Abstract
Objective
Higher hemoglobin A1c (HbA1c) is associated with lower cognitive function in type 2 diabetes. To determine if associations persist at lower levels of dysglycemia in patients who have established cardiovascular disease, cognitive performance was assessed in the Targeting Inflammation Using Salsalate in Cardiovascular Disease (TINSAL-CVD) trial.
Research Design and Methods
The age-adjusted relationships between HbA1c and cognitive performance measured by the Mini-mental State Examination (MMSE), Digit Symbol Substitution Test (DSST), Rey Auditory Verbal Learning Test (RAVLT), Trail Making Test (TMT), and Categorical Verbal Fluency (CVF) were assessed in 226 men with metabolic syndrome and established stable coronary artery disease.
Results
61.5% of participants had normoglycemia, 20.8% impaired fasting glucose, and 17.7% type 2 diabetes. HbA1c was associated with cognitive function tests of DSST, RAVLT, TMT and CVF (all P<0.02), but not MMSE. In an age-adjusted model, a 1% (11 mmol/mol) higher HbA1c value was associated with a 5.9 lower DSST score (95%CI: −9.58 to −2.21; P<0.0001); a 2.44 lower RAVLT score (95%CI: −4.00 to −0.87; P<0.0001); a 15.6 higher TMT score (95%CI: 5.73 to 25.6; P<0.0001); and a 3.71 lower CVF score (95%CI: −6.41 to −1.01; P<0.02). In multivariate model adjusting for age, education and cardiovascular covariates, HbA1c remains associated with cognitive function tests of RAVLT (R2=0.27, P<0.0001), TMT (R2=0.18, P<0.0001), and CVF (R2=0.20, P<0.0001) although association with DSST was reduced.
Conclusion
Higher HbA1c is associated with lower cognitive function performance scores across multiple domain tests in men with metabolic syndrome and coronary artery disease. Future studies may demonstrate whether glucose lowering within the normative range improves cognitive health.
Keywords: Cognitive Function, Hemoglobin A1c, glycemia, cardiovascular disease
Introduction
Mild cognitive impairment is common and may precede frank dementia. About 19% of persons above age 65 years and 29% above 85 years have mild cognitive impairment 1, representing a substantial population health issue among older persons. Persons with coronary artery disease and those with type 2 diabetes are both at higher risk of cognitive impairment 2–4. More patients with cardiovascular disease have dysglycemia, diabetes or prediabetes, than normoglycemia 5.
Cognitive function is associated with glycemia in patients with type 1 or type 2 diabetes 6–8. Cognitive function declines with acute hyperglycemia 9 or hypoglycemia 10, 11. Working memory may improve in patients with type 2 diabetes with improving metabolic control 12. The Memory in Diabetes (MIND) substudy of the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial established an association between higher age-adjusted HbA1c and lower cognitive function in patients with type 2 diabetes 13 at high cardiovascular risk and with HBA1c above 7.5% (58.5 mmol/mol) at study entry. As dysglycemia is highly prevalent in patients with cardiovascular disease, we sought to determine if the association between glucose and cognitive dysfunction was also present at lower levels of dysglycemia than in the ACCORD study population, as this could have substantial impact on general health of patients with coronary heart disease, including medication adherence and quality of life. Thus, we evaluated the relationship between HbA1c, and cognition in a complementary cohort to the ACCORD-mind with stable coronary artery disease and HbA1c below 7.5% (58.5 mmol/mol), spanning the range from normal to pre-diabetes and well-controlled diabetes.
Research Design and Methods
Study was approved by the Joslin Diabetes Center Institutional Review Board. Subjects provided informed written consent. This study was conducted as an ancillary investigation in the trial Targeting INflammation Using SALsalate in CardioVascular Disease (TINSAL-CVD, ClinicalTrials.gov Identifier: NCT00624923). The aim of the parent study is to determine efficacy of targeting inflammation using salsalate to reduce progression of non-calcified coronary artery plaque volume assessed by multi-detector computed tomography angiography over 30 months. A sub-aim of the study is to assess the effects of targeting inflammation on cognitive function. Only baseline data was used in this analysis.
Participants include community-dwelling adult males with metabolic syndrome, fluent in the English language, under the age of 75 years, with body mass index between 27–40 kg/m2, metabolic syndrome, and established coronary artery disease including previous myocardial infarction or coronary artery bypass, stable angina, abnormal cardiac exercise or pharmacologic stress test, or plaque by prior imaging in at least one coronary artery. All participants were using statin class agents, and had estimated Cockcroft-Gault creatinine clearance above 60 ml/min 14. Persons with prior stroke, malignancy, tinnitus, gastric bypass surgery, gastrointestinal bleeding, alcohol use exceeding 14 units/week, using chronic thiazolidinediones, insulin, glucagon-like peptide-1 agonists, corticosteroids, nonsteroidal anti-inflammatory drugs, warfarin, or uricosuric agents, were excluded from the parent study. Women represent under 6% of the parent study population, so were excluded from sub-study analysis. Participants with poor glycemic control (HbA1c above 7.5% (58.5 mmol/mol)) were excluded a priori to maintain focus of investigation on persons spanning normal to moderate dysglycemia. The mean of three blood pressure measurments was used. Blood was collected after overnight fast for HbA1c, glucose, lipids, and creatinine (Quest Laboratories, Cambridge, MA). Table 1 summarizes cognitive measurement tools performed by a trained study coordinator after participants had a light standardized meal.
Table 1. Cognitive Function Tests Administered.
A description of the cognitive function tools, functional domains evaluated in the tests, and scoring process is provided.29
| Cognitive Function Test | Acronym | Test Assessment | Scoring |
|---|---|---|---|
| Mini-Mental State Exam | MMSE | Brief screen for dementia-orientation to time and place, memory, attention, calculation, language and visual-spatial skills | Number of correctly completed questions of problems answered correctly out of possible total of 30 |
| Digit Symbol Substitution Test | DSST | Psychomotor performance including sustained attention, response speed and visuo-motor coordination | Number of symbols correctly matched with their corresponding digit in a minute |
| Rey Auditory Verbal Learning Test | RAVLT | Immediate verbal memory and learning | Average number of words recalled (0–15) over the immediate (reported as sum of four trials), short, and delayed recall trials |
| Trail Making Test | TMT | Complex visual scanning, attention and ability to shift between the tasks | Subject must first connect consecutively numbered circles (Part A) and then connect the same number of consecutively numbered and lettered circles alternating between the two sequences (Part B) |
| Categorical Verbal Fluency | CVF | Language, memory and fluency of speech | Number of items from each category (animals and supermarket items) named in 60 seconds |
| Short-Form (36) Health Survey | SF-36 | Patient Reported Outcomes of health reflecting aspects of physical function, mental health, and quality of life | Self-administered 36 questions survey |
(ref: Lezak MD: Neuropsychological Assessment. New York, NY, Oxford University Press, 2004)
Statistical Methods
Linear regression was used to assess the relationship of each measure of cognitive status with HbA1c, and control for potential confounding factors, including age, education, smoking status, body mass index (BMI0, blood pressure, non-high density lipoprotein (HDL) cholesterol, short form-36 (SF-36) Mental Score, and history of depression. The age-adjusted relationship between HbA1c and cognitive measure was the primary endpoint (Model 1). The age-adjusted analysis was repeated in a sub-set excluding those with type 2 diabetes (Model 2). Model 3 included age and education adjustment. Model 4 included all covariates listed above. β-coefficient estimates are provided with 95% confidence limits and as standardized estimates. P-values below 0.05 were considered significant. All analyses were performed using SAS 9.2 (SAS Institute, Cary, NC, USA).
