Abstract
Background
LIGHT, a ligand for lymphotoxin-β receptor (LTβR) and herpes virus entry mediator, is predominantly expressed on activated immune cells and LTβR signaling leads to the recruitment of lymphocytes. The interaction between LIGHT and LTβR has been previously shown in a virus induced tumor model to activate immune cells and result in tumor regression, but the role of LIGHT in tumor immunosuppression or in a prostate cancer setting, where self antigens exist, has not been explored. We hypothesized that forced expression of LIGHT in prostate tumors would shift the pattern of immune cell infiltration, would inhibit T regulatory cells (Tregs) and would induce prostate cancer tumor associated antigen (TAA) specific T cells that would eradicate tumors.
Methods
Real Time PCR was used to evaluate expression of forced LIGHT and various other genes in prostate tumors samples. Adenovirus encoding murine LIGHT was injected intratumorally into TRAMP C2 prostate cancer cell tumor bearing mice for in vivo studies. Chemokine and cytokine concentrations were determined by multiplex ELISA. Flow cytometry was used to phenotype tumor infiltrating lymphocytes and expression of LIGHT on the tumor cell surface. Tumor specific lymphocytes were quantified via an ELISpot assay. Treg induction and Treg suppression assays determined Treg functionality after LIGHT treatment.
Results
LIGHT expression peaked within 48 hours of infection, recruited effector T cells into the tumor microenvironment that recognized mouse prostate stem cell antigen (PSCA) and inhibited the infiltration of Tregs. Tregs isolated from tumor draining lymph nodes had impaired suppressive capability after LIGHT treatment. LIGHT in combination with a therapeutic vaccine, PSCA TriVax, reduced tumor burden.
Conclusion
Forced LIGHT treatment combined with PSCA TriVax therapeutic vaccination delays prostate cancer progression in mice by recruiting effector T lymphocytes to the tumor and inhibiting Treg mediated immunosuppression.
Keywords: PSCA TriVax, Tumor-Antigen Specificity, TNFSF14, Tumor immunosuppression
Introduction
Prostate cancer is the second most common cause of cancer related deaths in men in the United States. Approximately 32,000 deaths are expected annually in the United States, and 258,000 deaths are expected annually worldwide (1). While treatments for patients with early stage prostate cancer exist, many are accompanied by severe side effects such as impotence or incontinence (2,3). Options for patients with advanced stage disease are limited. The standard of care for metastatic prostate cancer patients is chemical castration, a hormone therapy that reduces androgen levels and removes necessary growth components for transformed prostate cancer cells, halting cell growth (4,5). Alternatively, hormone manipulation can be avoided when treating advanced prostate cancer with personalized therapeutic treatments such as Sipuleucel-T (Provenge®), which activates the immune system to attack cells expressing tumor associated antigens (TAA) (6). Sipuleucel-T extends median survival by 25.8 months as compared to 21.7 months with standard of care and has been recommended by the National Comprehensive Cancer Network as a category 1 drug indicating a first choice treatment for advanced diseases (7–9). Despite the development of new treatments such as this, mortality rates remain unchanged (1,10,11). The efficacy of therapeutic vaccines have been limited when used alone, but the discovery of adjuvants such as aluminum-based mineral salts, toll-like receptor agonists, check point inhibitors and other immune response stimulators have become key factors in vaccine development (12). Therefore, identifying novel adjuvants or checkpoint regulators for therapeutic vaccines is expected to improve the overall efficacy of generating an immune response against prostate cancer, ultimately increasing patient survival.
The goal of cancer immunotherapy is to stimulate the immune system to eradicate malignant tumors. One of the most common responses that effective therapeutic vaccines elicit is TAA specific T lymphocytes (13). However, a suppressive tumor microenvironment counteracts the efficacy of these vaccines (14), often times by preventing the elicited TAA specific T lymphocytes from migrating into the tumor or by inactivating tumor-infiltrating lymphocytes. There are a variety of mechanisms that may suppress immune responses in prostate cancer but Tregs appear to be central to tumor-mediated immune suppression (15).
