Abstract
TRPM1 is a spontaneously active non-selective cation channel that has recently been shown to play an important role in the depolarising light responses of ON bipolar cells. Consistent with this role, mutations in the TRPM1 gene have been identified as a principle cause of congenital stationary night blindness. However, previous microarray studies have shown that Trpm1 and Trpm3 are acutely regulated by light in the eye of mice lacking rods and cones (rd/rd cl), a finding consistent with a role in non-image forming photoreception. In this study we show that pupillary light responses are significantly attenuated in both Trpm1−/− and Trpm3−/− animals. Trpm1−/− mice exhibit a profound deficit in the pupillary response that is far in excess of that observed in mice lacking rods and cones (rd/rd cl) or melanopsin, and cannot be explained by defects in bipolar cell function alone. Immunolocalisation studies suggest that TRPM1 is expressed in ON-bipolar cells and also a subset of cells in the ganglion cell layer, including melanopsin expressing photosensitive retinal ganglion cells (pRGCs). We conclude that in addition to its role in bipolar cell signalling, TRPM1 is involved in non-image forming responses to light and may perform a functional role within pRGCs. By contrast, TRPM3−/− mice display a more subtle pupillary phenotype with attenuated responses under bright light and dim light conditions. Expression of TRPM3 is detected in Muller cells and the ciliary body but is absent from pRGCs, and thus our data supports an indirect role for TRPM3 in pupillary light responses.
Keywords: melanopsin, pRGCs, phototransduction, circadian, melastatin
Introduction
Transient receptor potential cation channel, subfamily M, member 1 (TRPM1) forms a spontaneously active non-selective cation channel (Duncan et al., 1998; Kraft & Harteneck, 2005) whose expression is restricted to the retina and skin (Koike et al., 2010). A remarkable body of recent research has confirmed that TRPM1 is expressed in ON bipolar cells and is responsible for carrying the inward current that drives the depolarising light responses in these cells (Morgans et al., 2009; Shen et al., 2009; Koike et al., 2010; Morgans et al., 2010). Loss of TRPM1 has been shown to abolish light responses from rod ON bipolar cells and dramatically reduce responses from cone ON bipolar cells in the mouse retina (Morgans et al., 2009; Koike et al., 2010). Furthermore, mutations of the Trpm1 gene have been identified as a primary cause of stationary night blindness in humans and horses (Audo et al., 2009; Li et al., 2009; van Genderen et al., 2009), a condition characterised by disruption of signalling via ON-bipolar cells. Thus it would seem that TRPM1 performs an essential role in ON bipolar cells and is necessary for the transmission of rod and cone information to the inner retina and transduction of the ON visual pathway (Morgans et al., 2010).
In addition to the rod and cone photoreceptors of the outer retina, research over the last decade has identified an additional class of inner retinal photoreceptor (Freedman et al., 1999; Lucas et al., 1999), consisting of a small subset of photosensitive retinal ganglion cells (pRGCs) expressing the photopigment melanopsin (Hattar et al., 2002; Hankins et al., 2008). These cells signal environmental irradiance, mediating a range of non-image forming responses to light including circadian entrainment, pupil constriction and the regulation of sleep (Freedman et al., 1999; Lucas et al., 2001; Lupi et al., 2008). Unlike the rods and cones, the molecular details of the phototransduction cascade employed by melanopsin pRGCs remain uncertain. Whilst studies have suggested the involvement of a Gαq/11–type G-protein, phospholipase C and activation of a Trp-type ion channel (Hankins et al., 2008; Do & Yau, 2010), the identification of the genes and proteins involved is unclear. We have previously used microarrays to investigate responses to light in the eye of mice lacking rods and cones (rd/rd cl), a system where all remaining responses to light are driven by melanopsin-dependent signalling. This study identified changes in expression of both Trpm1 and Trpm3 mRNA and thus identified these genes as potential components of the melanopsin signalling pathway (Peirson et al., 2007). It is therefore possible that TRPM1 channels perform multiple roles in the retina, including the classical image-forming visual pathway as well as non-image forming responses to light associated with melanopsin expressing pRGCs.
To test our hypothesis that TRPM1 and TRPM3 contribute to non-image forming responses to light we assessed pupillary responses in Trpm1−/− and Trpm3−/− mice, comparing these responses to those of wildtype mice with normal retina, mice lacking melanopsin (Opn4−/−) and mice lacking functional rod and cone photoreceptors (rd/rd cl). Our results demonstrate that Trpm1−/− and Trpm3−/− mice have attenuated pupillary responses consistent with a role for these channels in non image forming photoreception.
Methods
Animals
Trpm1−/− (NIH-1696: LexKO 428) and Trpm3−/− (NIH-1697: LexKO 380) mice (n=6, n=5, respectively) were obtained via a Wellcome Trust Knockout Mouse Resource application awarded to MWH & SNP. Mice were supplied via EMMA (www.emmanet.org). Opn4−/− (n=3) and rd/rd cl mice (n=4) were bred at the University of Oxford (UK) as described previously (Freedman et al., 1999; Hattar et al., 2002). Wildtype mice (n=5) used in these studies were on a C57BL/6x129 mixed background, the same as Trpm1−/−, Trpm3−/− and Opn4−/− animals. All mice were aged over 3 months and were accustomed to handling. All procedures were conducted in accordance with the Animals (Scientific Procedures) Act 1986 and the University of Oxford Policy on the Use of Animals in Scientific Research.
Microarray analysis
Light pulse experiments and microarray analysis were performed as reported previously (Peirson et al., 2007). Briefly, rd/rd cl animals (age 130 ± 16 days) were sacrificed at 0, 30, 60, and 120 min (n=4 per time point) after onset of a 15 min light pulse (fluorescent white light, 1.4 mW/cm2/s) or sham light pulse. Eyes were then collected under infrared light and immediately snap frozen on dry ice. Total RNA was isolated from whole eyes, in vitro transcribed and then hybridized to Mouse Genome 430 v2.0 Genechips (Affymetrix), and the resulting data analysed as previously described (Peirson et al., 2007).
Laser capture microscopy and PCR
Laser capture microscopy and PCR analysis were performed as described previously (Peirson et al., 2007). Briefly, wildtype eyes (ZT 6–12) were snap frozen and sectioned at 20 μm. Slides were briefly fixed in 70% ethanol at −20°C, stained with 20% cresyl violet, dehydrated, and dried at 40°C for 1–2 minutes. Sections of the retinal ganglion cell (RGC) layer were laser dissected with a PALM MicroBeam system (PALM-microlaser, Bernried, Germany), with each preparation containing approximately 30–40 cells. Total RNA was subsequently extracted with a PicoPure RNA extraction kit (Arcturus, Sunnyvale, CA), treated with 1 unit DNase (Sigma), reverse transcribed with random decamers with a RETROscript kit (Ambion), and tested for candidate gene expression with Sybr green I mastermix (Applied Biosystems) with 50 cycles of amplification. Primer sequences used were as follows (5′ to 3′): Trpm1 F GGGTTTGCTGATCTGGGTAA, Trpm1 R TGATGAAAGGTTCGGTGGTT, Trpm3 F TCCTGTCACTGGAGCATCTG, Trpm3 R CACAGCGGTAGCAGCAATAA, β-actin F ACCAACTGGGACGATATGGAGAAGA, β-actin R CGCACGATTTCCCTCTCAGC.