Results
Demographic and clinical characteristics of study participants are described in Table 2. 61.5% had normoglycemia, 20.8% impaired fasting glucose, and 17.7% type 2 diabetes. 97.3% of participants had normal cognition based on Mini Mental State Examination scores of 25 or above, and no participant had scores consistent with moderate or severe dementia. HbA1c was not associated with the Mini Mental State Examination score in any model. However, in bivariate analysis, HbA1c was associated with scores on Digit Symbol, RAVLT Word Learning, Trailmaking B and Categorical Verbal Fluency (all P<0.02) (Figure 1). In models including HbA1c and age (the primary endpoint) (Table 3, Model 1), the variance explained by the models for these four cognitive tests improved compared with HbA1c alone, and higher HbA1c remains associated with lower cognitive function. Specifically in the age-adjusted model for the full population a 1% higher HbA1c value was associated with a 5.9 lower Digit Symbol score (95% CI: −9.58 to −2.21; P<0.0001); 2.44 lower RAVLT Word Learning score (95%CI: −4.00 to −0.87; P<0.0001); 15.6 higher Trailmaking B score (95%CI: 5.73 to 25.6 P<0.0001); and 3.71 lower Categorical Verbal Fluency test score (95%CI: −6.41 to −1.01; P<0.02). Considering only the sub-cohort without diabetes, in age-adjusted models higher HbA1c remained associated with lower cognitive function in Digital Symbol, Rey Word Learning, and Trailmaking B scores, although significance was not retained for Categorical Verbal Fluency (Table 3, Model 2).
Table 2. Baseline Characteristics of TINSAL-CVD male participants with cognitive function tests.
Continuous data are presented as the mean and standard deviation or median and interquartile range and categorical data as counts and percentages.
| Variable | Result | Conventional Unit |
|---|---|---|
| N | 226 | |
| Male sex (%) | 226 (100.0) | |
| Race/Ethnicity | ||
| - Caucasian | 212 (93.8) | |
| - African American | 4 (1.8) | |
| - Asian | 5 (2.2) | |
| - Multi-Racial | 5 (2.2) | |
| Age (years) | 61 ± 6.9 | |
| Weight (kg) | 96.9 ± 12.0 | |
| BMI (kg/m2) | 31.4 ± 3.0 | |
| Waist Circumference (cm) | 107.7 ± 8.6 | |
| Blood Pressure | ||
| - Systolic (mmHg) | 128 ± 12.7 | |
| - Diastolic (mmHg) | 75 ± 8.0 | |
| - Mean Arterial Pressure (mmHg)a | 93 ± 8.5 | |
| - Heart Rate (bpm) | 61 ± 9.6 | |
| Glycemiab | ||
| - Normal Glucose Tolerance | 139 (61.5) | |
| - Impaired Fasting Glucose | 47 (20.8) | |
| - Type 2 Diabetes | 40 (17.7) | |
| Cardiac Risk Factor History | ||
| - Hypertension | 153 (67.7) | |
| - High LDL Cholesterol | 200 (88.5) | |
| - Low HDL Cholesterol | 173 (76.6) | |
| - High Triglycerides | 140 (62.0) | |
| - Smoking Statusc | ||
| ○ Current Smoker | 37 (16.4) | |
| ○ Former Smoker | 64 (28.3) | |
| ○ Non Smoker | 125 (55.3) | |
| Past Medical/Surgical History | ||
| - Coronary Heart Disease | ||
| ○ Previous Myocardial Infarction | 141 (62.4) | |
| ○ Stable Angina | 89 (39.4) | |
| ○ Angioplasty/Stent | 152 (67.3) | |
| ○ Previous Coronary Artery Bypass Surgery | 54 (23.9) | |
| ○ Abnormal Exercise Tolerance Test | 88 (38.9) | |
| ○ Significant Non-Calcified Plaque | 5 (2.21) | |
| - Vascular Disease | ||
| ○ Stroke | 4 (1.8) | |
| ○ Transient Ischemic Attack | 3 (1.3) | |
| ○ Carotid Vascular Disease | 6 (2.7) | |
| ○ Carotid Endartectomy | 3 (1.3) | |
| ○ Peripheral Vascular Disease | 9 (4.0) | |
| ○ Peripheral Artery Bypass Surgery | 3 (1.3) | |
| ○ Peripheral Artery Angioplasty | 3 (1.3) | |
| - Psychologicald | ||
| ○ Depression | 37 (16.4) | |
| ○ Counseling for Psychological Problems | 25 (11.1) | |
| ○ Medicines for Psychological Problems | 22 (9.8) | |
| ○ Anxiety | 9 (4.0) | |
| Years of School Completed | ||
| - 11–14 | 71 (31.7) | |
| - 15–18 | 116 (51.8) | |
| - 19–22 | 31 (13.8) | |
| - 23–26 | 6 (2.7) | |
| - Unknown | 2 (0.9) | |
| Laboratory Results | ||
| - Glucose (mmol/L) | 5.49 | 99.0± 18.1 (mg/dL) |
| - Hemoglobin A1c (mmol/mol) | 41.0 | 5.9 ± 0.49 (%) |
| - Lipid Profile (mmol/L) | ||
| ○ Total Cholesterol | 3.90 | 150.8 ± 31.1 (mg/dl) |
| ○ HDL Cholesterol | 1.14 | 44.1 ± 11.3 (mg/dl) |
| ○ LDL Cholesterol | 2.03 | 78.4 ± 25.7 (mg/dl) |
| ○ Triglycerides | 1.63 | 144.1 ± 90.3 (mg/dl) |
| ○ Non HDL Cholesterole | 5.9 | 106.7 ± 29.7 (mg/dl) |
| - Serum Creatinine (mg/dL) | 84.9 | 0.96 ± 0.17 (mg/dl) |
| - Estimated Creatinine Clearance (mL/s)f | 1.90 | 114.0 ± 27.4 (ml/min) |
| - Microalbumin Creatinine Ratio | ||
| ○ Normal (<3.39 mg/mmol creatinine) g | 214 (95.5) | |
| ○ Microalbuminia(3.39–33.8mg/mmol creatinine)h | 10 (4.5) | |
| SF-36 Health Survey Score | ||
| - Physical Health (0–100) | 81 (73–88) | |
| - Mental Health (0–100) | 86 (78–90) | |
| - Total SF-36 (0–100) | 86 (79–91) | |
| Cognitive Function | ||
| - Mini-Mental State Examination | 29 (28–30) | |
| - Digit Symbol Substitution Test | 61 (53–71) | |
| - Rey Auditory Verbal Learning Test | ||
| ○ Sum of the First 4 Trials on List A | 30 (26–35) | |
| ○ Short Delay for List A | 6 (5–8) | |
| ○ Delayed Recall of List A | 6 (5–8) | |
| ○ Delayed Recognition of List A | 23 (22–24) | |
| - Trail Making Tests | ||
| ○ Trail A (in seconds) | 29 (24–36) | |
| ○ Trail B (in seconds) | 72 (57–100) | |
| - Categorical Verbal Fluency | ||
| ○ Score 1 – Sum of Animals | 20 (17–24) | |
| ○ Score 2 – Sum of Supermarket Items | 25 (21–29) | |
| ○ Score 3 – Sum of Score 1 and Score 2 | 45 (39–52) | |
| ○ Score 4 – Average of Score 1 and Score 2 | 23 (20–26) | |
Data are means ± SD, n (%) or median (25th–75th percentile).
