Abstract
Prior exposure of vertebrate hosts to tick salivary proteins can induce specific immunity to tick infestation, as well as affording protection against tick-transmitted Borrelia burgdorferi infection in the mammalian host. Vaccination using an adenovirus expression system to deliver 4 tick salivary proteins (Ad-Salps) derived from Ixodes scapularis, Salp15, Salp25A, Salp25D, and Isac, was explored. Results indicate that vaccination with tick salivary proteins in an adenoviral vector can be used to modulate a Th1 response in the host and partially control spirochete load in immunized mice after infected tick challenge. Published by Elsevier GmbH.
Keywords: Tick salivary proteins, Anti-tick vaccine, Ixodes scapularis, Adenovirus
Introduction
The black-legged tick, Ixodes scapularis, is capable of transmitting several infectious agents including Borrelia burgdorferi, Babesia microti, and Anaplasma phagocytophilum (de la Fuente et al., 2008). Bo. burgdorferi sensu lato is the infectious agent of Lyme disease, the most prevalent tick-borne illness in the United States and certain areas of Eurasia, where the primary vectors are I. ricinus and I. persulcatus (Fikrig and Narasimhan, 2006). The saliva of blood-feeding arthropods, including ticks, is a mixture of pharmacologically active components which are capable of circumventing the host’s hemostatic system and altering the inflammatory and immune response of the host by inhibiting host antibody responses; complement activation; T-cell proliferation; and cytokine elaboration by macrophages and Th1 lymphocytes (Ribeiro, 1995; Wikel, 1999). Tick saliva is important in the transmission of tick-borne pathogens in that it is capable of enhancing pathogen transmission, which has been termed saliva-activated transmission (SAT) (Nuttall and Jones, 1991). One identified molecule from I. scapularis which is involved in enhancing transmission of pathogens is Isac, which can inhibit complement activity in the natural host (Valenzuela et al., 2000). Another identified salivary protein, Salp15, seemingly has several functions; one of which is a CD4+ T cell inhibitor (Anguita et al., 2002). Salp15 can also bind an outer surface protein, OspC, of Bo. burgdorferi, presumably facilitating spirochete survival in the host (Ramamoorthi et al., 2005).
Preventing infection of hosts with Bo. burgdorferi could in theory be prevented by generating a vaccine targeted to tick salivary proteins (de la Fuente et al., 2008; Havlikova et al., 2009; Willadsen, 2004). Previous studies in our laboratory have indicated that preventing a Th2 cytokine response at the time of tick feeding greatly decreases spirochete load in the host (Zeidner et al., 1997), while reconstituting the Th1 arm of the host immune response at the time of tick feeding was successful in blocking tick-transmitted infection (Zeidner et al., 2008). It is our hypothesis that appropriate vaccination with specific I. scapularis salivary proteins should modulate the tick’s ability to drive a potent Th2 inflammatory response and promote a productive Th1 response to block transmission of Bo. burgdorferi.
In this study, we chose to explore the use of replication-incompetent adenovirus (Ad) vectors for vaccine delivery to drive a strong Th1 cytokine response. Ad vectors have a number of advantages, including direct infection of antigen-presenting dendritic cells (Song et al., 1997). It is our belief that utilizing this vaccine vector system in combination with salivary gland proteins will promote a Th1 response which will block or reduce transmission of Bo. burgdorferi.