Herpes virus entry mediator receptor (HVEM) signaling plays an important role in either activating naïve T cells or inactivating T cell responses by enhancing Treg suppression depending on the bound ligand (16,17). TNFSF14/LIGHT, lymphotoxin-like inducible protein that competes with glycoprotein D for HVEM on T cells, is a membrane bound protein that is highly expressed during lymphogenesis for the recruitment of lymphocytes via chemokine signaling of LTβR (18,19). LIGHT has been shown to induce strong activating co-stimulatory signals on the recipient cell when bound to HVEM (20). In an HPV-induced cervical cancer model, forced intra-tumoral LIGHT expression induced naïve T cell recruitment into the tumor microenvironment, HPV-specific immunity, and increased overall survival in mice (21). The effects of forced LIGHT expression in prostate cancers where immunological self-tolerance exists has not been explored previously, and therefore was analyzed in this study.
Tumor immunosuppression and escape mechanisms have long been implicated as hurdles for successful immunotherapy, yet many vaccines focus solely on activating specific T cells and do not attempt to address the inhibitory aspects of the tumor microenvironment (14,22). HVEM binds B and T Lymphocyte Attenuator (BTLA), which has previously been shown to enhance Treg suppression and inactivate T cell responses (17), possibly contributing to the failure of therapeutic vaccines. In contrast, LIGHT has the opposite effect of BTLA on HVEM, potentially skewing the tumor microenvironment away from immunosuppression by tipping the balance towards activating co-stimulatory signals. Given the multiple mechanisms of LIGHT, including homing of T cells to the tumor microenvironment and induction of TAA-specific T cells in an HPV setting, we hypothesized that forced LIGHT expression in murine prostate cancer transgenic adenocarcinoma of the mouse prostate (TRAMP-C2) tumor model would increase prostate cancer survival by inducing prostate TAA specific T cells, would inhibit Tregs and would synergize with a TAA therapeutic vaccine.
Prostate Stem Cell Antigen (PSCA) is highly elevated in aggressive and established prostate tumors (23). It is therefore an excellent prostate TAA for the basis of a therapeutic vaccine. In this study, we have chosen to use PSCA TriVax, a vaccine that targets PSCA83-91 peptide and consist of two dendritic cell activators, anti-CD40 antibody (Ab) and Poly-ICLC. We explored LIGHT treatment alone and in combination with PSCA TriVax in an established TRAMP-C2 prostate tumor system as a new therapeutic approach. The results of our study can potentially improve the outcome for prostate cancer patients that are treated with a therapeutic vaccine.
Materials and Methods
2.1 Mice and cell lines
Specific pathogen free C57BL/6 mice and C3H mice, 6 to 8 weeks of age, were purchased from Taconic Farms (Germantown, NY). TRAMP-C2 (ATCC CRL-2731; originally derived from the prostate tumor of a TRAMP mouse on the C57BL/6 background) cells were used for tumor challenge studies. TRAMP-C2 cells were grown and expanded in vitro with IMDM medium supplemented with 5% Fetal bovine serum (FBS; Gemini, Sacramento, CA), 5% Nu Serum IV (BD Biosciences, San Jose, CA), 0.01 nM dihydrotestosterone (Sigma Chemical Co.), and 5 μg/ml insulin (Sigma Chemical Co.). All in vivo studies were in compliance and approved by University of Southern California Institutional Animal Care and Use Committee (USC IACUC).
2.2 Antibodies and Reagents
The following antibodies were purchased from BD Bioscience (San Jose, California): αmu-CD4 FITC, αmu-CD25 PE-Cy5, αmu-FoxP3 PE-Cy7, αmu-CD3 PE-Cy7, and αmu-CD8 PE. Goat αmu-IgG FITC antibodies were purchased from Biolegend (San Diego, CA). LTβR-Fc antibody was purchased from R&D Systems (Minneapolis, MN). Appropriate isotype controls were purchased from either BD Bioscience or Biolegend.
2.2 Tumor Challenge, Treatments and Immunizations
Groups of 6 to 8 week old C57BL/6 male mice were challenged subcutaneously with 5×105 TRAMP-C2 tumor cells in PBS. Tumor growth was measured three times per week with manual calipers by measuring tumor length, height, and depth to generate a tumor volume. Tumor volumes exceeding 1500 mm3 or ulcerated tumors resulted in euthanasia as per USC IACUC guidelines. For studies evaluating the effect of LIGHT in vivo, recombinant adenovirus carrying DNA encoding the murine LIGHT gene (Ad-LIGHT) were injected intratumorally using a 31 gauge insulin syringe (24). For every in vivo experiment with LIGHT treatment, injections were performed when average tumor volumes in randomized groups were approximately 30 mm3 (25–30 days post challenge). Ad-LIGHT treatment was given twice, three days apart with 2×1010 viral particles (vp) per intratumoral injection. Control adenovirus particles (Ad-Control) were used as a control. In studies evaluating the synergistic properties of both Ad-LIGHT and therapeutic vaccination PSCA TriVax, mice were treated with two doses of Ad-LIGHT given three day apart when average tumor volumes in randomized groups reached 30mm3, and were subsequently vaccinated i.m. with PSCA TriVax 7 days and 14 days after the first LIGHT injection. PSCA TriVax consist of a mixture of 50 μg of synthetic peptide PSCA83-91, 100 μg anti-CD40 mAb (BioXCell) and 50 μg of Poly-ICLC (Hiltonol, Oncovir, Inc.). Control immunizations were conducted with a mixture of 100 μg of anti-CD40 mAb and 50 μg of Poly-ICLC alone. Tumor burden was recorded three times per week. Euthanasia was conducted as per USC IACUC guidelines.