Mean arterial pressure: [(2*Diastolic) + Systolic]/3
Normal glucose tolerance determined by fasting glucose <5.55 mmol/L (100 mg/dl) and HbA1c <6.5% (47.5 mmol/mol); Impaired fasting glucose determined by fasting glucose between 5.55 mmol/L and 6.94 mmol/L (100 to 126 mg/dl) and HbA1c <6.5% (47.5 mmol/mol); and type 2 diabetes determined by medical history of diagnosis or fasting glucose ≥6.94 mmol/L (126 mg/dl) or HbA1c ≥6.5% (47.5 mmol/mol)
Smoking: if stopped 15 years or more then not a smoker
Self-reported: past medical history self-report of psychological conditions
Non-HDL Cholesterol = Total Cholesterol − HDL Cholesterol
Cockroft-Gault creatinine clearance = ((140-age) × weight (kg))/plasma creatinine × 72 for men (normal 95–145 ml/min)
less than 30 μg/mg creatinine
30–299 μg/mg creatinine
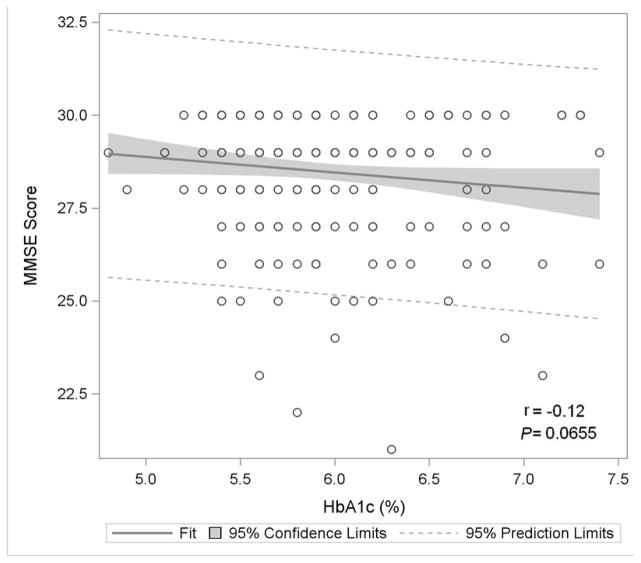
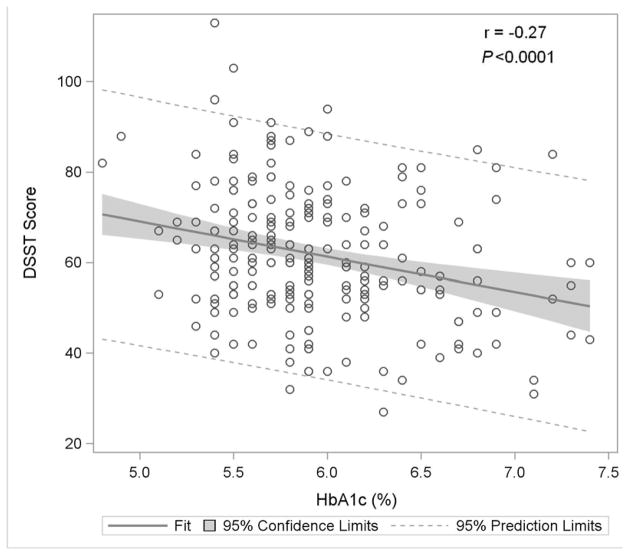
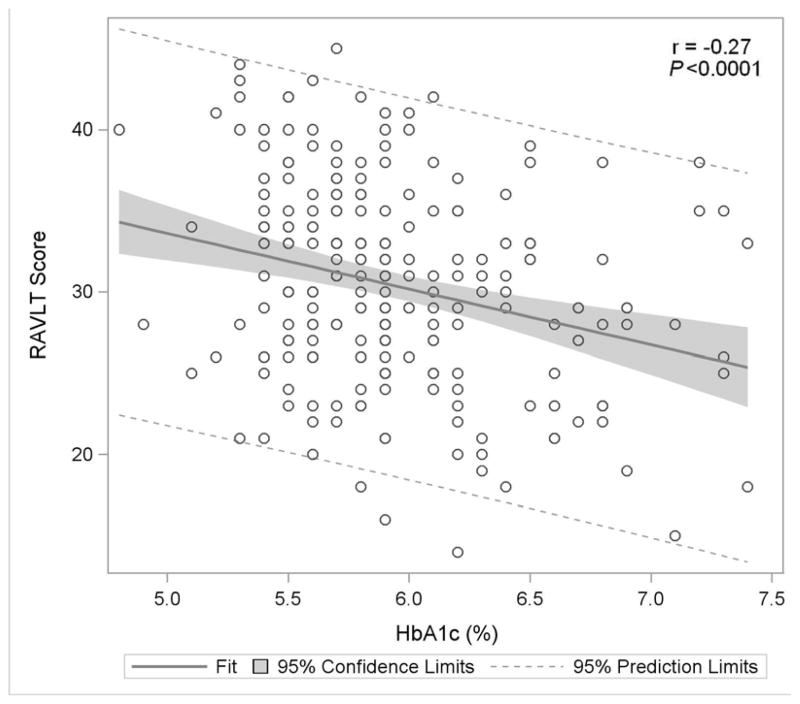
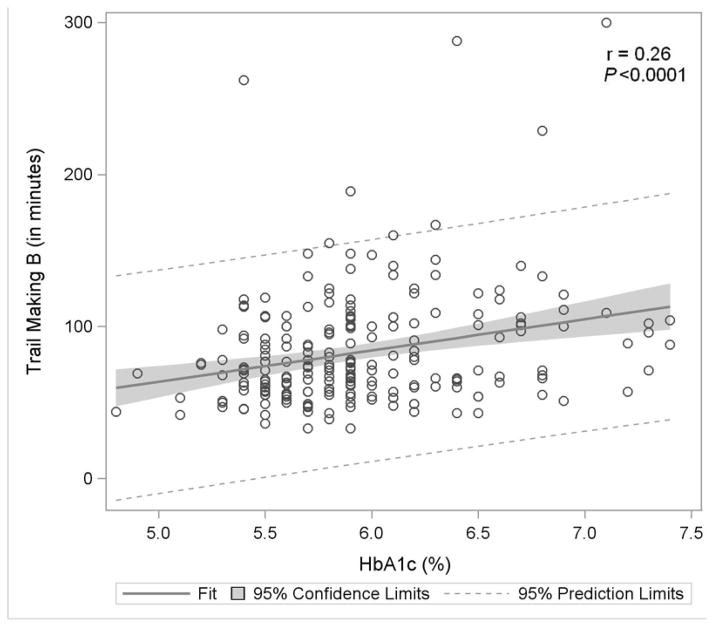
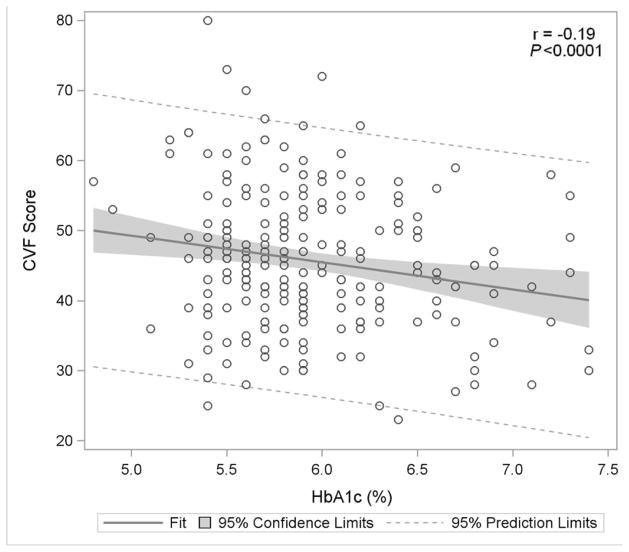
Figure 1. Association of Glycemia with Measures of Cognitive Function.
