for 1 h in 1% OsO4 in 0.1 M phosphate buffer at 4°C, dehydrated in graded methanol:water and propylene oxide, and embedded in epoxy resin. After the epoxy was polymerized, ACLAR coverslips were peeled away from the epoxy-embedded explant. The explants were sectioned on a LKB ultramicrotome and examined using a Hitachi H-7000 transmission electron microscope at 75 kV.
Verifying that explant pairs are matched
Explant-pairs that were not equal, that is symmetrical in size, shape and regional topography, were discarded during the dissection. To further verify that matched explant pairs were homologous, explant dimensions, as well as Purkinje cell numbers, were compared in matched explant pairs. Explant area was measured morphometrically as described before (23). Explant thickness was estimated by measuring the distance traveled by the fine-focusing adjustment (z axis) from the collagen substrate surface to the uppermost surface of the explant.
RESULTS
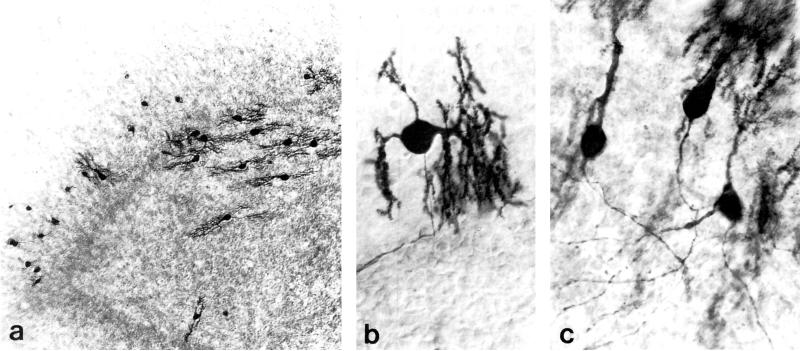
Purkinje cells in explants derived from 1 or 7-day-old mice displayed extensive dendritic development after 7-10 days in organotypic culture in the present study (Fig. 1) as previously reported (6,26,29). Calbindin-D28k antibodies immunostained Purkinje cells in their entirety, including the soma, dendrites and axon (Fig. 1). In cerebellar explant cultures, calbindin-D28k was selective for Purkinje cells. Morphine reportedly decreases calbindin-D28k immunoreactivity within some Purkinje cells in vivo (18), but the decreased immunoreactivity apparently does not occur in the deafferented cerebellum (R. Harlan, personal communication). Morphine did not affect the pattern or intensity of calbindin-D28k immunoreactivity within organotypic explant cultures in the present study.
FIG. 1.
Calbindin-D28k-immunoreactive Purkinje cells in cerebellar organotypic explant cultures. Bright-field photomicrographs of explants derived from 1 and 7-day-old mice at 7 to 10 days in vitro. (a). Dendritic size sometimes differed among Purkinje cells within the same explant (derived from a 1-day-old mouse). (b) Calbindin-D28k-immunoreactivity permitted visualization of Purkinje cell perikarya, dendrites and axons (explant derived from 1-day-old mouse). (c) Three relatively well differentiated Purkinje cells in an explant derived from a 7-day-old mouse. (a) 100 ×; (b & c) 480 ×.
Within matched pairs, explant area and thickness typically did not vary more than 5%. Purkinje cell numbers also were similar. For example, Purkinje cell numbers differed less than 10% in explant pairs where one explant was untreated (control), while the other explant was given a treatment having minimal effects (such as concurrent morphine and naloxone). Moreover, when untreated explants derived from 1-day-old mice (Figs. 2 & 3) are compared to their paired homologs treated concurrently with morphine and naloxone, the number of Purkinje cells is highly correlated (r = 0.798; P < 0.007). Collectively, this indicates that Purkinje cell numbers are similar within bilateral-matched explants, and suggests that the mirror-explant paradigm (63,64) is a valid model for examining the effects of opiates on Purkinje cell development.
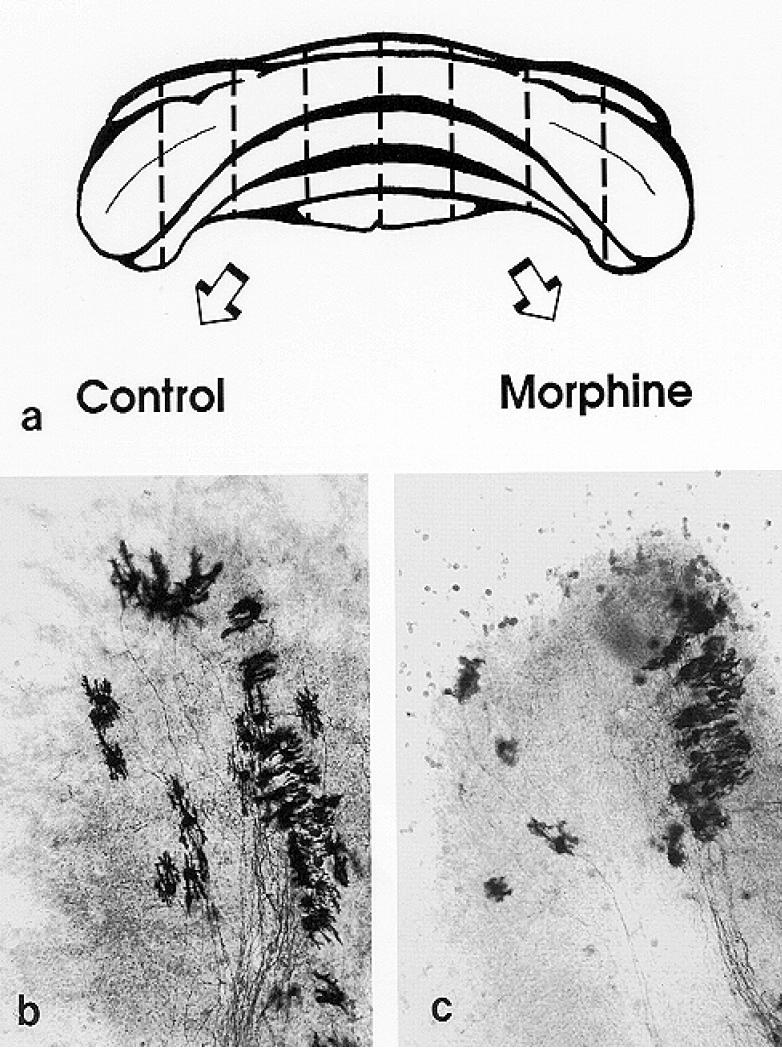
FIG. 2.
