Abstract
Background & Aims
Adequate protein intake and digestion are necessary to prevent muscle wasting in cystic fibrosis (CF). Accurate and easy-to-use methodology to quantify protein maldigestion is lacking in CF.
Objective
To measure protein digestibility and the response to pancreatic enzyme intake in CF by using a new stable isotope methodology.
Design
In 19 CF and 8 healthy subjects, protein digestibility was quantified during continuous (sip) feeding for 6 hours by adding 15N-labeled spirulina protein and L-[ring-2H5]phenylalanine (PHE) to the nutrition and measuring plasma ratio [15N]PHE to [2H5]PHE. Pancreatic enzymes were ingested after 2 h in CF and the response in protein digestibility was assessed. To exclude difference in mucosal function, postabsorptive whole-body citrulline (CIT) production rate was measured by L-[5-13C-5,5-2H2]-CIT pulse and blood samples were taken to analyze tracer-tracee ratios.
Results
Protein digestibility was severely reduced in the CF group (47% of healthy subjects; P<0.001). Intake of pancreatic enzymes induced a slow increase in protein digestibility in CF until 90% of values obtained by healthy subjects. Maximal digestibility was reached at 100 min and maintained for 80 min. Stratification into CF children (n=10) and adults showed comparable values for protein digestibility and similar kinetic responses to pancreatic enzyme intake. Whole-body citrulline production was elevated in CF indicating preserved mucosal function.
Conclusion
Protein digestibility is severely compromised in patients with CF as measured by this novel and easy-to-use stable isotope approach. Pancreatic enzymes are able to normalize protein digestibility in CF, albeit with a severe delay.
Keywords: Novel stable isotope method, Protein digestibility, Pancreatic enzymes, Whole-body citrulline production, Cystic Fibrosis
INTRODUCTION
Malabsorption of nutrients due to exocrine pancreatic insufficiency is viewed as the main factor contributing to weight loss/poor weight gain in patients with cystic fibrosis (CF) and may lead to deficiencies of essential nutrients. The term malabsorption is often used to describe the combined process of digestion and absorption as it is very difficult to distinguish these two processes. Although it is well established that fat digestibility is impaired in CF, the available methods to measure protein digestibility are limited and often not accurate. As impaired protein digestibility leads to an impaired anabolic response to a meal as well as to high loads of undigested proteins in the colon, which induce diarrhea and the production of harmful toxins by gut bacteria (1), accurate quantification of protein digestibility is of high clinical importance in CF.
Many decades ago, a diminished retention of dietary proteins in CF was demonstrated with nitrogen balance tests (2) and stool analyses (3), methods with limited accuracy that are too laborious and time consuming for routine diagnostic use. In 1952, a more simple and accurate method was developed using oral ingestion of 131I-labeled protein (4). In the last decade, several studies used 13C intrinsically labeled milk or egg proteins to assess protein digestibility by measuring the enrichment kinetics of labeled CO2 in the breath in response to meal intake (5–7). A limitation of this method is the difficulty to obtain large amounts of proteins with sufficiently high amino acid enrichment levels and with adequate labeling patterns, making production of these labeled proteins cumbersome and expensive. Furthermore, the accuracy of using 13C-intrinsically labeled milk or egg protein, or even uniformly 13C-labeled algal protein (8) and measuring 13CO2 production might be limited in CF as these patients are characterized by chronic and acute episodes of lung inflammation which contribute to CO2 production in the breath and changes in the CO2 pool size (9). Recently, 15N-labeled spirulina protein has become available enabling the measurement of protein digestibility by taking blood instead of breath samples. Spirulina protein is commercially available and much cheaper than the intrinsically labeled milk and egg protein. In addition, the digestion rate of spirulina is lower than that of labeled milk and egg protein and thus enables better the measurement of factors that negatively affect digestion such as pancreatic enzyme deficiency. This suggests that protein digestibility can be quantified in CF by providing the patients a meal containing 15N-labeled spirulina protein and 2H5-labeled phenylalanine and measuring the ratio between 15N and 2H5-enrichment of phenylalanine in plasma. This method is independent of further metabolism of phenylalanine.
In order to test this method in CF, we chose to study CF patients during continuous (sip) feeding of a nutritional supplement often used in CF care as it closely reflects the situation present in patients with a gastrostomy tube during overnight feeding and potential differences in the digestion and absorption rate of phenylalanine from the spirulina protein and the free amino acid tracer will not affect the method. As the optimal timing point of pancreatic enzyme replacement therapy in relation to feeding is still unclear in these patients, we also examined protein digestion kinetics in response to intake of a bolus of pancreatic enzymes in this study setting. If the method is able to assess acute changes in protein digestibility, it could be used in future studies to quantify and optimize protein digestibility in CF patients using different nutritional supplements or pancreatic enzyme regimens. In this way, the effectiveness of current and new nutritional approaches and pancreatic enzyme preparations as well as the impact of certain medications like acid blockers on protein digestibility could be assessed in CF in a relatively cheap and non-laborious way.
Citrulline is for a very large part produced in the gut and therefore citrulline production is viewed as an indirect measure of gut mucosa function (10). We recently did not find mucosal dysfunction in children with CF recovering from an acute exacerbation (11) using a primed constant continuous infusion of L-[ureido13C-5,5-2H2]-citrulline. However a more refined technique has become available which uses an IV pulse of L-[5-13C-2H2]-citrulline that more precisely measures intracellular citrulline production, reduces the invasiveness as it eliminates placement of a second catheter, and does not need careful estimation of the isotope priming dose. Furthermore, there is a reduced required sampling time although more sampling points are needed.
In the present study, we tested a combined methodology using intrinsically labeled protein and free amino acid tracers that was able to simultaneously quantify protein digestibility as well as whole-body citrulline production using the refined IV pulse technique in patients with CF as compared to healthy subjects. Furthermore, we examined the acute kinetic response in protein digestibility to pancreatic enzyme intake in CF, and whether aging affects protein digestibility and whole-body citrulline production by comparing a group of children and adults with CF.
SUBJECTS AND METHODS
Subjects
The study population consisted of 19 subjects with CF. Ten of them were pediatric subjects, age of 10 to 18 years, and admitted to Arkansas Children’s Hospital for pulmonary exacerbation. Nine of the CF patients were adults, age of 18 to 35 years at the time of enrollment. Three of the adult patients were admitted to the University of Arkansas for Medical Sciences for pulmonary exacerbation, and 6 were outpatients. All CF subjects had a diagnosis of CF based on universal diagnostic criteria, were clinically stable at enrollment, and were pancreatic insufficient. The CF inpatients were enrolled at the end of their hospital stay with improvement in lung function (FEV1) at the time of enrollment back to baseline values (determined as FEV1 in past 12 months). Exclusion criteria included established diagnosis of diabetes mellitus, unstable metabolic diseases (ie impaired renal failure), and chronic respiratory failure with cor pulmonale. In addition, 8 healthy subjects were recruited in the local community and studied as age-matched control subjects to the adult CF patients. Written informed consent was obtained and the study was approved by the Institutional Review Board of the University of Arkansas for Medical Sciences. All authors had access to the study data and had reviewed and approved the final manuscript.