Figure 1A: Mini-Mental State Exam
Figure 1B: Digit Symbol Substitution Test
Figure 1C: Rey Auditory Verbal Learning Test
Figure 1D: Trail Making Test B
Figure 1E: Categorical Verbal Fluency
Figure 1 displays in the full study cohort population scatterplots showing correlation, fitted regression, and 95% confidence intervals relating Hemoglobin A1c and cognitive function tests [A] Displays the fit plot for regression of Mini-mental state examination (MMSE) and Hemoglobin A1c (HbA1c). There is no association between HbA1c and Mini-Mental State Examination score (P=0.07). [B] Displays the fit plot regression for Digit Symbol Substitution Test (DSST) and HbA1c. The average DSST score of a patient changes by β̂ =−7.79 units for each unit change in HbA1c (r=−0.27, P<0.0001), [C] Displays the fit plot for regression of Rey Auditory Verbal Learning Test (RAVLT) and HbA1c. The average RAVLT score of a patient changes by β̂= −3.44 units for each unit change in HbA1c (r=−0.27, P <0.0001). [D] Displays the fit plot for regression of Trail Making B and HbA1c, The average Trail Making B score of a patient changes by β̂=20.6 units for each unit change in HbA1c (r=0.27, P<0.0001) and [E] Displays the fit plot for regression of Categorical Verbal Fluency (CVF) and HbA1c. The average CVF score of a patient changes by β̂= −3.82 units for each unit change in HbA1c (r=−0.19, P=0.0042). To convert HbA1c: HbA1c(%) = [0.09148 * HbA1c (mmol/mol)] + 2.152.
Table 3. Relationship between Cognitive Function Tests and Hemoglobin A1C in Multivariate Analysis Adjusted for Age, and Age and Education.
Association of glycemia with measures of cognitive function in
[A] Model 1: model adjusted for age for full study cohort population
[B] Model 2: model adjusted for age for population excluding type 2 diabetes
[C] Model 3: model adjusted for age and education for full study cohort population
| Model 1: Age Adjusted Model (full study cohort) | ||||||
|---|---|---|---|---|---|---|
| Outcome Variable | R2 | Model P-value | Covariates | β (95 CI) | Standardized Estimate | Covariate P-Value |
| MMSE | 0.06 | 0.0013 | HbA1c | −0.23 (−0.68, 0.22) | −0.07 | 0.313 |
| Age | −0.05 (−0.08, −0.02) | −0.21 | 0.002 | |||
| DSST | 0.13 | <0.0001 | HbA1c | −5.90 (−9.58, −2.21) | −0.20 | 0.002 |
| Age | −0.52 (−0.79, −0.26) | −0.26 | 0.0001 | |||
| RAVLT | 0.17 | <0.0001 | HbA1c | −2.44 (−4.00, −0.87) | −0.19 | 0.002 |
| Age | −0.28 (−0.39, −0.17) | −0.31 | <0.0001 | |||
| Trail Making B | 0.13 | <0.0001 | HbA1c | 15.6 (5.73, 25.6) | 0.20 | 0.002 |
| Age | 1.37 (0.67, 2.08) | 0.25 | 0.0001 | |||
| CVF | 0.04 | 0.0160 | HbA1c | −3.71 (−6.41, −1.01) | −0.18 | 0.007 |
| Age | −0.03 (−0.22, 0.16) | −0.02 | 0.750 | |||
| Model 2: Age Adjusted Model (study population excluding type 2 diabetes) | ||||||
|---|---|---|---|---|---|---|
| Outcome Variable | R2 | Model P-value | Covariates | β (95 CI) | Standardized Estimate | Covariate P-Value |
| MMSE | 0.04 | 0.036 | HbA1c | −0.48 (−1.28, 0.31) | −0.09 | 0.23 |
| Age | −0.03 (−0.07, 0.003) | −0.14 | 0.08 | |||
| DSST | 0.09 | 0.0002 | HbA1c | −7.28 (−14.2, −0.41) | −0.16 | 0.0378 |
| Age | −0.42 (−0.73, −0.11) | −0.20 | 0.0075 | |||
| RAVLT | 0.13 | <0.0001 | HbA1c | −3.51 (−6.4, −0.61) | −0.18 | 0.0179 |
| Age | −0.23 (−0.36, −0.10) | −0.26 | 0.0006 | |||
| Trail Making B | 0.10 | 0.0001 | HbA1c | 17.4 (0.29, 34.6) | 0.15 | 0.0463 |
| Age | 1.18 (0.41, 1.95) | 0.23 | 0.0029 | |||
| CVF | 0.02 | 0.22 | HbA1c | −4.07 (−9.11, 0.98) | −0.12 | 0.11 |
| Age | −0.02 (−0.25, 0.21) | −0.01 | 0.86 | |||
| Model 3: Age and Education Adjusted Model (full study cohort) | ||||||
|---|---|---|---|---|---|---|
| Outcome Variable | R2 | Model P-value | Covariates | β (95 CI) | Standardized Estimate | Covariate P-Value |
| MMSE | 0.09 | <0.0001 | HbA1c | −0.098 (−0.55, 0.36) | −0.03 | 0.673 |
| Age | −0.06 (−0.09, −0.03) | −0.24 | 0.001 | |||
| Education | 0.11 (0.03, 0.19) | 0.19 | 0.003 | |||
| DSST | 0.23 | <0.0001 | HbA1c | −3.88 (−7.46, −0.31) | −0.13 | 0.033 |
| Age | −0.60 (−0.85, −0.35) | −0.29 | <0.0001 | |||
| Education | 1.63 (1.03, 2.23) | 0.32 | <0.0001 | |||
| RAVLT | 0.24 | <0.0001 | HbA1c | −1.65 (−3.18, −0.13) | −0.13 | 0.033 |
| Age | −0.31 (−0.42, −0.22) | −0.35 | <0.0001 | |||
| Education | 0.58 (0.33, 0.84) | 0.27 | <0.0001 | |||
| Trail Making B | 0.15 | <0.0001 | HbA1c | 13.2 (3.17, 23.3) | 0.17 | 0.010 |
| Age | 1.51 (0.78, 2.19) | 0.27 | <0.0001 | |||
| Education | −2.08 (−3.96, −0.56) | −0.15 | 0.016 | |||
| CVF | 0.16 | <0.0001 | HbA1c | −2.34 (−4.93, 0.25) | −0.11 | 0.079 |
| Age | −0.10 (−0.28, 0.08) | −0.07 | 0.284 | |||
| Education | 1.27 (0.83, 1.70) | 0.36 | <0.0001 | |||
Likewise in models adjusting for age and education (Table 3, Model 3), the model predictive values are improved for these four cognitive tests compared with HbA1c alone, and HbA1c as a covariate remains associated with cognitive function, with the exception of Categorical Verbal Fluency where significance for HbA1c is reduced.