Bilaterally-matched explant pairs were grown in the presence and absence of morphine. (a). Schematic drawing of the cerebellum from a 1-day-old mouse illustrating the “homologous- or mirror-pair” paradigm (63,64) used to assess the experimental effects of opiates. Tissue dissection was guided by existing anatomical divisions within the cerebellum. Cerebella from 1-day-old mice were divided into rostral and caudal portions at the primary fissure, and further divided into parasagittally-oriented explant cultures using additional cerebellar landmarks. Cerebella from 7-day-old mice are larger and are subdivided using different anatomical landmarks for dissection (see text). (b & c). Bilaterally-matched explant pairs have similar size, shape, and cytoarchitecture which is maintained in organotypic culture. For example, Purkinje cells in the untreated control explants (b) had larger dendrites compared to their matched-pair counterparts that were treated with 10-6 M morphine (c) at 7 to 10 days in vitro. (b & c) 220 ×.
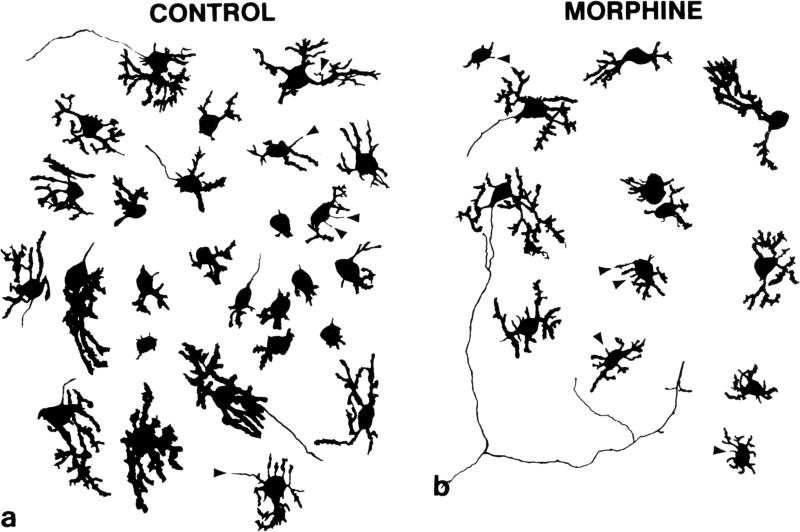
FIG. 3.
Composite camera lucida drawings of calbindin-D28k-immunoreactive Purkinje cells illustrating the effects of high concentrations (10−5 M) of morphine on Purkinje cell numbers. Ccompared to untreated controls (a), continuous exposure to morphine (b) for 7 to 10 days in vitro caused concentration-dependent reductions in Purkinje cell numbers and dendritic length in cultures derived from 1-day-old mice (see Figs. 4 & 5). Numerous filopodial processes (arrowheads) were seen extending from the cell body or dendrites of calbindin-D28k-immunoreactive Purkinje cells. Filopodia are slender, thread-like processes of consistent diameter that transiently appear on growing neurons. Some filopodia terminate in a growth cones. (a & b) 320 ×.
Morphine Reduced Purkinje Cell Dendritic Length
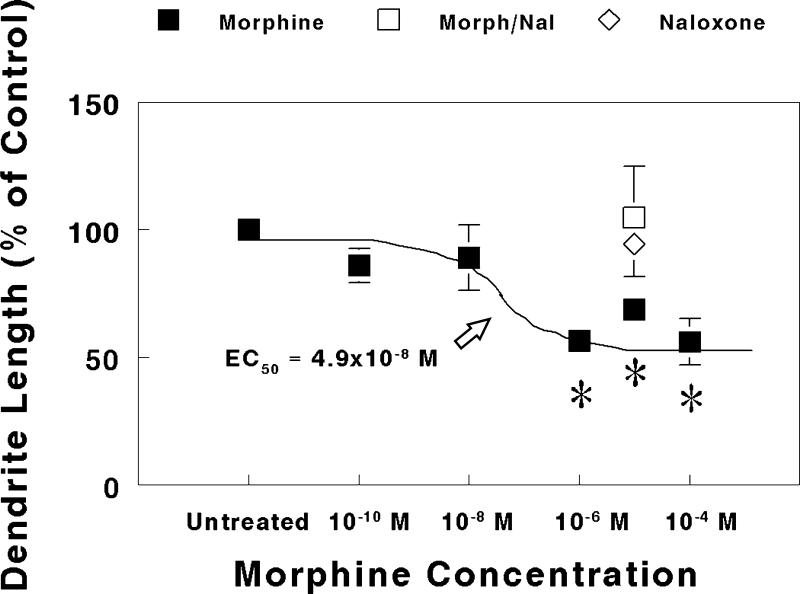
In explant cultures taken from 1-day-old mice, continuous exposure to morphine for 7 to 10 days resulted in a significant concentration-dependent reduction in Purkinje cell dendritic length (Fig. 4). At 10−6 M or greater concentrations, morphine caused a significant decrease in dendritic length compared to matched-pair controls. The mean dendritic length was 94 ± 6 μm for Purkinje cells in untreated explants. The concentration of morphine causing a half maximal reduction in dendritic length was estimated using non-linear regression to be 4.9 × 10−8 M (Fig. 4). Moreover, the reduction in dendritic length caused by 10−5 M morphine was completely prevented by concomitant treatment with 3 × 10−5 M naloxone, while 3 × 10−5 M naloxone treatment alone had no effect (Fig. 4). Numerous filopodial processes were seen extending from the cell body or dendrites of calbindin-D28k-immunoreactive Purkinje cells (Fig. 3). Filopodia are slender, thread-like processes of consistent diameter that appear on growing neurons. Filopodia may terminate in growth cones. Filopodia may or may not develop into dendrites, and were not included in measurements of dendritic length.
FIG. 4.
Effect of increasing concentrations of morphine on Purkinje cell dendritic length in explants derived from 1-day-old mice. Purkinje cell dendritic length was measured and compared in bilaterally-matched paired-explant cultures. One explant within a matched-pair was given nutrient medium alone, while the other explant was continuously treated with morphine, morphine plus naloxone, or naloxone alone for 7 to 10 days in vitro. Note that concurrent 3 × 10−5 M naloxone plus 10−5 M morphine (Morph/Nal) treatment prevented morphine-induced deficits in dendritic length, while 3 × 10−5 M naloxone alone had no effect. Eight to 16 Purkinje cells were randomly sampled from each explant in a matched-pair. Determinations were made from explant pairs sampled from 6 mice for each drug concentration or treatment group. *P < 0.05 vs. untreated control.
In explant cultures derived from 7-day-old mice, Purkinje cell dendritic length was unaffected by continuous morphine treatment. The mean dendritic length for Purkinje cells was 435 ± 87 μm in untreated explants and 513 ± 117 μm in morphine-treated explants.
