Abstract
Nowadays prostate cancer is the most common solid tumor in men from industrialized countries and the second leading cause of death. At the ages when PCa is usually diagnosed, mortality related to cardiovascular morbidity is high; therefore, men at risk for PCa frequently receive chronic lipid-lowering and antiplatelet treatment. The aim of this study was to analyze how chronic treatment with statins, aspirin, and their combination influenced the risk of PCa detection. The tumorigenic properties of these treatments were evaluated by proliferation, colony formation, invasion, and migration assays using different PCa cell lines, in order to assess how these treatments act at molecular level. The results showed that a combination of statins and aspirin enhances the effect of individual treatments and seems to reduce the risk of PCa detection (OR: 0.616 (95% CI: 0.467–0.812), P < 0.001). However, if treatments are maintained, aspirin (OR: 1.835 (95% CI: 1.068–3.155), P = 0.028) or the combination of both drugs (OR: 3.059 (95% CI: 1.894–4.939), P < 0.001) represents an increased risk of HGPCa. As observed at clinical level, these beneficial effects in vitro are enhanced when both treatments are administered simultaneously, suggesting that chronic, concomitant treatment with statins and aspirin has a protective effect on PCa incidence.
1. Introduction
Nowadays prostate cancer (PCa) is the most common solid tumor in men from industrialized countries and the second leading cause of death [1, 2]. For this reason, the discovery of modifiable risk determinants would provide an opportunity to prevent or delay the onset of this common disease. Established risk factors, such as ancestry or family history, are not modifiable, and the evidence for dietary and lifestyle risk factors is inconclusive [3].
At the ages when PCa is usually diagnosed, mortality related to cardiovascular morbidity is high [4]. Associations between coronary heart disease and PCa risk have even been suggested [5]; men at risk of PCa frequently receive chronic lipid-lowering and antiplatelet treatment [6, 7]. Statins and aspirin are two of the most frequently used drugs, and they both have been implicated in prostate carcinogenesis, albeit not without some controversy [8, 9].
Statins (3-hydroxy-3-methyl-glutaryl-coenzyme A reductase inhibitors) are the most commonly used drugs for lowering cholesterol [10]. In recent years, a growing amount of evidence on the anticancer effects of statins has become available from laboratory and epidemiological studies. In vitro and in vivo studies [11] suggest that statins are able to arrest cell cycle progression [12], induce apoptosis [13], reduce inflammation, and impede angiogenesis [14].
Currently, observational studies support that statin users have an unchanged overall risk of PCa; however, some well-designed epidemiological studies have reported a reduced incidence of advanced PCa in this population. Moreover, statins showed an inhibitory effect on the growth of PCa in both in vitro and in vivo studies [15]. On the other hand, in recent years the role of inflammation in cancer etiology has gained attention, and several studies have suggested that nonsteroidal anti-inflammatory drugs (NSAIDs) may have chemopreventive activity. NSAIDs have been associated with a reduced risk of detection for different malignancies: a reduction of the relative risk of 63% for colorectal cancer, 39% for breast cancer, 36% for lung cancer, and up to 39% for PCa [16]. In particular, the use of acetyl salicylic acid (ASA), the active metabolite of aspirin and a well-known NSAID, is associated with a 15%–55% risk reduction for PCa [17–20]; however, two other studies associated aspirin with increased risk of PCa [21, 22], while others reported no association [23, 24]. Although some controversy exists, the protective effect of aspirin treatment is reinforced by a recent meta-analyses study that showed a decreased overall mortality from cancer in a population using a continuous low dose of aspirin daily. This effect increased according to the treatment time [25]. This effect could be related to the potential role of the inflammation pathway in the development of PCa.
There are many studies based on the influence of statins and aspirin on PCa risk. However, even though it is common clinical practice [26], there is no report describing how a chronic treatment combining both treatments influences the risk of PCa detection. Therefore, the aim of our study was to analyze how the chronic treatment with statins, aspirin, and their combination influenced the risk of PCa detection in a cohort of patients undergoing prostate biopsy (PB) due to elevated prostate specific antigen (PSA) or abnormal digital rectal examination (DRE). We also evaluated the effect of these treatments on tumorigenic properties using different PCa cell lines, in order to corroborate clinical observations at in vitro level.
2. Materials and Methods
2.1. Design
We conducted a retrospective case-control study at a single academic center. All participants received detailed information about the study procedure and provided written informed consent before study entry. All procedures were in accordance with the ethical standards established in our country. Internal review board approval was not required for such a nonexperimental study.
2.2. Patients
Between January 2006 and December 2011, 2,408 consecutive Mediterranean men, who had been referred for prostate biopsy due to abnormal DRE and/or serum PSA levels higher than 4 ng/mL, were included in this study.
A total of 1,830 men (76%) were undergoing their first PB, while, for 578 of them, it was a repeat PB procedure. All patients were assessed by anamnesis about cardiovascular comorbidities and risk factors, life style (smoking habit and physical activity), and pharmacological treatments. A total of 1,504 men (62.5%) had not been treated with statins or aspirin, while 440 of them (18.3%) had been treated with statins, 160 (6.6%) with aspirin, and 304 (12.6%) with a combination of statins and aspirin. All of the men who received prior treatment had received it chronically for more than 1 year when this study began, and no significant differences existed among the groups, either in the initial PSA levels, in DRE results, or in the Gleason scores (see Table 1).
Table 1.
Characteristics of study groups.
| Nontreated cells | Statins | Aspirin | Statin + aspirin | |
|---|---|---|---|---|
| Men (n) | 1504 | 440 | 160 | 304 |
| Age (years) | 67 | 68 | 68 | 67 |
| BMI (Kg/m2) | 26.9 | 27.6 | 27.2 | 27.8 |
| Serum PSA (ng/mL) | 6.9 | 6.7 | 6.9 | 6.8 |
| Positive DRE (%) | 540 (36) | 160 (36) | 50 (31) | 106 (35) |
| Gleason score < 7 (%) | 112 (7.4) | 32 (7.3) | 14 (8.8) | 18 (6) |
| Gleason score = 7 (%) | 273 (18.2) | 76 (17.3) | 38 (23.8) | 45 (14.8) |
| Gleason score > 7 (%) | 155 (10.3) | 43 (9.8) | 18 (11.2) | 24 (7.9) |
BMI: body mass index, PSA: prostate specific antigen, and DRE: digital rectal examination.
2.3. Prostatic Biopsy Technique
The PB was done as an ambulatory procedure with local anesthesia. An end-fire ultrasound transducer (Falcon 2101, B-K Medical, Inc.) and a 16-gauge automatic biopsy needle (Bard, Inc.) were used to perform the biopsy. At least 10 cores, plus 2 to 8 additional ones, according to age and prostate volume, were obtained, according to a modified Vienna nomogram [27].