In a model adjusted for age, education, age and cardiovascular and depression covariates (Table 4, Model 4), HbA1c remains associated with cognitive function tests of Rey Word Learning, Trail Making, and Categorical Verbal Fluency (all P<0.0001), although association with Digital Symbol score was reduced. Furthermore, in standardized parameter estimates HbA1c was the top ranking covariate, after age and education, associated with cognitive function for each test.
Table 4. Relationship between Cognitive Function Tests and Hemoglobin A1C in Multivariate Analysis Adjusted for Age, Education and Coronary Risk Factors.
Association of glycemia with measures of cognitive function adjusted for (Model 4) age, education, mean arterial pressure, smoking status, body mass index (BMI), non-high density lipoprotein (HDL) Cholesterol, Short Form-36 (SF-36) mental health score, and past medical history of depression in the full study cohort population.
| Model 4: Fully Adjusted Model (full study cohort)
| ||||||
|---|---|---|---|---|---|---|
| Outcome Variable | R2 | Model P-value | Covariates | β (95 CI) | Standardized Estimate | Covariate P-Value |
| MMSE | 0.11 | <0.0035 | HbA1c | −0.15 (−0.63, 0.33) | −0.04 | 0.539 |
| Age | −0.06 (−0.09, −0.03) | −0.24 | 0.001 | |||
| Education | 0.10 (0.02, 0.18) | 0.17 | 0.014 | |||
| Mean Arterial Pressure | 0.01 (−0.02, 0.04) | 0.06 | 0.408 | |||
| Non-HDL-C | −0.00 (−0.01, −0.01) | −0.00 | 0.991 | |||
| Smoking Status | 0.03 (−0.28, 0.34) | 0.01 | 0.849 | |||
| BMI | −0.05 (−0.12, 0.02) | −0.09 | 0.179 | |||
| SF-36 Mental | −0.00 (−0.02, 0.01) | −0.03 | 0.646 | |||
| Depression | −0.06 (−0.66, 0.55) | −0.01 | 0.851 | |||
|
| ||||||
| DSST | 0.26 | <0.0001 | HbA1c | −3.60 (−7.31, 0.11) | −0.12 | 0.057 |
| Age | −0.66 (−0.92, −0.40) | −0.32 | <0.0001 | |||
| Education | 1.57 (0.94, 2.20) | 0.31 | <0.0001 | |||
| Mean Arterial Pressure | 0.13 (−0.08, 0.33) | 0.07 | 0.23 | |||
| Non-HDL-C | −0.05 (−0.11, 0.01) | −0.11 | 0.08 | |||
| Smoking Status | 0.69 (−1.68, 3.06) | 0.04 | 0.57 | |||
| BMI | 0.13 (−0.43, 0.68) | 0.03 | 0.66 | |||
| SF-36 Mental | 0.02 (−0.11, 0.14) | 0.02 | 0.81 | |||
| Depression | −2.45 (−7.13, 2.24) | −0.07 | 0.30 | |||
|
| ||||||
| RAVLT | 0.27 | <0.0001 | HbA1c | −1.58 (−3.16, −0.01) | −0.12 | 0.049 |
| Age | −0.32 (−0.43, −0.21) | −0.36 | <0.0001 | |||
| Education | 0.60 (0.33, 0.87) | 0.28 | <0.0001 | |||
| Mean Arterial Pressure | 0.11 (0.02, 0.20) | 0.14 | 0.018 | |||
| Non-HDL-C | 0.00 (−0.02, 0.03) | 0.02 | 0.706 | |||
| Smoking Status | −0.04 (−1.05, 0.97) | −0.00 | 0.941 | |||
| BMI | −0.13 (−0.37, 0.11) | −0.06 | 0.283 | |||
| SF-36 Mental | 0.03 (−0.03, 0.08) | 0.06 | 0.335 | |||
| Depression | −0.41 (−2.40, 1.59) | −0.03 | 0.688 | |||
|
| ||||||
| Trail Making B | 0.18 | <0.0001 | HbA1c | 12.0 (1.43, 22.5) | 0.15 | 0.026 |
| Age | 1.63 (0.90, 2.37) | 0.29 | <0.0001 | |||
| Education | −1.79 (−3.57, −0.01) | −0.13 | 0.049 | |||
| Mean Arterial Pressure | 0.42 (−0.16, 1.01) | 0.09 | 0.157 | |||
| Non-HDL-C | 0.13 (−0.03, 0.29) | 0.10 | 0.121 | |||
| Smoking Status | −1.50 (−8.22, 5.22) | −0.03 | 0.661 | |||
| BMI | −0.26 (−1.85, 1.32) | −0.02 | 0.743 | |||
| SF-36 Mental | −0.06 (−0.43, 0.30) | −0.02 | 0.730 | |||
| Depression | 0.36 (−12.9, 13.6) | 0.00 | 0.957 | |||
|
| ||||||
| CVF | 0.20 | <0.0001 | HbA1c | −2.84 (−5.5, −0.16) | −0.14 | 0.038 |
| Age | −0.11 (−0.30, 0.07) | −0.08 | 0.230 | |||
| Education | 1.44 (0.99, 1.89) | 0.41 | <0.0001 | |||
| Mean Arterial Pressure | 0.10 (−0.05, 0.24) | 0.08 | 0.211 | |||
| Non-HDL-C | 0.01 (−0.03, 0.06) | 0.04 | 0.509 | |||
| Smoking Status | −1.62 (−3.3, 0.09) | −0.12 | 0.063 | |||
| BMI | 0.16 (−0.24, 0.57) | 0.05 | 0.428 | |||
| SF-36 Mental | −0.02 (−0.11, 0.08) | −0.02 | 0.735 | |||
| Depression | −2.44 (−5.81, 0.94) | −0.09 | 0.156 | |||
In contrast, while there was an association in unadjusted analysis between HbA1c and cognitive functions captured by the Rey Auditory Verbal Learning Test immediate recall (sum of four trials, Figure 1C), Short Delay for List A (R2=0.0284, P=0.011), and Delay Recall for List A (R2=0.0216, P=0.027), the association between HbA1c and the delayed components did not remain significant when considering age, education, and/or cardiovascular and depression covariates.