Pupillometry
Animals were housed on a 12:12 light:dark cycle and were tested between ZT 4-8. All animals were dark adapted for 1–2 hours prior to testing. A xenon arc lamp (150W solar simulator, Lot Oriel, UK) with a 480 nm monochromatic filter (Andover, 10 nm half-bandwidth) was used to produce a light intensity of 14.6 log quanta/cm2/s (173 μW/cm2/s) (bright light) or 11.6 log quanta/cm2/s (0.17 μW/cm2/s) (dim light). Where stated a bright white light stimulus was used (16.6 log quanta/cm2/s, 13.6 mW/cm2/s). In all cases, light was transmitted to the eye via a liquid light pipe as an irradiant light stimulus using a 2″ integrating sphere (Pro-lite Technology, UK). Irradiance measurements were made using a radiometrically calibrated spectrophotometer (Ocean Optics, UK). The delivery of the light stimulus was controlled via software which regulated the opening and closing of a shutter in the light pathway (LSZ160 shutter, Lot Oriel UK; custom software supplied by BRSL, Newbury, UK). In order to determine the level of the pupillary light response, images were collected with a Prosilica NIR sensitive CCD video camera (BRSL, Newbury, UK) at a rate of 10 frames per second. The camera was positioned perpendicular to the contralateral eye which was illuminated by infra-red LEDs (850nm, 10nm half-bandwidth). In this way consensual pupil responses could be measured in response to an irradiant light stimulus. 5 minutes prior to recording, a 1% tropicamide was applied to the stimulated eye. During pupil measurements unanaesthetised animals were temporarily restrained using normal husbandry techniques for the duration of the recording (29 seconds). After brief baseline measurements of the dark adapted pupil (2 seconds), the left eye was exposed to light stimulus for 10 seconds. Recovery data was collected for a post-stimulus period of 17 seconds. Each animal was tested on multiple occasions to minimise any artefacts due to handling. Results were comparable across all tests. To assess the ability of the pupil to constrict fully, a topical solution of 1M carbachol (Sigma) in sterile PBS (pH 7.4) was applied to the cornea. Pupil size was measured after 1 hour of dark adapting before carbachol was administered, and again 15 minutes after application of the miotic. All images were analysed using ImageJ (http://rsbweb.nih.gov/ij).
Immuocytochemistry of retinal sections
Eyes were removed (ZT 6-10) and the lens punctured with a fine needle prior to fixation in 4% paraformaldehyde in PBS at 4°C for 16 hrs. Eyes were then cryoprotected in 30% (w/v) sucrose in PBS at 4°C for 48 hours before embedding in OCT medium (Sakura Finetek) and stored at −80°C prior to use. 18μm tissue sections were prepared at −23oC using a Leica CM1850 cryostat (Leica Microsystems) and collected on Poly-L-Lysine coated slides (Thermo Scientific). Fluorescent immunolabelling was performed using standard techniques. Briefly, retinal sections were permeabilised in PBS with 0.2% Triton X at RT for 20 min and blocked in PBS with 10% normal goat serum (Sigma) with 0.2% Triton X for 1 hour at RT. Primary antibodies were incubated for 16 hrs at 4°C diluted in 2.5% goat serum in PBS with 0.2% Triton X; rabbit polyclonal anti-melanopsin antibody recognising the N-terminus of murine Opn4 (UF006, Advanced Targeting Systems, San Diego, CA, US), 1:2500 (Provencio et al., 2002; Berson et al., 2010; Ecker et al., 2010); chicken polyclonal anti-β-gal antibody (ab9361, Abcam) 1:1000, (Pires et al., 2009). Goat anti-rabbit and Goat anti-chicken Alexa-488 and Alexa-555 labelled secondary antibodies (Life Technologies) were incubated for 2 hrs at RT diluted 1:200 in 2.5% goat serum in PBS with 0.2% Triton X. For double-labelling primary antibodies were incubated simultaneously and secondary antibodies were incubated sequentially. All wash steps were performed using PBS with 0.05% Tween-20. Sections were mounted in Prolong Gold anti-fade reagent containing DAPI (Life Technologies). Immunolabelling of retinal flatmounts with the UF006 melanopsin antibody was performed using similar protocols, with the exception that primary antibody was incubated for 72 hrs, secondary antibody incubated for 16 hrs and Triton-X concentrations increased to 1% for all solutions. Fluorescent images were acquired using an inverted LSM 710 laser scanning confocal microscope (Zeiss) with Zen 2010 image acquisition software (Zeiss). Excitation was 405nm, 488nm and 561nm with emissions collected between 440-480, 505-550 and 600-700nm for DAPI, green and red fluorescence respectively. Collected image stacks typically comprised 6-8 focal planes. Unless stated images show a single focal plane. Pixel size was typically 0.2, 0.2, 1.0μm (x, y, z). For all images, enhancements of brightness and contrast were performed using ImageJ software.
Statistical analysis
All data are shown ± standard error of the mean. Microarray data were analysed by one-way ANOVA and corrected for multiple tests (Benjamini Hochberg FDR correction) as described previously (Peirson et al., 2007). Statistical comparisons for pupillometry data were performed using a 2-tailed Student’s t-test using MS Excel.
Results
Trpm1 and Trpm3 as candidate genes in melanopsin signalling
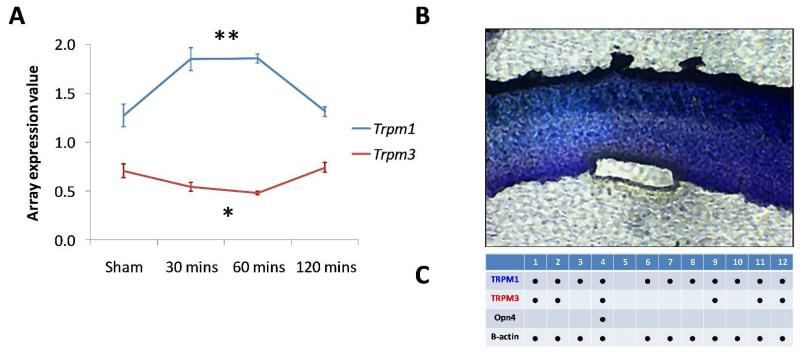
Microarray studies have identified Trpm1 and Trpm3 as potential candidate genes in melanopsin pRGC signalling (Peirson et al., 2007). Trpm1 mRNA expression is up-regulated 1.4 fold in response to acute light stimulation in mice lacking rods and cones (rd/rd cl) (F3,12 =13.4, P=0.00039, P=0.0054 with FDR correction), a model where the only remaining responses to light are driven by melanopsin pRGC-dependent signalling. By contrast, Trpm3 mRNA is negatively regulated by light in the rd/rd cl eye (F3,12 =7.38, P= 0.0046, P=0.0079 with FDR correction) (Figure 1A). Laser capture microscopy was used to isolate small groups of retinal ganglion cells (typically 30–40 cells) for PCR analysis (Figure 1B). Trpm1 mRNA expression was detected in all ganglion cell samples in which housekeeping gene expression (β-actin) could also be detected (11 of 12 samples). Trpm3 expression was detected in 5 of 12 samples, whereas melanopsin was detected in only 1 sample (Figure 1C). Combined, these results demonstrate that both Trpm1 and Trpm3 mRNA are expressed in the ganglion cell layer, and suggest that both TRPM1 and TRPM3 are involved in non-image forming responses to light, potentially participating in the melanopsin signalling pathway. To further investigate the role of TRPM1 and TRPM3 in non-image forming responses to light we compared pupillary light responses (PLR) in Trpm1−/− and Trpm3−/− mice against Opn4−/− mice lacking melanopsin and rd/rd cl mice lacking rods and cones.
Figure 1.