2.4. Cell Culture
All cell lines were obtained from the American Type Culture Collection (Rockville, MD). PCa cell lines PC3 (isolated from bone metastasis of human prostate carcinoma) and LNCaP (established from a biopsy of a lymph node metastasis of hormone refractory PCa) were maintained in RPMI 1640 medium (Life Technologies, Inc., Grand Island, NY) supplemented with 10% fetal bovine serum (FBS), 2 mM l-glutamine, 100 U of penicillin/mL, 100 μg of streptomycin/mL, and 0.1 mM nonessential amino acids. Cells were grown at 37°C in an atmosphere of 5% CO2-95% air and 99% humidity.
2.5. Drug Concentrations
Doses of the drugs used in this study were chosen based on previous studies by Murtola et al. [28], where, moreover, the same prostate cancer cell lines were used. Standard doses of simvastatin (STA) have been reported to obtain a peak serum value in the range of 10–100 nM; a concentration slightly above the therapeutic range had been chosen for this study (1 μM) (Sigma-Aldrich). Acetylsalicylic acid (ASA) (Sigma-Aldrich) was used at a concentration of 1 mM based on common plasma salicylate levels with therapeutic doses of aspirin [29].
2.6. Proliferation Analysis
A number of 5 × 104 cells per well were seeded on six-well plates with the appropriate medium. The cells were allowed to attach and were subsequently treated with the indicated drugs (STA, ASA, or STA + ASA) for 7 days, renewing growth medium and treatments daily. Cells were counted by using Neubauer chamber.
2.7. Colony Formation Assay
For the colony formation assay, cells were seeded at low density (250 cells/well in a 12-well plate) and were allowed to grow for 10 days, renewing growth medium and treatments every 2 days. Then, the cells were fixed in paraformaldehyde 4% and stained with crystal violet. Colony formation was quantified by OD reading at 590 nm after 2-hour extraction of the crystal violet stain.
2.8. Migration Assays
Wound Healing. The PC3 cells were allowed to reach confluent monolayer in 24-well plates and were incubated overnight. A straight line (wound) was then gently performed at the bottom of the dish with a 0.5 mm plastic pipette tip. Afterwards, cells were washed, incubated in medium with 2% FBS, with or without treatments, and kept in a computer controlled mini-incubator, which provided a stabilized temperature of 37°C with 95% humidity, 5% CO2, and optical transparency for microscopic observations. The incubator was fastened to an inverted microscope (Live Cell Imaging CellR, Olympus, Japan) to monitor cell migration. Images were taken with the 4x objective every 30 minutes at predetermined wound sites (3 sites per condition, performed in triplicate) and were analyzed using the Image J software (Wright Cell Imaging Facility, USA). Initial wound area (μm2) and time needed to close the wound (hr) were the variables used to calculate the migration speed of the cells.
Transwell Migration Assay. LNCaP cells were seeded (105 cells/insert) onto transwell inserts (BD Bioscience, USA) with 8 μm diameter pore membranes in medium supplemented with 2% FBS in the presence or absence of treatments. After 48 hours, cells from inserts were fixed with 4% paraformaldehyde (PFA) and stained with 0.1% crystal violet. Migration was quantified by OD reading at 590 nm after 2-hour extraction of the crystal violet stain.
2.9. Transwell Matrigel Invasion Assay
PCa cells were seeded (104/insert) onto Matrigel Invasion Chamber inserts (BD Bioscience, USA) with 8 μm diameter pore membranes in triplicate in medium supplemented with 2% FBS in the presence or absence of treatments and with the lower chamber containing medium supplemented with 10% FBS. After 48 hours, the number of invading cells was determined as described above (transwell migration assay).
2.10. Western Immunoblotting
Cultured cells, washed with cold PBS, were scratched with RIPA buffer (Tris 20 mM pH 8.8, NaCl 150 mM, EDTA 5 mM, Triton X-100 1%, and protease inhibitors) and supernatant boiled at 100°C for 5 min with Laemmli buffer. The same amount of protein was resolved by 10% SDS-PAGE and transferred onto a PVDF membrane (Immobilon PSQ, Millipore, Massachusetts, USA). Membranes were blocked in TBS with 5% nonfat dried milk and 0.1% Tween 20 for 1 h prior to incubation at 4°C overnight with the appropriate primary antibody (1 : 200 α2-integrin, Santa Cruz Biotechnology, Santa Cruz, CA, USA; 1 : 400 cyclin D1, Santa Cruz Biotechnology, Santa Cruz, CA, USA; 1 : 50 vimentin, Sigma-Aldrich, Saint Louis, Missouri, USA; 1 : 2000 E-cadherin, BD Transduction Laboratories, Palo Alto, CA, USA; 1 : 1000 α-tubulin, Sigma-Aldrich, Saint Louis, Missouri, USA). Detection was done using horseradish-peroxidase-conjugated secondary antibodies and enhanced chemiluminescence reagents (Amersham ECL, GE Healthcare, Buckinghamshire, UK).
2.11. Statistical Analysis
Quantitative variables were expressed as medians, interquartile range, and range. Qualitative variables were expressed as percentages. Mann-Whitney U test, Wilcoxon test, and Kruskal-Wallis test were used to evaluate the significance of differences, comparing quantitative variables in two or more groups. Chi-square and Cochran tests were used to assess the normal distribution of patients, according to categorical variables and their correlation. A multivariate analysis through a binary logistic regression was performed to detect independent PC or HGPCa predictors. Odds ratios and 95% confidence interval were estimated. All analyses were calculated using SPSS v.20 (IBM, Inc.) statistical software. Statistical analysis of the cell culture data was performed by means of one-way analysis of variance (ANOVA) (Tukey's posttest).
3. Results
3.1. Observational Clinical Study
Once the final diagnosis was obtained, PCa was detected in 848 of the 2,408 patients included in this study (35.2%), 240 of whom presented with high grade PCa (HGPCa) (28.3%).
The distribution of PCa and, specifically, the HGPCa cases in relation to aspirin and statins treatments is shown in Table 2. Among the 1,504 nontreated men, PCa was detected in 552 (36.7%) without statistical differences when compared to men who were only treated with statins or those who were only treated with aspirin. In the statin-only subset, PCa was detected in 152 of 440 patients (34.5%) (P value = 0.879), and in the aspirin-only subset 64 PCa cases were detected (40%) (P value = 0.536). Interestingly, in the group of 304 patients that received concomitant treatment with statins and aspirin, PCa was detected in significantly fewer cases (26.3%) when compared to the nontreated group (P value = 0.003).