Fasting glucose on the morning of testing was correlated with Digit Symbol Substitution Test (R2=0.032, P=0.006) and Trail Making A score (R2=0.025, P=0.02), but not the other test components or the Mini Mental State Exam. In age-adjusted models, fasting glucose on the morning of testing remained associated with Digit Symbol Substitution Test score (95%CI: −0.21 to −0.01; P=0.028); but the association was lost when other covariates were added.
Discussion
We demonstrate an association between cognitive function and glycemia assessed by HbA1c in men with stable coronary artery disease spanning a range of normal to moderately abnormal glucose metabolism. Age and education are important determinants of cognitive function 15 and the association between cognitive function and glycemia remains significant in age-, and age- and education-adjusted models. HbA1c remains associated with cognitive function when cardiovascular risk factors, depression, and SF-36 mental status are also included in the model. These findings are important given the increased prevalence of pre-diabetes and diabetes, cardiovascular disease, and cognitive impairment ranging from mild to frank dementia in the elderly, and the negative role cognitive impairment in patients with mild dysglycemia could play on individual capacity to adhere with complex cardiovascular treatment recommendations, together providing substantial importance to identify therapeutic targets for treatment and prevention of cognitive decline.
Vascular dementia may contribute substantially to cognitive decline, both in those with coronary artery disease and type 2 diabetes 2, 3. Additionally, about 5% of adults aged 65–74 and 50% 85 years and older in the United States have Alzheimer’s disease 16. About 22% of the same population (aged 65–74) has been diagnosed with diabetes, and the prevalence of abnormal glucose tolerance is substantially higher when including those with undiagnosed diabetes and pre-diabetes 17. The two disorders frequently co-occur and type 2 diabetes has been associated with cognitive impairment 6–8, 13, accelerated cognitive decline 18–20, and higher risk of Alzheimer’s disease 21–23. Furthermore, cognitive impairment less severe than dementia may impair quality of life and independence. Thus, it is of public health importance to better understand the relationship between glycemia and cognitive function, especially in persons with coronary artery disease, in whom multiple mechanisms may contribute to impaired function.
Acute hypoglycemia has been associated with reduced mental function 10. Likewise, increased glycemia has been associated with poorer cognitive function. In longitudinal analysis, self-reported diabetes was associated with incident all cause, amnestic, and non-amnestic mild cognitive impairment 24. Longer duration and severity of diabetes are important determinants of mild cognitive impairment 8. The ACCORD-Mind demonstrated an age-adjusted association between HbA1c and cognitive function in patients with mean diabetes duration of 10 years and HbA1c above 7.5% (58.5 mmol/mol) at study entry, with mean of 8.3% (67.2 mmol/mol) 13. Our studies extend the association between HbA1c and cognitive dysfunction into more modest degrees of dysglycemia (below 7.5%, 58.5 mmol/mol) in men with metabolic syndrome and stable coronary artery disease, to levels that would be considered non-diabetic to medically well controlled.
We found associations between age and education with cognitive function, consistent with studies in the general population and in those with diabetes 15, 25, 26. Our studies are also consistent with those showing association between HbA1c and cognitive function in type 2 diabetes 8, 13, 15, 27, and in pre-diabetes and well glycemic controlled diabetes 28, but extend these findings into a population with established coronary heart disease. Our study demonstrates the similar strength of association after adjustment for age and education between HbA1c multiple cognitive domains as captured by scores for Digital Symbol, Rey Word Learning Test, and Trail Making B, but less strong association with Categorical Verbal Fluency. Additionally, between 72–96% of the strength of association between HbA1c and cognitive function in unadjusted analysis is retained when adding age to the model, and 48–64% retained when both age and education are considered. Moreover, in the sub-cohort without diabetes, HbA1c remained associated with Digital Symbol Substitution Test, Rey Auditory Verbal Learning Test, and Trail Making B, although did not remain associated with Categorical Verbal Fluency. This may be due to reduced power in this smaller group, suggested by the relatively similar beta and standardized estimates compared with the full cohort. It is also possible cognitive performance in this test of verbal production, semantic memory and language 29 is not associated with HbA1c, as suggested by reduced association in the model including age, education and cardiometabolic variables and the analysis limited solely to the non-diabetic HbA1c glycemic range.
Multiple cognitive tests were administered, and higher HbA1c was related to poorer performance across multiple functional domains including aspects of executive function, speed of processing, and language. While digit substitution and the auditory-verbal learning component Rey Auditory Verbal Learning Test were associated with glycemia, we found only weak association between HbA1c and the memory component in the Rey Auditory Verbal Learning Test (short delay or delay recall) which did not remain significant when adjusted for covariates, and no association was found between HbA1c and the memory component of the Mini-Mental State Examination. These findings are consistent with studies showing strongest associations between poor glucose tolerance and lower verbal fluency, although others have not found this relationship in persons with impaired glucose tolerance 30.
Importantly, our study cohort did not have dementia, so associations with mild to moderate dementia would not be detectable. The ranges of cognitive tests scores in our cohort are similar to those considered to be a cognitively normal, non-diabetic US sample 31. The Mini-Mental State Examination was not associated with glycemia in our cohort similar to studies in persons without dementia 30. It is possible associations would be found in cohorts including greater proportion with compromised cognition.
Association between HbA1c and cognitive function does not establish causality. It is plausible patients with better cognition also adhere to or make better lifestyle choices and thus have lower HbA1c. It is also possible HbA1c is a biomarker for severity of vascular disease and/or other factor(s) influencing cognition. We found stronger association between HbA1c, than fasting glucose on the morning of testing. Our study was limited by the measure of fasting blood sugar and administration of cognitive function testing after a meal, such that immediate measure of immediate glucose concentration during testing is not available. There is no evidence dietary composition of a preceding meal influences cognitive function 32. Our findings may not be applicable to women. Statins may be associated with cognitive dysfunction. All participants were using statins, but type and dose varied. Finally our study was cross-sectional, and we cannot infer on decline.
In our cohort with established coronary heart disease, we found HbA1c associated with cognitive function tests of Digit Symbol Substitution Test, Rey Auditory Verbal Learning Test, Trail Making and Categorical Verbal Fluency but not Mini-Mental State Examination. Associated tests mainly measure speed of processing, memory and executive functions 33. These findings are consistent with reduced neuronal functional connectivity in patients with type 2 diabetes compared with non-diabetic controls in the frontal-parietal and temporal areas of the brain 34 anatomic areas mainly relate to cognitive functions of speed of processing, memory and executive functions 33, and in white matter and the default-mode network, an area that includes the posterior cingulate cortex and temporoparietal posterior association cortical regions of the brain 34–36. Higher HbA1c also correlates with reduced hippocampal volume and microstructure 28. Longer disease duration and elevated fasting blood glucose levels are associated with lower grey matter volume in T2D patients 20. Our study did not measure brain structure, so whether associations between HbA1c and cognitive function are mediated by structural changes needs further confirmation. However, if hyperglycemia leads to differences in brain structure, it is important to consider it may not be possible to recover function following chronic exposure that has caused structural change to the adult brain.