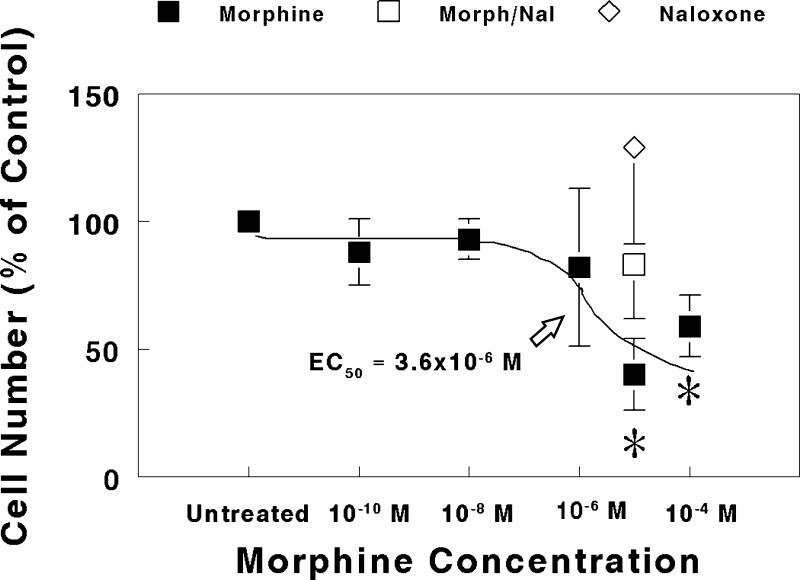
High Concentrations of Morphine Reduced Purkinje Cell Numbers
In explants derived from 1-day-old mice, high concentrations of morphine (10−5 or 10−4 M) significantly reduced Purkinje cell numbers at 7-10 days in vitro (Fig. 5). The mean number of Purkinje cells was 127 ± 29 in untreated explants. The concentration of morphine estimated to cause a half maximal reduction in Purkinje cell numbers was 3.6 × 10−6 M. The reduction in Purkinje cell numbers caused by 10−5 M morphine was prevented by co-administering 3 × 10−5 M naloxone, while 3 × 10−5 M naloxone alone had no effect on Purkinje cell numbers.
FIG. 5.
Effect of increasing concentrations of morphine on Purkinje cell numbers in explants derived from 1-day-old mice. Purkinje cells were counted and their numbers compared in bilaterally-matched paired-explant cultures. One explant within a matched-pair was given nutrient medium alone, while the other explant was continuously treated with morphine, morphine plus naloxone, or naloxone alone for 7 to 10 days in vitro. Note that the reduction in cell numbers caused by 10-5 M morphine was prevented by concurrent treatment with 3 × 10−5 M naloxone (Morph/Nal), while naloxone (3 × 10−5 M) alone had no effect compared to untreated controls. Each determination was made from multiple matched-explant pairs sampled from 12 mice. *P < 0.05 vs. untreated control.
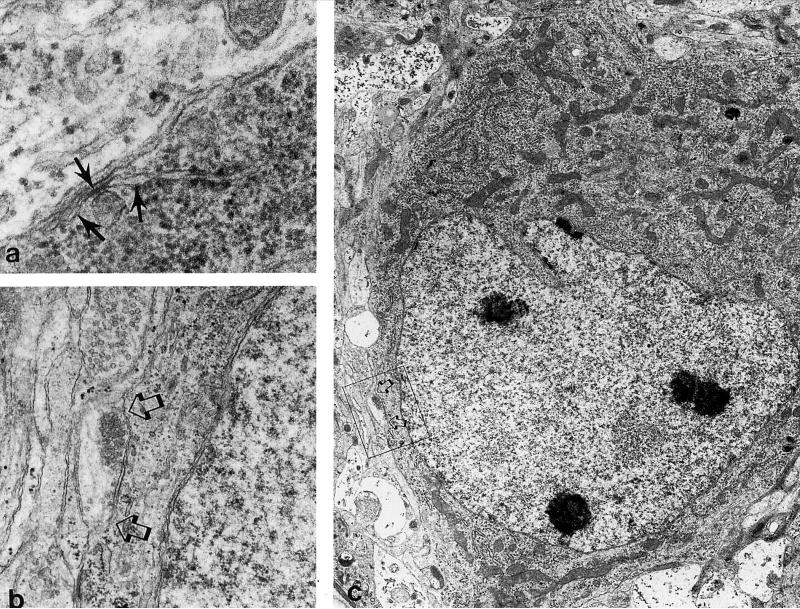
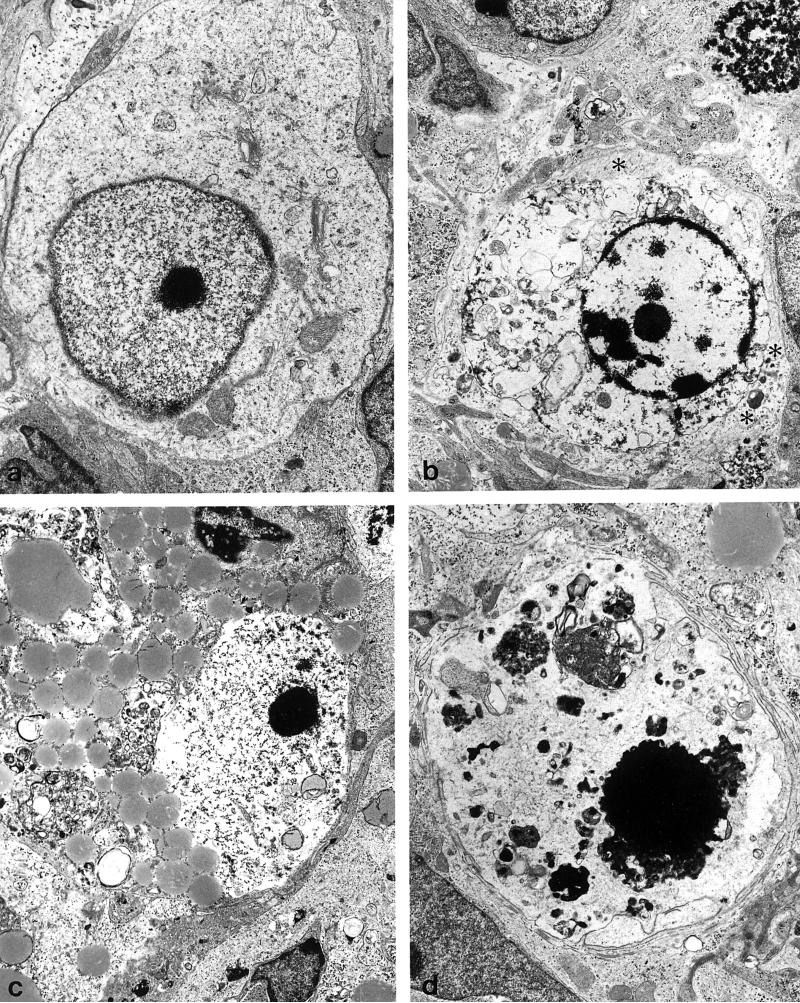
Electron microscopy revealed that compared to untreated explants, degenerating cells were frequently present in 10−5 M morphine treated explants at 7-days-in vitro (Figs. 6,7). When the degenerating cells could be identified with some certainty, they appeared to be small EGL-derived neurons or larger neurons resembling Purkinje cells. EGL cells and their progeny have a distinct size (about 7 μm in diameter) (19,22), ultrastructural morphology (47,55), and distribution within explants (19). The somata of Purkinje cells were often larger than other cell types, and have hypolemmal cisternae and axosomatic synapses. Some cells also had perisomatic processes that are characteristic of the stellate stage of Purkinje cell development. Pathophysiological changes within the cytoplasm included vacuolar degeneration, and the accumulation of both lipids and glycogen. With more progressive degeneration, there was a near-complete dissolution of organelles within the cytoplasm, as well as nuclear pyknosis. Many severely degenerated cells that resembled Purkinje cells could not be identified with certainty due to a loss of ultrastructural features (Fig. 7). Few degenerating astrocytes were seen. Often, the processes of astroglia were seen surrounding phagocytosed cellular debris or degenerating cells (Fig. 7). Astrocytes were identified by clusters of intermediate filaments, as well as a relative abundance of ribosomes and glycogen, in the cytoplasm (47,55).