Study design
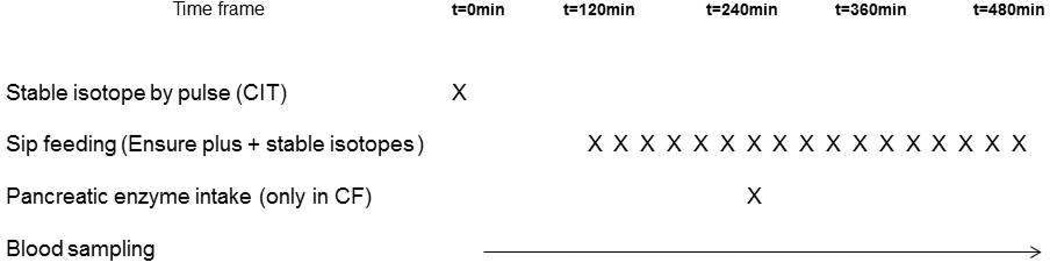
The study day was performed in the patient’s hospital room during the last days of antibiotic treatment for a CF exacerbation at Arkansas Children’s Hospital (CF children, n=10) or University of Arkansas for Medical Sciences (CF adults, n=3). Moreover, 6 adults with CF and 8 healthy adults were studied at the outpatient clinical research center at University of Arkansas for Medical Sciences. The study day started in the early morning after overnight fasting and lasted for 8 hours. If present, a central-venous port or peripheral line already in place for clinical care was used; otherwise, a catheter was placed in a superficial vein of the lower arm. This line was used for background blood sampling, the bolus infusion of the stable isotope of citrulline (L-[5-13C-5,5-2H2]-Cit) (Sigma-Aldrich; St. Louis, MO) as well as for subsequent blood sampling. Two hours after the IV citrulline bolus, each subject ingested orally or received enterally (when feeding tube was present in CF) a commercially available nutritional supplement (see Table 1) according to a sip feeding protocol (each 20 min) during 6 hours. The oral isotopes of 15N-spirulina and L-[ring- 2H5]Phenylalanine (Cambridge Isotopic Laboratories, Andover, MA) were added to the nutritional supplement. After 2 hours of sip feeding, one serving of pancreatic enzymes (Creon®, 4000u lipase/g fat intake) (Abbott; Abbott Park, IL) was ingested by the CF subjects. No pancreatic enzymes were taken by the healthy adults. Arterialized-venous blood samples were taken every 20 minutes throughout the study and just before intake of the sip feeds for analysis of concentrations and tracer-tracee ratios (TTR) of amino acids. For an overview of the study design, see Figure 1.
Table 1.
Nutrition, pancreatic enzyme and oral isotope dose used in children and adults with Cystic Fibrosis and healthy subjects
| Healthy subjects (n=8) |
CF adults (n=9) |
CF children (n=10) |
||
|---|---|---|---|---|
| Ensure plus | ml | 260.1 ± 36.9 | 224.3 ± 37.3 | 170.8 ± 39.4 |
| Creon 12000 | U | 0 ± 0 | 3.56 ± 0.53 | 2.5 ± 0.53 |
| Creon 6000 | U | 0 ± 0 | 0.11 ± 0.33 | 0.50 ± 0.53 |
| 15N-Spirulina | mg | 4572.8 ± 648.5 | 3943.3 ± 656.2 | 3003.0 ± 693.1 |
| 2H5-Phenylalanine | mg | 228.6 ± 32.5 | 197.2 ± 32.8 | 150.1 ± 34.6 |
Values are means ± SEM
Figure 1.
Overview of the study design.
Composition of the nutritional supplement and pancreatic enzyme dose
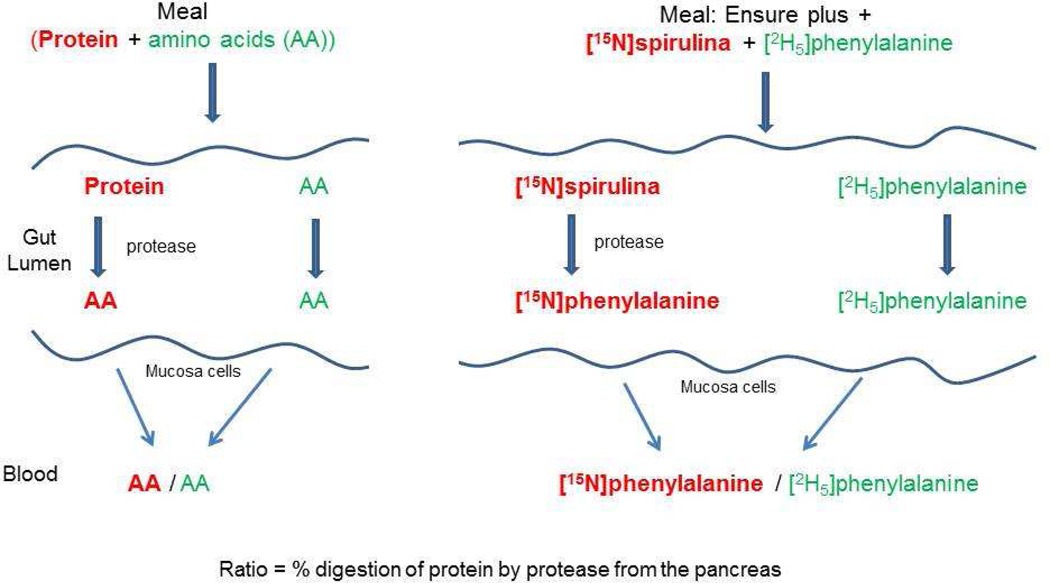
All subjects received a commercially available nutritional supplement, Ensure Plus® (Abbott Nutrition; Abbott Park, IL), which is often used in CF care. The subjects ingested the nutritional supplement orally according to a sip feeding protocol (every 20 min) or enterally when a gastrostomy tube was present (CF children, n=3). The dosage of the nutritional supplement for each subject was based on 1/3rd of their total daily protein needs, estimated using Dietary Reference Intakes (DRI) (for composition see Table 1). We added the stable isotopes 15N-spirulina protein (63 mg/kg BW Spirulina protein that contains about 1.7 mg L-[15N]phenylalanine (PHE)) and L-[ring-2H5]PHE (3.14 mg/kg BW) to the nutritional supplement (3.6 g/kg BW Ensure Plus®) to measure protein digestion rate (=ratio [15N]PHE to [2H5]PHE in plasma); the principle physiology is explained in Figure 2. The nutritional supplement was mixed with the oral stable isotopes and divided into equal portions and stored in the refrigerator before use. A sample of the supplement was stored for composition and PHE isotope ratio analysis.
Figure 2.
The basic principle of measuring protein digestibility with 15N-labelled protein and 2H5-labelled phenylalanine.
Pancreatic enzyme (Creon®) intake in CF took place at 2 h into feeding, and the dose was based on the fat content of the meal (4000u lipase/g fat) (Table 1). The pancreatic enzymes for the children and adults with CF were obtained from the Arkansas Children Hospital and University of Arkansas for Medical Sciences pharmacy, respectively.
Anthropometric data and body composition
In the early morning of the study day, body weight and height were measured in all subjects by a digital beam scale and stadiometer, respectively. BMI was calculated by dividing body weight by squared height and expressed in kg/m2 for the adults. Height, weight, and BMI percentiles of the CF children were calculated in accordance with the CF consensus report (12). Whole-body fat mass (FM) and fat-free mass (FFM) were obtained by dual-energy X-ray absorptiometry (DXA) (Hologic QDR 4500/ Version 12.7.3.1; Bedford, MA) when the subjects were in supine position. The DXA procedure was conducted in the outpatient research setting on the study day, or obtained during hospital stay in case of hospital admission.
The anthropometric and body composition data were standardized for height (13) to obtain BMI, fat-free mass index (FFMI), and fat mass index (FMI). FFMI and FMI were expressed as percentage of published reference data (14–17). Nutritional failure was defined as FFMI<5th percentile in accordance to previous studies in CF (18–23) and/or BMI <10th percentile (age≤20 years) or BMI < 18 kg/m2 (age 21 years and older) using the Cystic Fibrosis Foundation selected BMI cut-off point (24).
Lung function
Forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC) were measured by spirometry (nSpire Health; Longmont, CO) in all CF participants and reference equations (25, 26) were used to calculate FEV1 and FVC % predicted values.
Biochemical analysis
Blood samples were put in Li-heparinized tubes, immediately put on ice and instantly frozen and stored at −80°C until further analyses ( 11). Samples of the nutritional supplement were hydrolyzed in 6N HCl solution for 24 h at 110°C (27). All samples obtained were analyzed in a batch. Analysis for enrichment and concentrations was done by LC-ESI-MS (QTrap 5500MS) (AB Sciex; Foster City, CA) with ExpressHT Ultra LC (Eksigent AB Sciex; Foster City, CA) after derivatization with 9-fluorenylmethoxycarbonyl (Fmoc). Fmoc-PHE and Fmoc-CIT were fragmented to obtain specific and high sensitivity fragments (11).