2.4 IFN-γ Enzyme Linked Immunospot Assay
96-well ELISpot plates (Millipore Multiscreen HTS IP) were coated with 10 μg/ml IFNγ capture Ab (IFNγ R406A2, BD Pharmingen) in sterile PBS overnight at 4°C. Plates were washed once with 0.5% PBS-T and then twice with sterile PBS. Complete RMPI medium was then used to block plates for 2 hours at 37°C. Splenocytes isolated from treated mice were plated in serial dilutions ranging from 5×105 to 1.25×105 cells per well in medium containing either 50 μg/mL of PSCA83-91 peptide, DMSO control or 10 μg/ml of PHA-L. After 48 h of incubation at 37°C, plates were washed 6 times with 0.05% PBST and were incubated with 1 μg/ml of biotinylated IFN-γ antibody (BD Pharmingen) in 0.05% PBST/1% BSA for 2 h at room temperature. Plates were washed 6 times with 0.05% PBST and wells were subsequently incubated with 100 μl of 1:4000 diluted streptavidin-horseradish peroxidase (Sigma Chemical Co.) for 1 h at room temperature. Spots were developed with 3-amino-9-ethylcarbazole (Sigma Chemical Co.) for 5 minutes and reactions were quenched with deionized water. A Zeiss KS ELISPOT microscope was used to determine the number of spots per well. Numbers of spots were normalized to background control (DMSO control) then each treatment group was further compared to the untreated study arm.
2.5 Treg Suppression Assay
Tumor draining lymph nodes from individual treatment groups were pooled together and isolated for CD4+CD25hi (suppressive cells) populations via a CD4+CD25hi Regulatory T cell magnetic activated cell separation (MACS) kit (Miltenyi Biotec). CD4+CD25− (responder cells) population was isolated from splenocytes of naïve C57BL/6 mice. 5×104 Responder cells were co-cultured with a decreasing ratio of suppressor cells (Tresp:Treg ratios: 1:1, 2:1, 4:1 and 8:1), 1 μg/ml anti-CD3, 2 μg/ml anti-CD28 and 5×105 irradiated accessory cells (allogenic T cell cells) that were isolated from C3H mice. After 48 h, 1 μg of 3H-thymidine was added into each well for an additional 24 h. Responder cell proliferation was measured by thymidine incorporation using a TopCount NXT microplate scintillation counter (PerkinElmer, Shelton, CT). The proliferation index was calculated for each Tresp:Treg ratio and was normalized to maximum proliferation (Tresp cultured in the absence of Tregs).
2.6 Treg Induction Assay
Naïve CD4+ T cells were isolated from splenocytes of a naïve C57BL/6 mouse via the Mouse CD4+CD62L+ T cell MACS kit (Miltenyi Biotec). Untreated TRAMP-C2 cells or TRAMP-C2 cells infected with 2×103 vp/cell of Ad-LIGHT were irradiated at 30 Gray prior to co-cultures. 5×105 naïve CD4+ T cells were plated out into each well of a 6 well plate in complete T cell medium (RPMI medium supplemented with 10% FBS). Treg-inducing factors and tumor cells were added to appropriate wells; 100 units/mL rhIL-2, 5 ng/ml rhTGF-b, 1:1 bead to cell ratio of CD3/CD28 dynabeads (Invitrogen, Oslo, Norway), and 1:1 ratio of naïve T cells to tumor cells. Cultures were incubated for 5 days at 37°C prior to Treg phenotyping via flow cytometry.
