Abstract
Introduction
Hypermethylation of key tumor suppressor genes plays an important role in lung carcinogenesis. The purpose of this study is to explore the therapeutic potential of regional administration (via the airways) of the demethylating agent 5-azacytidine (5-Aza) for the treatment of early lung cancer.
Patients and Methods
We administered 5-Aza solution directly into the trachea in imprinting control region (ICR) mice (to study its toxicity) and in nude mice bearing orthotopic human lung cancer xenografts (to assess its antitumor activity).
Results
In vitro, 5-Aza inhibited the growth of human lung cancer cell lines H226, H358, and H460 in a dose-dependent manner. The concentrations to inhibit cell growth by 50% (IC50) were about 0.6-4.9 μg/mL. 5-Azacytidine reversed hypermethylation in the promoter of tumor suppressor gene RASSF1a in the H226 cells at a 6000-fold lower concentration than its IC50. In animal studies, intratracheal (I.T.) administration of 90 mg/kg 5-Aza (the maximum tolerated dose of 5-Aza intravenous injection [I.V.]) resulted in moderate pulmonary toxicity and 5-fold reduced myelosuppression compared with the same dose of I.V. 5-Aza. Using an optimized multiple dose schedule, I.T. 5-Aza was about 3-fold more effective than I.V. 5-Aza in prolonging the survival of mice bearing orthotopic H460 and H358 xenografts, and did not cause any detectable toxicity.
Conclusion
5-Azacytidine can reverse the hypermethylation in the human lung cancer cell lines at a nontoxic dose. Regional administration to the airways enhances the therapeutic index of 5-Aza by 75-fold. The potential of regional administration of 5-Aza (including by aerosolization) for the treatment of advanced bronchial premalignancy deserves further investigation.
Keywords: Aerosol administration, Airway epithelium, Demethylation
Introduction
Lung cancer is the number one cause of cancer-related death causing over one million deaths worldwide each year.1 About 90% of such cases are the end result of cumulative aberrant epigenetic changes and genetic damage to the respiratory epithelium chronically exposed to tobacco carcinogens.2,3,4
One of the well accepted mechanisms of carcinogenesis in lung cancer is aberrant methylation of CpG islands in the promoter regions of tumor suppressor genes (TSGs) leading to underexpression or absence of the proteins of those genes thus propagating tumorigenesis.5 For example, the p16INK4a (p16) gene is inactivated in > 70% of cell lines derived from all histologic subtypes of non–small-cell lung cancer (NSCLC)6,7,8; hypermethylation of PTEN and RASSF1 promoters is associated with shorter time to recurrence of the patients with stage I and II NSCLC receiving surgical operation.9 Hypermethylation of death-associated protein kinase (DAPK) promoter directly reduces the sensitivity to tumor necrosis factor–related apoptosis-inducing ligand (TRAIL)-induced apoptosis in human NSCLC cell lines,10 and may result in downregulation of p53 responsive genes11; hypermethylation of the E-cadherin promoter was found in 34% of resected NSCLC tumors12; DLEC1 and MLH1 promoter hypermethylation are associated with poor prognosis in NSCLC patients.13
Similar to the genes mentioned above, the expression of other important TSGs can be downregulated because of hypermethylation. Thus, it is conceivable that DNA-methyltransferase inhibitors (DMTI) such as 5-Aza, 5-Aza-2′-deoxycytidine, and Zebularine could be used14,15,16 as therapeutic agents to re-express TSGs by causing hypomethylation of the CpG islands in the promoter region; ie, demethylation could be used as a clinically effective method to reactivate the function of silenced TSGs and to gain tumor suppression function.
Inhalation of carcinogens, mainly as a result of tobacco exposure, causes a field cancerization effect thereby placing the entire bronchial epithelium at risk for developing bronchogenic carcinoma. Any strategies that aim at decreasing the incidence of lung cancer or decreasing the incidence of a second primary in a patient with a history of lung cancer would have to have an effect on the entire bronchial epithelium. In the case of a pharmacologic agent, this would be possible and feasible by inhalation of aerosolized solution of the drug. DMTI agents such as 5-Aza have the potential to re-express tumor suppressor genes, which might lead to reversal of premalignant changes, slow the carcinogenesis process, and eventually decrease the incidence of bronchogenic carcinoma.17 Systemic administration of these drugs has been explored in patients with advanced NSCLC but not pursued because of significant systemic toxicity.18 No preclinical studies of the use of these agents by regional administration through the airways for advanced bronchial premalignancy or endobronchial lung cancer have been previously reported.
In this study, we tested the toxicity and antitumor activity of the DMTI 5-Aza administered directly in the upper airways in mice bearing endobronchial human lung cancers secondary to intratracheal inoculation of human NSCLC cells. Results were compared with animals treated with 5-Aza intravenously.
Patients and Methods
Cell Lines
Human NSCLC cell lines H226 (squamous cell carcinoma), H358 (bronchoalveolar carcinoma), and H460 (large cell carcinoma) were purchased from American Type Culture Collection (ATCC, Manassas, VA). Cell lines were cultured in 10% fetal bovine serum and 90% RPMI 1640 medium (Invitrogen Corporation, Carlsbad, CA) and maintained in a 37°C incubator with 5% CO2 and 95% air.