Calculations for protein digestibility and whole body citrulline production
The principle of the proposed technique to measure protein digestibility is based on the measurement of the hydrolysis rate of proteins in the gut lumen by proteases that originate from the pancreas (Figure 22). Proteins are broken down to amino acids that are subsequently taken up (absorbed) by the mucosa and released into the portal vein to be available for the rest of the body. In 15N-spirulina protein, all nitrogen atoms of the amino acids in the protein are 15N. Therefore, phenylalanine in the spirulina protein will be broken down to [15N]-phenylalanine after digestion. In addition to the stable isotope of 15N-spirulina protein, we also added 2H5-phenylalanine to the supplement that does not need digestion before absorption (digestibility=100%) and therefore makes it possible to calculate digestibility by measuring the ratio [15N]PHE to [2H5]PHE in plasma and the nutrition.
Protein digestibility was calculated by dividing the plasma [15N]PHE to [2H5]PHE ratio by the [15N]PHE to [2H5]PHE ratio in the nutritional supplement. Protein digestion rate during feeding was calculated as the average value at 200 min to 240 min into the study (=80–120 min after start of sip feeding). Highest plateau value of protein digestion rate after intake of pancreatic enzymes was calculated as the average value at 340 min to 400 min into the study (=100–160 min after intake of pancreatic enzymes in CF).
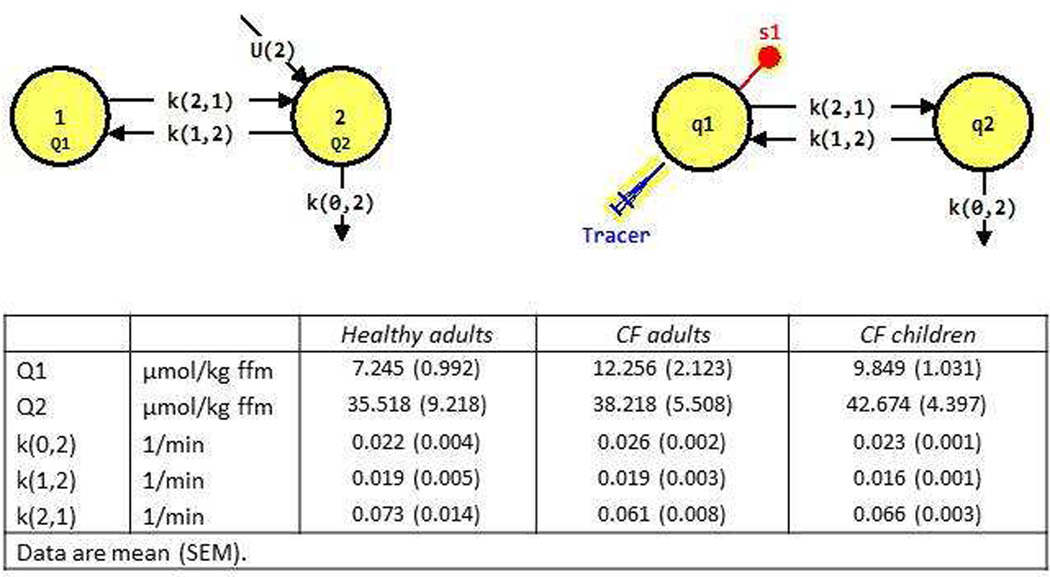
To assess whole-body citrulline production in the postabsorptive state with a pulse of citrulline isotope, we first used non-compartmental analysis (Graphpad Prism, Version 6.01) and observed that there are 2 compartments. Therefore, we created a 2 compartment model for citrulline production. The model used is a commonly used model for amino acid kinetics. We used the program SAAM II (Version 2.2) (The Epsilon Group; Charlottesville, VA) to calculate the k values and pool sizes in the two compartmental model (see Figure 3). The k values were converted to whole-body rate of appearance (WbRa) or intracellular production. The compartmental modeling estimates the parameters to calculate WbRa (the amount of citrulline that appears in pool 1 (=plasma)) and intracellular production rates (the amount that is produced in pool 2) in relation to the actual intracellular precursor pool enrichment. These rates are higher than when estimated with primed-constant infusion production, and in that case, the precursor pool enrichment in arterial plasma is used as a proxy of intracellular enrichment, which is higher than the intracellular enrichment as the production takes place intracellularly.
Figure 3.
We used the compartmental model (tracee model; upper left panel) of a plasma pool (pool 1) and an intracellular pool (pool 2) of unlabeled citrulline. In this model, the amount of unlabeled citrulline in each compartment is denoted by Q (Q1 and Q2 for the pool sizes of compartments 1 and 2) and its intracellular appearance (U(2)). We used a citrulline tracer experiment (upper right panel) to calculate the rate constants and pool sizes. Thus, citrulline tracer is introduced in plasma (Pool 1 or Q1) and samples (S1) are taken from plasma. Q2 is most likely the intracellular citrulline pool. The arrows represent transfer of citrulline from one compartment to another. k(0,2) is the fractional rate constant for irreversible loss from pool 2, k(1,2) is the fractional rate of transfer of citrulline (per unit time) from pool 2 to pool 1, and k(2,1) from pool 1 to pool 2 (38). Intracellular appearance (production) of citrulline is shown entering pool 2 (U(2)=F(0,2)) and is calculated as k(0,2)*Q2. The % irreversible loss of citrulline from pool 2 is the rate of citrulline irreversible lost divided by the rate that citrulline exits pool 2 by all routes and calculated as F(0,2)/(F(0,2)+F(1,2)), and (1-% irreversible loss) is the citrulline that appears in pool 1. WbRa is therefore calculated by multiplying (1-% irreversible loss) by F(0,2). WbRa is therefore lower than the total production rate to the extent that citrulline does not appear into plasma. The obtained data for Q1, Q2, k(0,2), k(1,2) and k(0,1) for the healthy adults, CF adults and CF children are provided in the lower panel.
Statistical analysis
Results are expressed as mean ± standard error (SE). Data failing the normality or equal variance test were log-transformed where appropriate. One-way ANOVA was used to determine differences between the children and adults with CF and the healthy subjects, and Newman-Keuls was used as post hoc analysis. Unpaired Student’s t test was used to determine differences in clinical changes between the children and adults with CF group, and in the CF group with and without nutritional failure. The level of significance was set at p<0.05. The statistical package within Graphpad Prism (Version 6.01) and SPSS (Version 20) was used for data analysis.
RESULTS
The group consisted of 10 children with CF (age: 14.9 ± 0.2 y), 9 adults with CF (age: 28.6 ± 0.8 y), and 8 healthy adults (age: 29.2 ± 1.2 y, Table 2). Age of the CF adults was not different from the healthy adults. The homozygous DF 508 gene was present in 90% of the children and in 25% of the adults with CF.
Table 2.
General characteristics of the children and adults with Cystic Fibrosis and healthy subjects
| Parameter | Healthy subjects (n=8) |
CF adults (n=9) |
P | CF children (n=10) |
P | |
|---|---|---|---|---|---|---|
| Gender | m/f | 4 / 4 | 5 / 4 | NS | 6 / 4 | |
| Age | y | 29.2 ± 1.2 | 28.6 ± 0.8 | NS | 14.9 ± 0.2 | <0.001a |
| BMI | Kg/m2 Percentile | 23.5 ± 0.4 | 21.6 ± 0.5 | NS | 46.4 ± 3.0 | |
| FFMI | %norm | 102.3 ± 1.6 | 90.7 ± 1.5 | 0.05 | 92.8 ± 1.1 | |
| FMI | %norm | 113.0 ± 17.3 | 99.4 ± 18.1 | NS | 96.8 ± 10.0 | |
| Nutritional failure | y/n | 0 / 8 | 3 / 6 | 3 / 7 | ||
| FEV1 | %pred | 63.9 ± 3.3 | NS | 81.6 ± 1.3 | 0.08a | |
| FVC | %pred | 76.6 ± 3.6 | NS | 98.0 ± 1.6 | 0.04a | |
| CF genotype | DF508/DF508,DF50 8/G542x, unknown: n=2, DF508/2184delA, DF508/G1244: n=1 |
DF508/DF508: n=9; DF508/1717-1GtoA: n=1 |
||||
Values are means ± SEM. CF: Cystic Fibrosis, BMI: Body mass index, FFMI: Fat-free mass index, FEV1: forced expiratory volume in one second, FMI: fat mass index, FVC: forced vital capacity. The P value represents a comparison with healthy subjects.