Expression of Trpm1 and Trpm3 mRNA. (A) Microarray data show that Trpm1 and Trpm3 are acutely regulated by light in the eye of mice lacking rods and cones (rd/rd cl). Trpm1 was significantly upregulated in response to light (**=F3,12 =13.4, P=0.00039, P=0.0054 with FDR correction), whereas Trpm3 was significantly downregulated (*=F3,12 =7.38, P= 0.0046, P=0.0079 with FDR correction). (B, C) Both Trpm1 and Trpm3 mRNA are detected in the retinal ganglion cell layer of wildtype mice, based upon PCR analysis of cDNA from isolated ganglion cell layer preparations. (B) Retinal section stained with 20% cresyl violet, following the isolation of a retinal ganglion cell layer preparation using laser capture microdissection (typically 30-40 cells/preparation). (C) Summary table showing the expression of Trpm1, Trpm3, Opn4 and β-actin in 12 ganglion cell layer preparations.
Trpm1−/− and Trpm3−/− mice show attenuated pupillary responses to light
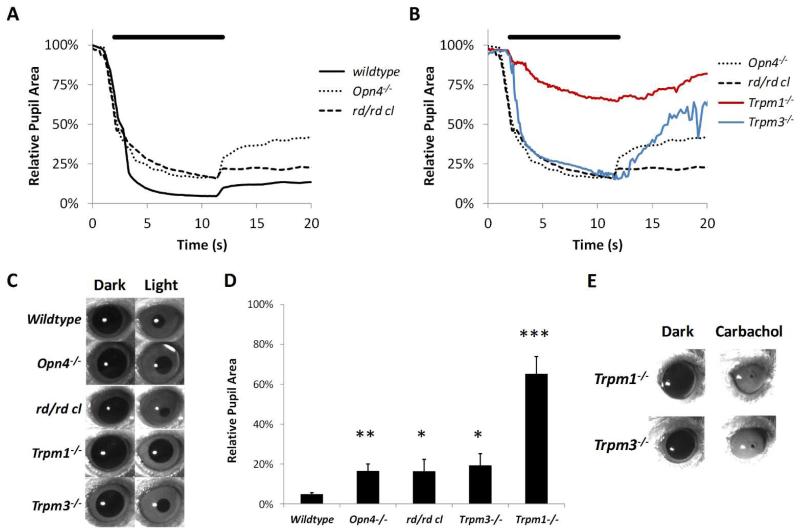
In order to demonstrate the overlapping contributions of the classical rod cone photoreceptors and the melanopsin system we assessed pupillary responses in rd/rd cl mice lacking functional rods and cones and Opn4−/− mice lacking melanopsin. Consistent with previous reports (Lucas et al., 2001; Lucas et al., 2003) our data show that loss of only rod and cones or melanopsin based photoreception in isolation has only a subtle effect on pupillary responses when compared with wildtype mice in which all classes of photoreceptors are functional (Figure 2). In wildtype mice a rapid constriction of the pupil was observed following the onset of bright light stimulation (480nm, 14.6 log quanta/cm2/s), with pupil area reduced to ~5-10% of dark adapted values. Pupil constriction was maintained throughout illumination and following termination of the light stimuli, with a sustained constriction after the cessation of stimulus presentation (post-illumination pupil response) (Kankipati et al., 2010). Opn4−/− mice have a relatively modest defect in the PLR. The initial phase of constriction was similar in Opn4−/−and wildtype mice yet Opn4−/−mice failed to achieve full pupil constriction with the pupil area constricted to ~20% of dark adapted values compared to ~5% for wildtype mice (P=0.0053, t=7.33, df=3). In addition Opn4−/−mice showed a less pronounced post-illumination pupil response with a more rapid recovery of pupil size compared to wildtype mice. The maintenance of significant pupil constriction in Opn4−/− mice shows that rods and cones are capable of driving near complete pupil constriction in the absence of melanopsin function. Consistent with the known role of melanopsin expressing pRGCs, significant pupil constriction was also observed in rd/rd cl mice (3-6 months) lacking functional rods and cones. Again the initial phase of rapid constriction was similar between rd/rd cl and wildtype mice. Whilst rd/rd cl mice failed to achieve full constriction with the 480nm stimulus used, full constriction was achieved using a bright white light stimulus (16 log quanta/cm2/s, full data not shown). With the 480nm stimulus a reduction in pupil area to ~15% of dark adapted values was achieved (P=0.040, t=3.49, df=3), consistent with the reduction in sensitivity previously described (Lucas et al., 2001). The post-stimulus response was also apparent in rd/rd cl mice, with pupil constriction maintained at ~25% of dark adapted values following termination of the light stimulus, consistent with a strong melanopsin contribution to this response (Dacey et al., 2005; Kankipati et al., 2010). In all respects, pupillary responses observed in wildtype, Opn4−/− and rd/rd cl mice are entirely consistent with previously published data (Lucas et al., 2001; Lucas et al., 2003).
Figure 2.
Analysis of the pupillary light response (PLR) in Trpm1−/− and Trpm3−/− mice. (A) Pupil constriction profiles recorded from normal wildtype, Opn4 −/− and rd/rd cl mice in response to bright light illumination (480nm, 14.6 log quanta/cm2/s). (B) Pupil constriction profiles recorded from Trpm1−/− and Trpm3−/− mice in response to bright light illumination (14.6 log quanta/cm2/s). Responses from Opn4−/− and rd/rd cl mice are plotted for comparison. Light stimuli from 2-12 seconds (black bar above traces). Profiles shown are mean of responses from n=3-6 mice. (C) Representative images of pupil size recorded before and after bright light stimulation. (D) Graph shows the maximal pupil constriction observed following bright light exposure. * = p<0.05, **=P<0.01, ***=P<0.001. (E) Images of pupil size observed for Trpm1−/− and Trpm3−/− mice before and after application of 1M carbachol.
By contrast to both rd/rd cl and Opn4−/− mice, Trpm1−/− mice showed a profound attenuation in pupillary responses to light (Figure 2). The initial phase of rapid pupil constriction was entirely absent with only a small and slow response observed resulting in a maximal pupil constriction of only ~75-80% of dark adapted values after 10 seconds of illumination (480nm, 14.6 log quanta/cm2/s) (P=0.00015, t=14.04, df=4). Rapid or significant pupil constriction was not observed for any individual Trpm1−/− mouse investigated during any trial, with similar results observed on multiple days with multiple handlers. Furthermore, a significant pupil constriction was also absent following stimulation with bright white light (16.6 log quanta/cm2/s, full data not shown). Overall, the defect observed for Trpm1−/− mice is far greater than that observed in mice lacking functional rods and cones or mice lacking melanopsin, but is not completely abolished as has been described in triple knockout mice lacking all retinal photoreceptors (Hattar et al., 2003). Given that either rods and cones or melanopsin pRGCs alone are capable of driving near complete pupil constriction, the large deficit observed in Trpm1−/− mice suggests a disruption of both rod/cone signalling (mediated by ON bipolar cells) and melanopsin driven signalling in these mice. Trpm3−/− mice also displayed a defect in the PLR when compared to wildtype controls, yet this defect was not as severe as that observed for Trpm1−/− mice. Trpm3−/− mice exhibited a rapid pupil constriction in response to light stimulation that was maintained throughout illumination and similar to that observed for wildtype, rd/rd cl and Opn4−/− mice. However, Trpm3−/− mice also failed to reach full pupil constriction with a maximum reduction in pupil size of ~80% observed (P=0.041, t=2.98, df=4). This value is similar to that observed for Opn4−/− mice and interestingly Trpm3−/− mice also displayed a profoundly attenuated post-stimulus response following the termination of light stimulation. This defect was more pronounced in Trpm3−/− mice than observed in Opn4−/− mice. Application of carbachol (1M) resulted in a complete constriction of the pupil in Trpm1−/− and Trpm3−/− mice suggesting that the functioning of the ciliary muscle in these mice is grossly unaffected and is physically capable of driving full pupil constriction (Figure 2).