Methods and materials
Recombinant adenoviruses containing Salp15, Salp25A, Salp25D, or Isac were generated, amplified, and purified by ViraQuest (ViraQuest, North Liberty, IA). Adenovirus vector constructs lacking inserts (AdEmpty) were also acquired (ViraQuest, North Liberty, IA). Four experimental groups of 7 female C3H/HeJ mice, 5–6 weeks of age (Jackson Laboratory, Bar Harbor, ME, USA), each were immunized with either 100 µl of injection buffer (20 mM HEPES, 3% sucrose) (buffer control), 1 × 1010 PFU of AdEmpty (cassette control) in 100 µl injection buffer, 1 × 1010 of AdSalp15 in 100 µl injection buffer, or 1 × 1010 of AdIsac in 100 µl of injection buffer subcutaneously, between the scapulae. Alternatively, 3 experimental groups of 7 C3H/HeJ mice each were given either 100 µl of injection buffer (20 mM HEPES, 3% sucrose) for the buffer control group, 4 × 1010 PFU AdEmpty virus in 100 µl injection buffer, or 1 × 1010 of AdSalp15, AdSalp25A, AdSalp25D, and AdIsac each pooled in 100 µl of injection buffer subcutaneously, between the scapulae. Two weeks post immunization, 5 Bo. burgdorferi B31-infected I. scapularis ticks (Piesman, 1993) were allowed to feed to repletion per mouse. Two weeks post tick drop-off, ear biopsies were taken and cultured to determine the number of infected mice (Sinsky and Piesman, 1989). Blood was collected for Western blot analysis, and sera from individual mice were analyzed with recombinant Salp15 (courtesy of Andrias Hojgaard) or recombinant Salp25A (courtesy of Erol Fikrig) with 1:5000 anti-IgG mouse antibody. SuperSignal West Femto Maximum Sensitivity Chemiluminescent Substrate (Pierce, Rockford, IL) was utilized for staining, and bands were visualized utilizing UVP. Six weeks post tick drop-off, blood was collected for Western blot analysis, and heart and bladder were taken and divided between BSK culture for Borrelia and quantitative PCR for Bo. burgdorferi fliD normalized with mouse "-actin (Zeidner et al., 2001). Spleens from animals were harvested and individual spleens prepared for stimulation and cytokine production as described previously (Zeidner et al., 1997). 1 × 106 splenocytes were stimulated with 2 µg of concanavalin A (Con A), and supernatants were collected at 24 h post stimulation. IL-2, IL-4, IL-5, IFN-γ, and TNF-alpha concentration were determined by cytometric bead array (CBA) for mouse Th1/Th2 cytokines (BD Biosciences, San Jose, CA) on a FACSCaliber flow cytometer (BD Biosciences, San Jose, CA) per manufacturer’s instructions. Data were analyzed with FCAP array software (Soft Flow, Hungary Ltd.) and normalized with unstimulated control splenocytes. The significance of difference of the means of qPCR and CBA results were evaluated using Student’s t-test with GraphPad Prism software (GraphPad Software Inc.). The significance of difference of the means of CBA results was analyzed by one-way ANOVA (GraphPad Software Inc.).
Results
To elucidate what effect individual immunization with AdSalp15 and AdIsac had on spirochete load and cytokine profiles, mice were inoculated with either injection buffer (buffer control), AdEmpty cassette, AdSalp15, or AdIsac. Infected I. scapularis nymphal ticks were placed on each mouse, and replete ticks were pooled by mouse and cultured in BSK media. Cultures were read at 7 days with all tick pools being positive. Ear biopsies of mice were taken at 2 weeks post drop-off, all of which were positive for Bo. burgdorferi.