P value represents a comparison with CF adults
The patients with CF were characterized by mild to severe airflow obstruction. Mean FEV1 tended to be lower (P=0.08) and FVC was significantly (P<0.05) lower in the CF adults than in the CF children. The CF adult group was characterized by reduced values for FFMI (as percentage of control values; P<0.05), but no difference in FFMI was found between the children and adults with CF. Mean BMI (in kg/m2) was not different between the adults with CF and the healthy subjects. The mean BMI of the CF children was at the 46 percentile indicating lower than the CF recommended BMI of 50%. Three adults (33%) and 3 children with CF (30%) were characterized by nutritional failure.
Protein digestibility
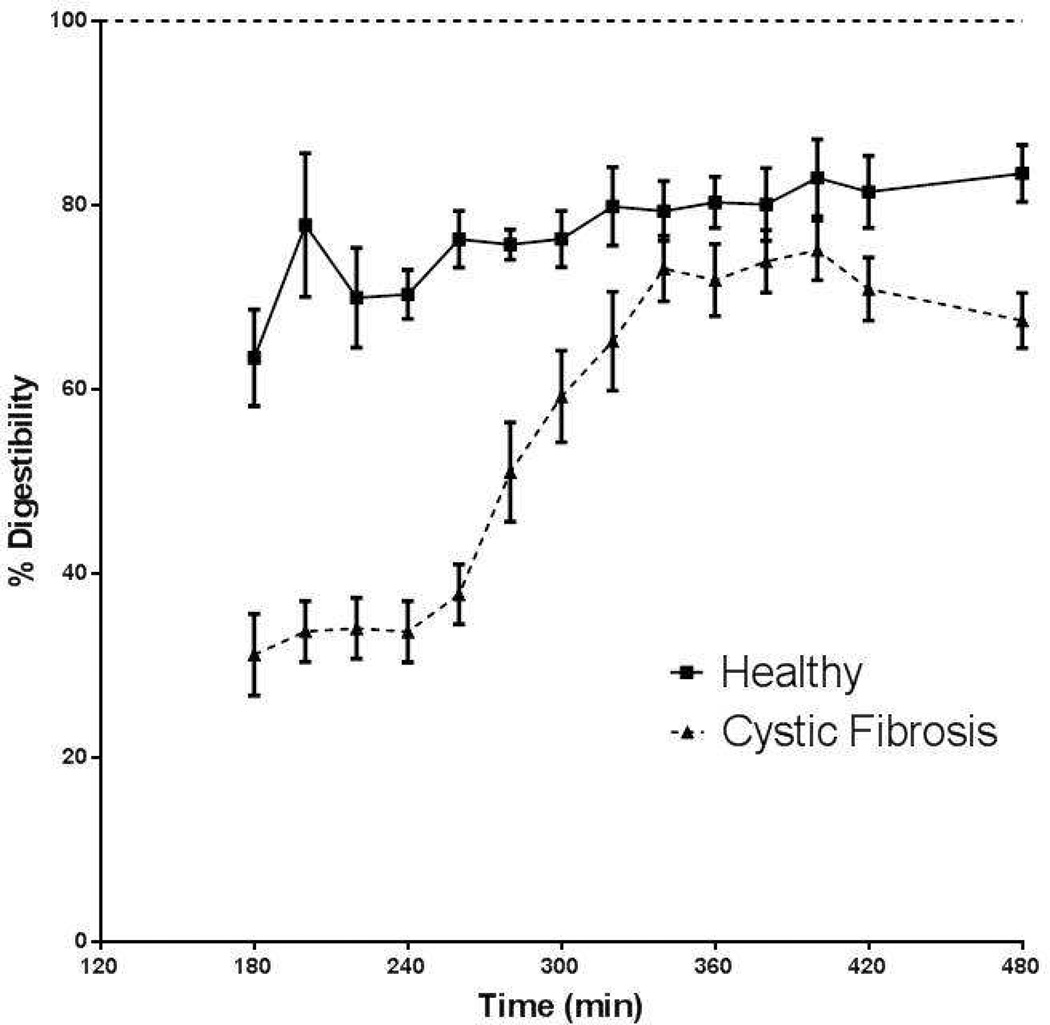
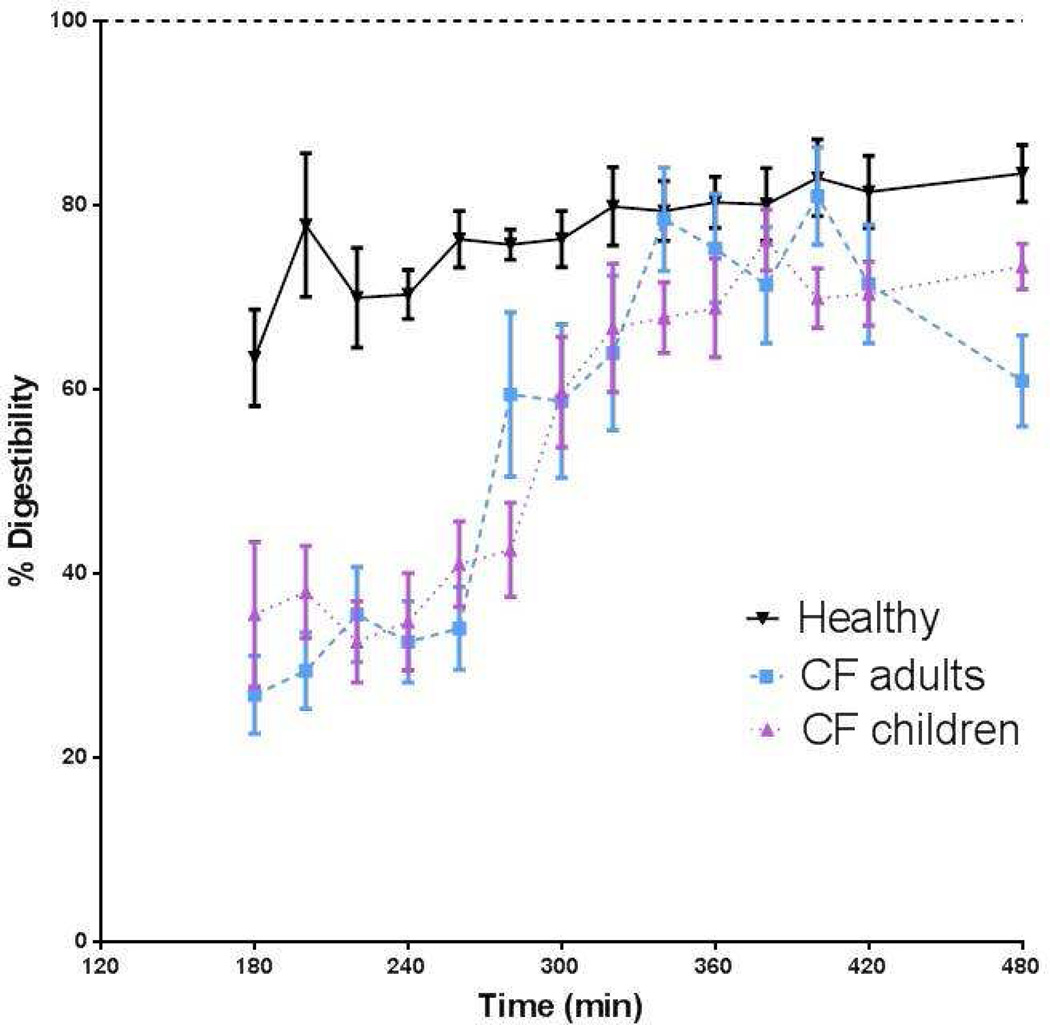
We found that in healthy young adults (Figure 4), protein digestibility was about 80% in line with reported rates of spirulina protein (28). The average protein digestibility during feeding and before the pancreatic enzyme intake (t=200–240 min) was significantly lower in the total CF group as compared to the healthy subjects (p<0.001; Figure 4a) which corresponded to 46.5% of the healthy subjects. After pancreatic enzyme intake protein digestibility increased in CF and reached its maximal value at 344 min (=104 min) of 90.3% of the healthy subjects and a plateau in digestibility occurred for approximately 80 minutes.