Table 2.
Distribution of patients according to the diagnosis of PCa and HGPCa and the treatment with statins, aspirin, and the combination of both treatments.
| PCa detection (%) | P value | HGPCa detection (%) | P value | |
|---|---|---|---|---|
| Nontreated cells | 552/1504 (37) | — | 136/552 (24.6) | — |
| Statins | 152/440 (34.5) | 0.879 | 40/152 (26.3) | 0.673 |
| Aspirin | 64/160 (40) | 0.536 | 24/64 (37.5) | 0.034 |
| Statins + aspirin | 80/304 (26.3) | 0.003 | 40/80 (50) | <0.001 |
Regarding the rate of PCa patients who presented with HGPCa, we observed that, in the nontreated group, 24.6% of the PCa patients presented with HGPCa (136/552), whereas the aspirin group presented a significantly higher incidence rate of HGPCa, 37.5% (24/64) (P value = 0.034). This increase was especially noted in men who had been administrated the concomitant treatment with statins and aspirin, achieving a rate of 50% (40/80) (P value < 0.001). On the other hand, no statistical difference was observed in PCa patients treated with statins with HGPCa cases having a rate of 26.3% (P value = 0.673).
After the analysis of the above results, a binary logistic regression was conducted to evaluate if the treatment with statins and aspirin could be an independent predictor of the PCa risk and HGPCa incidence. Age, serum PSA, DRE and body mass index (BMI), statins, aspirin, and the combination between statins and aspirin treatments as well as the duration of those were included as predictive variables in this analysis (Table 3). As expected, age and serum PSA were PCa predictors. The reduction in overall PCa incidence was statistically significant for current users of both drugs. Statins and aspirin used as chronic concomitant treatment were an independent predictor factor of reduction of PCa detection risk, OR: 0.616 (95% CI: 0.467–0.812), P < 0.001.
Table 3.
Multivariate analysis of PC risk and HGPCa risk according to the treatment with statins and aspirin respect no treatment.
| Treatment | PCa detection* | P value | HGPCa detection* | P value |
|---|---|---|---|---|
| Age (years) | 1.066 (1.049–1.084) | 0.001 | 1.100 (1.071–1.130) | 0.001 |
| BMI (Kg/m2) | 1.018 (0.001–1.047) | 0.189 | 0.989 (0.948–1.032) | 0.616 |
| PSA (ng/mL) | 1.024 (1.008–1.041) | 0.004 | 1.038 (1.027–1.049) | 0.001 |
| DRE (positive versus negative) | 1.219 (1.028–1.428) | 0.001 | 1.266 (0.879–1.822) | 0.205 |
| Statin (yes versus no) | 0.910 (0.728–11.137) | 0.430 | 1.092 (0.725–1.646) | 0.637 |
| Aspirin (yes versus no) | 1.150 (0.824–1.604) | 0.439 | 1.835 (1.068–3.155) | 0.028 |
| Statins + aspirin (yes versus no) | 0.616 (0.467–0.812) | <0.001 | 3.059 (1.894–4.939) | <0.001 |
| Time on statins (months) | 0.998 (0.955–1.000) | 0.063 | 1.005 (1.000–1.010) | 0.034 |
| Time on aspirins (months) | 0.984 (0.979–0.990) | <0.001 | 1.033 (1.020–1.047) | <0.001 |
BMI: body mass index, PSA: prostate specific antigen, and DRE: digital rectal examination. *Values expressed as odds ratio (95% confidence interval).
Regarding the risk of high grade tumors detection, treatment with aspirin, OR: 1.835 (95% CI: 1.068–3.155), P = 0.028, and combined therapy, OR: 3.059 (95% CI: 1.894–4.939), P < 0.001, were independent factors of HGPCa. Furthermore, the length of aspirin treatment was a significant predictor of PCa, OR: 0.998 (95% CI 0.979–0.990), P < 0.001, while the length of statins treatment, OR: 1.005 (1.000–1.010), P = 0.034, and aspirin, OR: 1.033 (1.020–1.047), P < 0.001, was a predictor of HGPCa.
3.2. In Vitro Effects of Simvastatin, Acetylsalicylic Acid, and Their Combination
Considering the clinical observations that have been mentioned above, STA and ASA were used to treat different PCa cell lines, in order to unveil by which mechanisms the chronic treatment with these drugs significantly decreased the PCa incidence.
3.2.1. Effect on the Proliferation of Prostatic Cancer Cells
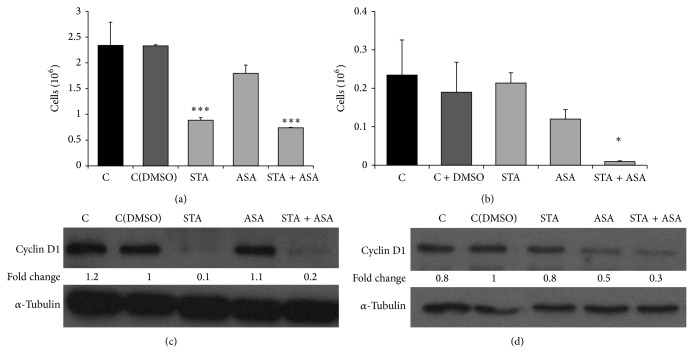
Firstly, after 7 days of treatment the effects of STA, ASA, and their combination on proliferation were studied in PC3 and LNCaP PCa cell lines. The results showed a significant decrease in the proliferation rate of PC3 treated with STA (62%; P value = 0.001) and with the combination of both (68%; P value < 0.001); however, no effects were observed due to the presence of ASA in the culture media compared to control cells (cells treated only with DMSO). On the other hand, only the combination of STA and ASA was able to markedly reduce the proliferation in LNCaP cells (90%; P value = 0.002) (Figures 1(a)-1(b)).
Figure 1.
Effect of STA, ASA, and the combination of both treatments on prostate cancer cell lines proliferation. Values for proliferation are presented as number of cells (∗106) after 7 days of treatment for (a) PC3 and (b) LNCaP cell lines with different conditions: nontreated cells, control (C) and control with DMSO (C(DMSO)), and cells treated with statin (STA), aspirin (ASA), and a combination of both statin and aspirin (STA + ASA) (for further details, see Section 2). The bars represent the mean ± SEM. Values that are significantly different by one-way analysis of variance (ANOVA) from the C(DMSO) group are indicated by * P < 0.05, *** P < 0.001. Western blots of cyclin D1 (37 KDa) and the control α-tubulin (52 KDa) performed on total cell extracts after 7 days of treatment are presented for PC3 (c) and LNCaP (d) cell lines. Densitometry analysis using Image J software is represented below bands as fold change values (versus C(DMSO)).