Formulation
For the in vitro studies, 5-Aza (Sigma, St Louis, MO) stored at −20°C as dry power was dissolved in Lactated Ringer's Injection (Hospira, Inc., Lake Forest, IL) immediately before use (5-Aza).
MTT Assay
Growth inhibition of 5-Aza was determined as described previously.19 Briefly, approximately 10,000 cells in 0.135 μL RPMI 1640 per well were seeded in 96-well plates. After 24 hours of culture, 5-Aza at various concentrations was added to the cells. Three days later, the cells were stained with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and lysed. The absorbance of each well was measured in a microplate reader at 570 nm. The percent growth inhibition of the cells was calculated as the absorbance of treated cells normalized to no treatment cells.
Methylation-Specific PCR
H226 human NSCLC cells were treated with 5-Aza at 0.1, 1, and 10 ng/mL. On day 4 after the treatment, the cells were harvested. About 8 × 104 cells were used to detect the methylation status of the RASSF1a promoter using EZ DNA Methylation-Direct Kit™ (Zymo Research Corporation, Orange, CA), according to the manufacturer's instructions. The bisulfate-converted DNA was then used as a template for methylation-specific polymerase chain reactions (PCRs) using primers specific for either the modified methylated or modified unmethylated promoter sequences of the genes evaluated in this study. The primers used have been described previously by others.20 The sequences are: methylated: 5′-GGG TTT TGC GAG AGC GCG-3′ (forward) and 5′-GCT AAC AAA CGC GAA CCG-3′ (reverse); unmethylated: 5′-GGT TTT GTG AGA GTG TGT TTA G-3′(forward), and 5′-CAC TAA CAA ACA CAA ACC AAA C-3′ (reverse). Briefly, PCR reactions contained 1-4 μL of bisulfate-converted DNA, purified as above, 300 ng each of forward and reverse primers, 45 μL of Platinum® PCR SuperMix (Invitrogen), and H2O to a final reaction volume of 50 μL. PCR amplification conditions were as described in the references,20 unless otherwise noted. The PCR products were separated on 2% agarose gels containing ethidium bromide and were visualized under ultraviolet illumination.
Animals
Male and female ICR and NCRNU-M nude mice, 6-7 weeks old, were purchased from Taconic Farms (Germantown, NY).
5-Azacytidine Toxicity in Mice
The ICR mice were used to evaluate and compare the acute toxicities of 5-Aza by the intratracheal or intravenous routes. Two groups of mice (5-8 mice per group) were treated with 90 mg/kg of 5-Aza via intravenous injection (I.V.) or intratracheal injection (I.T.), respectively. The dose of 90 mg/kg is the maximum tolerated dose (MTD) of I.V. 5-Aza in mice. The I.V. and I.T. injection methods were described previously.21,22 Briefly, for the I.T., the mice were anesthetized with intraperitoneal injection of 30-50 mg/kg of Nembutal. The drug solution or cell suspension was carefully injected into the trachea through mouth via a 22-gauge feeding needle attached to a 1 mL syringe. The injection volume did not exceed 100 μL per mouse. For myelotoxicity assessment, blood (100 μL per mouse, 5 mice per group) was drawn from the tail vein before treatment (on day 0) and on days 4, 7, 14, and 28 after treatment. Red blood cells (RBCs) were removed from the blood samples using RBC lysis buffer (eBioscience, Inc., San Diego, CA). White blood cells (WBCs) were collected as per the manufacturer's protocol and counted with a hemocytometer under a microscope. Blood samples from untreated mice were used as controls. In the organ toxicity studies, groups of mice given intratracheal injections of Lactated Ringer's Solution or without treatment were used as vehicle and normal controls, respectively. Creatinine levels, and liver function tests were determined at ANTECH Diagnostics (Lake Success, NY). Organ pathologic examinations were performed at different time points. Briefly, 5 mice in each group were euthanized on days 4, 7, 14, and 28 days after administration of the drug. Blood was drawn from the caudal vena cava, and lungs, livers and kidneys were resected and fixed with 10% buffered Formalin. The fixed tissues were processed with standard procedure for hemotoxylin and eosin (H&E) staining. The toxicity levels were determined by giving toxicity grade to each tissue sample. The grading based on the general pathology guidelines was 0-4, they reflect a percentage of damaged tissue of 0, < 10 (mild), 10-30 (moderate), 30-60 (severe), > 60 (life threatening), respectively. Dr. Rani Sellers, Director, Histopathology Core Facility at the Albert Einstein Cancer Center examined the organ toxicity.
Antitumor Efficacy
Male and female nude mice (NCRNU; 5-6 weeks old) were anesthetized with intraperitoneal injection of Nembutal (40 mg/kg). The human NSCLC cancer cells (H358, or H460) in suspension were carefully injected into the trachea (approximately 3 × 106 cells/mouse) using the method described above. Ten days later, the mice were randomly divided into 3 groups of 5 mice each, and were treated with daily I.V. 5-Aza at 6.25 mg/kg per day × 6 doses or I.T. 5-Aza at 2.5 mg/kg per every other day × 3 doses. These optimal doses for the therapeutic study were determined in previous dose-ranging studies. A group of mice without treatment was used as control. Survival was used as the major endpoint to evaluate efficacy.