Figure 4.
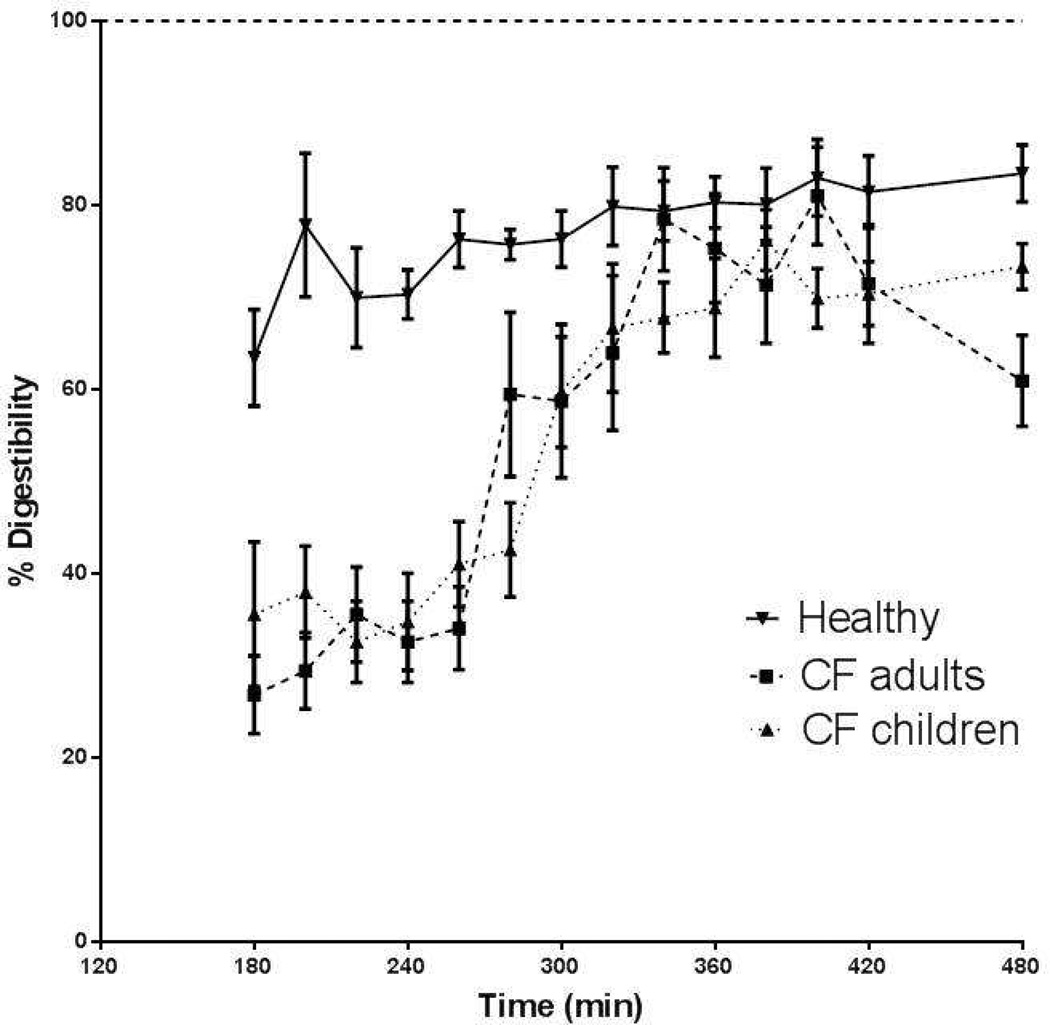
Response in protein digestibility during feeding and after intake of pancreatic enzymes (only in CF) in the whole CF group (dashed line) and healthy adult group (solid line) (left panel), and in the healthy group (solid line) and after stratification of the whole CF group into adults (dashed line) and children (dotted line) (right panel).
Stratification of the CF group into adults and children showed comparable values for protein digestibility during feeding (as % of the healthy subjects) in both groups (44.7% vs. 48.3%, ns) (Figure 4b) and a similar kinetic response to pancreatic enzyme intake. Between 340 and 420 min, protein digestibility reached average (plateau) values of 93.4% vs. 87.4% of the healthy subjects in the CF children vs. CF adults, resp. (not significant). No difference was found in average protein digestibility during feeding or after pancreatic enzyme intake between CF patients with and without the homozygous DF 508 gene, or between CF patients with or without nutritional failure (data not shown). Furthermore, no significant relationship was found with lung function in the CF group.
Proton-pump inhibitors (PPI)
Use of PPI may influence our data a pronounced and long-lasting reduction of gastric acid increases the activity of exogenous pancreatic enzymes. In the present study, 84% of the CF patients were using PPI on a daily basis, and none of them were using H2 blockers. Protein digestion during feeding as well as maximum digestibility after pancreatic enzyme intake were comparable between the PPI users and non-users groups but the non-user group was very small (n=3). As the dose of PPI varied in the CF group between 15 and 80 mg/day, we examined whether daily PPI dosage was related to protein digestibility or maximum digestibility after pancreatic enzyme intake, but no significant relationships were found.
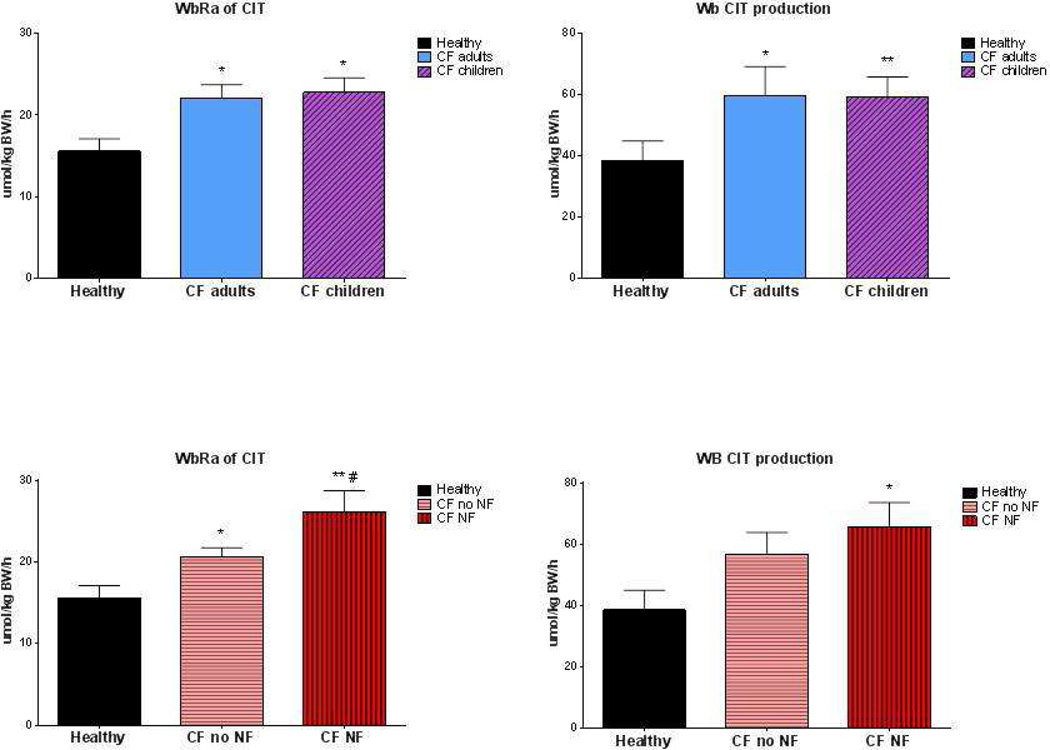
Whole-body rate of appearance and production rate of citrulline
Whole-body citrulline rate of appearance and production rate were significantly higher in the CF patients as compared to the healthy subjects in the postabsorptive state (p<0.05; Figure 5). However, no difference was present between the adults and children with CF. Plasma citrulline concentration was not significantly different in CF as compared to the healthy group (41 ± 4 µmol/L vs. 33 ± 2 µmol/L; p=0.1). Significant higher values were found for whole-body citrulline rate of appearance in the CF patients with nutritional failure as compared to those without nutritional failure (P<0.05). Whole-body citrulline rate of appearance and production rate were not related to lung function or protein digestibility during feeding (data not shown).