Multiple cellular and molecular mechanisms may underlie structural changes in brain and/or the relationship between HbA1c and cognitive impairment, including direct or indirect effects of dysglycemia on vascular disease, glycation products which may alter signal transduction pathways or metabolic intermediates 37, 38, neuronal mitochondrial function or oxidative stress, endoplasmic reticulum stress, or inflammation, insulin resistance, or the effect of insulin degrading enzyme activity on clearance of brain amyloid β 39–41, or other factors associated with HbA1c.
Accelerated cognitive decline is dependent on both duration of diabetes and glycemic control 20. Effects of glycemic improvement on cognitive function remain incompletely understood. One study demonstrated improvement over 24 weeks treatment with sulfonylurea or metformin 12. In contrast, neither the ACCORD-mind or the Anglo–Danish–Dutch Study of Intensive Treatment in People with Screen-Detected Diabetes in Primary Care (ADDITION) study demonstrated improved cognitive function in the intensive compared with standard treatment groups 27, 42. Hypoglycemia, which was more common in the ACCORD-mind intensive treatment group compared with standard-of-care, might have confounded potential benefits of glucose-lowering. Conceivably, slower rates of cognitive decline might occur using anti-hyperglycemic approaches not associated with hypoglycemia. In the ADDITION trial, both intensive and routine treatment groups had improvement in HbA1c (7.3% (56.3 mmol/mol) to 6.2% (44.3 mmol/mol) intensive, and 7.3% (56.3 mmol/mol) to 6.5% (47.5 mmol/mol) control, at baseline and final visit respectively). The glycemic difference between treatment groups may be insufficient to demonstrate effects of glycemic lowering on cognitive decline. There were multi-factorial metabolic interventions in the ADDITION trial, including antihypertensive and lipid lowering medications. Statin addition or other factors could confound cognitive improvement. Once cognitive function is lost over extended time it may not be regained in older adults, so understanding factors associated with and efforts to prevent early loss remain highly important.
In conclusion, higher HbA1c concentrations, even across the range from normal to pre-diabetes and well controlled diabetes, are associated with lower cognitive function performance scores across multiple domains in men with metabolic syndrome and cardiovascular disease. Lower cognitive function may impact quality of life and adherence to complex treatment regimins. Future studies may demonstrate whether glucose lowering within the normative range improves cognitive health or prevents progressive decline.
Clinical Significance.
Higher HbA1c, a measure of average glucose concentrations over 2 months, is associated with lower cognitive function in type 2 diabetes.
The association between HBA1c and cognitive function extends into the glycemic range that would be considered non-diabetic to well controlled disease, in men with metabolic syndrome and stable coronary artery disease.
Demonstrating that this relationship occurs is important to understand the pathophysiology and develop novel therapeutic approaches.
Acknowledgments
Funding Source: P50HL083813 and P30DK036836
Footnotes
Conflict of interest: None
Author Contributions: ABG, RA, KF, MA, KW researched data. RA, KF, CB, ST, WW, CP participated in patient visits and performed data entry. ABG, RA, TH, KW analyzed data, ABG, RA, KF, MA, KW, TH wrote manuscript. All co-authors reviewed/edited manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lopez OL, Jagust WJ, DeKosky ST, et al. Prevalence and classification of mild cognitive impairment in the Cardiovascular Health Study Cognition Study: part 1. Archives of neurology. 2003;60(10):1385–9. doi: 10.1001/archneur.60.10.1385. [DOI] [PubMed] [Google Scholar]
- 2.Exalto LG, Whitmer RA, Kappele LJ, Biessels GJ. An update on type 2 diabetes, vascular dementia and Alzheimer’s disease. Experimental gerontology. 2012;47(11):858–64. doi: 10.1016/j.exger.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 3.Gorelick PB, Scuteri A, Black SE, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke; a journal of cerebral circulation. 2011;42(9):2672–713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Craft S. The role of metabolic disorders in Alzheimer disease and vascular dementia: two roads converged. Archives of neurology. 2009;66(3):300–5. doi: 10.1001/archneurol.2009.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartnik M, Ryden L, Ferrari R, et al. The prevalence of abnormal glucose regulation in patients with coronary artery disease across Europe. The Euro Heart Survey on diabetes and the heart. European heart journal. 2004;25(21):1880–90. doi: 10.1016/j.ehj.2004.07.027. [DOI] [PubMed] [Google Scholar]
- 6.Cox DJ, Kovatchev BP, Gonder-Frederick LA, et al. Relationships between hyperglycemia and cognitive performance among adults with type 1 and type 2 diabetes. Diabetes care. 2005;28(1):71–7. doi: 10.2337/diacare.28.1.71. [DOI] [PubMed] [Google Scholar]
- 7.Shorr RI, de Rekeneire N, Resnick HE, et al. Glycemia and cognitive function in older adults using glucose-lowering drugs. The journal of nutrition, health & aging. 2006;10(4):297–301. [PubMed] [Google Scholar]
- 8.Roberts RO, Geda YE, Knopman DS, et al. Association of duration and severity of diabetes mellitus with mild cognitive impairment. Archives of neurology. 2008;65(8):1066–73. doi: 10.1001/archneur.65.8.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sommerfield AJ, Deary IJ, Frier BM. Acute hyperglycemia alters mood state and impairs cognitive performance in people with type 2 diabetes. Diabetes care. 2004;27(10):2335–40. doi: 10.2337/diacare.27.10.2335. [DOI] [PubMed] [Google Scholar]
- 10.Strachan MW, Ewing FM, Frier BM, et al. Effects of acute hypoglycaemia on auditory information processing in adults with Type I diabetes. Diabetologia. 2003;46(1):97–105. doi: 10.1007/s00125-002-0950-2. [DOI] [PubMed] [Google Scholar]
- 11.Draelos MT, Jacobson AM, Weinger K, et al. Cognitive function in patients with insulin-dependent diabetes mellitus during hyperglycemia and hypoglycemia. The American journal of medicine. 1995;98(2):135–44. doi: 10.1016/S0002-9343(99)80397-0. [DOI] [PubMed] [Google Scholar]
- 12.Ryan CM, Freed MI, Rood JA, et al. Improving metabolic control leads to better working memory in adults with type 2 diabetes. Diabetes care. 2006;29(2):345–51. doi: 10.2337/diacare.29.02.06.dc05-1626. [DOI] [PubMed] [Google Scholar]
- 13.Cukierman-Yaffe T, Gerstein HC, Williamson JD, et al. Relationship between baseline glycemic control and cognitive function in individuals with type 2 diabetes and other cardiovascular risk factors: the action to control cardiovascular risk in diabetes-memory in diabetes (ACCORD-MIND) trial. Diabetes care. 2009;32(2):221–6. doi: 10.2337/dc08-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poggio ED, Wang X, Greene T, et al. Performance of the modification of diet in renal disease and Cockcroft-Gault equations in the estimation of GFR in health and in chronic kidney disease. Journal of the American Society of Nephrology : JASN. 2005;16(2):459–66. doi: 10.1681/ASN.2004060447. [DOI] [PubMed] [Google Scholar]
- 15.Guerrero-Berroa E, Ravona-Springer R, Schmeidler J, et al. Age, gender, and education are associated with cognitive performance in an older Israeli sample with type 2 diabetes. International journal of geriatric psychiatry. 2014;29(3):299–309. doi: 10.1002/gps.4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.http://www.cdc.gov/aging/aginginfo/alzheimers.htm. [December 2, 2013]; Available from: http://www.cdc.gov/aging/aginginfo/alzheimers.htm.