Statistical Analysis
Differences among groups were analyzed by 2-side log-rank assay. A difference was considered statistically significant when P < .05.
Results
Growth Inhibition of 5-Azacytidine in Human NSCLC Cell Lines
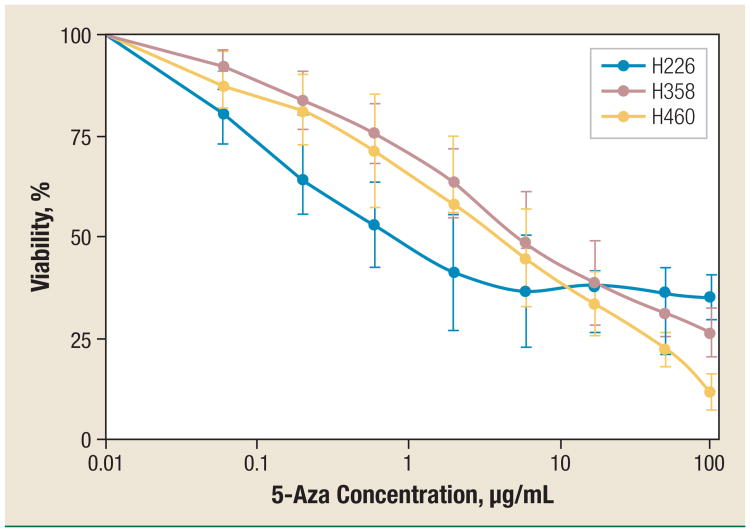
5-Azacytidine has both functions: cell growth inhibition and demethylation. In order to know whether 5-Aza's demethylation effect can function at a nontoxic concentration, we measured its cell growth inhibition in different human NSCLC cell lines. As shown in Figure 1, 5-Aza inhibited the growth of the NSCLC cells in a dose-dependent manner. The 50% inhibitory concentrations (IC50) of 5-Aza were 0.6, 3.4, and 4.9 μg/mL in H226, H358, and H460 cells, respectively. In this study, 5-Aza at a concentration below 0.6 μg/mL did not cause significant growth inhibition in all tested cell lines.
Figure 1. Growth Inhibition of 5-Azacytidine on Human NSCLC Cell Lines.
H226, H358, and H460 cells were treated with (5-fold) increasing concentrations of 5-Aza. The percentage of growth inhibition was measured with MTT Assay. The data for each cell line are mean ± standard deviation obtained from 3 independent experiments.
Abbreviations: 5-Aza = 5-azacytidine; NSCLC = non–small-cell lung cancer
The Demethylation Function of 5-Azacytidine in the NSCLC Cell Line
We used methylation-specific PCR method to detect the demethylation function of 5-Aza in the H226 NSCLC cell line at a very low concentration range (0.1-10 ng/mL). As shown in Figure 2, the unmethylated band (#5) of the promoter of RASSF1a gene was found in the H226 cells at the lowest concentration of 0.1 ng/mL, which is 6000-fold lower than the IC50 of 5-Aza in the same cell line. This indicates that when directly exposing lung cancer cells to 5-Aza, 5-Aza can function as an effective demethylation agent at an extremely low concentration without causing any direct cytotoxicity.
Figure 2. The Demethylation Function of 5-Azacytidine in the NSCLC Cells.
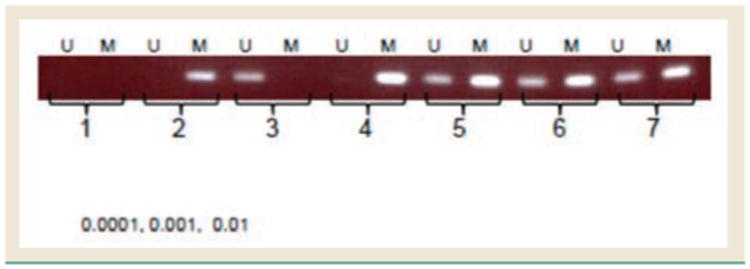
H226 human NSCLC cells (8 × 104 cells) were treated with 5-Aza at 0.1, 1, and 10 ng/mL. The methylation status of the RASSF1a promoter was detected using the EZ DNA Methylation-Direct Kit and methylation-specific PCR. Bands 1 to 3 are samples of water, methylated DNA control, and unmethylated DNA control; bands 4 to 7 are samples of H226 cells treated with 5-Aza at 0, 0.1, 1, and 10 ng/mL, respectively.