2.7 Isolating tumor Infiltrating Lymphocytes (TIL), Ad-LIGHT infected TRAMP-C2 cells and Flow Cytometry
Tumors were extracted and weighed from TRAMP-C2 bearing C57BL6 mice that were treated with Ad-LIGHT and/or immunized with PSCA TriVax. Tumor tissues were minced into small pieces prior to using the Miltenyi Tumor Dissociation Kit and GentleMACS Dissociator. Cell suspension was passed through a 70 μm nylon strainer to generate a single cell population and separated in a Lympholyte-M gradient (Cedarlane) for the isolation of TIL from debris. TIL were then washed 3 times with PBS, stained with antibodies and analyzed by flow cytometry to determine the phenotype of infiltrating lymphocytes. For Treg population, we first gated on CD4+ cells and then gated on CD25+ and Foxp3+ cells. Effector T cells were gated on CD8+ and CD3+ double positive population and helper T cells were gated on CD4+ and CD3+ double positive populations.
TRAMP-C2 cells infected with Ad-LIGHT (1×103 or 2×103 viral particles per cell) were collected 24, 48, 72, 96 and 120 h post infection, washed twice with FACS Buffer, prior to being stained with primary antibody, LTβR-Fc recombinant protein, and then with secondary, goat anti-mouse FITC. TRAMP-C2 cells were gated on FITC expressing cells and the mean fluorescence intensity was recorded.
2.8 Quantitative real-time polymerase chain reaction
Tumors harvested from treated mice were weighed and stored in RNAlater solution. Fixed tumors were homogenized with the PolyTron PT2100 homogenizer in RLT buffer solution at 4°C. Ad-LIGHT treated TRAMP-C2 cells were isolated subsequent to Ad-LIGHT infection with either 1×103 or 2×103 viral particles per cell. Total RNA was isolated using the QIAGEN RNAeasy Plus kit following manufacturer’s instructions. The iScript cDNA synthesis kit (Bio-Rad, Hercules, CA) was used to reverse transcribe the isolated RNA to cDNA. RNA and cDNA concentrations (ng/ml) were quantified using a NanoDrop 2000 (Waltham, MA). Quantitative real time PCRs were performed on a CFX-96 real time PCR machine (Bio-Rad) using the Sensi-fast SYBR NO-ROX kits (BioLine, Taunton, MA), following manufacturers protocol. Genes including GAPDH, mLIGHT, NOS, Arg2 and IDO were analyzed. The relative expression of each gene was normalized to the expression of GAPDH (ΔΔCq) and results from each treatment groups were compared to the untreated control study arm. All primers were synthesized by IDT (Coralville, IA) or the USC DNA Core.
GAPDH qPCR primers: forward 5′-TCA ATG AAG GGG TCG TTG AT-3′; reverse 5′-CGT CCC GTA GAC AAA ATG GT-3. ′
mLIGHT qPCR primers: forward 5′-CAA CCC AGC AGC ACA TCT TA-3′; reverse 5′-GCT CAG CTG CAC TTT GGA G-3. ′
NOS qPCR primers: forward 5′-GTC GAT GTC ACA TGC AGC TT-3′; reverse 5′-GAA GAA AAC CCC TTG TGC TG-3. ′
Arg2 qPCR primers: forward 5′-AGG GAT CAT CTT GTG GGA CA-3′; reverse 5′-AGA AGC TGG CTT GCT GAA GA-3. ′
IDO qPCR primers: forward 5′-GTG GGC AGC TTT TCA ACT TC-3′; reverse 5′-GGG CTT TGC TCT ACC ACA TC-3′
2.9 Measuring intratumoral cytokines
Tumors harvested from treated mice were weighed and homogenized with the PolyTron PT2100 homogenizer (Kinematica AG, Switzerland) in a 1x Halt Proteinase Inhibitor Cocktail (Pierce, Rockford, IL)/PBS solution for 20 minutes at 4°C. Homogenate was centrifuged and supernatants were collected to quantify cytokine levels with a custom 22-plex Milliplex mouse cytokine immunoassay (Millipore, Billerica, MA) using the Bio-Plex multiplex system (Bio-Rad).
2.10 Statistical Analysis
All statistical analyses were performed on Prism, Graphpad 5.0 (GraphPad Software Inc., San Diego, CA). Tumor growth, ELISpots, Cytokine levels and flow cytometry results were assessed with either a student t test, one-way ANOVA or a two-way ANOVA comparing data to untreated controls. Significance was defined at p≤0.05 for all experiments.