To further study the effect of these treatments on the cell cycle, we analyzed the levels of cyclin D1 protein, a key protein in regulation of cell cycle. As expected, cyclin D1 showed a significant decrease in protein levels in those groups in which we also obtained a most marked decrease in cell proliferation (Figures 1(c)-1(d)).
3.2.2. Effect on Colony Formation Capacity
PC3 cells were grown at low cell density (250 cells/well) in the absence or presence of the indicated concentration of STA, ASA, and the combination of both STA and ASA for 10 days. PC3 cells formed colonies, as described by others [30]; however LNCaP cells did not show the same ability to grow from so low cell density. Regarding treatment effects, long-term ASA treatment inhibited cell colony formation ability compared to control cells which had been receiving DMSO in 36% (P value < 0.001); furthermore, this effect was even higher in those cells treated with STA (91%; P value < 0.001) and the combination of both drugs (98%; P value < 0.001) (Figure 2).
Figure 2.
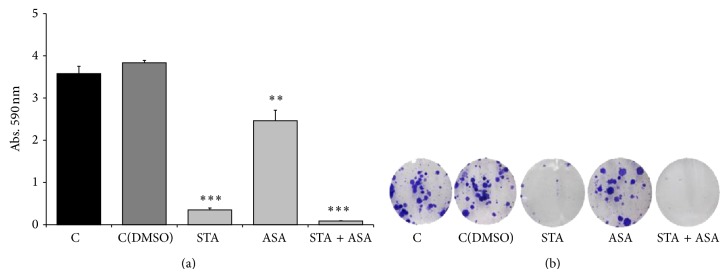
Effect of STA, ASA, and the combination of both treatments on PC3 cell line colony formation capacity. Values for colony formation capacity are presented as crystal violet absorbance/well after 10 days of treatment for PC3 cell line with different conditions: nontreated cells, control (C) and control with DMSO (C(DMSO)), and cells treated with statin (STA), aspirin (ASA), and a combination of both statin and aspirin (STA + ASA) (for further details, see Section 2). (a) The bars represent the mean ± SEM. Values that are significantly different by one-way analysis of variance (ANOVA) from the C(DMSO) group are indicated by ** P < 0.01, *** P < 0.001. (b) An inverted microscope with phase contrast at 10x magnification was used to take images of stained colonies.
3.2.3. Effect on Migration and Invasion Capacity
In addition to cell growth and proliferation, cell mobility was studied as an important step in tumor progression; therefore, we analyzed the invasion and migration abilities of PC3 and LNCaP cells.
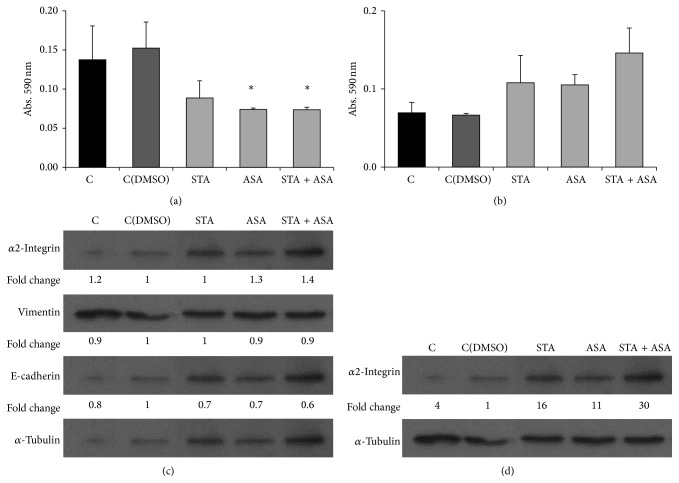
First, using transwell assay the invasion capacity was assessed on PC3 and LNCaP cell lines. The number of PC3 that migrated to the bottom of the transwell chambers significantly decreased compared to the nontreated group (cells with DMSO) after being treated for 48 h with ASA (48%) and STA + ASA (48%) (Figures 3(a) and 3(c)). Unexpectedly, no evident changes were found for LNCaP invasiveness (Figures 3(b) and 3(d)).
Figure 3.
Effect of STA, ASA, and the combination of both treatments on prostate cancer cell lines invasion capacity. Values for invasion are presented as crystal violet absorbance/well for PC3 (a) and LNCaP (b) cell lines after 48 h of treatment with different conditions: nontreated cells, control (C) and control with DMSO (C(DMSO)), and cells treated with statin (STA), aspirin (ASA), and a combination of both statin and aspirin (STA + ASA) (for further details, see Section 2). The bars represent the means ± SEM. Values that are significantly different by one-way analysis of variance (ANOVA) from the control (C = nontreated) group are indicated by * P < 0.05 (versus C(DMSO)). Western blot of α2-integrin (150 KDa), vimentin (58 KDa), E-cadherin (120 KDa), and the control α-tubulin (52 KDa) was performed on total cell extracts after 7 days of treatment for PC3 cell line (c) and α2-integrin (150 KDa) and the control α-tubulin (52 KDa) for LNCaP (d). Densitometry analysis using Image J software is represented below bands as fold change values (versus C(DMSO)).
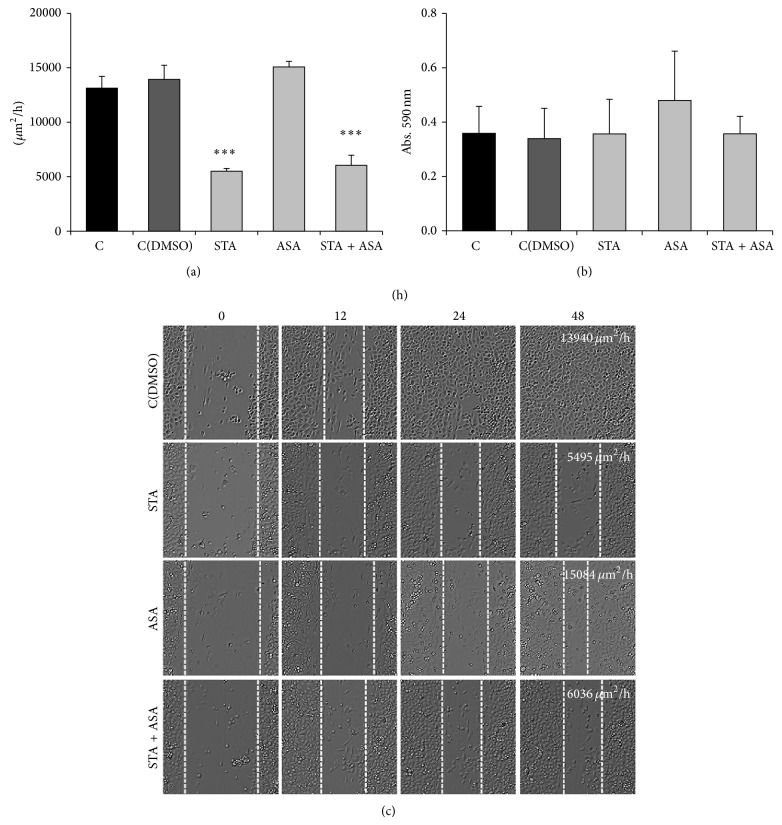
Second, the migration ability of both tumoral cell lines was analyzed by using wound-healing assays in PC3 line because of their ability to form a monolayer and transwell assay in LNCaP. STA and the combination of STA and ASA caused significant inhibition of PC3 migration after 48 h (61% and 57%, resp.) (Figure 4(a)) as these treatments do not allow the cells to close the wound (Figure 4(c)). On the contrary, no effect for the different treatments was observed in LNCaP cell lines (Figure 4(b)).