FIG. 6.
Electron micrographs of Purkinje cells in morphine-treated explants. Many Purkinje cells in morphine-treated explants show no morphologic signs of degeneration. (a) Subsurface or hypolemmal cisternae (arrows) are a characteristic cytoplasmic feature of Purkinje cells (47,55) (64,500×). (b) Detail of an axosomatic synapse (arrows in b & c) present on the Purkinje cell in (c) (19,100×). (c) Developing Purkinje cell (5,460×).
FIG. 7.
Degenerating cells in morphine-treated explants at 7 to 10 days in vitro. With progressive degeneration and loss of cellular morphology, it can be difficult to identify Purkinje cells with certainty. (a) A Purkinje cell with a deficit in cytoplasmic organelles and abnormally dense marginal heterochromatin (8,770×). (b) A dying cell surrounded by astrocytic processes containing numerous intermediate filaments (asterisks) (6,150×). (c) A degenerating cell with accumulated lipid and glycogen in the cytoplasm and partial destruction of the nuclear membrane. This cell had remnents of hypolemmal cisternae (not shown) (5,390×). (d) A large pyknotic cell positioned within the Purkinje cell layer that is difficult to identify with certainty (6,750×).
In explants derived from 7-day-old mice, Purkinje cell numbers were unaffected by continuous treatment with 10−5 M morphine treatment for 7 to 10 days in vitro. There were 40 ± 14 Purkinje cells in untreated explants and 34 ± 12 Purkinje cells in morphine-treated explants.
DISCUSSION
The results show that morphine per se can inhibit Purkinje cell dendritic development and cell survival through a direct action on the developing cerebellum. However, the effects of morphine on Purkinje cell morphogenesis are complex. Dendritic development is more sensitive to opiates than cell survival. Ten to 100-fold greater concentrations of morphine were needed to reduce Purkinje cell numbers than were needed to inhibit dendritic development. Therefore, within a range of concentrations, morphine may affect the dendritic size without affecting cell viability. Perhaps, morphine affects cell differentiation and survival by separate mechanisms. Alternatively, there may be a threshold where dendritic loss results in a failure to thrive. Morphine's efficacy was also age-dependent additionally suggesting there is a critical period when Purkinje cell dendritic development and survival is vulnerable to this narcotic. These findings collectively suggest that the type and severity of the developmental defect depends on the amount, duration, and timing of opiate exposure.
Dendritic Development
Endogenous opioids and/or opiate drugs (presumably by acting through similar mechanisms) regulate dendritic growth in neurons in the cerebral cortex, hippocampus, and cerebellum during normal development (21). However, based on in vivo studies alone, it is unclear whether opioids per se directly affect dendritic growth. Opiates influence many non-opioid systems that may indirectly affect dendritic development (review (24)), including nutrition (69), many endocrine systems (for example, vassopressin, insulin, prolactin, growth, thyroid and adrenocorticotrophic hormones (5,48,56)), and respiration (57). Alterations in nutrition (72) and thyroid hormone (10), for example, can in turn affect dendritic differentiation. Psychosocial factors can also affect dendritic development (13,68), suggesting that the psychosocial problems associated with opiate drug abuse (7,12,32,33,71) could indirectly affect dendrogenesis. Despite the potential importance of indirect opiate effects, our findings show that opiates, through a direct action on the cerebellum, can intrinsically affect the morphogenesis of Purkinje cell dendrites.
Cell Survival
Purkinje cells are postmitotic before birth in mice (28,43). Therefore, a reduction in the number of calbindin-D28k-immunoreactive Purkinje cells in morphine-treated explants cannot be explained by a reduced rate of cell proliferation, but instead indicates a decreased rate of survival. Ultrastructural studies provided more direct evidence for decreased survival, since degenerating Purkinje cells were seen as a result of morphine treatment. Similar neurotoxic effects of high concentrations (10−4 M) of methadone have been seen in organotypic cultures of dorsal root ganglia (15). Yet, the presence of dying cells in organotypic cultures needs to be interpreted with caution. Some neurons die from trauma at explantation (55), and poor diffusion of nutrients can occur in explants that are too large causing central necrosis. Avoiding the above caveats, stabile neural populations (with few degenerating neurons) are found in untreated cultures at 7 to 10 days in vitro. This is consistent with other investigations of the mouse cerebellum in organotypic culture after 1 to 2 weeks in culture (26,55). In general, we find that the conditions present in organotypic culture, unlike dissociated culture, supports the long-term (greater than 3-6 months) growth and survival of neurons. Thus, despite the potential for artifactual loss of cells in culture, our findings suggest that high concentrations of morphine are toxic to Purkinje cells in vitro.