- 17.http://www.cdc.gov/diabetes/statistics/incidence_national.htm. [December 2, 2013]; Available from: http://www.cdc.gov/diabetes/statistics/incidence_national.htm.
- 18.Allen KV, Frier BM, Strachan MW. The relationship between type 2 diabetes and cognitive dysfunction: longitudinal studies and their methodological limitations. European journal of pharmacology. 2004;490(1–3):169–75. doi: 10.1016/j.ejphar.2004.02.054. [DOI] [PubMed] [Google Scholar]
- 19.Cukierman T, Gerstein HC, Williamson JD. Cognitive decline and dementia in diabetes--systematic overview of prospective observational studies. Diabetologia. 2005;48(12):2460–9. doi: 10.1007/s00125-005-0023-4. [DOI] [PubMed] [Google Scholar]
- 20.Tuligenga RH, Dugravot A, Tabak AG, et al. Midlife type 2 diabetes and poor glycaemic control as risk factors for cognitive decline in early old age: a post-hoc analysis of the Whitehall II cohort study. The lancet Diabetes & endocrinology. 2014;2(3):228–35. doi: 10.1016/S2213-8587(13)70192-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leibson CL, Rocca WA, Hanson VA, et al. Risk of dementia among persons with diabetes mellitus: a population-based cohort study. American journal of epidemiology. 1997;145(4):301–8. doi: 10.1093/oxfordjournals.aje.a009106. [DOI] [PubMed] [Google Scholar]
- 22.Luchsinger JA, Tang MX, Stern Y, et al. Diabetes mellitus and risk of Alzheimer’s disease and dementia with stroke in a multiethnic cohort. American journal of epidemiology. 2001;154(7):635–41. doi: 10.1093/aje/154.7.635. [DOI] [PubMed] [Google Scholar]
- 23.Ott A, Stolk RP, van Harskamp F, et al. Diabetes mellitus and the risk of dementia: The Rotterdam Study. Neurology. 1999;53(9):1937–42. doi: 10.1212/wnl.53.9.1937. [DOI] [PubMed] [Google Scholar]
- 24.Luchsinger JA, Reitz C, Patel B, et al. Relation of diabetes to mild cognitive impairment. Archives of neurology. 2007;64(4):570–5. doi: 10.1001/archneur.64.4.570. [DOI] [PubMed] [Google Scholar]
- 25.Beeri MS, Schmeidler J, Sano M, et al. Age, gender, and education norms on the CERAD neuropsychological battery in the oldest old. Neurology. 2006;67(6):1006–10. doi: 10.1212/01.wnl.0000237548.15734.cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gladsjo JA, Schuman CC, Evans JD, et al. Norms for letter and category fluency: demographic corrections for age, education, and ethnicity. Assessment. 1999;6(2):147–78. doi: 10.1177/107319119900600204. [DOI] [PubMed] [Google Scholar]
- 27.Koekkoek PS, Ruis C, van den Donk M, et al. Intensive multifactorial treatment and cognitive functioning in screen-detected type 2 diabetes--the ADDITION-Netherlands study: a cluster-randomized trial. Journal of the neurological sciences. 2012;314(1–2):71–7. doi: 10.1016/j.jns.2011.10.028. [DOI] [PubMed] [Google Scholar]
- 28.Kerti L, Witte AV, Winkler A, et al. Higher glucose levels associated with lower memory and reduced hippocampal microstructure. Neurology. 2013;81(20):1746–52. doi: 10.1212/01.wnl.0000435561.00234.ee. [DOI] [PubMed] [Google Scholar]
- 29.Lezak MD. Neuropsychological Assessment. 4. New York, NY: Oxford University Press; 2004. [Google Scholar]
- 30.Lamport DJ, Lawton CL, Mansfield MW, Dye L. Impairments in glucose tolerance can have a negative impact on cognitive function: a systematic research review. Neuroscience and biobehavioral reviews. 2009;33(3):394–413. doi: 10.1016/j.neubiorev.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 31.Weintraub S, Salmon D, Mercaldo N, et al. The Alzheimer’s Disease Centers’ Uniform Data Set (UDS): the neuropsychologic test battery. Alzheimer disease and associated disorders. 2009;23(2):91–101. doi: 10.1097/WAD.0b013e318191c7dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lamport DJ, Dye L, Mansfield MW, Lawton CL. Acute glycaemic load breakfast manipulations do not attenuate cognitive impairments in adults with type 2 diabetes. Clin Nutr. 2013;32(2):265–72. doi: 10.1016/j.clnu.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 33.Zhang H, Sachdev PS, Wen W, et al. Neuroanatomical correlates of cognitive performance in late life. Dementia and geriatric cognitive disorders. 2011;32(3):216–26. doi: 10.1159/000333372. [DOI] [PubMed] [Google Scholar]
- 34.Hoogenboom WS, Marder TJ, Flores VL, et al. Cerebral white matter integrity and resting-state functional connectivity in middle-aged patients with type 2 diabetes. Diabetes. 2013 doi: 10.2337/db13-1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Musen G, Jacobson AM, Bolo NR, et al. Resting-state brain functional connectivity is altered in type 2 diabetes. Diabetes. 2012;61(9):2375–9. doi: 10.2337/db11-1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reijmer YD, Leemans A, Brundel M, et al. Disruption of the cerebral white matter network is related to slowing of information processing speed in patients with type 2 diabetes. Diabetes. 2013;62(6):2112–5. doi: 10.2337/db12-1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith MA, Sayre LM, Perry G. Diabetes mellitus and Alzheimer’s disease: glycation as a biochemical link. Diabetologia. 1996;39(2):247. doi: 10.1007/BF00403972. [DOI] [PubMed] [Google Scholar]
- 38.Brownlee M. Lilly Lecture 1993. Glycation and diabetic complications. Diabetes. 1994;43(6):836–41. doi: 10.2337/diab.43.6.836. [DOI] [PubMed] [Google Scholar]
- 39.Farris W, Mansourian S, Chang Y, et al. Insulin-degrading enzyme regulates the levels of insulin, amyloid beta-protein, and the beta-amyloid precursor protein intracellular domain in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(7):4162–7. doi: 10.1073/pnas.0230450100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qiu WQ, Walsh DM, Ye Z, et al. Insulin-degrading enzyme regulates extracellular levels of amyloid beta-protein by degradation. The Journal of biological chemistry. 1998;273(49):32730–8. doi: 10.1074/jbc.273.49.32730. [DOI] [PubMed] [Google Scholar]
- 41.Selkoe DJ. The origins of Alzheimer disease: a is for amyloid. JAMA : the journal of the American Medical Association. 2000;283(12):1615–7. doi: 10.1001/jama.283.12.1615. [DOI] [PubMed] [Google Scholar]
- 42.Launer LJ, Miller ME, Williamson JD, et al. Effects of intensive glucose lowering on brain structure and function in people with type 2 diabetes (ACCORD MIND): a randomised open-label substudy. Lancet neurology. 2011;10(11):969–77. doi: 10.1016/S1474-4422(11)70188-0. [DOI] [PMC free article] [PubMed] [Google Scholar]