Figure 5.
Postabsorptive whole body rate of appearance (WbRa, panel a + c) and production rate (panel b+d) of citrulline in the healthy group (solid bar) and after stratification of the whole CF group into adults (open bar) and children (cross striped bar), and after stratification of the CF group into nutritional failure (NF, vertical striped bar) and no nutritional failure (no NF, horizontal striped bar). Mean values ± SE are shown. Significance of difference as compared to the healthy group (*: P<0.05, **: P<0.01) and as compared to the CF group with no nutritional failure (#: P<0.05).
DISCUSSION
In the present study, we have tested a novel method that is able to quantify protein digestibility accurately and in a convenient way in the clinical setting in patients with CF. Protein digestibility in CF was reduced to 47% of healthy subjects, and it took 100 minutes before pancreatic enzymes were able to increase protein digestion rate to 90%.
Novel methodology to measure protein digestibility in CF
It is generally known that exocrine pancreatic insufficiency in CF impairs fat digestion, but the extent of protein maldigestion during feeding was not clear in CF. The presently observed severely compromised protein digestibility in CF may explain why very high amounts of protein are necessary to stimulate protein accretion when pancreatic enzymes are not provided (9). However, intake of high amounts of protein results in an excess protein content in the colon in CF whereas when smaller amounts of protein are provided it may result in protein deficiency. Therefore, to unravel the digestion kinetics of ingested protein and the effectiveness of pancreatic enzyme replacement in CF, accurate and non-laborious methods to measure protein digestibility are of clinical importance. So far, two major categories of indirect tests are routinely used to measure maldigestion in CF; 1) analysis of undigested and unabsorbed food components in feces or 2) measurement of pancreatic enzymes/their products in the serum or stool (29). Measurement of fecal nitrogen losses accounts poorly for the true digestibility of protein because of the bacterial use of protein and amino acids in the colon. Amino acids can be transformed into ammonia, which makes detection of any perturbation of protein absorption difficult. Limitations of the fecal nitrogen determination might explain why a recent study showed that protein maldigestion was mostly undetectable using standard fecal tests in patients with pancreatic exocrine insufficiency (30) and that an improved effect of enzyme therapy on fecal protein digestibility was not detectable over a 4-week period (31) in patients with chronic pancreatitis.
Stable isotope techniques as used in the present study are safe methods for in vivo study of protein digestibility. Labeled amino acids must be incorporated into the ingested protein to adequately represent its fate. A strong relationship was found between duodenal trypsin output and 13CO2 excretion in the breath after intake of proteins intrinsically labeled with 13C-leucine (32). When proteins or amino acids are labeled with 13C, their oxidation following digestion and absorption can be evaluated by measuring 13CO2 excretion in the breath. However, measurement of the enrichment of labeled CO2 in breath does not take into account disease-related changes in CO2 production due to pulmonary inflammation as present in CF (9). Only a few studies are available using stable isotopes to measure protein digestibility in CF. Oral ingestion of 131I-labeled protein showed a 10% to 40% fecal excretion of the isotope in children with CF (vs <6% in control subjects) (4). When pancreatic enzymes (pancreatin) were ingested to hydrolyze the test meal, fecal excretion of isotope resulted in a 50% decrease in CF, but pancreatic enzymes were not equally effective in all patients (4). However, study of the metabolic fate of dietary 15N using 15N-labeled casein, enabling transfer of dietary nitrogen, showed increases to some extent in plasma amino acids, proteins, and the deamination pool (i.e., dietary N is still present in body urea and excreted through urinary urea) (30). Although not optimal, this method was able to reveal protein maldigestion in patients with pancreatic exocrine insufficiency as well as evaluate the efficacy of enzyme substitution although a high variation was observed between subjects (30). The absorptive capacity of the gut and small bowel transit time are also important factors in the overall process of protein assimilation but those processes do not affect the calculated protein digestibility as assessed by our stable isotope method.
Impaired protein digestibility in CF during feeding and response to pancreatic enzyme intake
According to the Cystic Fibrosis Foundation patient registry data based recommendations (33), achieving and maintaining normal weight for adults and typical growth for children is expected when appropriate nutrition and pancreatic enzyme replacement therapy are given. Inadequate treatment of pancreatic insufficiency in CF may have serious consequences for nutritional status, which has been directly correlated with lung function and survival for adults and children (33). In the present study, we used sip feeds as a model that reflects supplemental enteral feeding by gastrostomy tube as often used to improve the nutritional status of undernourished CF patients (34). Tube feeding is administered mostly at night at continuous infusion over 6 to 12 hours and pancreatic enzymes are given at the start of the feeding. The observed protein digestibility during feeding of 47% of normal in CF indicates a severe reduction of protein digestibility that was comparable in adults and children with CF.
In the present study, the dose of pancreatic enzymes was determined on an individual basis taking the fat content and rate of administration of the sip feeding into consideration. We observed that 100 min was needed after intake of the pancreatic enzymes before protein digestibility reached its maximal value of 90% of normal in CF. A comparable response in protein digestibility after pancreatic enzyme intake was present in children and adults with CF. It is unclear whether increasing the dose of pancreatic enzymes just before intake of a bolus meal will improve protein digestibility sufficiently.
Approaches to circumvent the impaired protein digestibility in CF
There are several ways to circumvent the reduced protein digestibility in CF patients while they are on pancreatic enzyme replacement therapy, i.e., adjusting the type of protein intake into more slowly digested proteins, intake of hydrolyzed proteins or free dietary amino acids, changing the timing of pancreatic enzyme intake, and/or by modifying the dose/composition (lipase, protease, amylase) of the pancreatic enzyme capsules. Our results indicate that when meals are consumed consisting of proteins that are slowly digested (e.g., casein protein), which mimics the sip feeding protocol used in the present study, it takes nearly 2 hours before enzyme activity has its maximal effect in CF patients. On the other hand, supplements with hydrolyzed proteins and particularly with free amino acids might be of interest to circumvent reduced protein digestibility and at the same time to meet the increased need for amino acids for building muscle and acute phase proteins in CF related to their inflammatory state (20). Recently, we have shown (35) that an oral mixture of free dietary essential amino acids are highly anabolic in patients with CF and that the amount of essential amino acids that after splanchnic extraction comes available to the peripheral compartment determines the anabolic capacity of a supplement in CF.
The normalization of protein digestibility that occurred after pancreatic enzyme intake in the studied CF patients suggests that the amount of protease in the pancreatic enzymes is sufficient. However, normalization of protein digestibility was severely delayed as it took nearly 2 hours before protein digestibility was normalized, suggesting that the pancreatic enzyme capsules need to be modified during continuous feeding to make the enzymes more quickly available and active. Pancreatic enzyme products are labeled according to the amount of lipase they contain. All products also contain protease and amylase, but the labeled and actual amounts of these two enzymes may differ from product to product even when labeled lipase amounts are the same. Our data suggest that the pancreatic enzyme products need to work faster during meal intake and that the labeling of the products should also contain the protease activity. For pancreatic enzymes to be effective proteases, it is crucial that they are available when protein in the food reaches the proximal small intestine, which is the place where amino acid uptake mainly takes place. Our results indicate that a fast response is essential for the proteases, and therefore the capsules need to be opened to release the proteases in the last part of the duodenum when pH is less acidic, while for the lipases a much slower response is needed as they are irreversibly inactivated at pH 4.0 or lower. The dose and timing of administered pancreatic enzymes in CF should therefore be based on multiple factors like the composition of the diet (amount fat/protein) in order to improve both fat and protein digestibility simultaneously and on factors like gastric acid secretion, pH at different levels of the small and large intestine, patterns of gastric emptying, and bile acid composition and concentration (36).