Figure 4.
Effect of STA, ASA, and the combination of both treatments on prostate cancer cell lines invasion capacity. Values for PC3 cell line migration capacity (a) are presented as the difference between the wound-healing speed (μm2/h) before and after 48 h of treatment calculated using Image J software and values for LNCaP migration (b) are presented as crystal violet absorbance/well after 48 h of treatment. Both cell lines were treated with different conditions: nontreated cells, control (C) and control with DMSO (C(DMSO)), and cells treated with statin (STA), aspirin (ASA), and a combination of both statin and aspirin (STA + ASA) (for further details, see Section 2). The bars represent the mean ± SEM. Values that are significantly different by one-way analysis of variance (ANOVA) from the C(DMSO) group are indicated by *** P < 0.001. (c) Phase contrast 10x images from migration mode during wound-healing assay at 48 h after treatment for PC3 cell line.
The lethal consequences of HGPCa are related to its metastasis to other organ sites, especially bones. Epithelial-to-mesenchymal transition (EMT) has received considerable attention as a paradigm to explain invasive and metastatic behavior during PCa progression as well as other tumors. Cells undergoing EMT lose their epithelial morphology, reorganize their cytoskeleton, and acquire a motile phenotype through the up- and downregulation of several molecules including tight and adherent junctions proteins and mesenchymal markers [31]. Bearing this in mind, the final aim of the present investigation was to see which proteins could be related to these effects on cell invasion and migration. The levels of some of those proteins, which play an important role during EMT, were analyzed (α2-integrin, vimentin, and E-cadherin for PC3 and α2-integrin for LNCaP). The results showed that, after 7 days of treatment with ASA and the combination of STA and ASA, the levels of α2-integrin increased slightly in PC3 cells. In the case of LNCaP, α2-integrin slightly increased in cells treated with ASA and increased more markedly with the combination of both treatments compared to the control cells (Figures 3(c) and 3(d)). On the other hand, no change was observed in the other proteins analyzed.
4. Discussion
PCa predominantly affects older men, who are likely to have comorbidities, such as cardiovascular conditions, that often indicate treatment with aspirin and statins. This find is in keeping with some authors, who have reported a significant correlation between PCa and coronary disease prevalence [5] as well as the fact that cardiovascular events are usually the first cause of death in patients with PCa [4].
In the last years some publications on this field investigated the relation between the uptake of statins and aspirin and PCa incidence; however, the impact of chronic treatment with statins and aspirin in prostate carcinogenesis is controversial. In 2008, a meta-analysis that included 6 randomized trials, 6 cohort studies, and 6 case-control studies concluded that there was neither association between statin therapy and PCa risk nor the time of treatment administration [9]. However, other studies observed a protective effect of statins on diagnosing PCa in advanced stages [9]. A similar study, where 4,202 men who had undergone PB were analyzed [32], showed that 24% of the patients that had been treated with statins at the time of PB presented a PCa detection rate of 45% compared to 42% in those patients who had not received statins. Furthermore, the Gleason score was 7 or higher in 72% of the tumors detected in chronic statins users compared to 62% in those patients not using this drug. However, the rate of patients with a Gleason score 8 or higher in radical prostatectomy specimens was 14% in both groups. This study did not take into account treatment with aspirin. With the same objective, Murtola et al. [33] analyzed the impact of statins in a series of 23,320 men (28% of them received statins) who underwent PSA screening between 1996 and 2004. The authors concluded that this treatment reduced the overall incidence of PCa, even after removing the bias produced by its effect on serum PSA. Moreover, they observed an association between time of statins therapy and the risk of PCa detection, without any relation to tumor aggressiveness. This study also monitored the use of other drugs, such as aspirin (17% of patients) and other potential confounders of PCa risk, which did not change the benefit of statins. However, the impact of combination therapy with statins and aspirin was not analyzed in this study. Aspirin has been associated with a reduced risk of PCa by some authors, while others have questioned this correlation. The chemopreventive effect of aspirin and other nonsteroidal anti-inflammatory drugs has been reported in recent data. This effect ranges from 10 to 55% [17, 18, 20, 34–37]. A recent meta-analysis that included 24 studies, 9 case controls with 5,795 men, and 15 cohorts with 31,657 patients has suggested a chemopreventive effect of aspirin on PCa [38].
With the aim of clarifying these controversial studies, we conducted a clinical observational study on 1,504 men undergoing PCa screening. Our study shows that more than a third of the population at screening of PCa was receiving some treatment to prevent cardiovascular disease. Specifically in this cohort, around 38% were chronic patients with 48% receiving statins, 18% receiving aspirin, and 34% receiving both drugs.
We observed that the detection rates of PCa in patients treated with statins or aspirin were statistically similar to the rate observed in patients who were not receiving any of these drugs. However, the detection rate of PCa observed in patients that were treated simultaneously with statins and aspirin was significantly lower (P value = 0.003). Furthermore, with relation to histological grade, our study suggests that chronic treatment with aspirin could be an independent predictor of HGPCa detection as well as the combination therapy with statins and aspirin. This is because, according to our analysis, there was a higher incidence of HGPCa in chronic aspirin (P value = 0.034) users or men receiving concomitant treatment (P value < 0.001).
This is not the first time that an epidemiological study has related the increase of HGPCa tumors in chronic users of aspirin [38]. However, other authors have found an inverse correlation between aspirin and HGPCa rates [39, 40]. Besides that, some studies have shown that statins are able to decrease serum PSA levels [41]. This could be considered as one possible explanation that could clarify the controversy on the effect of statins in PCa detection. The serum PSA level directly impacts the indication for PB [42, 43], and it may cause a masking bias on the possible protective effect of statins, since a population subgroup would not have biopsy criterion. In this case, the treatments may decrease the risk of PCa but also delay the diagnosis, which could explain the increased detection of HGPCa.