Abbreviations: 5-Aza = 5-azacytidine; M = methylated detection; NSCLC = non–small-cell lung cancer; U = unmethylated detection
Intratracheal Administration of 5-Azacytidine Results in Significantly Reduced Myelotoxicity
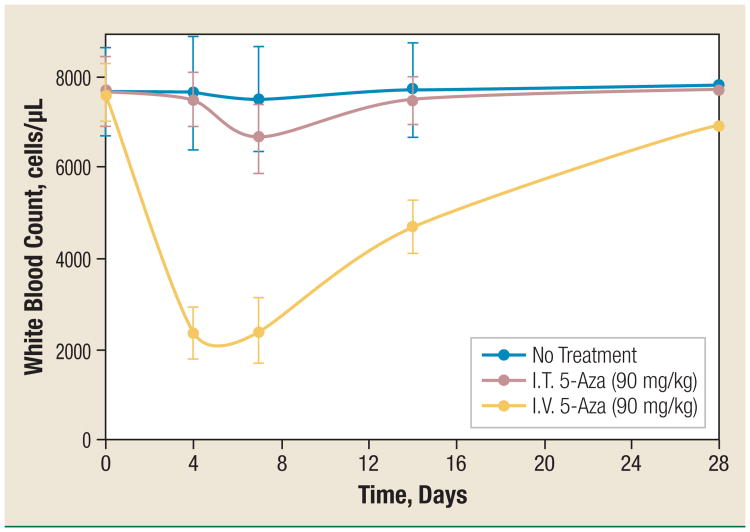
Myelosuppression is the dose-limiting toxicity of intravenously administered 5-Aza when used clinically. In this study, we compared the myelotoxicity of I.T. and I.V. 5-Aza at the same dose (90 mg/kg, which is the MTD when using I.V. administration). Intratracheal injection 5-Aza produced significantly less myelotoxicity than I.V. 5-Aza at the MTD of I.V. 5-Aza. As shown in Figure 3, I.V. 5-Aza significantly reduced the total WBC by > 68% on days 4 and 7 (P < .004) and > 38% on day 14 (P < .006), the WBC count recovered to about 90% of the normal level on day 28. The only detectable WBC reduction in I.T. 5-Aza–treated mice was about 13% on day 7 (P < .01). The recovery was faster (on day 14) and complete (> 97% of the normal level; P > .5) compared with I.V. 5-Aza (Figure 3).
Figure 3. Intratracheal Injection Administration of 5-Azacytidine Decreases by 5-fold the Reduction in the WBC Count as Compared With Intravenous Injection 5-Azacytidine.
5-Aza was administered I.T. (red dots) or I.V. (yellow dots) at a dose of 90 mg/kg. Control mice were not given any treatment (blue dots). Blood was drawn on days 0, 4, 7, 14, and 28 after treatment. White blood cells were counted after removal of red blood cells. The data of each group (5-8 mice each) are mean ± standard deviation.
Abbreviations: 5-Aza = 5-azacytidine; I.T. = intratracheal injection; I.V. = intravenous injection; WBC = white blood cells
Organ Toxicity
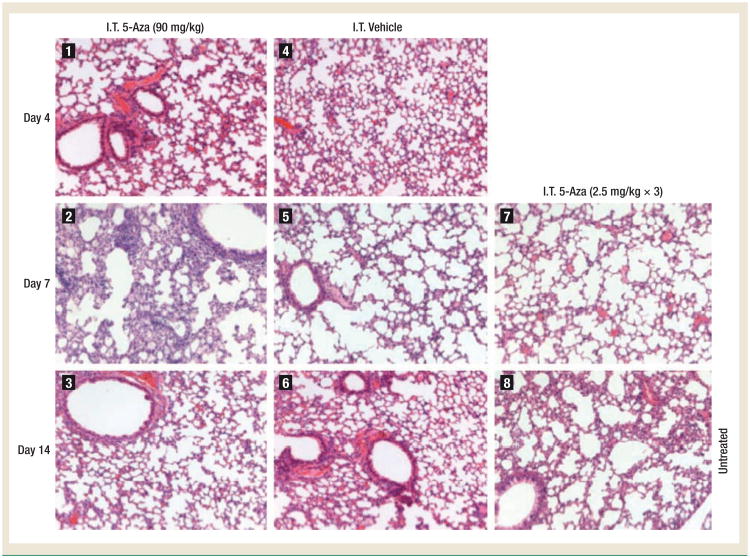
At the MTD, the results of serum liver function tests and serum creatinine measurements were normal for all mice and there were no differences among the groups of I.V. 5-Aza, I.T. 5-Aza, and no treatment (data not shown). On histopathologic evaluation, no liver or kidney toxicities were identified in any treatment group (data not shown). By lung histologic evaluation, moderate pulmonary toxicity was observed in all 5 animals in the I.T. 5-Aza group on day 7 but not at the other time points. Of note is that the I.T. dose used in these experiments is a 12-fold higher dose than the optimized total dose used in the therapeutic experiments. The lung toxicity was described as moderate pneumonitis, characterized by type II pneumocyte hypertrophy, neutrophilic infiltration, and lymphohistiocytic inflammation (Figure 4, photograph 2). As stated before, no pulmonary toxicity was observed in animals treated with I.T. 5-Aza on days 4 or 14. At the optimal therapeutic dose (2.5 mg/kg, every other day × 3), I.T. 5-Aza did not cause lung toxicity (Figure 4, photograph 7) or any other toxicity (data not shown). The pulmonary toxicity grades are listed in Table 1. These results indicate that the pulmonary toxicity caused by I.T. 5-Aza at supratherapeutic doses (the MTD of I.V. 5-Aza) is moderate and reversible within 2 weeks. I.T. 5-Aza at the therapeutic dose, I.T. vehicle, and I.V. 5-Aza (data not shown) did not cause detectable pulmonary toxicity.
Figure 4. High-Dose Intratracheal Injection 5-Azacytidine Produces Recoverable Pulmonary Toxicity.