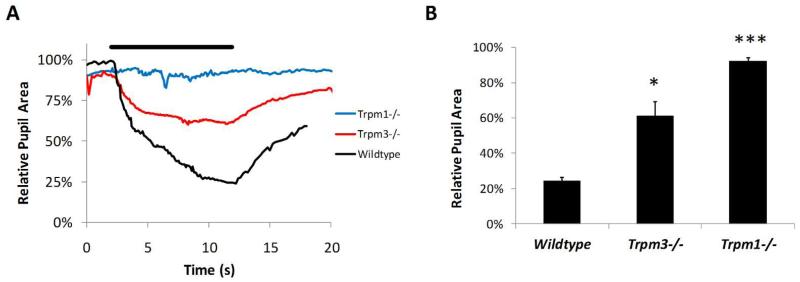
In addition to stimulation with bright light we also examined the pupillary light responses of Trpm1−/− and Trpm3−/− mice in response to dim light illumination (480nm, 11.6 log quanta/cm2/s) (Figure 3). At this intensity of light, the majority of pupil constriction is driven by rod/cone photoreceptors with only a minimal, if any, contribution from melanopsin pRGCs (Lucas et al., 2003; Lall et al., 2010). In keeping with the known role of TRPM1 in ON bipolar cell function and transmission of rod/cone driven signals, pupil constriction was completely absent in Trpm1−/− mice following dim light stimulation (P=7.4E-9, df=4, t=168.91). By contrast, Trpm3−/− mice showed a notable pupil constriction in response to dim light with pupil area reduced to ~40% of dark adapted values. However, this value was significantly attenuated compared to wildtype mice (P=0.0054, df=4, t=5.47).
Figure 3.
Analysis of the pupillary light response (PLR) in Trpm1−/− and Trpm3−/− mice. (A) Pupil constriction profiles recorded from Trpm1−/−, Trpm3−/− and normal wildtype mice in response to dim light illumination (11.6 log quanta/cm2/s). Profiles shown are mean of responses from n=3-6 mice. (B) Graph shows the mean maximal pupil constriction observed following dim light exposure. * = p<0.01, ***=P< 0.5E-8.
In summary, the data presented shows an attenuation of the pupillary light response in both TRPM1−/− and TRPM3−/− mice. The phenotype observed in Trpm1−/− mice is consistent with defects in both ON bipolar cell function and also melanopsin driven responses. Trpm3−/− mice show attenuated constriction under bright light and dim light conditions when compared to wildtype controls.
Expression of TRPM1 and TRPM3 in the retina and pRGCs
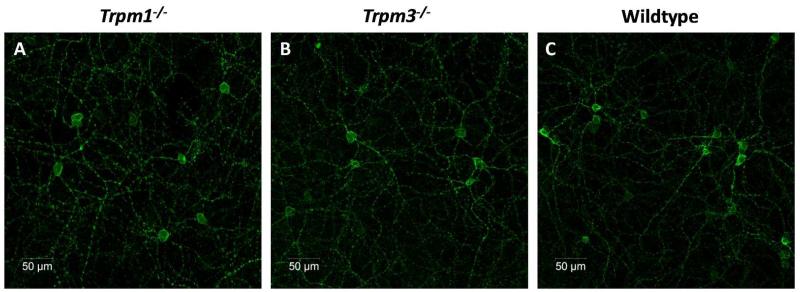
The structure and morphology of Trpm1−/− and Trpm3−/− retinae appear anatomically normal, with no obvious indications of retinal degeneration (data not shown). Immunolabelling with an anti-melanopsin antibody revealed that the melanopsin system also appears normal in the retina of both Trpm1−/− and Trpm3−/− mice. The levels of melanopsin staining and the morphology of pRGCs were indistinguishable from wildtype controls (Figure 4). No melanopsin labelling was observed in retina of Opn4−/− mice (data not shown).
Figure 4.
Melanopsin expression in Trpm1−/− and Trpm3−/− mice. Confocal images, showing the levels of melanopsin immunoreactivity observed in Trpm1−/− (A), Trpm3−/− (B) and normal wildtype whole mounted retina (C). Note that no differences were observed, with levels of expression, number of cells and distribution of melanopsin expressing cells similar for all strains of mice.
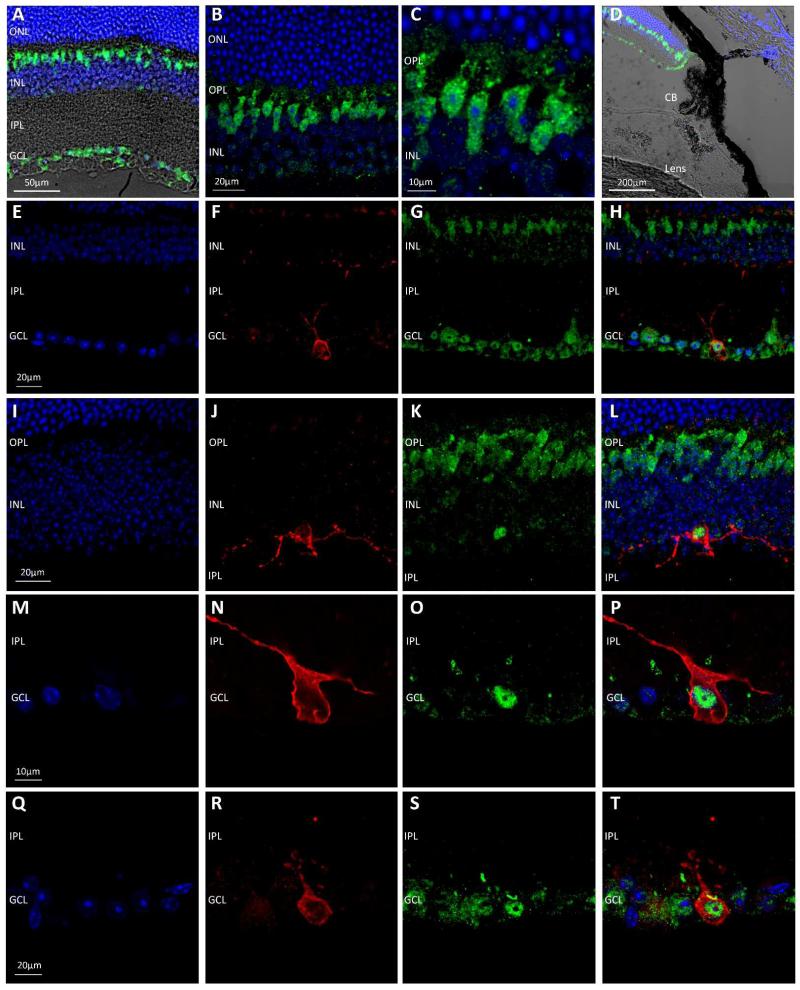
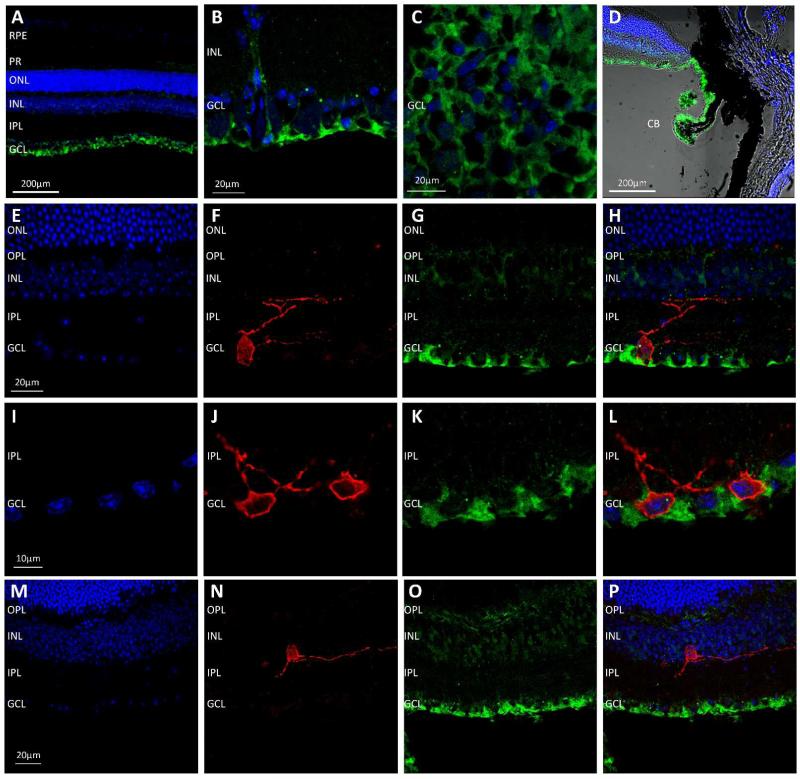
The Trpm1−/− and Trpm3−/− transgenic mouse models used in this study both incorporate a β-gal reporter encoded by the insertion of a Lac-Z gene into the reading frame of the target genes. We performed immunolabelling with an anti-β-gal antibody on retina from Trpm1−/− and Trpm3−/− mice in order to visualise the transgene product and determine the pattern of TRPM1 and TRPM3 expression in the retina. Detection of the β-gal reporter in the Trpm1−/− retina revealed expression in cells located within the inner nuclear layer (INL) resembling bipolar cells. However, in addition expression of the β-gal reporter was also detected in a subset of cells in the ganglion cell layer (GCL) (Figure 5). No expression of the β-gal reporter was detected in the ciliary body in Trpm1−/− mice. Double labelling with the anti-β-gal antibody and an anti-melanopsin antibody revealed the consistent expression of the β-gal reporter (TRPM1) within melanopsin expressing pRGCs (>90% of all pRGCs observed), with β-gal expression consistently detected in M1 and M2 type pRGCs and also displaced M1 type pRGCs located in the INL (Figure 5). No appreciable staining was observed following incubation of the β-gal antibody with normal wildtype retina not incorporating a β-gal transgene (data not shown). Immunolabelling of the β-gal reporter in Trpm3−/− mice revealed expression primarily in structures resembling the end-feet of Muller cells in the GCL, with weaker levels of staining observed in a subset of cells in the INL consistent with the cell bodies of Muller cells (Figure 6). In addition to expression in the retina, strong expression of the β-gal reporter was also observed in the ciliary body of TRPM3−/− mice. Double labelling with an anti-melanopsin antibody shows a lack of β-gal expression within pRGCs of TRPM3−/− mice (Figure 6).
Figure 5.
Localisation of TRPM1 in the mouse retina. (A-C) Localisation of TRPM1 expression via detection of the β-gal reporter (green) in the Trpm1−/− retina demonstrates expression in cells of the inner nuclear layer (INL) resembling bipolar cells, and in a subpopulation of cells in the ganglion cell layer (GCL). (D) TRPM1 (β-gal) expression was not detected in the cells of the ciliary body. (E-T) Double staining for β-gal (green) and melanopsin (red) in the Trpm1−/− retina demonstrates the consistent co-expression of TRPM1 (β-gal) within melanopsin expressing pRGCs, including M1 type pRGCs, M2 type pRGCs and displaced pRGCs. DAPI nuclear counter stain is shown in blue. Outer nuclear layer (ONL), outer plexiform layer (OPL), inner nuclear layer (INL), inner plexiform layer (IPL), ganglion cell layer (GCL), ciliary body (CB).