Results
Ad-LIGHT infected TRAMP-C2 cells are capable of expressing membrane bound LIGHT
Prior to in vivo studies with intratumoral LIGHT injections, we wanted to determine whether TRAMP-C2 prostate cancer cells were capable of taking up an adenovirus vector coding for LIGHT DNA and then expressing membrane bound LIGHT on the tumor cell surface. To examine this, TRAMP-C2 tumors were incubated with either 1×103 viral particles/cell (vp/cell) or 2×103 vp/cell for 24 h, 48 h, 72 h, 96 h and 120 h. Adenovirus encoding for no foreign gene (Ad-control) was used for controls. TRAMP-C2 cells infected with 1×103 vp/cell and 2×103 vp/cells were harvested at various time points and analyzed for mRNA expression and surface expression of LIGHT. LIGHT mRNA level peaked within the first 24–48 h, with a 1 fold increase compared to untreated controls at a dose dependent manner (Fig. 1a). Expression levels tapered off 72 h post-infection. Similar results were detected by flow cytometry using the LIGHT ligand, LTβR-Fc recombinant protein, to assess LIGHT expression on the cell surface. Membrane bound LIGHT peaked within the first 24–48 h post-infection (Fig. 1b). TRAMP-C2 cells infected with 2×103 vp/cell Ad-LIGHT showed a 2-fold increase in mean fluorescence intensity as compared to cells infected with 1×103 vp/cell Ad-LIGHT at 24 h, indicating a dose-dependent increase in membrane bound LIGHT expression. These results demonstrate the ability of TRAMP-C2 cells to take up Ad-LIGHT and express membrane bound LIGHT on the tumor cell surface.
Fig. 1.
Membrane bound LIGHT expression in TRAMP-C2 cells peaks within the first 48 hours of infection. (A) 5×105 TRAMP-C2 cells were infected with either 1×103 or 2×103 Ad-LIGHT viral particles per cell. mRNA was isolated and showed a higher mRNA level of LIGHT with 2×103 viral particles as compared to 1×103 viral particles. Expression peaked at 24 and 48 hours. Shown is the relative expression of LIGHT mRNA normalized to GAPDH (± SD) in Ad-LIGHT infected cells measured by RT-qPCR. (B) Membrane bound LIGHT was detected via flow cytometry with the LTβR-Fc ligand. Expression of LIGHT protein correlates with the mRNA expression level, where 24 hours shows the highest levels of LIGHT expression. All experiments were repeated once and representative data are shown.
LIGHT treatment increases Teffector:Treg ratio amongst tumor infiltrating lymphocytes
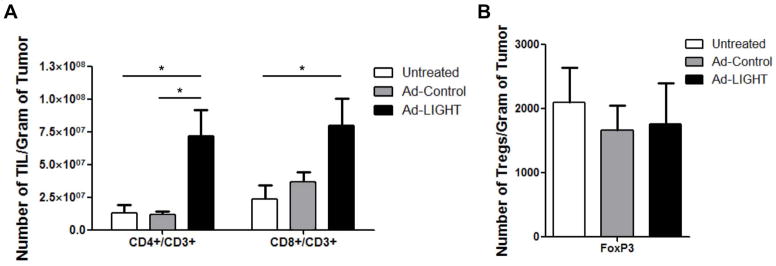
We investigated whether chemokine signaling as a result of LIGHT-LTβR signaling in the stroma of the TRAMP-C2 prostate cancer tumor model was able to recruit T cells to the microenvironment. To this end, mice were challenged subcutaneously with 5×105 TRAMP-C2 cells. When tumors were palpable and large enough to inject, mice were randomized into treatment groups with an average tumor volume of 30 mm3. Mice were then given two intratumoral injections of either Ad-LIGHT or Ad-Control given three days apart. Tumors were harvested one week after the last treatment. TIL were released from tumors using a tumor dissociation kit and phenotyped by flow cytometry. An increase in the mean number of infiltrating CD8+/CD3+ and CD4+/CD3+ T cells per gram of tumor was observed in Ad-LIGHT treated tumors compared to untreated controls (Fig. 2a). In contrast, the mean number of suppressive Treg per gram of tumor was not statistically significantly different between any of the three treatment groups (Fig. 2b). These data show that expression of intratumoral LIGHT increases the number of infiltrating effector T cells but does not increase the total number of Tregs in the tumor microenvironment, shifting the balance of the intratumoral Teffector:Treg ratio to a more favorable state.
Fig. 2.