It is uncertain whether reductions in Purkinje cell dendritic length or cell numbers occur in the offspring of opiate-dependent mothers. Our study focused on events occurring in the postnatal mouse cerebellum. Corresponding stages of Purkinje cell development occur prenatally in humans (3). Moreover, it is difficult to extrapolate what might occur in utero or early childhood in humans based on in vitro findings. Nevertheless, the present culture model accurately mimics the effects of opioids seen in the dendrites of preweaning rats (21) using relatively low (~ 5 × 10−8 M) concentrations of morphine. It is more difficult to extrapolate the effects of high concentrations of morphine in culture to what might occur in vivo. As mentioned, opiates are not reported to induce Purkinje cell loss. The maturation of Purkinje cell nucleoli and ribosomes are delayed in rats whose mothers were exposed to morphine during pregnancy and/or lactation (44,45). While this suggests delayed maturation, morphine toxicity was not noted. In these studies, however, the actual dosage of morphine to the pups is difficult to estimate since morphine was administered both intraperitoneally, as well as orally ad libidum (44,45). Methadone treatment significantly reduces the area of the molecular layer, an indirect measurement of Purkinje cell dendritic size (76). Daily administration (from birth) of 1 mg/kg of the opioid antagonist naltrexone, a dosage reported to exacerbate endogenous opioid actions, significantly decreases Purkinje cell dendritic area in 21-day-old rats (21). Treatment with 1 mg/kg/day of naltrexone during the first 3 postnatal weeks did not affect Purkinje cell content, width, and packing density within the pyramis (77), despite significant decreases in both the area of the pyramis (31% of controls) and in cerebellar weight (77). Furthermore, it is uncertain whether 10−6 to 10−5 M concentrations of morphine occur in the fetal nervous system in utero--even with chronic drug abuse. Yet, even at high concentrations, morphine affected Purkinje cells in explants derived from 1-day-old mice, but had no effect on Purkinje cells derived from 7-day-old mice in the present study. This age-related selectivity suggests a developmentally regulated mechanism, rather than generalized neurotoxicity, although prolonged exposure or higher concentrations of morphine might cause neurotoxicity in Purkinje cells derived from 7-day-old mice. Additional studies need to assess the effects of morphine on Purkinje cell survival in vivo, as well as the potential mechanisms (e.g, apoptosis, necrosis) by which opiates induce cell loss.
In humans, therapeutic concentrations of morphine in serum are normally 65 ± 80 ng/ml during surgical anesthesia (31). Analgesic concentrations of morphine can range from 16 to 364 ng/ml in the treatment of cancer (31,49). However, opiate addicts are reported to tolerate opiate blood levels that are 2.5 to 100-fold greater than therapeutic concentrations (30). For example, blood levels of methadone, a congener of morphine with similar potency, can be as high as 6 × 10−6 M at 2 h post ingestion in addicts on methadone maintenance programs (15). Opiates such as morphine or methadone readily cross the placenta (38) and can cause neurobehavioral impairment in offspring (12). Morphine concentrations in some regions of the nervous system can approach 1500 ng/g tissue weight following a 10 mg/kg i.v. dosage in normal rats (4). In organotypic cultures, higher concentrations of substances (63,64), including morphine (23), are often required to elicit a response comparable to that seen in vivo. This is, in part, because bioactive substances reach cell targets without a vascular system and without normal endothelial transport mechanisms. Therefore, despite difficulties comparing drug dosages between experimental paradigms and species, it is possible to speculate that sustained high opiate levels could affect Purkinje cell development in children.
Cellular Site of Action
It is uncertain whether morphine is acting directly on Purkinje cells themselves. Morphine also can modify the rate of DNA synthesis in EGL neuroblasts (granule, basket and stellate cell precursors) (19), as well as the growth of astrocytes (23), in cerebellar explants. Granule cell afferent synapses may affect the growth and orientation of Purkinje cell dendrites (1,2,8,50,51,53). Conversely, Purkinje cells may affect the genesis of granule cells (27). Thus, the development of Purkinje cells and granule cells may be, in part, interdependent, and it is not surprising that both Purkinje and EGL cells respond to opioids in vitro. However, because of the developmental interdependence of these two cell types, it is not possible to determine the primary target for opiate action in the present study.
Opioid binding sites have been demonstrated biochemically (41,65,73) and autoradiographically within the developing cerebellum (34). In autoradiographic studies of the developing human, [3H]-naloxone binding is localized over the EGL, but is not associated with the Purkinje cell layer (34). Binding studies suggest that EGL cells, but not Purkinje cells, express opioid receptors and are a cellular target for opioid action.
A reduction in Purkinje cell dendritic length and/or cell numbers may affect cerebellar function. Alterations in the linear dimensions and topography of Purkinje cell dendrites are proposed to result in changes in stimulus integration (53). A relationship between the structural complexity of dendrites and behavior has been hypothesized (25), and more recently proposed as a mechanism by which endogenous opioids influence neurobehavioral development (21). The timing and amount of opiate exposure may be essential in determining developmental outcome. Defining critical periods of vulnerability will be especially important toward understanding the developmental mechanisms of opiate action, as well as for potentially preventing and/or correcting developmental abnormalities resulting from opiate drug abuse.
ACKNOWLEDGMENTS