Figure 6.
Localisation of TRPM3 in the retina. (A-C) Localisation of TRPM3 expression via detection of the β-gal reporter (green) in the Trpm3−/− retina demonstrates strong expression in structures located in the ganglion cell layer (GCL) resembling Muller cell end feet (D) Strong expression of the β-gal reporter is detected in the cillary body. (E-L) Double staining for β-gal (green) and melanopsin (red) demonstrates the lack of TRPM3 (β-gal) expression within melanopsin expressing pRGCs located in the GCL. (M-P) Expression of TRPM3 (β-gal) is absent in displaced pRGCs in the Trpm3−/− retina. DAPI nuclear counter stain is shown in blue. Photoreceptors (PR), outer nuclear layer (ONL), outer plexiform layer (OPL), inner nuclear layer (INL), inner plexiform layer (IPL), ganglion cell layer (GCL), ciliary body (CB).
Overall the results of our immunolocalisation studies, suggest that TRPM1 is expressed in ON-bipolar cells and also a subset of cells in the GCL, including pRGCs, whereas TRPM3 is expressed in Muller cells and also cells of the ciliary body but is absent from pRGCs.
Discussion
A remarkable body of recent research has confirmed a role for TRPM1 in the depolarising light responses of ON bipolar cells, and shown that mutations in TRPM1 appear to account for about half of all cases of complete congenital stationary night blindness (CSNB1) (Morgans et al., 2010). In addition to this role in the ON visual pathway, our microarray studies suggest that TRPM1 and TRPM3 may play a potential role in responses to light in the absence of rods and cones, presumably mediated by melanopsin expressing pRGCs (Peirson et al., 2007). The visual phenotype of the Trpm1−/− mice used in this study has been investigated previously (Morgans et al., 2009). ERG recordings from Trpm1−/− mice show a normal a-wave but the b-wave (a measure of ON-bipolar cell depolarisation) is absent (Morgans et al., 2009; Shen et al., 2009). Trpm1−/− mice also have visual defects, including a relatively modest reduction in spatial frequency threshold (10%) and reduced contrast threshold (3-fold) compared to normal wildtype controls (Morgans et al., 2009). These authors conclude that Trpm1−/− mice have significant but not profound visual impairment, similar to those observed for CSNB. However, to date no study has investigated non-image forming responses to light in Trpm1−/− animals. In this study we have used the pupillary light response to assess the function of both rod cone and melanopsin based signalling pathways in Trpm1−/− and Trpm3−/− mice.
A role for Trpm1 in non-image forming responses to light
Consistent with previous reports, our data demonstrates that both the rod/cone photoreceptors and melanopsin pRGCs contribute significantly to pupillary responses to light. Only subtle defects were observed in mice lacking melanopsin, or mice lacking rods and cones, demonstrating that whilst melanopsin is required to attain full pupil constriction in response to bright light (Lucas et al., 2003), either pathway is capable of driving significant pupillary constriction. Previous studies have shown that removal of all three classes of photoreceptors is necessary to completely abolish the pupillary light response in mice (Hattar et al., 2003). Remarkably Trpm1−/− mice show a profound attenuation of pupillary responses to light, with only very limited levels of pupil constriction observed in response to bright light illumination, and a complete lack of constriction observed under dim light conditions. Given that existing data indicates that expression of Trpm1 mRNA is restricted to skin and retina, and is absent from the brain (Morgans et al., 2009; Koike et al., 2010), it would seem unlikely that the pupil defect observed in Trpm1−/− mice is due to downstream changes in the neural circuits that regulate pupil constriction, but is instead mediated by changes in retinal function. The pupillary defect observed in Trpm1−/− mice was significantly greater than that observed in rd/rd cl animals and cannot therefore be explained by the loss of ON-bipolar cell function and disruption of rod and cone driven signals alone. In fact, the defect observed in Trpm1−/− mice is most consistent with a defect in both ON bipolar function and also melanopsin driven responses in the retina of these mice. In support of this conclusion, it is worth noting that mGluR6-deficient mice, which are defective in the same ON bipolar signalling pathway as Trpm1−/− mice, are still able to attain pupil constriction down to ~25% area (compared with preceding pupil area in darkness) (Iwakabe et al., 1997). Taken together, the profound defects in pupillary responses over and above those observed in the absence of rods/cones or in other transgenic mouse lines with defective ON bipolar cell signalling (Iwakabe et al., 1997; Thompson et al., 2011) strongly suggests a substantial contribution of TRPM1 to pupillary light responses in a manner that is independent of bipolar cell function.