Increase in intratumoral CD4+ and CD8+ T cells following forced expression of membrane bound LIGHT in a prostate cancer tumor model. (A) Tumor infiltrating lymphocytes were released from untreated or treated tumors 7 days after Ad-Control or Ad-LIGHT injection. Cells were stained with CD4, CD8 and CD3 Ab and analyzed via flow cytometry. The number of TIL/gram of tumor from CD8+/CD3+ and CD4+/CD3+ T cells were significantly higher in Ad-LIGHT treated mice compared to untreated. (p<0.05, one-way ANOVA). (B) The number of CD4+CD25+Foxp3+ Tregs per gram of tumor were not significantly differently, despite the increase in total number of infiltrating lymphocytes in the Ad-LIGHT samples. Shown is the average number of FoxP3+ TIL (±SD) from 5 treated mice/group. Data are representative of two individual experiments.
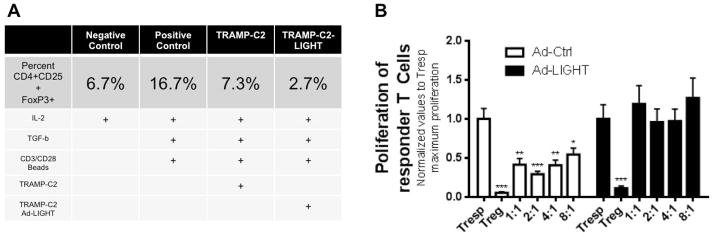
LIGHT prevents the maturation of Tregs from naïve CD4+ T cells and compromises the suppressive functions of existing Tregs
Induced Tregs (iTregs) are derived from naïve CD4+ T cells that receive stimulatory signals of IL-2, TGF-β and a weak co-stimulation, as is commonly found in the tumor microenvironment. However, since LIGHT provides a strong co-stimulatory signal we hypothesized that intratumoral LIGHT expression would prevent the induction of Tregs from an infiltrating naïve population, potentially explaining the data presented in Figure 2. To investigate this possibility, we harvested spleens from naïve C57BL/6 mice and isolated naïve CD4+CD62L+ T cells to use in an iTreg induction assay. We then forced the maturation of naïve CD4+ T cells to Tregs by providing IL-2, TGF-β and CD3/CD28 stimulation, and introduced irradiated TRAMP-C2 cells or TRAMP-C2-LIGHT expressing cells to appropriate samples. After 5 days of co-culture with stimulants, the percentage of naïve CD4+ T cells that had been induced to become CD4+CD25+FoxP3+ Tregs was quantitated by flow cytometry. In the positive control group, 16.7% of naïve T cells had been converted to iTregs under normal iTreg-inducing conditions. Using the same conditions but with the addition of TRAMP-C2-LIGHT cells; the frequency of iTregs was reduced to 2.7%, suggesting that LIGHT expression prevented the maturation of naïve T cells to iTregs (Fig. 3a).
Fig. 3.
Intratumoral expression of LIGHT reduces the frequency of induced Tregs and causes existing Tregs to lose their suppressive capacity. (A) Naïve CD4+CD62L+ T cells were cultured with Treg inducing factors. Flow cytometry data represented in table. Results in each column show the frequency of Tregs induced in different treatment arms and growth factors and cytokines that were added. Results demonstrate that the presence of LIGHT reduces the frequency of iTregs. Experiment was repeated once and representative frequency is shown. (B) Tresp cells alone (1:0 Tresp:Treg ratio) was taken as 100% proliferation (Set to 1). Tregs isolated from Ad-Control treated mice suppressed Tresp proliferation at all co-culture ratios. Tregs isolated from Ad-LIGHT treated mice lose the ability to suppress Tresp proliferation. Tregs isolated from untreated tumor-bearing mice or naïve mice showed statistically similar suppressive capacity to Ad-Control treated mice (Supplemental Fig. A). N=10 per experiment, one-way ANOVA, *p<0.05, **p<0.01, ***p<0.001). Experiment was repeated twice.