We thank Dr. Dan Goldowitz and Dr. Sylvia Christakos for advice on Purkinje cell markers, Dr. Jim Hyde for comments on experimental design, and Dr. Marilyn Duncan for the use of the non-linear regression software (GraphPad Inplot, GraphPad Software) on her computer. Appreciation is extended to Ms. Mary Gail Engel and Cynthia Kinzey for technical help. Preliminary aspects of these studies have been reported elsewhere (20,24). Supported by the National Institute on Drug Abuse (DA 06204).
REFERENCES
- 1.ALTMAN J. Experimental reorganization of the cerebellar cortex. VII. Effects of late X-irradiation schedules that interfere with cell acquisition after stellate cells are formed. J.Comp.Neurol. 1976;165:65–76. doi: 10.1002/cne.901650106. [DOI] [PubMed] [Google Scholar]
- 2.BAPTISTA CA, HATTEN ME, BLAZESKI R, MASON CA. Cell-cell interactions influence survival and differentiation of purified Purkinje cells in vitro. Neuron. 1994;12:243–260. doi: 10.1016/0896-6273(94)90268-2. [DOI] [PubMed] [Google Scholar]
- 3.BAYER SA, ALTMAN J, RUSSO RJ, ZHANG X. Timetables of neurogenesis in the human brain based on experimentally determined patterns in the rat. Neurotoxicol. 1993;14:83–144. [PubMed] [Google Scholar]
- 4.BHARGAVA HN, VILLAR VM, RAHMANI NH, LARSEN AK. Time course of the distribution of morphine in brain regions, spinal cord and serum following intravenous injection to rats of differing ages. Pharmacology. 1993;47:13–23. doi: 10.1159/000139073. [DOI] [PubMed] [Google Scholar]
- 5.BRUNI JF, VANVUGT D, MARSHALL S, MEITES J. Effects of naloxone, morphine and methionine enkephalin on serum prolactin, luteinizing hormone, follicle stimulating hormone, thyroid stimulating hormone and growth hormone. Life Sci. 1977;21:461. doi: 10.1016/0024-3205(77)90528-8. [DOI] [PubMed] [Google Scholar]
- 6.CALVET M-C, CALVET J. Computer-assisted analysis of the developing Purkinje neuron. I. Effects of the age of the animal at the moment of explantation on the subsequent dendritic development in organotypic cultures. Brain Res. 1988;462:321–333. doi: 10.1016/0006-8993(88)90560-4. [DOI] [PubMed] [Google Scholar]
- 7.CASADO-FLORES J, BANO-RODRIGO A, ROMERO E. Social and medical problems in children of heroin-addicted parents: A study of 75 patients. Am.J.Dis.Child. 1990;144:977–979. doi: 10.1001/archpedi.1990.02150330037017. [DOI] [PubMed] [Google Scholar]
- 8.COHEN-CORY S, DREYFUS CF, BLACK IB. NGF and excitatory neurotransmitters regulate survival and morphogenesis of cultured cerebellar Purkinje cells. J.Neurosci. 1991;11:462–471. doi: 10.1523/JNEUROSCI.11-02-00462.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.CROFFORD M, SMITH AA. Growth retardation in young mice treated with dl-methadone. Science. 1973;181:947–949. doi: 10.1126/science.181.4103.947. [DOI] [PubMed] [Google Scholar]
- 10.DUSSAULT JH, RUEL J. Thyroid hormones and brain development. Ann.Rev.Physiol. 1987;49:321–334. doi: 10.1146/annurev.ph.49.030187.001541. [DOI] [PubMed] [Google Scholar]
- 11.FELDMAN SC, CHRISTAKOS S. Vitamin D-dependent calcium binding protein in rat brain: biochemical and immunocytochemical characterization. Endocrinology. 1983;112:290–302. doi: 10.1210/endo-112-1-290. [DOI] [PubMed] [Google Scholar]
- 12.FINNEGAN LP. The effects of narcotics and alcohol on pregnancy and the newborn. Ann.N.Y.Acad.Sci. 1981;362:136–157. doi: 10.1111/j.1749-6632.1981.tb12802.x. [DOI] [PubMed] [Google Scholar]
- 13.FLOETER MK, GREENOUGH WT. Cerebellar plasticity: Modification of Purkinje cell structure by differential rearing in monkeys. Science. 1979;206:227–229. doi: 10.1126/science.113873. [DOI] [PubMed] [Google Scholar]
- 14.GHADIRIAN A. A tissue culture study of morphine dependence on the mammalian CNS. Can.Psychiatr.Ass.J. 1969;14:607–615. doi: 10.1177/070674376901400609. [DOI] [PubMed] [Google Scholar]
- 15.GRODE ML, MURRAY MR. Effects of methadone-HCl on dorsal root ganglia in organotypic culture. Exp.Neurol. 1973;40:68–81. doi: 10.1016/0014-4886(73)90124-6. [DOI] [PubMed] [Google Scholar]
- 16.HAMMER RP, JR., HAUSER KF. Consequences of early exposure to opioids on cell proliferation and neuronal morphogenesis. In: MILLER M, editor. Development of the Central Nervous System: Effects of Alcohol and Opiates. Wiley-Liss; New York: 1992. pp. 319–339. [Google Scholar]
- 17.HAMMER RP, JR., RICALDE AA, SEATRIZ JV. Effects of opiates on brain development. Neurotoxicol. 1989;10:475–484. [PubMed] [Google Scholar]
- 18.HARLAN RE, GILSTER J, GARCIA MM. Morphine decreases calbindin D28K immunoreactivity in cerebellar Purkinje neurons. Soc.Neurosci.Abstr. 1993;19:484. [PubMed] [Google Scholar]
- 19.HAUSER KF. Morphine regulates DNA synthesis in cerebellar neuroblasts in vitro. Dev. Brain Res. 1992;70:291–297. doi: 10.1016/0165-3806(92)90210-n. [DOI] [PubMed] [Google Scholar]
- 20.HAUSER KF. High concentrations of morphine inhibit Purkinje cell morphogenesis and survival in organotypic cultures of the mouse cerebellum. Soc.Neurosci.Abstr. 1992;18:769. [Google Scholar]
- 21.HAUSER KF, MCLAUGHLIN PJ, ZAGON IS. Endogenous opioid systems and the regulation of dendritic growth and spine formation. J.Comp.Neurol. 1989;281:13–22. doi: 10.1002/cne.902810103. [DOI] [PubMed] [Google Scholar]
- 22.HAUSER KF, OSBORNE JG, STIENE-MARTIN A, MELNER MH. Cellular localization of proenkephalin mRNA and enkephalin peptide products in cultured astrocytes. Brain Res. 1990;522:347–353. doi: 10.1016/0006-8993(90)91482-v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.HAUSER KF, STIENE-MARTIN A. Characterization of opioid-dependent glial development in dissociated and organotypic cultures of mouse central nervous system: Critical periods and target specificity. Dev.Brain Res. 1991;62:245–255. doi: 10.1016/0165-3806(91)90172-f. [DOI] [PubMed] [Google Scholar]
- 24.HAUSER KF, STIENE-MARTIN A. Opiates and the regulation of nervous system development: Evidence from in vitro studies. In: HAMMER RP JR., editor. Neurobiology of Opiates. CRC Press; Boca Raton, Florida.: 1992. pp. 23–61. [Google Scholar]
- 25.HEBB DO. The Organization of Behavior. Wiley; New York: 1949. [Google Scholar]