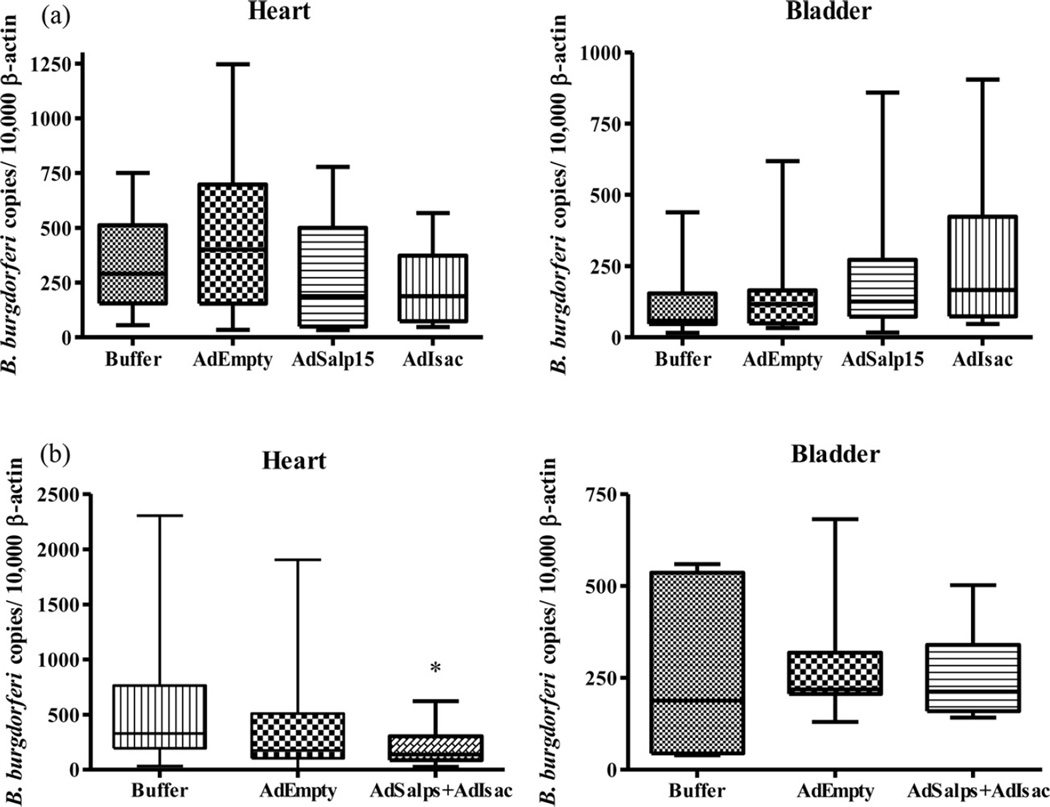
Six weeks post infection; heart and bladder were collected for qPCR and BSK culture. No significant difference was observed in spirochete numbers in heart (p = 0.2714) or bladder (p = 0.2674) between these experimental groups. However, a trend was observed in a reduction of the spirochete load in the hearts and bladders of AdIsac-vaccinated mice as compared to buffer control, AdEmpty control, or AdSalp15 (Fig. 1). The cytokine profiles of AdEmpty and AdSalp15 were similar to that of the buffer control. AdIsac, however, demonstrated a large increase in IFN-γ as compared to all other experimental groups (p = 0.0003), as well as TNF-α (p < 0.0001) and IL-2 (p < 0.0001) (Table 1).
Fig. 1.
qPCR results from heart and bladder 6 weeks post infected ticks fed on mice vaccinated with (a) individual adenovirus constructs challenged with infected tick bite. No results were significantly different. (b) A combination of AdSalp15, AdSalp25A, AdSalp25D, and AdIsac challenged with B. burgdorferi-infected I. scapularis; *p = 0.0121.
Table 1.
Cytokine profiles of splenocytes from mice inoculated with buffer control, AdEmpty control, AdSalp15, AdIsac, or pooled AdSalps at 48 h post stimulation with ConA. AdSalp15 levels were similar to that of the buffer control. AdIsac, however, demonstrated a large and statistically significant increase in IFN-γ, TNF-α, and IL-2 as compared to controls. A statistically significant increase in IFN-γ and TNF–α was observed in pooled AdSalps as compared to controls.
| Buffer | AdEmpty | AdSalp15 | AdIsac | Pooled AdSalps |
|
|---|---|---|---|---|---|
| IFN-γ (pg/mL) | 1289.6 | 1052.4 | 2121.3 | 3147.7** | 2958.7*** |
| TNF-α (pg/mL) | 59.4 | 95.80 | 175.7 | 493.5*** | 385.4* |
| IL-2 (pg/mL) | 172.6 | 196.1 | 67.1 | 635.5*** | 210.1 |
p = 0.0036.
p = 0.0003.
p < 0.0001.