In addition, we evaluated the suppressive capacity of Tregs isolated from Ad-LIGHT treated mice through a Treg suppression assay. We sought to determine whether the co-stimulatory interaction of LIGHT to HVEM acted as a competitive inhibitor to the suppressive BTLA-HVEM interaction. Tregs were isolated from tumor draining lymph nodes of tumor-bearing mice (treated with either Ad-Control, Ad-LIGHT, or left untreated) and naïve mice (untreated and tumor-free). We performed co-culture experiments with various ratios using isolated Tregs from Ad-LIGHT treated animals and naïve T responder cells (Tresp) isolated from naïve mice. The proliferation of Tresp in these co-cultures was expected to inversely correlate to the suppressive function of Tregs. The proliferation index of cells at all Tresp:Treg ratios were compared to the maximum proliferation of Tresp (i.e., Tresp cells cultured alone, without the influence of Treg). As expected, the proliferation of co-cultures containing Tregs isolated from naïve, untreated and Ad-Control mice were inhibited proportionally to the number of Tregs in the co-cultures. (Fig. 3b; supplemental Fig. 1A). In contrast, Tregs isolated from Ad-LIGHT treated mice were incapable of suppressing the proliferation of Tresp; maximum proliferation was observed at all Tresp:Treg ratios from 1:1 to 8:1 indicating that Tregs isolated from Ad-LIGHT treated mice had lost their suppressive capacity.
LIGHT expression and adenovirus-vector alters the suppressive tumor microenvironment to a pro-inflammatory setting
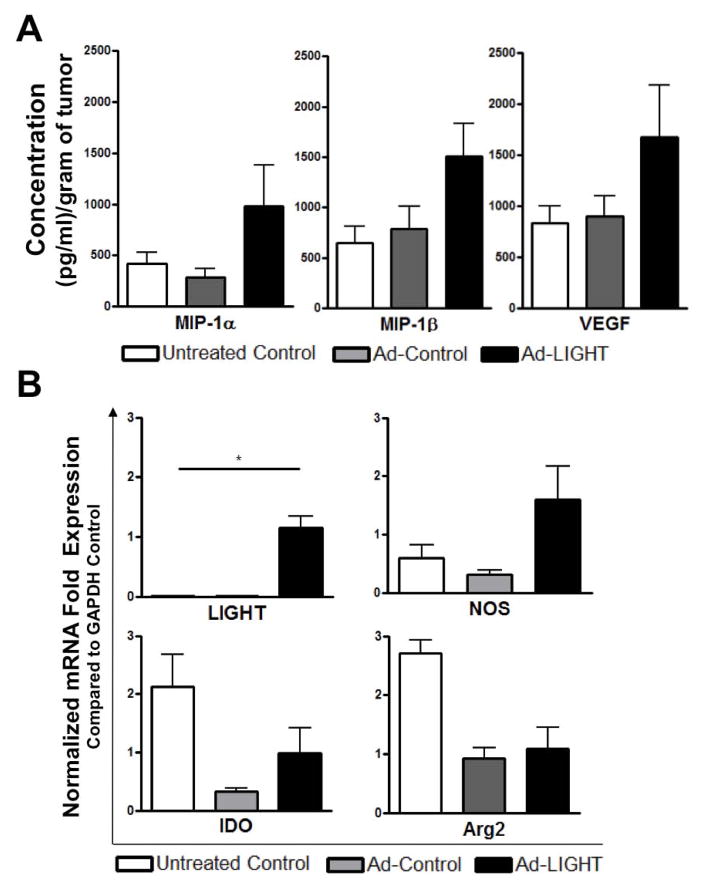
Next, we wanted to determine the mRNA expression of several immunosuppressive and tumor-promoting factors including nitric oxide synthase (NOS), indolamine 2,3-dioxygenase (IDO) and arginase-2 (Arg-2) in treated tumors. Tumors from Ad-LIGHT or Ad-control treated mice were isolated one week after treatment and RNA isolated to analyze gene expression in the tumors. First we wanted to confirm that the presence of LIGHT expression was only evident within LIGHT treated tumors with the relative expression of LIGHT mRNA normalized to GAPDH. As expected, LIGHT expression was only present within LIGHT treated tumors while untreated and vector-control treated animals showed no expression of LIGHT (Fig. 4). Relative mRNA expression of NOS, normalized to GAPDH, was increased in LIGHT treated mice compared to untreated and vector control. The relative mRNA levels of both IDO and Arg-2 also were decreased in both LIGHT and adenovirus-vector control groups, suggesting an adenovirus-vector effect. As a result, we see a reduction in immunosuppressive/tumor-promoting factors (Arg2, IDO) due to this adenovirus-vector effect while an increase in the anti-tumoral factor NOS is enhanced upon LIGHT expression.
Fig. 4.