The ICR mice were intratracheally injected with 90 mg/kg of 5-Aza, 2.5 mg/kg every other day × 3 of 5-Aza, or the equal volume of vehicle (Lactated Ringer's Injection). The lungs of mice were resected on days 4, 7, and 14 after injection. Standard hematoxylin and eosin staining of lung tissues was used to assess pulmonary toxicity. Photographs 1-6 are the lungs from mice receiving 90 mg/kg of I.T. 5-Aza (1-3) or the same volume of I.T. vehicle (4-6). Photograph 7 is the lung from mice receiving the therapeutic dose of I.T. 5-Aza (2.5 mg/kg, every other day × 3) on day 7 post the final injection. Photograph 8 is the lung from untreated mice. Moderate toxicity was found in the lungs of mice receiving 90 mg/kg of I.T. 5-Aza on 7 days post the injection (photograph 2) but not any other samples.
Abbreviations: 5-Aza = 5-azacytidine; ICR = imprinting control region; I.T. = intratracheal injection
Table 1. Toxicity Grade of Lungs of the Mice Treated With Intratracheal Injection 5-Azacytidine.
| Days | 4 | 7 | 14 | 28 |
|---|---|---|---|---|
| I.T. 5-Aza (90 mg/kg) | 0a | 2a | 0a | 0 |
| I.T. 5-Aza (2.5 mg/kg × 3) | 0 | 0a | 0 | 0 |
| I.T. Vehicle | 0a | 0a | 0a | 0 |
| I.V. 5-Aza (90 mg/kg) | 0 | 0 | 0 | 0 |
| No Treatment | 0 | 0a | 0 | 0 |
Histopathologic photographs are shown in Figure 4.
Abbreviations: 5-Aza = 5-azacytidine; I.T. = intratracheal injection
Intratracheal Administration of 5-Azacytidine Significantly Prolonged the Survival of Mice Bearing Orthotopic Human NSCLC Xenografts
To evaluate the efficacy of I.T. 5-Aza in clinically relevant NSCLC models, we inoculated the human NSCLC cell lines H460 and H358 into the lungs of nude mice via the trachea. These models mimic closely orthotopic human NSCLC. In mice, small mucosal tumor nodules are evident at 1-3 weeks after the inoculation of tumor cells. In the absence of any intervention, the mice succumb to the tumor in 7-10 weeks. The survival curve in these models closely correlates with the tumor burden,23 and can be used as an endpoint for the evaluation of treatment efficacy.
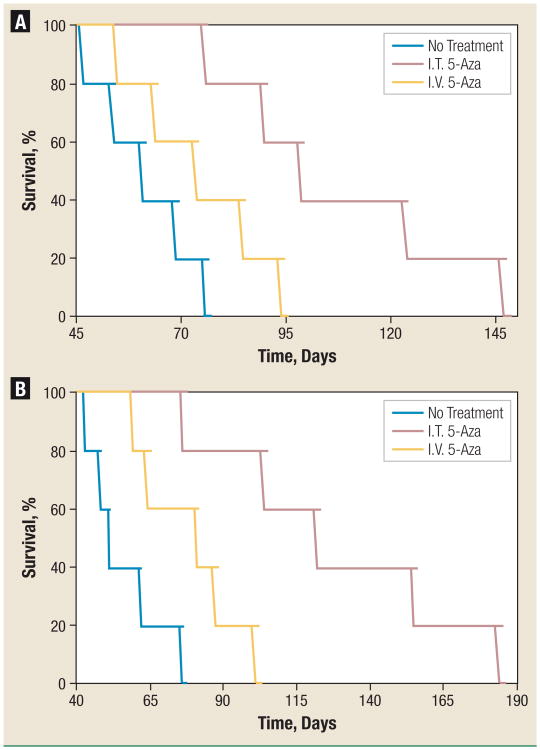
Treatments were initiated on day 10 post tumor inoculation. The survival observed in mice treated with I.T. 5-Aza was compared with that in mice treated with I.V. 5-Aza and untreated tumorbearing mice. Animals in each treatment group were given multiple injections; these doses and schedules were optimized in a preliminary study (data not shown). The total dose was 7.5 mg/kg for I.T. 5-Aza (2.5 mg/kg, every other day × 3) and 37.5 mg/kg for I.V. 5-Aza (6.25 mg/kg, every day × 6). Both dose levels are significantly lower than the corresponding MTDs. Results are shown in Figure 5. I.V. 5-Aza had limited efficacy against both lung cancer models at the optimal therapeutic dose: the median survival increased by 22% in the H358 model (73 days vs. 60 days; P > .05) and 60% in the H460 model (80 days vs. 50 days; P < .01), whereas I.T. 5-Aza demonstrated significantly increased efficacy: the median survival increased by 63% in the H358 model (98 days vs. 60 days; P < .006) and by 142% in the H460 model (121 days vs. 50 days; P < .002). The increased lifespan (ILS)24 of I.T. 5-Aza treated mice bearing H358 or H460 lung tumors was > 3.2-fold higher than that of I.V. 5-Aza treated mice (75.8% vs. 21.5%; 131.3% vs. 40.7%). The efficacy of each treatment is summarized in Table 2.
Figure 5. Intratracheal Administration of Low-Dose 5-Azacytidine Significantly Prolongs Survival of Mice Bearing Orthotopic Human NSCLC Xenografts.