- 26.HENDELMAN WJ, AGGERWAL AS. The Purkinje neuron: I. A Golgi study of its development in the mouse and in culture. J.Comp.Neurol. 1980;193:1063–1079. doi: 10.1002/cne.901930417. [DOI] [PubMed] [Google Scholar]
- 27.HILLMAN DE, CHEN S. Regulation of granule cell number by a predetermined number of Purkinje cells in development. Dev.Brain Res. 1989;45:137–147. doi: 10.1016/0165-3806(89)90015-1. [DOI] [PubMed] [Google Scholar]
- 28.INOUYE M, MARAKAMI U. Temporal and spatial patterns of Purkinje cell formation in the mouse cerebellum. J.Comp.Neurol. 1980;194:499–503. doi: 10.1002/cne.901940302. [DOI] [PubMed] [Google Scholar]
- 29.JAEGER CM, KAPOOR R, LLINÁS R. Cytology and organization of rat cerebellar organ cultures. Neuroscience. 1988;26:509–538. doi: 10.1016/0306-4522(88)90165-0. [DOI] [PubMed] [Google Scholar]
- 30.JAFFE JH. Drug addition and drug abuse. In: GILMAN AG, GOODMAN LS, RALL TW, MURAD F, editors. The Pharmacological Basis of Therapeutics. MacMillan Pub. Co.; New York: 1985. pp. 532–581. [Google Scholar]
- 31.JAFFE JH, MARTIN WR. Opioid analgesics and antagonists. In: GILMAN AG, GOODMAN LS, RALL TW, MURAD F, editors. The Pharmacological Basis of Therapeutics. MacMillan Publishing Co.; New York: 1985. pp. 491–531. [Google Scholar]
- 32.KALTENBACH KA, FINNEGAN LP. Prenatal narcotic exposure: perinatal and developmental effects. Neurotoxicology. 1989;10:597–604. [PubMed] [Google Scholar]
- 33.KANDALL SR, GARTNER LM. Late presentation of drug withdrawal symptoms in newborns. Amer.J.Dis.Child. 1974;127:58–61. doi: 10.1001/archpedi.1974.02110200060008. [DOI] [PubMed] [Google Scholar]
- 34.KINNEY HC, WHITE WF. Opioid receptors localize to the external granular cell layer of the developing human cerebellum. Neuroscience. 1991;45:13–21. doi: 10.1016/0306-4522(91)90099-a. [DOI] [PubMed] [Google Scholar]
- 35.KORNBLUM HI, LOUGHLIN SE, LESLIE FM. Effects of morphine on DNA synthesis in neonatal rat brain. Dev.Brain Res. 1987;31:45–52. doi: 10.1016/0165-3806(87)90081-2. [DOI] [PubMed] [Google Scholar]
- 36.KUHN C, IGNAR D, WINDH R. Methodological Issues in Controlled Studies on the Effects of Prenatal Exposure to Drug Abuse. NIDA Res. Monog. Vol. 114. USPHS, NIDA; Rockville, MD.: 1991. Endocrine function as a target of perinatal drug effects: methodological issues. pp. 206–232. [PubMed] [Google Scholar]
- 37.LARSELL O. The morphogenesis and adult pattern of the lobules and fissures of the cerebellum of the white rat. J.Comp.Neurol. 1952;97:281–356. doi: 10.1002/cne.900970204. [DOI] [PubMed] [Google Scholar]
- 38.LEE CC, CHIANG CN. Maternal-fetal transfer of abused substances: pharmaocokinetic and pharmacodynamic data. In: CHIANG CN, LEE CC, editors. Prenatal Drug Exposure: Kinetics and Dynamics. NIDA Res. Monogr. Vol. 60. USPHS, NIDA; Rockville, MD: 1985. pp. 110–147. [PubMed] [Google Scholar]
- 39.LEGRAND C, THOMASSET M, PARKES CO, CLAVEL MC, RABIE A. Calcium-binding protein in the developing rat cerebellum. An immunocytochemical study. Cell Tissue Res. 1983;33:389–402. doi: 10.1007/BF00238305. [DOI] [PubMed] [Google Scholar]
- 40.LORBER BA, FREITAG SK, BARTOLOME JV. Effects of beta-endorphin on DNA synthesis in brain regions of preweanling rats. Brain Res. 1990;531:329–332. doi: 10.1016/0006-8993(90)90795-d. [DOI] [PubMed] [Google Scholar]
- 41.LOUGHLIN SE, AN A, LESLIE FM. Opioid receptor changes in weaver mouse striatum. Brain Res. 1992;585:149–155. doi: 10.1016/0006-8993(92)91200-x. [DOI] [PubMed] [Google Scholar]
- 42.MERINEY SD, GRAY DB, PILAR G. Morphine-induced delay of normal cell death in the avian ciliary ganglion. Science. 1985;228:1451–1453. doi: 10.1126/science.2990029. [DOI] [PubMed] [Google Scholar]
- 43.MIALE IL, SIDMAN RL. An autoradiographic analysis of histogenesis in the mouse cerebellum. Exp.Neurol. 1961;4:277–296. doi: 10.1016/0014-4886(61)90055-3. [DOI] [PubMed] [Google Scholar]
- 44.NOSAL G. Influence exercée sur la progéniture de rat par l'exposition à la morphine maternelle. I. Exposition foetale et neuronogénèse dans le cervelet néonatal. Acta Neurol. Latinoamer. 1979;25:27–46. [PubMed] [Google Scholar]
- 45.NOSAL G. Influence exercée sur la progéniture de rat par l'exposition a la morphine maternelle. II. Exposition pré- et postnatale et maturation cérébelleuse chez le raton. Acta Neurol. Latinoamer. 1979;25:151–166. [PubMed] [Google Scholar]
- 46.OSBORNE JG, KINDY MS, SPRUCE BA, HAUSER KF. Ontogeny of proenkephalin mRNA and enkephalin peptide expression in the cerebellar cortex of the rat: Spatial and temporal patterns of expression follow maturational gradients in the external granular layer and in Purkinje cells. Dev.Brain Res. 1993;76:1–12. doi: 10.1016/0165-3806(93)90117-s. [DOI] [PubMed] [Google Scholar]
- 47.PALAY SL, CHAN-PALAY V. The Cerebellar Cortex, Cytology and Organization. Springer-Verlag; New York: 1974. [Google Scholar]
- 48.PFEIFFER A, HERZ A. Endocrine actions of opioids. Int.Rev.Neurobiol. 1985;26:1–83. doi: 10.1016/s0074-7742(08)60072-0. [DOI] [PubMed] [Google Scholar]
- 49.PORTENOY RK, KHAN E, LAYMAN M, LAPIN J, MALKIN MG, FOLEY KM, THALER HT, CERBONE DJ, INTURRISI CE. Chronic morphine therapy for cancer pain: Plasma and cerebrospinal fluid morphine and morphine-6-glucuronide concentrations. Neurology. 1991;41:1457–1461. doi: 10.1212/wnl.41.9.1457. [DOI] [PubMed] [Google Scholar]
- 50.RAKIC P. Genetic and epigenetic control of local neuronal circuits in the mammalian central nervous system. In: SCHMITT FO, WORDEN FG, editors. The Neurosciences—Fourth Study Program. The MIT Press; Cambridge, MA.: 1979. pp. 109–127. [Google Scholar]
- 51.RAMÓN Y, CAJAL S. Studies on Vertebrate Neurogenesis. Charles C. Thomas; Springfield, Il.: 1960. [Google Scholar]
- 52.RICALDE AA, HAMMER RP., JR. Perinatal opiate treatment delays growth of cortical dendrites. Neurosci.Lett. 1991;115:137–143. doi: 10.1016/0304-3940(90)90444-e. [DOI] [PubMed] [Google Scholar]
- 53.SADLER M, BERRY M. Link-vertex analysis of Purkinje cell dendritic trees from the murine cerebellum. Brain Res. 1988;474:130–146. doi: 10.1016/0006-8993(88)90676-2. [DOI] [PubMed] [Google Scholar]