PCR analysis of isolated ganglion cell preparations indicates that Trpm1 mRNA is expressed in cells of the GCL, although this expression is seemingly not restricted to melanopsin pRGCs (as expression is detected in samples lacking Opn4 expression). In support of a direct role for TRPM1 in the melanopsin signalling pathway we consistently detected the expression of the TRPM1 β-gal reporter in melanopsin-expressing pRGCs, including M1 and M2 type pRGCs and also displaced pRGCs with cell bodies located in the INL (Berson et al., 2010; Schmidt et al., 2011). However, previous reports detailing the expression of TRPM1 in the retina (in situ hybridisation and immunocytochemistry) have reported that expression of TRPM1 is confined to ON-bipolar cells (Morgans et al., 2009; Koike et al., 2010), although one study has reported low but detectable transcript levels in the GCL (Hackler et al., 2010). The reason for the discrepancy between these previous studies and our results are unclear. It is plausible that the detection of the β-gal transgene reporter offers a more sensitive method for determining expression of TRPM1 than those used in previous studies. However, it is worth noting that on close examination of the images shown by Morgans et al, (2009) a case can be argued for a weak level of labelling of the TRPM1-L antibody in the GCL, albeit at lower levels than observed in bipolar cells. Alternatively, it is possible that the β-gal transgene in Trpm1−/− mice may report the expression of additional TRPM1 splice variants that are not recognised by the specific probes used previously (Oancea et al., 2009). A further possibility is that our β-gal results are an artefact of the transgenic mouse line used in this study, and that expression of the β-gal reporter is aberrantly expressed in other cell types that do not express TRPM1. It is also possible that changes in the pattern of TRPM1 expression are induced by functional changes in Trpm1 deficient mice, potentially due to developmental abnormalities or disruption of the transcription control elements that regulate Trpm1 expression. However, such possibilities fail to explain the light induction of Trpm1 expression in the rd/rd cl retina, the presence of Trpm1 mRNA in isolated GCL preparations from wildtype mice, or the results of our pupillometry studies where a clear and striking defect in non-image forming responses is evident in Trpm1−/− mice. We suggest that low levels of TRPM1 are expressed in a subset of cells in the GCL, which includes melanopsin-expressing pRGCs.
Combined our data suggest that TRPM1 plays a previously uncharacterised role in non-image forming responses to light that is distinct from its role in ON bipolar cells, and is consistent with a role in melanopsin pRGCs as previously predicted (Peirson et al., 2007). However, the potential cellular functions of TRPM1 in pRGCs (and other ganglion cells) are currently unclear. Stimulation of the melanopsin signalling pathway itself is known to result in activation of a Gnαq/11 type G-protein signalling pathway leading to the activation of PLC-β isoforms and ultimately the influx of Ca2+ through an as yet unidentified TRP like channel in the cell membrane (Hankins et al., 2008; Do & Yau, 2010). Previous studies have indicated that the most likely candidates for this TRP-like channel are members of the TRPC channel subfamily, potentially TRPC3, TRPC6 or TRPC7 (Sekaran et al., 2003; Warren et al., 2006; Graham et al., 2008), although recent evidence has shown that intrinsic photoresponses persist in pRGCs lacking each of these channels (in isolation) and suggests only a non-essential role for TRPC6 in melanopsin signalling (Perez-Leighton et al., 2011). Despite the identification of a profound attenuation of pupillary responses to light in Trpm1−/− mice, and the consistent detection of the β-gal reporter within melanopsin pRGCs, based on the biophysical and pharmacological properties of the light induced current in pRGCs TRPM1 would appear unlikely to be the as-yet unidentified channel mediating the primary depolarising responses to light in these cells. The depolarising Trp channel in pRGCs is known to be activated by Gαq/11 type G-proteins and is largely insensitive to agents that influence the Gαo pathway, whereas by contrast TRPM1 is spontaneously active and negatively regulated by the Gαo signalling pathway (Lambert et al.; Graham et al., 2008; Do et al., 2009; Morgans et al., 2009; Koike et al., 2010). However, it is possible that multiple TRP-like channels contribute to the cellular functions of pRGCs (Perez-Leighton et al., 2011), including TRPM1, and this channel may regulate resting membrane potential, depolarisation and/or intrinsic photoresponses, potentially in a Gαo dependant manner similar to that seen in ON-bipolar cells (Morgans et al., 2009; Koike et al., 2010; Morgans et al., 2010). It is also possible that TRPM1 contributes to intracellular calcium homeostasis within pRGCs, as has been suggested in melanocytes (Devi et al., 2009). Further electrophysiological studies are required to determine the specific role played by TRPM1 in pRGCs. Moreover, expression of TRPM1 does not appear to be restricted to pRGCs, but was also detected in a significant proportion of other cells in the GCL. We cannot therefore rule out a more generalised role of TRPM1 in ganglion cell function, which is in some way necessary for pRGC signalling. However, the relatively modest reported effects on spatial vision in the Trpm1−/− mice suggest that any defect in the visual retinal ganglion cells is minor. Selective deletion of TRPM1 expression within pRGCs, potentially via conditional knockouts, will be necessary to confirm the specific role of TRPM1 in pRGC function and melanopsin phototransduction.
Irrespective of whether or not TRPM1 is indeed a direct component of the melanopsin signalling pathway or influences the pupillary light response via an indirect mechanism, the data described here does demonstrate a significant role for TRPM1 in non-image forming responses to light. The implications of our findings are that human CSNB1 patients with mutations in the TRPM1 gene may also show defects in such responses, potentially impacting on not only pupil constriction but also circadian entrainment and regulation of sleep. Indeed, this may provide a potential diagnostic tool for CSNB1 resulting from TRPM1 mutations. Assessment of pupillary light responses in these patients in combination with sleep monitoring studies will be required to confirm this hypothesis. However it should be noted that loss of TRPM1 exerts a greater deficit on rod ON-bipolar cell function than cone ON-bipolar function (Morgans et al., 2009), and as humans have a significantly higher proportion of cones compared to the rod-dominated mouse retina, the defects observed in pupil responses from human patients may not be as severe as those observed in Trpm1−/− mice.