The present study had some limitations, which were also common in several other studies, where the effects of statins and aspirin were evaluated, for example, not being a prospective study or the effect of statins in the serum PSA hindering the evaluation of the influence of this drug in PCa. Then, there is the idea of the chronic treatment concept or not analyzing each drug dose separately, which would not allow us to draw distinct conclusions.
As a secondary objective, the tumorigenic properties of statins and aspirin on different PCa cell lines were studied, as a way to better understand our clinical observations. Thus, taking into account the results obtained in vitro in which the proliferation decreased dramatically as well as colony formation capacity after treating the cells with STA and ASA, we were able to hypothesize that statins and aspirin do have antitumoral effects in PCa, as other authors have observed previously [44–46]. Specifically, it could be that either aspirin treatment alone and/or simultaneous treatment may enhance the preventive effect of statins on prostate carcinogenesis at the expense of an increase in the detection of HGPCa. As we mentioned before, we have seen a direct effect of statins on proliferation, which can probably be explained by a marked decrease of cyclin D1 levels. The combination of both treatments could enhance this effect. These results, in addition to those based on the significant effect of the three treatments being higher when the cells received a concomitant treatment and on colony formation capacity, could be related to clinical observations of patients referred for PB and the incidence of PCa.
The mechanism by which statins influence prostate carcinogenesis could be mediated by the reduction of serum cholesterol and, consequently, by a decrease of intraprostatic dihydrotestosterone levels. Prostate tissues lose homeostatic control over cholesterol levels with age [47]. This effect could explain the observed differences in the results for each cell line used in our study because, as it is well known, PC3 prostate cancer cells are hormone independent and LNCaP prostate cancer cells are hormone dependent and these properties may influence the cell line response although several studies would be needed to clarify this question. The overall consequence of this cholesterol accumulation on prostate physiology is unknown, but a role for high levels of serum cholesterol in PCa incidence and progression has been suggested by a number of epidemiological and preclinical studies [10–14, 48].
In order to find an explanation for the increased incidence of HGPCa in patients treated chronically with aspirin and simultaneously with statins and aspirin, we should perform more extensive studies. However, in cancer, it is known that the expression of integrins, which are involved in cell adhesion, is frequently altered, leading to cell proliferation, migration, and metastasis. Previous studies have shown that integrin expression levels were correlated to the different stages of human cancer progression [49–51], and it has also been suggested that α2-integrin may be a metastasis suppressor [50]. In contrast, in PCa, α2-integrin was found to induce PCa cell metastasis to the bone [51, 52]. Therefore, integrin function is cell type and context dependent. Keeping in mind what we obtained, a moderate increase of these protein levels in vitro after ASA and concomitant treatment of STA and ASA in PC3 cell line, it could be related to those patients with a higher incidence of HGPCa. On the other hand, after performing invasion and migration assays on both PCa cell lines, the concomitant treatment seemed to present antitumorigenic effects. No changes in vimentin and E-cadherin were observed in vitro, which makes us think that the decrease in migration might not be related to changes in phenotype EMT. Therefore, a different approach is necessary to clarify the role of integrin and how other proteins could be implicated in the positive properties of statins and aspirin in PCa and HGPCa.
5. Conclusions
Although only a randomized, prospective trial that controls for patient age, comorbidities, adjuvant treatments, and dose stratification will prove the benefits of statins and aspirin treatment, the collection of all of the results presented in this study suggests that chronic concomitant treatment with statins and aspirin does have a protective effect for PCa incidence. Statins and aspirin have antitumorigenic properties, and these beneficial effects are enhanced when both treatments are administered simultaneously.
Acknowledgments
M. Olivan is the recipient of a postdoctoral fellowship from the Instituto de Salud Carlos III (CD12/00475). The authors acknowledge Movember Foundation, Instituto de Salud Carlos III (PI13/00173, PI11/02486, and CP10/00355), Ministerio de Ciéncia e Innovación (RD12/0036/0035), and Fundación para la Investigación en Urología and Lisa Piccione for reviewing the document.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Authors' Contribution
A. Doll and J. Morote contributed equally to this work.
References
- 1.Center M. M., Jemal A., Lortet-Tieulent J., et al. International variation in prostate cancer incidence and mortality rates. European Urology. 2012;61(6):1079–1092. doi: 10.1016/j.eururo.2012.02.054. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R., Naishadham D., Jemal A. Cancer statistics, 2013. CA Cancer Journal for Clinicians. 2013;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 3.Hsing A. W., Devesa S. S. Trends and patterns of prostate cancer: what do they suggest? Epidemiologic Reviews. 2001;23(1):3–13. doi: 10.1093/oxfordjournals.epirev.a000792. [DOI] [PubMed] [Google Scholar]
- 4.Albertsen P. C. Screening for prostate cancer is neither appropriate nor cost-effective. Urologic Clinics of North America. 1996;23(4):521–530. doi: 10.1016/S0094-0143(05)70332-3. [DOI] [PubMed] [Google Scholar]
- 5.Thomas J.-A., II, Gerber L., Bañez L. L., et al. Prostate cancer risk in men with baseline history of coronary artery disease: results from the reduce study. Cancer Epidemiology Biomarkers and Prevention. 2012;21(4):576–581. doi: 10.1158/1055-9965.EPI-11-1017. [DOI] [PubMed] [Google Scholar]