Whole-body citrulline production as measure of gut function
In the present study, plasma citrulline concentration was elevated in the CF as compared to the healthy subjects, which was in line with our previous data in CF (11). Therefore, both in CF patients at the end of hospitalization for an acute exacerbation as in stable CF outpatients, gut mucosal function seem to be unaffected. Citrulline is an amino acid released almost exclusively from the small bowel enterocyte mucosal mass as it is not a protein component and as such not incorporated in enteral food or endogenous proteins. Serum citrulline levels were significantly lower in short bowel patients compared to healthy controls and correlated well with both small bowel length/area and digestive protein absorption, calculated as percentage of protein representing the proportion of oral intake not recovered in fecal output (10, 37). Recently we have shown that besides plasma citrulline concentration also whole-body production of citrulline was elevated in CF patients and particularly in those with nutritional failure (11). In that study, whole-body citrulline production was measured in CF patients using a primed, constant, and continuous infusion of L-[ureido-13C-2H2]citrulline whereas in the present study a single pulse of L-[5-13C-2H2]-Citrulline was used. We have no reason to assume that measuring citrulline production will differ between the isotopes. In line, in the present study, whole-body citrulline rate of appearance and production rate were also elevated in CF but not related to protein digestion rate during feeding or with the average protein digestion rate obtained after pancreatic enzyme intake in CF. Also, no association was found between whole-body citrulline rate of appearance or production and lung function or age in CF.
Limitations of the study
Our stable isotope-based approach to measure protein digestibility has some limitations. As we used spirulina protein and not the actual proteins in the nutritional supplement, we are not completely certain that the digestibility as measured with the spirulina protein represents protein digestibility in general. The observed digestibility, however, is in line with digestibility observed in animals (28). We therefore have no reason to assume that the digestibility of spirulina protein is not correctly represented by our results. However, to measure overall digestibility of meals with proteins, some or all proteins should be labeled with 15N. Isotope ratio measurements are necessary to be able to calculate the protein digestion rate, but these analytical techniques can be easily implemented on available GC-MS or LC-MS machines in the clinic. As our stable isotope-based method measures protein digestibility, we did not get information on amino acid absorption. As the elevated whole-body citrulline production indirectly indicates that mucosa function is normal in CF, a direct measurement of amino acid absorption could be made by using marker amino acids that are not metabolized in the body (e.g., labeled amino acid analogues), assuming that the marker absorption rates are representative for the amino acid absorption rate in general. This needs to be studied more in detail in CF. Furthermore, in order to test this new method, we chose to study CF patients during continuous (sip) feeding as it closely reflects the situation present in patients with a gastrostomy tube during overnight feeding. We acknowledge that the percentage of CF patients with a gastrostomy tube is small and therefore may not satisfactorily reflect the large CF population with a normal bolus eating pattern. Studies are needed that use our new method to measure protein digestion kinetics during bolus feeding with/without pancreatic enzyme intake as present in daily life of most patients with CF. Furthermore, to investigate whether daily use of proton-pump inhibitors has an effect on protein digestibility, the CF group was stratified into PPI users and non-users. As 87% of the patients were on daily PPI, the number of non-users was too small to do accurate statistics. Still no association was found between the daily dosage of PPI in the PPI using group and protein digestibility during feeding and after intake of the pancreatic enzymes.
In conclusion, protein digestibility during continuous feeding as measured by our novel and easy-to-use stable isotope technique is severely compromised in patients with CF and normalization is possible but delayed after pancreatic enzyme intake. Gut (mucosa) function as measured by whole-body citrulline production is not affected in CF.
ACKNOWLEDGEMENT
The contribution of the authors to the manuscript is as follows: MPKJ Engelen: study design, data collection, data analysis and writing of the manuscript, G Com and P Anderson: study design, data collection and review of manuscript, NEP Deutz: study design, data analysis and writing of the manuscript
SOURCES OF FUNDING
This study is supported by Award Number 1UL1RR029884 from the National Center for Research Resources and by NIH S10RR027047.
Abbreviation list
- AA
Amino acids
- BMI
Body mass index
- CIT
Citrulline
- CF
Cystic Fibrosis
- FEV1
Forced expiratory volume in 1 second
- FMI
Fat mass index
- FVC
Forced vital capacity
- FFMI
Fat-free mass index
- NF
Nutritional failure
- PHE
Phenylalanine
- PPI
Proton-pump inhibitor
- TR
Tracer-tracee ratio
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
There is no Conflict of Interest to declare.
STATEMENT OF AUTHORSHIP
Each author has participated sufficiently, intellectually or practically, in the work to take public responsibility for the content of the article, including the concept, design, and conduction of the experiment and for data interpretation (authorship).
REFERENCES
- 1.Ferrone M, Raimondo M, Scolapio JS. Pancreatic enzyme pharmacotherapy. Pharmacotherapy. 2007;27(6):910–920. doi: 10.1592/phco.27.6.910. [DOI] [PubMed] [Google Scholar]
- 2.Shohl AT. Further studies of nitrogen and fat metabolism in infants and children with fibrosis of the pancreas. The Journal of pediatrics. 1948;32(2):180–183. doi: 10.1016/s0022-3476(48)80009-0. [DOI] [PubMed] [Google Scholar]
- 3.Andersen DH. Celiac syndrome. II fecal excretion in congenital pancreatic deficiency at various ages and with various diets, with discussion of the optimal diet. Am J Dis Child. 1945;69:221. [Google Scholar]
- 4.Lavik PS, Matthews LW, Buckaloo GW, Lemm FJ, Spector S, Friedell HL. Use of I131 - labeled protein in the study of protein digestion and absorption in children with and without cystic fibrosis of the pancreas. Pediatrics. 1952;10(6):667–676. [PubMed] [Google Scholar]
- 5.Boirie Y, Gachon P, Corny S, Fauquant J, Maubois JL, Beaufrere B. Acute postprandial changes in leucine metabolism as assessed with an intrinsically labeled milk protein. Am J Physiol. 1996;271(6 Pt 1):E1083–E1091. doi: 10.1152/ajpendo.1996.271.6.E1083. [DOI] [PubMed] [Google Scholar]
- 6.van Loon LJ, Boirie Y, Gijsen AP, Fauquant J, de Roos AL, Kies AK, et al. The production of intrinsically labeled milk protein provides a functional tool for human nutrition research. J Dairy Sci. 2009;92(10):4812–4822. doi: 10.3168/jds.2009-2317. [DOI] [PubMed] [Google Scholar]
- 7.Geboes KP, Bammens B, Luypaerts A, Malheiros R, Buyse J, Evenepoel P, et al. Validation of a new test meal for a protein digestion breath test in humans. J Nutr. 2004;134(4):806–810. doi: 10.1093/jn/134.4.806. [DOI] [PubMed] [Google Scholar]