Mice intratracheally inoculated with H358 (A) or H460 (B) human NSCLC cell lines were treated with I.V. or I.T. 5-Aza on day 10 at a dose of 6.25 mg/kg daily × 6 for I.V. and 2.5 mg/kg every other day × 3 for I.T. The control was a group of untreated mice (thin line).
Abbreviations: 5-Aza = 5-azacytidine; I.T. = intratracheal injection; I.V. = intravenous injection; NSCLC = non–small-cell lung cancer
Table 2. Efficacy Summary.
| Efficacy Measure | No Treatmenta | I.V. | I.T. | I.T./I.V. | |
|---|---|---|---|---|---|
| H358 | Median survival, days | 60 | 73 | 98 | 1.34 |
| Survival range, days | 46-75 | 54-93 | 75-146 | 1.4-1.6 | |
| ILS, % | 0 | 21.5 | 75.8 | 3.52 | |
| H460 | Median survival, days | 50 | 80 | 121 | 1.51 |
| Survival range, days | 42-75 | 58-100 | 75-183 | 1.3-1.8 | |
| ILS, % | 0 | 40.7 | 131.3 | 3.23 | |
Untreated mice or mice that did nor receive therapeutic treatment.
Abbreviations: ILS = increased lifespan; I.T. = intratracheal injection; I.V. = intravenous injection; NA = not available
Discussion
5-Azacytidine is currently approved by the Food and Drug Administration for the treatment of myelodysplastic syndromes,14 a preleukemic condition, and has potential for the treatment of other cancers and premalignant conditions as a result of a cytotoxic effect, a DNA demethylating effect, or both. As a cytotoxic agent in proliferating cells, 5-Aza can disrupt RNA metabolism, DNA synthesis, and protein synthesis. Particularly, 5-Aza is incorporated into DNA and inhibits DNA methyltransferases and causes hypomethylation of replicating DNA,25,26 which can result in re-expression of tumor suppressor genes silenced by hypermethylation. From 1973 to 1977, there were at least 9 clinical studies in solid tumor patients with I.V. 5-Aza, for which included 78 lung cancer patients.27 The therapeutic efficacy observed was limited, possibly because of 2 major reasons: first, the studies were performed in advanced lung cancer patients where reversal of hypermethylation per se may not be sufficient to have a therapeutic effect; second, all the studies were done using systemic administration or a suboptimal dose schedule, which limits the use of these agents as a result of systemic toxicity. The studies presented here were designed to provide the foundation for the potential use of a regional demethylation strategy for malignant or premalignant conditions of the bronchial epithelium in which DNA hypermethylation plays an important role. We used 5-Aza as a model compound and tested its toxicity and antitumor efficacy by direct delivery in the respiratory airways via the trachea in models of endobronchial human NSCLC. Our studies demonstrate that I.T. 5-Aza produced a 5-fold reduced myelosuppression (as assessed by WBC nadir) than I.V. 5-Aza at a dose equivalent to the I.V. MTD and 3-fold higher antitumor efficacy (as assessed by ILS) at a dose 5-fold lower than that of I.V. 5-Aza, the end result being a 75-fold increased therapeutic index. These results justify continuing the exploration of regional demethylating therapy for the treatment of malignant or premalignant conditions of the lungs that are easily accessible through the airways, including advanced premalignancy, bronchoalveolar carcinoma, and small parenchymal metastatic disease.
Lung cancers develop in the epithelium in direct contact with the airways because carcinogens reach the lungs through inhalation. Bronchial premalignancy, carcinoma in situ, small primary or metastatic tumors, and some cases of bronchioloalveolar carcinoma are theoretically more accessible via the endobronchial space than through the bloodstream. Aerosol approaches to the treatment or prevention of these conditions are therefore a more logical therapeutic strategy than systemic treatment. However, in the present studies we used intratracheal administration rather than aerosol administration because the purpose was proof of concept and administration of drugs by aerosolization to mice is inefficient. The major difference between these 2 types of drug administration (I.T. vs. aerosol) would be a higher distribution of the drug to the alveolar space with aerosol administration. We are currently conducting studies to validate the results presented here using the clinically available formulation of 5-Aza administered by aerosolization to mice.
Our toxicity studies demonstrate, as expected, that 5-Aza given I.T. results in a 5-fold reduced myelosuppression, which is the dose-limiting toxicity of I.V. 5-Aza. Most importantly, I.T. 5-Aza at 90 mg/kg only caused mild pulmonary inflammation on day 7 after I.T. administration. It was encouraging to see that there was no evidence of lung inflammation on day 14 post I.T. 5-Aza, even when the dose used for I.T. was as high as the MTD using the I.V. route. In the efficacy experiments, the optimal total I.T. dose used was 12-fold lower, a dose that did not cause any pulmonary toxicity. To confirm this, we are currently performing more refined lung toxicity studies in the context of our current therapeutic experiments using aerosolized administration.