- 6.Gonyeau M. J., Yuen D. W. A clinical review of statins and cancer: helpful or harmful? Pharmacotherapy. 2010;30(2):177–194. doi: 10.1592/phco.30.2.177. [DOI] [PubMed] [Google Scholar]
- 7.McGettigan P., Henry D. Cardiovascular risk and inhibition of cyclooxygenase: a systematic review of the observational studies of selective and nonselective inhibitors of cyclooxygenase 2. Journal of the American Medical Association. 2006;296(13):1633–1644. doi: 10.1001/jama.296.13.jrv60011. [DOI] [PubMed] [Google Scholar]
- 8.Mahmud S. M., Franco E. L., Aprikian A. G. Use of nonsteroidal anti-inflammatory drugs and prostate cancer risk: a meta-analysis. International Journal of Cancer. 2010;127(7):1680–1691. doi: 10.1002/ijc.25186. [DOI] [PubMed] [Google Scholar]
- 9.Bonovas S., Filioussi K., Sitaras N. M. Statin use and the risk of prostate cancer: a metaanalysis of 6 randomized clinical trials and 13 observational studies. International Journal of Cancer. 2008;123(4):899–904. doi: 10.1002/ijc.23550. [DOI] [PubMed] [Google Scholar]
- 10.Mann D., Reynolds K., Smith D., Muntner P. Trends in statin use and low-density lipoprotein cholesterol levels among US adults: impact of the 2001 National Cholesterol Education Program guidelines. Annals of Pharmacotherapy. 2008;42(9):1208–1215. doi: 10.1345/aph.1L181. [DOI] [PubMed] [Google Scholar]
- 11.Jain M. K., Ridker P. M. Anti-inflammatory effects of statins: clinical evidence and basic mechanisms. Nature Reviews Drug Discovery. 2005;4(12):977–987. doi: 10.1038/nrd1901. [DOI] [PubMed] [Google Scholar]
- 12.Carlberg M., Dricu A., Blegen H., et al. Mevalonic acid is limiting for N-linked glycosylation and translocation of the insulin-like growth factor-1 receptor to the cell surface. Evidence for a new link between 3-hydroxy-3-methylglutaryl-coenzyme A reductase and cell growth. Journal of Biological Chemistry. 1996;271(29):17453–17462. doi: 10.1074/jbc.271.29.17453. [DOI] [PubMed] [Google Scholar]
- 13.Wong W. W.-L., Dimitroulakos J., Minden M. D., Penn L. Z. HMG-CoA reductase inhibitors and the malignant cell: the statin family of drugs as triggers of tumor-specific apoptosis. Leukemia. 2002;16(4):508–519. doi: 10.1038/sj/leu/2402476. [DOI] [PubMed] [Google Scholar]
- 14.Dulak J., Józkowicz A. Anti-angiogenic and anti-inflammatory effects of statins: relevance to anti-cancer therapy. Current Cancer Drug Targets. 2005;5(8):579–594. doi: 10.2174/156800905774932824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murtola T. J., Visakorpi T., Lahtela J., Syvälä H., Tammela T. L. J. Statins and prostate cancer prevention: where we are now, and future directions. Nature Clinical Practice Urology. 2008;5(7):376–387. doi: 10.1038/ncpuro1146. [DOI] [PubMed] [Google Scholar]
- 16.Harris R. E., Beebe-Donk J., Doss H., Burr Doss D. Aspirin, ibuprofen, and other non-steroidal anti-inflammatory drugs in cancer prevention: a critical review of non-selective COX-2 blockade (review) Oncology Reports. 2005;13(4):559–583. [PubMed] [Google Scholar]
- 17.Habel L. A., Zhao W., Stanford J. L. Daily aspirin use and prostate cancer risk in a large, multiracial cohort in the US. Cancer Causes and Control. 2002;13(5):427–434. doi: 10.1023/A:1015788502099. [DOI] [PubMed] [Google Scholar]
- 18.Rodríguez L. A. G., González-Pérez A. Inverse association between nonsteroidal anti-inflammatory drugs and prostate cancer. Cancer Epidemiology Biomarkers and Prevention. 2004;13(4):649–653. [PubMed] [Google Scholar]
- 19.Jacobs E. J., Rodriguez C., Mondul A. M., et al. A large cohort study of aspirin and other nonsteroidal anti-inflammatory drugs and prostate cancer incidence. Journal of the National Cancer Institute. 2005;97(13):975–980. doi: 10.1093/jnci/dji173. [DOI] [PubMed] [Google Scholar]
- 20.Dasgupta K., Di Cesar D., Ghosn J., Rajan R., Mahmud S., Rahme E. Association between nonsteroidal anti-inflammatory drugs and prostate cancer occurrence. Cancer Journal. 2006;12(2):130–135. [PubMed] [Google Scholar]
- 21.Neugut A. I., Rosenberg D. J., Ahsan H., et al. Association between coronary heart disease and cancers of the breast, prostate, and colon. Cancer Epidemiology Biomarkers & Prevention. 1998;7(10):869–873. [PubMed] [Google Scholar]
- 22.Bosetti C., Talamini R., Negri E., Franceschi S., Montella M., La Vecchia C. Aspirin and the risk of prostate cancer. European Journal of Cancer Prevention. 2006;15(1):43–45. doi: 10.1097/01.cej.0000180665.04335.de. [DOI] [PubMed] [Google Scholar]
- 23.Leitzmann M. F., Stampfer M. J., Ma J., et al. Aspirin use in relation to risk of prostate cancer. Cancer Epidemiology Biomarkers and Prevention. 2002;11(10 I):1108–1111. [PubMed] [Google Scholar]
- 24.Siemes C., Visser L. E., Coebergh J. W. W., Hofman A., Uitterlinden A. G., Stricker B. H. C. Protective effect of NSAIDs on cancer and influence of COX-2 C-765G genotype. Current Cancer Drug Targets. 2008;8(8):753–764. doi: 10.2174/156800908786733414. [DOI] [PubMed] [Google Scholar]
- 25.Rothwell P. M., Wilson M., Price J. F., Belch J. F. F., Meade T. W., Mehta Z. Effect of daily aspirin on risk of cancer metastasis: a study of incident cancers during randomised controlled trials. The Lancet. 2012;379(9826):1591–1601. doi: 10.1016/S0140-6736(12)60209-8. [DOI] [PubMed] [Google Scholar]
- 26.Algotar A. M., Thompson P. A., Ranger-Moore J., et al. Effect of aspirin, other NSAIDs, and statins on PSA and PSA velocity. Prostate. 2010;70(8):883–888. doi: 10.1002/pros.21122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Remzi M., Fong Y. K., Dobrovits M., et al. The Vienna nomogram: validation of a novel biopsy strategy defining the optimal number of cores based on patient age and total prostate volume. Journal of Urology. 2005;174(4) part 1:1256–1261. doi: 10.1097/01.ju.0000173924.83392.cc. [DOI] [PubMed] [Google Scholar]
- 28.Murtola T. J., Pennanen P., Syvälä H., Bläuer M., Ylikomi T., Tammela T. L. J. Effects of simvastatin, acetylsalicylic acid, and rosiglitazone on proliferation of normal and cancerous prostate epithelial cells at therapeutic concentrations. Prostate. 2009;69(9):1017–1023. doi: 10.1002/pros.20951. [DOI] [PubMed] [Google Scholar]
- 29.Wu K. K. Aspirin and salicylate: an old remedy with a new twist. Circulation. 2000;102(17):2022–2023. doi: 10.1161/01.CIR.102.17.2022. [DOI] [PubMed] [Google Scholar]