A role for Trpm3 in non-image forming responses to light
By contrast to TRPM1, the role of TRPM3 in the mammalian retina has not previously been investigated, although in situ hybridisation experiments have identified expression of Trpm3 in the retinal pigment epithelium, inner nuclear layer and ganglion cell layer, in addition to the ciliary body and lens epithelial cells (Karali et al., 2007). We show that Trpm3−/− mice exhibit attenuated pupillary responses to bright light, yet in contrast to Trpm1−/− animals this defect was relatively subtle and more closely resembles the phenotype observed in Opn4−/− mice with a failure to reach full pupil constriction and an exaggerated post-stimulus response (Lucas et al., 2003). However, Trpm3−/− mice show an attenuated pupil constriction under both bright light and dim light conditions. This property is not shared by Opn4−/− mice that show diminished PLR only at higher light intensities (Lucas et al., 2003). This data indicates that Trpm3−/− mice are not a phenocopy of Opn4−/− mice and that the mechanisms underlying the PLR defect in these mice are most likely different. This conclusion is supported by the observation that convincing expression of the β-gal reporter was not detected within melanopsin expressing pRGCs in Trpm3−/− mice. Furthermore, in agreement with previous in situ studies (Karali et al., 2007), high levels of TRPM3 expression were detected in the ciliary body and therefore a defect in muscle function cannot be eliminated as the basis of the pupillary phenotype observed in these mice. Studies in bovine ciliary muscle cells suggest that muscarinic stimulation by carbachol involves two types of non-selective cation channel, and Trp channels have been suggested as potential candidates (Takai et al., 2004). We suggest that TRPM3 may be one of the unidentified channels involved in regulating pupil constriction, but may also perform other roles in the retina. Future studies are required to clarify the level at which TRPM3 is involved in mediating pupillary responses to light.
Conclusions
In summary, here we demonstrate that both Trpm1−/− and Trpm3−/− mice show attenuated pupillary light responses. The profound nature of the defect observed for Trpm1−/− mice suggests a defect in both rod/cone and melanopsin pRGC-mediated signalling. The expression of TRPM1 in pRGCs indicates that TRPM1 may play a functional role in melanopsin signalling and therefore participate in both the classical ON visual pathway and also non-imaging forming response to light. The pupillary defect observed in Trpm3−/− mice has similarities to that observed in Opn4−/− mice, although the lack of TRPM3 expression in pRGCs and the strong expression in Muller cells and cells of the ciliary body suggest that this channel may influence pupillary responses and or pRGC function via an indirect mechanism. This conclusion is seemingly supported by responses to dim light illumination, where attenuated pupil constriction is observed for Trpm3−/− mice compared to wildtype controls, a property not consistent with the phenotype of Opn4−/− mice. Further experiments will be needed to clarify the roles of both channels in pRGC function and their contributions to non-image forming responses to light.
Acknowledgements
Trpm1−/− (NIH-1696: LexKO 428) and Trpm3−/− (NIH-1697: LexKO 380) mice were obtained via a Wellcome Trust Knockout Mouse Resource application awarded to MWH & SNP. This work was funded by a Wellcome Trust Programme Grant awarded to RGF, SNP and MWH. SH was funded by F. Hoffmann-La Roche during part of this work. AJ was funded by F. Hoffmann-La Roche. We would like to thank Laurence Brown and Lei Zheng for assistance with analysis of pupillometry images.
Abbreviations
- β-gal
β-galactosidase
- CCD
Charge couple device
- DAPI
4′,6-diamidino-2-phenylindole
- GCL
Ganglion cell layer
- INL
Inner nuclear layer
- OCT
Optimum Cutting Temperature
- PBS
Phosphate buffered saline
- pRGC
Photosensitive retinal ganglion cell
- RGC
Retinal ganglion cell
- TRPM
Transient receptor potential cation channel, subfamily M
Footnotes
None of the authors have any competing interests
References
- Audo I, Kohl S, Leroy BP, Munier FL, Guillonneau X, Mohand-Said S, Bujakowska K, Nandrot EF, Lorenz B, Preising M, Kellner U, Renner AB, Bernd A, Antonio A, Moskova-Doumanova V, Lancelot ME, Poloschek CM, Drumare I, Defoort-Dhellemmes S, Wissinger B, Leveillard T, Hamel CP, Schorderet DF, De Baere E, Berger W, Jacobson SG, Zrenner E, Sahel JA, Bhattacharya SS, Zeitz C. TRPM1 is mutated in patients with autosomal-recessive complete congenital stationary night blindness. Am J Hum Genet. 2009;85:720–729. doi: 10.1016/j.ajhg.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berson DM, Castrucci AM, Provencio I. Morphology and mosaics of melanopsin-expressing retinal ganglion cell types in mice. The Journal of comparative neurology. 2010;518:2405–2422. doi: 10.1002/cne.22381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacey DM, Liao HW, Peterson BB, Robinson FR, Smith VC, Pokorny J, Yau KW, Gamlin PD. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature. 2005;433:749–754. doi: 10.1038/nature03387. [DOI] [PubMed] [Google Scholar]
- Devi S, Kedlaya R, Maddodi N, Bhat KM, Weber CS, Valdivia H, Setaluri V. Calcium homeostasis in human melanocytes: role of transient receptor potential melastatin 1 (TRPM1) and its regulation by ultraviolet light. Am J Physiol Cell Physiol. 2009;297:C679–687. doi: 10.1152/ajpcell.00092.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do MT, Kang SH, Xue T, Zhong H, Liao HW, Bergles DE, Yau KW. Photon capture and signalling by melanopsin retinal ganglion cells. Nature. 2009;457:281–287. doi: 10.1038/nature07682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do MT, Yau KW. Intrinsically photosensitive retinal ganglion cells. Physiol Rev. 2010;90:1547–1581. doi: 10.1152/physrev.00013.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan LM, Deeds J, Hunter J, Shao J, Holmgren LM, Woolf EA, Tepper RI, Shyjan AW. Down-regulation of the novel gene melastatin correlates with potential for melanoma metastasis. Cancer Res. 1998;58:1515–1520. [PubMed] [Google Scholar]
- Ecker JL, Dumitrescu ON, Wong KY, Alam NM, Chen SK, LeGates T, Renna JM, Prusky GT, Berson DM, Hattar S. Melanopsin-expressing retinal ganglion-cell photoreceptors: cellular diversity and role in pattern vision. Neuron. 2010;67:49–60. doi: 10.1016/j.neuron.2010.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman MS, Lucas RJ, Soni B, von Schantz M, Munoz M, David-Gray Z, Foster R. Regulation of mammalian circadian behavior by non-rod, non-cone, ocular photoreceptors. Science. 1999;284:502–504. doi: 10.1126/science.284.5413.502. [DOI] [PubMed] [Google Scholar]
- Graham DM, Wong KY, Shapiro P, Frederick C, Pattabiraman K, Berson DM. Melanopsin ganglion cells use a membrane-associated rhabdomeric phototransduction cascade. J Neurophysiol. 2008;99:2522–2532. doi: 10.1152/jn.01066.2007. [DOI] [PubMed] [Google Scholar]