To determine the effect of vaccination with a combination of AdSalp15, AdSalp25A, AdSalp25D, and AdIsac collectively on transmission of Bo. burgdorferi by I. scapularis, mice were inoculated with injection buffer, AdEmpty cassette, or a cocktail of AdSalp15, AdSalp25A, AdSalp25D, and AdIsac. Infected I. scapularis nymphal ticks were placed on each mouse and allowed to feed to repletion. Recovered ticks were weighed, and no significant difference in tick weight or feeding duration was observed. Ticks were subsequently pooled by mouse and were cultured in BSK media. Cultures were read at 7 days with all tick pools being positive. Ear biopsies of mice were taken at 2 weeks post drop-off, all of which were positive for Bo. burgdorferi.
Six weeks post infection; heart and bladder were collected for qPCR and BSK culture. Significant differences in spirochete numbers in the heart (p = 0.0121) were observed at a rate of 59.7% as compared to buffer controls, but not in the bladder (p = 0.9058) (Fig. 1). At six weeks post infection, spleens were collected for cytokine profiles, and serum was collected. Cytokine profiles were similar to what was seen previously, with an increase of IFN-γ in mice inoculated with AdEmpty cassette versus buffer control, and a greater and statistically significant increase in IFN-γ inAdSalp15, AdSalp25A, AdSalp25D, and AdIsac versus buffer control and AdEmpty cassette at 48 h post stimulation (p < 0.0001) as well as TNF-α (p = 0.0036) (Table 1).
Discussion
Earlier unpublished work in our laboratory demonstrated that a host immune response to tick recombinant antigens could affect subsequent tick feeding. Mice were immunized with a combination of recombinant Salp14, Salp15, Salp25A, and Salp25D. A 60% reduction in fed tick weight was observed, but no effect on transmission of Bo. burgdorferi was seen (unpublished data).
Recently published research utilizing passive transfer of immunity to Salp15 from rabbits into mice demonstrated 40% protection from Bo. burgdorferi infection. Active immunization with recombinant Salp15 also demonstrated 40% protection in mice (Dai et al., 2009). In these current studies, we demonstrate a 60% reduction in the number of spirochetes using a single dose of Advectored Salp proteins including Salp15 and Isac. When Salp15 and Isac were examined individually, AdSalp15 did not reduce the spirochete burden as compared with injection buffer control or AdEmpty control, but AdIsac showed a trend in the reduction of spirochetes. Although Salp15 is a seemingly obvious target molecule for vaccination given the multitude of effects it has on host immunity and in the transmission of Bo. burgdorferi in our hands with the current system, it does not have an effect in the reduction of spirochetes in the vertebrate host, and may have impaired induction of a Th1 response. Isac, however, may be of greater interest and is worth exploring with other delivery mechanisms or boosting strategies. When the ability of AdSalp15 and AdIsac to stimulate an inflammatory response as measured by IFN-γ and TNF-α levels from cultured splenocytes was measured, we observed a marked increase of these 2 cytokines as compared to buffer control and AdEmpty control. When AdSalp15 and AdIsac were investigated individually, AdIsac demonstrated the largest increase of pro-inflammatory cytokines. This result encourages further work with Isac in combination with other molecules that drive a Th1 response as an anti-tick vaccine candidate.