- 54.SAKELLARIDIS N, MANGOURA D, VERNADAKIS A. Effects of opiates on the growth of neuron-enriched cultures from chick embryonic brain. Int.J.Dev.Neurosci. 1986;4:293–302. doi: 10.1016/0736-5748(86)90066-3. [DOI] [PubMed] [Google Scholar]
- 55.SEIL FJ, HERNDON RM, TIEKOTTER AND, BLANK NK. Reorganization of organotypic cultures of mouse cerebellum exposed to cytosine arabinoside: a timed ultrastructural study. J.Comp.Neurol. 1991;313:193–212. doi: 10.1002/cne.903130202. [DOI] [PubMed] [Google Scholar]
- 56.SHARP B, MORLEY JE, CARLSON HE, GORDON J, BRIGGS J, MELMED S, HERSHMAN JM. The role of opiates and endogenous opioid peptides in the regulation of TSH secretion. Brain Res. 1981;219:335. doi: 10.1016/0006-8993(81)90296-1. [DOI] [PubMed] [Google Scholar]
- 57.SHOOK JE, WATKINS WD, CAMPORESI EM. Differential roles of opioid receptors in respiration, respiratory disease, and opiate-induced respiratory depression. Am.Rev.Respir.Dis. 1990;142:895–909. doi: 10.1164/ajrccm/142.4.895. [DOI] [PubMed] [Google Scholar]
- 58.SLOTKIN T. Perinatal exposure to methadone: how do early biochemical alterations cause neurofunctional disturbances? Prog.Brain Res. 1988;73:265–279. doi: 10.1016/S0079-6123(08)60509-9. [DOI] [PubMed] [Google Scholar]
- 59.SOTELO C, ALVARADO-MALLART RM. Embryonic and adult neurons interact to allow Purkinje cell replacement in mutant cerebellum. Nature. 1987;327:421–423. doi: 10.1038/327421a0. [DOI] [PubMed] [Google Scholar]
- 60.SOTELO C, WASSEF M. Cerebellar Purkinje cell development: afferent organization and Purkinje cell heterogeneity. Phil.Trans.R.Soc.Lond.B. 1991;331:307–313. doi: 10.1098/rstb.1991.0022. [DOI] [PubMed] [Google Scholar]
- 61.SPRUCE BA, CURTIS R, WILKIN GP, GLOVER DM. A neuropeptide precursor in cerebellum: Proenkephalin exists in subpopulations of both neurons and astrocytes. EMBO J. 1990;9:1787–1795. doi: 10.1002/j.1460-2075.1990.tb08303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.STEELE WJ, JOHANNESSON T. Effects of prenatally-administered morphine on brain development and resultant tolerance to the analgesic effect of morphine in offspring of morphine treated rats. Acta Pharmacol.Toxicol. 1975;36:243–256. doi: 10.1111/j.1600-0773.1975.tb00792.x. [DOI] [PubMed] [Google Scholar]
- 63.TORAN-ALLERAND CD. Sex steroids and the development of the newborn mouse hypothalamus and preoptic area in vitro: Implications for sexual differentiation. Brain Res. 1976;106:407–412. doi: 10.1016/0006-8993(76)91038-6. [DOI] [PubMed] [Google Scholar]
- 64.TORAN-ALLERAND CD. Sex steroids and the development of the newborn mouse hypothalamus and preoptic area in vitro: II. morphological correlates and hormonal specificity. Brain Res. 1980;189:413–427. doi: 10.1016/0006-8993(80)90101-8. [DOI] [PubMed] [Google Scholar]
- 65.TSANG D, NG SC, HO KP, HO WKK. Ontogenesis of opiate binding sites and radioimmunoassayable beta- endorphin and enkephalin in regions of rat brain. Dev.Brain Res. 1982;5:257–261. doi: 10.1016/0165-3806(82)90124-9. [DOI] [PubMed] [Google Scholar]
- 66.VERNADAKIS A, ESTIN C, GIBSON DA, AMOTT S. Effects of methadone on ornithine decarboxylase and cyclic nucleotide phosphohydrolase in neuronal and glial cell cultures. J.Neurosci.Res. 1982;7:111–117. doi: 10.1002/jnr.490070203. [DOI] [PubMed] [Google Scholar]
- 67.VÉRTES Z, MELEGH G, VÉRTES M, KOVÁCS S. Effect of naloxone and D-Met2-Pro5-enkephalinamide treatment on the DNA synthesis in the developing rat brain. Life Sci. 1982;31:119–126. doi: 10.1016/0024-3205(82)90423-4. [DOI] [PubMed] [Google Scholar]
- 68.VOLKMAR FR, GREENOUGH WT. Rearing complexity affects branching of cortical dendrites in the visual cortex of the rat. Science. 1972;176:1445–1447. doi: 10.1126/science.176.4042.1445. [DOI] [PubMed] [Google Scholar]
- 69.WATSON RR, MOHS ME. Effects of morphine, cocaine, and heroin on nutrition. Prog.Clin.Biol.Res. 1993;1990;325:413–418. [PubMed] [Google Scholar]
- 70.WILLSON NJ, SCHNEIDER JF, ROIZIN L, FLEISS JF, RIVERS W, DEMARTINI JE. Effects of methadone HCl on the growth of organotypic cerebellar cultures prepared from methadone tolerant and control rats. J.Pharmacol.Exp.Ther. 1976;199:368–374. [PubMed] [Google Scholar]
- 71.WILSON GS. Clinical studies of infants and children exposed prenatally to heroin. Ann. NY Acad. Sci. 1989;562:183–193. doi: 10.1111/j.1749-6632.1989.tb21017.x. [DOI] [PubMed] [Google Scholar]
- 72.WINICK M. Malnutrition and Brain Development. Oxford Univ. Press; New York: 1976. [Google Scholar]
- 73.ZAGON IS, GIBO DM, MCLAUGHLIN PJ. Zeta (ζ), a growth-related opioid receptor in developing rat cerebellum: Identification and characterization. Brain Res. 1991;551:28–35. doi: 10.1016/0006-8993(91)90909-f. [DOI] [PubMed] [Google Scholar]
- 74.ZAGON IS, MCLAUGHLIN PJ. Morphine and brain growth retardation in the rat. Pharmacol. 1977;15:276–282. doi: 10.1159/000136699. [DOI] [PubMed] [Google Scholar]
- 75.ZAGON IS, MCLAUGHLIN PJ. Comparative effects of postnatal undernutrition and methadone exposure on protein and nucleic acid contents of the brain and cerebellum in rats. Dev.Neurosci. 1982;5:385–393. doi: 10.1159/000112698. [DOI] [PubMed] [Google Scholar]
- 76.ZAGON IS, MCLAUGHLIN PJ. Neuronal cell deficits following maternal exposure to methadone in rats. Experientia. 1982;38:1214–1216. doi: 10.1007/BF01959747. [DOI] [PubMed] [Google Scholar]
- 77.ZAGON IS, MCLAUGHLIN PJ. Opioid antagonist (naltrexone) modulation of cerebellar development: histological and morphometric studies. J.Neurosci. 1986;6:1424–1432. doi: 10.1523/JNEUROSCI.06-05-01424.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.ZAGON IS, MCLAUGHLIN PJ. Endogenous opioid systems regulate cell proliferation in the developing rat brain. Brain Res. 1987;412:68–72. doi: 10.1016/0006-8993(87)91440-5. [DOI] [PubMed] [Google Scholar]
- 79.ZAGON IS, MCLAUGHLIN PJ. Ultrastructural localization of enkephalin-like immunoreactivity in developing rat cerebellum. Neurosci. 1990;34:479–489. doi: 10.1016/0306-4522(90)90156-x. [DOI] [PubMed] [Google Scholar]