- Hackler L, Jr., Wan J, Swaroop A, Qian J, Zack DJ. MicroRNA profile of the developing mouse retina. Invest Ophthalmol Vis Sci. 2010;51:1823–1831. doi: 10.1167/iovs.09-4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankins MW, Peirson SN, Foster RG. Melanopsin: an exciting photopigment. Trends Neurosci. 2008;31:27–36. doi: 10.1016/j.tins.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Hattar S, Liao HW, Takao M, Berson DM, Yau KW. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science. 2002;295:1065–1070. doi: 10.1126/science.1069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattar S, Lucas RJ, Mrosovsky N, Thompson S, Douglas RH, Hankins MW, Lem J, Biel M, Hofmann F, Foster RG, Yau KW. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature. 2003;424:76–81. doi: 10.1038/nature01761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwakabe H, Katsuura G, Ishibashi C, Nakanishi S. Impairment of pupillary responses and optokinetic nystagmus in the mGluR6-deficient mouse. Neuropharmacology. 1997;36:135–143. doi: 10.1016/s0028-3908(96)00167-0. [DOI] [PubMed] [Google Scholar]
- Kankipati L, Girkin CA, Gamlin PD. Post-illumination pupil response in subjects without ocular disease. Invest Ophthalmol Vis Sci. 2010;51:2764–2769. doi: 10.1167/iovs.09-4717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karali M, Peluso I, Marigo V, Banfi S. Identification and characterization of microRNAs expressed in the mouse eye. Invest Ophthalmol Vis Sci. 2007;48:509–515. doi: 10.1167/iovs.06-0866. [DOI] [PubMed] [Google Scholar]
- Koike C, Obara T, Uriu Y, Numata T, Sanuki R, Miyata K, Koyasu T, Ueno S, Funabiki K, Tani A, Ueda H, Kondo M, Mori Y, Tachibana M, Furukawa T. TRPM1 is a component of the retinal ON bipolar cell transduction channel in the mGluR6 cascade. Proc Natl Acad Sci U S A. 2010;107:332–337. doi: 10.1073/pnas.0912730107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft R, Harteneck C. The mammalian melastatin-related transient receptor potential cation channels: an overview. Pflugers Arch. 2005;451:204–211. doi: 10.1007/s00424-005-1428-0. [DOI] [PubMed] [Google Scholar]
- Lall GS, Revell VL, Momiji H, Al Enezi J, Altimus CM, Guler AD, Aguilar C, Cameron MA, Allender S, Hankins MW, Lucas RJ. Distinct contributions of rod, cone, and melanopsin photoreceptors to encoding irradiance. Neuron. 2010;66:417–428. doi: 10.1016/j.neuron.2010.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert S, Drews A, Rizun O, Wagner TF, Lis A, Mannebach S, Plant S, Portz M, Meissner M, Philipp SE, Oberwinkler J. Transient receptor potential melastatin 1 (TRPM1) is an ion-conducting plasma membrane channel inhibited by zinc ions. J Biol Chem. doi: 10.1074/jbc.M110.202945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Sergouniotis PI, Michaelides M, Mackay DS, Wright GA, Devery S, Moore AT, Holder GE, Robson AG, Webster AR. Recessive mutations of the gene TRPM1 abrogate ON bipolar cell function and cause complete congenital stationary night blindness in humans. Am J Hum Genet. 2009;85:711–719. doi: 10.1016/j.ajhg.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas RJ, Douglas RH, Foster RG. Characterization of an ocular photopigment capable of driving pupillary constriction in mice. Nat Neurosci. 2001;4:621–626. doi: 10.1038/88443. [DOI] [PubMed] [Google Scholar]
- Lucas RJ, Freedman MS, Munoz M, Garcia-Fernandez JM, Foster RG. Regulation of the mammalian pineal by non-rod, non-cone, ocular photoreceptors. Science. 1999;284:505–507. doi: 10.1126/science.284.5413.505. [DOI] [PubMed] [Google Scholar]
- Lucas RJ, Hattar S, Takao M, Berson DM, Foster RG, Yau KW. Diminished pupillary light reflex at high irradiances in melanopsin-knockout mice. Science. 2003;299:245–247. doi: 10.1126/science.1077293. [DOI] [PubMed] [Google Scholar]
- Lupi D, Oster H, Thompson S, Foster RG. The acute light-induction of sleep is mediated by OPN4-based photoreception. Nat Neurosci. 2008;11:1068–1073. doi: 10.1038/nn.2179. [DOI] [PubMed] [Google Scholar]
- Morgans CW, Brown RL, Duvoisin RM. TRPM1: the endpoint of the mGluR6 signal transduction cascade in retinal ON-bipolar cells. Bioessays. 2010;32:609–614. doi: 10.1002/bies.200900198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgans CW, Zhang J, Jeffrey BG, Nelson SM, Burke NS, Duvoisin RM, Brown RL. TRPM1 is required for the depolarizing light response in retinal ON-bipolar cells. Proc Natl Acad Sci U S A. 2009;106:19174–19178. doi: 10.1073/pnas.0908711106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oancea E, Vriens J, Brauchi S, Jun J, Splawski I, Clapham DE. TRPM1 forms ion channels associated with melanin content in melanocytes. Sci Signal. 2009;2:ra21. doi: 10.1126/scisignal.2000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirson SN, Oster H, Jones SL, Leitges M, Hankins MW, Foster RG. Microarray analysis and functional genomics identify novel components of melanopsin signaling. Curr Biol. 2007;17:1363–1372. doi: 10.1016/j.cub.2007.07.045. [DOI] [PubMed] [Google Scholar]
- Perez-Leighton CE, Schmidt TM, Abramowitz J, Birnbaumer L, Kofuji P. Intrinsic phototransduction persists in melanopsin-expressing ganglion cells lacking diacylglycerol-sensitive TRPC subunits. Eur J Neurosci. 2011;33:856–867. doi: 10.1111/j.1460-9568.2010.07583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pires SS, Hughes S, Turton M, Melyan Z, Peirson SN, Zheng L, Kosmaoglou M, Bellingham J, Cheetham ME, Lucas RJ, Foster RG, Hankins MW, Halford S. Differential expression of two distinct functional isoforms of melanopsin (Opn4) in the mammalian retina. J Neurosci. 2009;29:12332–12342. doi: 10.1523/JNEUROSCI.2036-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provencio I, Rollag MD, Castrucci AM. Photoreceptive net in the mammalian retina. This mesh of cells may explain how some blind mice can still tell day from night. Nature. 2002;415:493. doi: 10.1038/415493a. [DOI] [PubMed] [Google Scholar]
- Schmidt TM, Chen SK, Hattar S. Intrinsically photosensitive retinal ganglion cells: many subtypes, diverse functions. Trends Neurosci. 2011 doi: 10.1016/j.tins.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekaran S, Foster RG, Lucas RJ, Hankins MW. Calcium imaging reveals a network of intrinsically light-sensitive inner-retinal neurons. Curr Biol. 2003;13:1290–1298. doi: 10.1016/s0960-9822(03)00510-4. [DOI] [PubMed] [Google Scholar]
- Shen Y, Heimel JA, Kamermans M, Peachey NS, Gregg RG, Nawy S. A transient receptor potential-like channel mediates synaptic transmission in rod bipolar cells. J Neurosci. 2009;29:6088–6093. doi: 10.1523/JNEUROSCI.0132-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai Y, Sugawara R, Ohinata H, Takai A. Two types of non-selective cation channel opened by muscarinic stimulation with carbachol in bovine ciliary muscle cells. J Physiol. 2004;559:899–922. doi: 10.1113/jphysiol.2004.065607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson S, Stasheff SF, Hernandez J, Nylen E, East JS, Kardon RH, Pinto LH, Mullins RF, Stone EM. Different inner retinal pathways mediate rod-cone input in irradiance detection for the pupillary light reflex and regulation of behavioral state in mice. Investigative ophthalmology & visual science. 2011;52:618–623. doi: 10.1167/iovs.10-6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Genderen MM, Bijveld MM, Claassen YB, Florijn RJ, Pearring JN, Meire FM, McCall MA, Riemslag FC, Gregg RG, Bergen AA, Kamermans M. Mutations in TRPM1 are a common cause of complete congenital stationary night blindness. Am J Hum Genet. 2009;85:730–736. doi: 10.1016/j.ajhg.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren EJ, Allen CN, Brown RL, Robinson DW. The light-activated signaling pathway in SCN-projecting rat retinal ganglion cells. Eur J Neurosci. 2006;23:2477–2487. doi: 10.1111/j.1460-9568.2006.04777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]