- 30.Beaver C. M. The effect of culture conditions on colony morphology and proliferative capacity in human prostate cancer cell lines. Cell Biology and Toxicology. 2012;28(5):291–301. doi: 10.1007/s10565-012-9224-z. [DOI] [PubMed] [Google Scholar]
- 31.Nauseef J. T., Henry M. D. Epithelial-to-mesenchymal transition in prostate cancer: paradigm or puzzle? Nature Reviews Urology. 2011;8(8):428–439. doi: 10.1038/nrurol.2011.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tan N., Klein E. A., Li J., Moussa A. S., Jones J. S. Statin use and risk of prostate cancer in a population of men who underwent biopsy. Journal of Urology. 2011;186(1):86–90. doi: 10.1016/j.juro.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 33.Murtola T. J., Tammela T. L. J., Määttänen L., et al. Prostate cancer and PSA among statin users in the finnish prostate cancer screening trial. International Journal of Cancer. 2010;127(7):1650–1659. doi: 10.1002/ijc.25165. [DOI] [PubMed] [Google Scholar]
- 34.Perron L., Bairati I., Moore L., Meyer F. Dosage, duration and timing of nonsteroidal antiinflammatory drug use and risk of prostate cancer. International Journal of Cancer. 2003;106(3):409–415. doi: 10.1002/ijc.11250. [DOI] [PubMed] [Google Scholar]
- 35.Platz E. A., Rohrmann S., Pearson J. D., et al. Nonsteroidal anti-inflammatory drugs and risk of prostate cancer in the Baltimore Longitudinal Study of Aging. Cancer Epidemiology Biomarkers & Prevention. 2005;14(2):390–396. doi: 10.1158/1055-9965.EPI-04-0532. [DOI] [PubMed] [Google Scholar]
- 36.Liu X., Plummer S. J., Nock N. L., Casey G., Witte J. S. Nonsteroidal antiinflammatory drugs and decreased risk of advanced prostate cancer: modification by lymphotoxin alpha. The American Journal of Epidemiology. 2006;164(10):984–989. doi: 10.1093/aje/kwj294. [DOI] [PubMed] [Google Scholar]
- 37.Bosetti C., Rosato V., Gallus S., la Vecchia C. Aspirin and urologic cancer risk: an update. Nature Reviews Urology. 2012;9(2):102–110. doi: 10.1038/nrurol.2011.219. [DOI] [PubMed] [Google Scholar]
- 38.Bosetti C., Rosato V., Gallus S., Cuzick J., La Vecchia C. Aspirin and cancer risk: a quantitative review to 2011. Annals of Oncology. 2012;23(6):1403–1415. doi: 10.1093/annonc/mds113. [DOI] [PubMed] [Google Scholar]
- 39.Brasky T. M., Velicer C. M., Kristal A. R., Peters U., Potter J. D., White E. Nonsteroidal anti-inflammatory drugs and prostate cancer risk in the VITamins and Lifestyle (VITAL) cohort. Cancer Epidemiology Biomarkers and Prevention. 2010;19(12):3185–3188. doi: 10.1158/1055-9965.EPI-10-0942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dhillon P. K., Kenfield S. A., Stampfer M. J., Giovannucci E. L. Long-term aspirin use and the risk of total, high-grade, regionally advanced and lethal prostate cancer in a prospective cohort of health professionals, 1988–2006. International Journal of Cancer. 2011;128(10):2444–2452. doi: 10.1002/ijc.25811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brookhart M. A., Patrick A. R., Dormuth C., et al. Adherence to lipid-lowering therapy and the use of preventive health services: an investigation of the healthy user effect. American Journal of Epidemiology. 2007;166(3):348–354. doi: 10.1093/aje/kwm070. [DOI] [PubMed] [Google Scholar]
- 42.Berg C. D., Andriole G. L., Crawford E. D., et al. Mortality results from a randomized prostate-cancer screening trial. The New England Journal of Medicine. 2009;360(13):1310–1319. doi: 10.1056/NEJMoa0810696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schröder F. H., Hugosson J., Roobol M. J., et al. Screening and prostate-cancer mortality in a randomized european study. The New England Journal of Medicine. 2009;360(13):1320–1328. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- 44.Brown M., Hart C., Tawadros T., et al. The differential effects of statins on the metastatic behaviour of prostate cancer. British Journal of Cancer. 2012;106(10):1689–1696. doi: 10.1038/bjc.2012.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hoque A., Chen H., Xu X.-C. Statin induces apoptosis and cell growth arrest in prostate cancer cells. Cancer Epidemiology Biomarkers and Prevention. 2008;17(1):88–94. doi: 10.1158/1055-9965.EPI-07-0531. [DOI] [PubMed] [Google Scholar]
- 46.Murtola T. J., Syvälä H., Pennanen P., et al. Comparative effects of high and low-dose simvastatin on prostate epithelial cells: the role of LDL. European Journal of Pharmacology. 2011;673(1–3):96–100. doi: 10.1016/j.ejphar.2011.10.022. [DOI] [PubMed] [Google Scholar]
- 47.O'Shaughnessy M., Konety B., Warlick C. Prostate cancer screening: issues and controversies. Minnesota Medicine. 2010;93(8):39–44. [PubMed] [Google Scholar]
- 48.Amling C. L., Blute M. L., Bergstralh E. J., Seay T. M., Slezak J., Zincke H. Long-term hazard of progression after radical prostatectomy for clinically localized prostate cancer: continued risk of biochemical failure after 5 years. Journal of Urology. 2000;164(1):101–105. doi: 10.1016/S0022-5347(05)67457-5. [DOI] [PubMed] [Google Scholar]
- 49.Mitchell K., Svenson K. B., Longmate W. M., et al. Suppression of integrin α3β1 in breast cancer cells reduces cyclooxygenase-2 gene expression and inhibits tumorigenesis, invasion, and cross-talk to endothelial cells. Cancer Research. 2010;70(15):6359–6367. doi: 10.1158/0008-5472.CAN-09-4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ramirez N. E., Zhang Z., Madamanchi A., et al. The α2β1 integrin is a metastasis suppressor in mouse models and human cancer. The Journal of Clinical Investigation. 2011;121(1):226–237. doi: 10.1172/JCI42328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hall C. L., Dubyk C. W., Riesenberger T. A., Shein D., Keller E. T., Van Golen K. L. Type I collagen receptor (α2β1) signaling promotes prostate cancer invasion through RhoC GTPase. Neoplasia. 2008;10(8):797–803. doi: 10.1593/neo.08380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Van Slambrouck S., Jenkins A. R., Romero A. E., Steelant W. F. A. Reorganization of the integrin α2 subunit controls cell adhesion and cancer cell invasion in prostate cancer. International Journal of Oncology. 2009;34(6):1717–1726. doi: 10.3892/ijo_00000302. [DOI] [PMC free article] [PubMed] [Google Scholar]