In the efficacy experiments, the efficacy ILS of I.T. 5-Aza at a 5-fold lower dose was 3.2- to 3.5-fold superior to that of I.V. 5-Aza in mice with endobronchial H358 or H460 tumors. These results indicate that the regional administration route into the airways is more efficient than the intravenous route for the treatment of endobronchial tumors. The main therapeutic potential of airway-administered 5-Aza is secondary prevention of NSCLC because of the field cancerization effect of inhaled carcinogens via tobacco smoke. The proposed mechanism would be hypomethylation of CpG islands of the promoter regions of tumor suppressor genes thereby inhibiting development of dysplasia and progression of dysplasia to cancer. In these studies, we used a mouse model of endobronchial tumors but not dysplasia. We are currently developing an animal model of lung premalignancy in mice by exposing them to tobacco carcinogens directly into the upper airways. We plan to test the ability of aerosolized 5-Aza in reversing tumor suppressor gene hypermethylation in this model.
In these studies, the efficacy endpoint was survival secondary to antitumor effect in models of malignancy. In the anticipated clinical scenario, the intermediate efficacy endpoint would be changes in hypermethylation patterns or effective gene re-expression. In this study, the optimal therapeutic dose was 12-fold lower than the MTD. This finding suggests that this strategy may have a large therapeutic window and that the risk of acute or chronic side effects might be very low if these agents were used at optimal doses rather than MTD. Therefore, determining optimal doses based on pharmacodynamic assessments in patients enrolled in clinical studies with this new therapeutic strategy are essential to minimize the potential side effects. Particularly, the potential carcinogenicity of this approach could become an important limitation if benefit was demonstrated but required chronic administration of unnecessarily high doses of these agents. Therefore, in the context of our initial phase I clinical study of inhaled 5-Aza we intend to monitor methylation patterns and gene re-expression in the target tissue pre and post therapy to establish an optimal dose based on target effects rather than MTD.
In vitro, we have proved that 5-Aza can effectively demethylate the hypermethylation in the promoter of tumor suppressor gene at a nontoxic concentration. In vivo, we found that I.T. 5-Aza is effective against experimental lung cancer by prolonging the life of the mice bearing orthotopic lung tumors without causing any detectable systemic or locoregional toxicity. Here the functions of both the epigenetic effect and the locoregional administration played an important role. We believe that the lung-specific epigenetic treatment with 5-Aza has great potential to reduce the tumor burden by reversing the hypermethylation in the promoters of the tumor suppressor genes and therefore reactivating the silenced genes. This is an important project to be further studied.
Conclusion
5-Aza can reverse the hypermethylation in the human lung cancer cell lines at a nontoxic dose. Regional administration to the airways enhances the therapeutic index of 5-Aza by 75-fold. The potential of regional administration of 5-Aza (including by aerosolization) for the treatment of advanced bronchial premalignancy deserves further investigation.
Acknowledgments
Grant Support: U.S. National Cancer Institute grant 5R21 CA104297-02 (Yiyu Zou).
Disclosures: Dr. Perez-Soler has received research funding from the National Institutes of Health, and has served as a paid consultant and is a stock shareholder for Amgen; AstraZeneca; Genentech, Inc.; Eli Lilly and Company; Novartis Pharmaceuticals Corporation; and TRANSAVE, Inc.; and is also a member of the Speaker's Bureau for Genentech, Inc. and Eli Lilly and Company. All other authors have no relevant relationships to disclose.
References
- 1.Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Williams MD, Sandler AB. The epidemiology of lung cancer. Cancer Treat Res. 2001;105:31–52. doi: 10.1007/978-1-4615-1589-0_2. [DOI] [PubMed] [Google Scholar]
- 3.Belinsky SA. Gene-promoter hypermethylation as a biomarker in lung cancer. Nat Rev Cancer. 2004;4:707–17. doi: 10.1038/nrc1432. [DOI] [PubMed] [Google Scholar]
- 4.Zöchbauer-Müller S, Minna JD, Gazdar AF. Aberrant DNA methylation in lung cancer: biological and clinical implications. Oncologist. 2002;7:451–7. doi: 10.1634/theoncologist.7-5-451. [DOI] [PubMed] [Google Scholar]
- 5.Tsou JA, Hagen JA, Carpenter CL, et al. DNA methylation analysis: a powerful new tool for lung cancer diagnosis. Oncogene. 2002;21:5450–61. doi: 10.1038/sj.onc.1205605. [DOI] [PubMed] [Google Scholar]
- 6.Otterson GA, Kratzke RA, Coxon A, et al. Absence of p16INK4 protein is restricted to the subset of lung cancer lines that retains wild-type RB. Oncogene. 1994;9:3375–8. [PubMed] [Google Scholar]