References
- 1.Anguita J, Ramamoorthi N, Hovius JW, Das S, Thomas V, Persinski R, Conze D, Askenase PW, Rincon M, Kantor FS, Fikrig E. Salp15, an Ixodes scapularis salivary protein, inhibits CD4(+) T cell activation. Immunity. 2002;16:849–859. doi: 10.1016/s1074-7613(02)00325-4. [DOI] [PubMed] [Google Scholar]
- 2.Dai JF, Wang PH, Adusumilli S, Booth CJ, Narasimhan S, Anguita J, Fikrig E. Antibodies against a tick protein, Salp15, protect mice from the Lyme disease agent. Cell Host and Microbe. 2009;6:482–492. doi: 10.1016/j.chom.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de la Fuente J, Kocan KM, Almazan C, Blouin EF. Targeting the tick-pathogen interface for novel control strategies. Front. Biosci. 2008;13:6947–6956. doi: 10.2741/3201. [DOI] [PubMed] [Google Scholar]
- 4.Fikrig E, Narasimhan S. Borrelia burgdorferi– traveling incognito? Microbes Infect. 2006;8:1390–1399. doi: 10.1016/j.micinf.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 5.Havlikova S, Roller L, Koci J, Trimnell AR, Kazimirova M, Klempa B, Nuttall PA. Functional role of 64P, the candidate transmission-blocking vaccine antigen from the tick,Rhipicephalus appendiculatus. Int. J. Parasitol. 2009;39:1485–1494. doi: 10.1016/j.ijpara.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 6.Nuttall PA, Jones LD. Non-viraemic tick-borne virus transmission: mechanisms and significance. In: Dusbabek F, Bukva V, editors. Modern Acarology. The Hague: Academia Prague and SPB Academic Publishing; 1991. pp. 3–6. [Google Scholar]
- 7.Piesman J. Standard system for infecting ticks (Acari: Ixodidae) with the Lyme disease spirochete,Borrelia burgdorferi. J. Med. Entomol. 1993;30:199–203. doi: 10.1093/jmedent/30.1.199. [DOI] [PubMed] [Google Scholar]
- 8.Ramamoorthi N, Narasimhan S, Pal U, Bao F, Yang XF, Fish D, Anguita J, Norgard MV, Kantor FS, Anderson JF, Koski RA, Fikrig E. The Lyme disease agent exploits a tick protein to infect the mammalian host. Nature. 2005;436:573–577. doi: 10.1038/nature03812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ribeiro JM. Blood-feeding arthropods: live syringes or invertebrate pharmacologists? Infect. Agents Dis. 1995;4:143–152. [PubMed] [Google Scholar]
- 10.Sinsky RJ, Piesman J. Ear punch biopsy method for detection and isolation of Borrelia burgdorferi from rodents. J. Clin. Microbiol. 1989;27:1723–1727. doi: 10.1128/jcm.27.8.1723-1727.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song W, Kong HL, Carpenter H, Torii H, Granstein R, Rafii S, Moore MA, Crystal RG. Dendritic cells genetically modified with an adenovirus vector encoding the cDNA for a model antigen induce protective and therapeutic antitumor immunity. J. Exp. Med. 1997;186:1247–1256. doi: 10.1084/jem.186.8.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Valenzuela JG, Charlab R, Mather TN, Ribeiro JM. Purification, cloning, and expression of a novel salivary anticomplement protein from the tick,Ixodes scapularis. J. Biol. Chem. 2000;275:18717–18723. doi: 10.1074/jbc.M001486200. [DOI] [PubMed] [Google Scholar]
- 13.Wikel SK. Tick modulation of host immunity: an important factor in pathogen transmission. Int. J. Parasitol. 1999;29:851–859. doi: 10.1016/s0020-7519(99)00042-9. [DOI] [PubMed] [Google Scholar]
- 14.Willadsen P. Anti-tick vaccines. Parasitology. 2004;129(Suppl.):S367–S387. doi: 10.1017/s0031182003004657. [DOI] [PubMed] [Google Scholar]
- 15.Zeidner N, Mbow ML, Dolan M, Massung R, Baca E, Piesman J. Effects of Ixodes scapularis and Borrelia burgdorferi on modulation of the host immune response: induction of a TH2 cytokine response in Lyme disease-susceptible (C3H/HeJ) mice but not in disease-resistant (BALB/c) mice. Infect. Immun. 1997;65:3100–3106. doi: 10.1128/iai.65.8.3100-3106.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zeidner NS, Schneider BS, Dolan MC, Piesman J. An analysis of spirochete load, strain, and pathology in a model of tick-transmitted Lyme borreliosis. Vector Borne Zoonotic Dis. 2001;1:35–44. doi: 10.1089/153036601750137642. [DOI] [PubMed] [Google Scholar]
- 17.Zeidner NS, Schneider BS, Rutherford JS, Dolan MC. Suppression of Th2 cytokines reduces tick-transmitted Borrelia burgdorferi load in mice. J. Parasitol. 2008;94:767–769. doi: 10.1645/GE-1416.1. [DOI] [PubMed] [Google Scholar]