- 8.Berthold HK, Hachey DL, Reeds PJ, Thomas OP, Hoeksema S, Klein PD. Uniformly 13C–labeled algal protein used to determine amino acid essentiality in vivo. Proceedings of the National Academy of Sciences of the United States of America. 1991;88(18):8091–8095. doi: 10.1073/pnas.88.18.8091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kien CL, Zipf WB, Horswill CA, Denne SC, McCoy KS, O’Dorisio TM. Effects of feeding on protein turnover in healthy children and in children with cystic fibrosis. Am J Clin Nutr. 1996;64(4):608–614. doi: 10.1093/ajcn/64.4.608. [DOI] [PubMed] [Google Scholar]
- 10.Crenn P, Messing B, Cynober L. Citrulline as a biomarker of intestinal failure due to enterocyte mass reduction. Clin Nutr. 2008;27(3):328–339. doi: 10.1016/j.clnu.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 11.Engelen MP, Com G, Luiking YC, Deutz NE. Stimulated Nitric Oxide Production and Arginine Deficiency in Children with Cystic Fibrosis with Nutritional Failure. The Journal of pediatrics. 2013 doi: 10.1016/j.jpeds.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borowitz D, Baker RD, Stallings V. Consensus report on nutrition for pediatric patients with cystic fibrosis. J Pediatr Gastroenterol Nutr. 2002;35(3):246–259. doi: 10.1097/00005176-200209000-00004. [DOI] [PubMed] [Google Scholar]
- 13.VanItallie TB, Yang MU, Heymsfield SB, Funk RC, Boileau RA. Height-normalized indices of the body’s fat-free mass and fat mass: potentially useful indicators of nutritional status. Am J Clin Nutr. 1990;52(6):953–959. doi: 10.1093/ajcn/52.6.953. [DOI] [PubMed] [Google Scholar]
- 14.Demerath EW, Schubert CM, Maynard LM, Sun SS, Chumlea WC, Pickoff A, et al. Do changes in body mass index percentile reflect changes in body composition in children? Data from the Fels Longitudinal Study. Pediatrics. 2006;117(3):e487–e495. doi: 10.1542/peds.2005-0572. [DOI] [PubMed] [Google Scholar]
- 15.Kyle UG, Genton L, Slosman DO, Pichard C. Fat-free and fat mass percentiles in 5225 healthy subjects aged 15 to 98 years. Nutrition. 2001;17(7–8):534–541. doi: 10.1016/s0899-9007(01)00555-x. [DOI] [PubMed] [Google Scholar]
- 16.Schutz Y, Kyle UU, Pichard C. Fat-free mass index and fat mass index percentiles in Caucasians aged 18–98 y. Int J Obes Relat Metab Disord. 2002;26(7):953–960. doi: 10.1038/sj.ijo.0802037. [DOI] [PubMed] [Google Scholar]
- 17.Kalkwarf HJ, Zemel BS, Gilsanz V, Lappe JM, Horlick M, Oberfield S, et al. The bone mineral density in childhood study: bone mineral content and density according to age, sex, and race. The Journal of clinical endocrinology and metabolism. 2007;92(6):2087–2099. doi: 10.1210/jc.2006-2553. [DOI] [PubMed] [Google Scholar]
- 18.Bolton CE, Ionescu AA, Evans WD, Pettit RJ, Shale DJ. Altered tissue distribution in adults with cystic fibrosis. Thorax. 2003;58(10):885–889. doi: 10.1136/thorax.58.10.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ionescu AA, Evans WD, Pettit RJ, Nixon LS, Stone MD, Shale DJ. Hidden depletion of fat-free mass and bone mineral density in adults with cystic fibrosis. Chest. 2003;124(6):2220–2228. doi: 10.1378/chest.124.6.2220. [DOI] [PubMed] [Google Scholar]
- 20.Ionescu AA, Nixon LS, Evans WD, Stone MD, Lewis-Jenkins V, Chatham K, et al. Bone density, body composition, and inflammatory status in cystic fibrosis. Am J Respir Crit Care Med. 2000;162(3 Pt 1):789–794. doi: 10.1164/ajrccm.162.3.9910118. [DOI] [PubMed] [Google Scholar]
- 21.Ionescu AA, Nixon LS, Luzio S, Lewis-Jenkins V, Evans WD, Stone MD, et al. Pulmonary function, body composition, and protein catabolism in adults with cystic fibrosis. Am J Respir Crit Care Med. 2002;165(4):495–500. doi: 10.1164/ajrccm.165.4.2104065. [DOI] [PubMed] [Google Scholar]
- 22.Allan PF, Thomas KV, Ward MR, Harris AD, Naworol GA, Ward JA. Feasibility study of noninvasive ventilation with helium-oxygen gas flow for chronic obstructive pulmonary disease during exercise. Respir Care. 2009;54(9):1175–1182. [PubMed] [Google Scholar]
- 23.Engelen MP, Schroder R, Van der Hoorn K, Deutz NE, Com G. Use of body mass index percentile to identify fat-free mass depletion in children with cystic fibrosis. Clinical nutrition (Edinburgh, Scotland) 2012 doi: 10.1016/j.clnu.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 24.Milla CE. Nutrition and lung disease in cystic fibrosis. Clinics in chest medicine. 2007;28(2):319–330. doi: 10.1016/j.ccm.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 25.Wang X, Dockery DW, Wypij D, Fay ME, Ferris BG., Jr Pulmonary function between 6 and 18 years of age. Pediatric pulmonology. 1993;15(2):75–88. doi: 10.1002/ppul.1950150204. [DOI] [PubMed] [Google Scholar]
- 26.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159(1):179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 27.Wolfe RR, Chinkes DL. Isotope Tracers in Metabolic Research: Principles and Practice of Kinetic Analysis. New York: Wiley, New York, New York; 2005. p. 274. [Google Scholar]
- 28.Peiretti PG, Meineri G. Effects of diets with increasing levels of Spirulina platensis on the performance and apparent digestibility in growing rabbits. Livestock Science. 2008;118(1):173–177. [Google Scholar]
- 29.Walkowiak J. Assessment of maldigestion in cystic fibrosis. The Journal of pediatrics. 2004;145(3):285–287. doi: 10.1016/j.jpeds.2004.06.033. [DOI] [PubMed] [Google Scholar]
- 30.Airinei G, Gaudichon C, Bos C, Bon C, Kapel N, Bejou B, et al. Postprandial protein metabolism but not a fecal test reveals protein malabsorption in patients with pancreatic exocrine insufficiency. Clin Nutr. 2011;30(6):831–837. doi: 10.1016/j.clnu.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 31.Van Hoozen CM, Peeke PG, Taubeneck M, Frey CF, Halsted CH. Efficacy of enzyme supplementation after surgery for chronic pancreatitis. Pancreas. 1997;14(2):174–180. doi: 10.1097/00006676-199703000-00010. [DOI] [PubMed] [Google Scholar]
- 32.Evenepoel P, Hiele M, Geypens B, Geboes KP, Rutgeerts P, Ghoos Y. 13C–egg white breath test: a non-invasive test of pancreatic trypsin activity in the small intestine. Gut. 2000;46(1):52–57. doi: 10.1136/gut.46.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stallings VA, Stark LJ, Robinson KA, Feranchak AP, Quinton H, et al. Clinical Practice Guidelines on G. Evidence-based practice recommendations for nutrition-related management of children and adults with cystic fibrosis and pancreatic insufficiency: results of a systematic review. J Am Diet Assoc. 2008;108(5):832–839. doi: 10.1016/j.jada.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 34.Rosenfeld M, Casey S, Pepe M, Ramsey BW. Nutritional effects of long-term gastrostomy feedings in children with cystic fibrosis. J Am Diet Assoc. 1999;99(2):191–194. doi: 10.1016/S0002-8223(99)00046-2. [DOI] [PubMed] [Google Scholar]
- 35.Engelen MP, Com G, Wolfe RR, Deutz NE. Dietary essential amino acids are highly anabolic in pediatric patients with cystic fibrosis. Journal of cystic fibrosis : official journal of the European Cystic Fibrosis Society. 2013 doi: 10.1016/j.jcf.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dodge JA. Pancreatic enzyme therapy in cystic fibrosis. Expert review of respiratory medicine. 2008;2(6):681–683. doi: 10.1586/17476348.2.6.681. [DOI] [PubMed] [Google Scholar]
- 37.Jianfeng G, Weiming Z, Ning L, Fangnan L, Li T, Nan L, et al. Serum Citrulline Is A Simple Quantitative Marker for Small Intestinal Enterocytes Mass and Absorption Function in Short Bowel Patients. Journal of Surgical Research. 2005;127(2):177–182. doi: 10.1016/j.jss.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 38.Wolfe RR, Chinkes DL. Isotope Tracers in Metabolic Research. Second ed. John Wiley & Sons, Inc; 2005. Chap4: Calculation of substrate kinetics: Multiple pool-models; pp. 51–76. [Google Scholar]