- 7.Merlo A, Herman JG, Mao L, et al. 5′ CpG island methylation is associated with transcriptional silencing of the tumour suppressor p16/CDKN2/MTS1 in human cancers. Nat Med. 1995;1:686–92. doi: 10.1038/nm0795-686. [DOI] [PubMed] [Google Scholar]
- 8.Georgiou E, Valeri R, Tzimagiorgis G, et al. Aberrant p16 promoter methylation among Greek lung cancer patients and smokers: correlation with smoking. Eur J Cancer Prev. 2007;16:396–402. doi: 10.1097/01.cej.0000236260.26265.d6. [DOI] [PubMed] [Google Scholar]
- 9.Buckingham L, Penfield Faber L, Kim A, et al. PTEN, RASSF1 and DAPK site-specific hypermethylation and outcome in surgically treated stage I and II non small cell lung cancer patients. Int J Cancer. 2010;126:1630–9. doi: 10.1002/ijc.24896. [DOI] [PubMed] [Google Scholar]
- 10.Tang X, Wu W, Sun SY, et al. Hypermethylation of the death-associated protein kinase promoter attenuates the sensitivity to TRAIL-induced apoptosis in human non-small cell lung cancer cells. Mol Cancer Res. 2004;2:685–91. [PubMed] [Google Scholar]
- 11.Raveh T, Kimchi A. DAP kinase-a proapoptotic gene that functions as a tumor suppressor. Exp Cell Res. 2001;264:185–92. doi: 10.1006/excr.2000.5134. [DOI] [PubMed] [Google Scholar]
- 12.Kim DS, Kim MJ, Lee JY, et al. Aberrant methylation of E-cadherin and H-cadherin genes in non small cell lung cancer and its relation to clinicopathologic features. Cancer. 2007;110:2785–92. doi: 10.1002/cncr.23113. [DOI] [PubMed] [Google Scholar]
- 13.Seng TJ, Currey N, Cooper WA, et al. DLEC1 and MLH1 promoter methylation are associated with poor prognosis in non-small cell lung carcinoma. Br J Cancer. 2008;99:375–82. doi: 10.1038/sj.bjc.6604452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaminskas E, Farrell AT, Wang YC, et al. FDA drug approval summary: azacitidine (5-azacytidine, Vidaza) for injectable suspension. Oncologist. 2005;10:176–82. doi: 10.1634/theoncologist.10-3-176. [DOI] [PubMed] [Google Scholar]
- 15.Momparler RL. Epigenetic therapy of cancer with 5-aza-2′-deoxycytidine (decitabine) Semin Oncol. 2005;32:443–51. doi: 10.1053/j.seminoncol.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 16.Marquez VE, Kelley JA, Agbaria R, et al. Zebularine: a unique molecule for an epigenetically based strategy in cancer chemotherapy. Ann N Y Acad Sci. 2005;1058:246–54. doi: 10.1196/annals.1359.037. [DOI] [PubMed] [Google Scholar]
- 17.Mufti G, List AF, Gore SD, et al. Myelodysplastic syndrome. Hematology (Am Soc Hematol Educ Program) 2003:176–99. doi: 10.1182/asheducation-2003.1.176. [DOI] [PubMed] [Google Scholar]
- 18.Momparler RL, Bouffard DY, Momparler LF, et al. Pilot phase I-II study on 5-aza-2′-deoxycytidine (Decitabine) in patients with metastatic lung cancer. Anticancer Drugs. 1997;8:358–68. doi: 10.1097/00001813-199704000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Carmichael J, DeGraff WG, Gazdar AF, et al. Evaluation of a tetrazolium-based semi automated colorimetric assay: assessment of chemosensitivity testing. Cancer Res. 1987;47:936–42. [PubMed] [Google Scholar]
- 20.Wang Y, Yu Z, Wang T, et al. Identification of epigenetic aberrant promoter methylation of RASSF1A in serum DNA and its clinicopathological significance in lung cancer. Lung Cancer. 2007;56:289–94. doi: 10.1016/j.lungcan.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 21.Zou Y, Zong G, Ling YH, et al. Effective treatment of early endobronchial cancer with regional administration of liposome-p53 complexes. J Natl Cancer Inst. 1998;90:1130–7. doi: 10.1093/jnci/90.15.1130. [DOI] [PubMed] [Google Scholar]
- 22.Zou Y, Ling YH, Van NT, et al. Antitumor activity of free and liposome-entrapped annamycin, a lipophilic anthracycline antibiotic with non-cross-resistance properties. Cancer Res. 1994;54:1479–84. [PubMed] [Google Scholar]
- 23.Zou Y, Zong G, Ling YH, et al. Development of cationic liposome formulations for intratracheal gene therapy of early lung cancer. Cancer Gene Ther. 2000;7:683–96. doi: 10.1038/sj.cgt.7700156. [DOI] [PubMed] [Google Scholar]
- 24.Zou Y, Yamagishi M, Horikoshi I, et al. Enhanced therapeutic effect against liver W256 carcinosarcoma with temperature-sensitive liposomal adriamycin administered into the hepatic artery. Cancer Res. 1993;53:3046–51. [PubMed] [Google Scholar]
- 25.Jones PA, Taylor SM. Cellular differentiation, cytidine analogs and DNA methylation. Cell. 1980;20:85–93. doi: 10.1016/0092-8674(80)90237-8. [DOI] [PubMed] [Google Scholar]
- 26.Jüttermann R, Li E, Jaenisch R. Toxicity of 5-aza-2′-deoxycytidine to mammalian cells is mediated primarily by covalent trapping of DNA methyltransferase rather than DNA demethylation. Proc Natl Acad Sci USA. 1994;91:11797–801. doi: 10.1073/pnas.91.25.11797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Digel W, Lübbert M. DNA methylation disturbances as novel therapeutic target in lung cancer: preclinical and clinical results. Crit Rev Oncol Hematol. 2005;55:1–11. doi: 10.1016/j.critrevonc.2005.02.002. [DOI] [PubMed] [Google Scholar]