Expression of intratumoral LIGHT alters gene expression in the tumor microenvironment. Tumors were isolated from TRAMP-C2 challenged mice that were given Ad-Control, Ad-LIGHT or left untreated. Various gene were examined by RT-qPCR including LIGHT, NOS, IDO and Arg2. Shown is the relative gene expression ± S.D. (Two-way ANOVA, *p<0.05) All experiments were repeated once and representative data are shown.
Ad-LIGHT expression in prostate tumor shows an increased trend in pro-inflammatory chemokines
We evaluated the change in tumor milieu by assessing chemokine and cytokine levels in untreated, Ad-Control and Ad-LIGHT treated tumors. Tumors lysates from treated mice were prepared and analyzed using a 22-plex chemokine and cytokine ELISA. Macrophage inflammatory protein (MIP)-1α, MIP-1β, and vascular endothelial growth factor (VEGF) showed an increased trend in Ad-LIGHT treated tumors (Fig. 5). All other analytes tested (GM-CSF, IFN-γ, IL-1α, IL1β, IL-2, IL-4, IL-5, IL-6, IL-9, IL-10, IL-12(p70), IL-13, IL-15, IL-17, KC, MCP-1, M-CSF, MIP-2, TNF-α) were equivalent between treatment groups (data not shown). These results suggest that LIGHT skews the tumor microenvironment from an immunosuppressive setting to an immune-stimulating milieu.
Fig. 5.
Ad-LIGHT treatment modulates the tumor microenvironment to an immunostimulatory environment. There is an increase trend in concentration of MIP-1α, MIP-1β and VEGF. MIP-1α is statistically higher in Ad-LIGHT treated groups when compared to combined controls of untreated and Ad-Control (student t test, p=0.46).
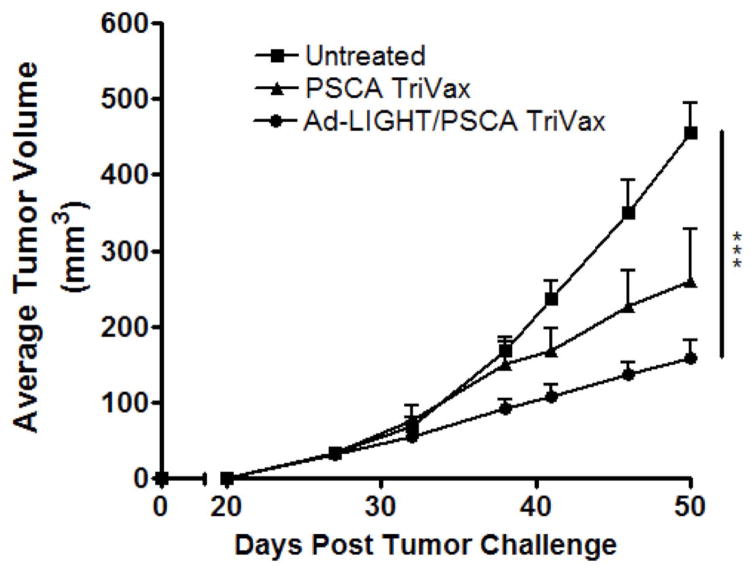
Synergistic therapeutic approach with Ad-LIGHT and PSCA TriVax reduces tumor burden
One of the goals of this study was to evaluate the synergistic approach of using Ad-LIGHT in conjunction with a therapeutic prostate cancer vaccine, PSCA TriVax, and determine the effects on tumor burden. Three groups of C57BL/6 mice were challenged and then treated as follows: Ad-LIGHT with PSCA TriVax, PSCA TriVax, or untreated. When compared to untreated and PSCA TriVax, Ad-LIGHT with PSCA TriVax showed a sustained reduction in tumor burden throughout day 50 (Fig. 6). These results indicate that LIGHT and PSCA TriVax treatment acted synergistically in reducing tumor burden. Ad-LIGHT treatment alone was not significantly different from the untreated control (Supplemental Fig. 1B).
Fig. 6.
Ad-LIGHT and mPSCA TriVax therapeutic vaccination delays TRAMP-C2 tumor growth. Mice were first treated with two doses of Ad-LIGHT (or Ad-control) prior to receiving mPSCA TriVax. 2 weeks post treatment, animals whom received Ad-LIGHT followed by mPSCA TriVax showed a delay in tumor growth. (Two-way ANOVA on single time-point, ***p<0.001). All experiments were repeated once and representative data are shown.
Ad-LIGHT synergizes with PSCA TriVax by increasing TIL
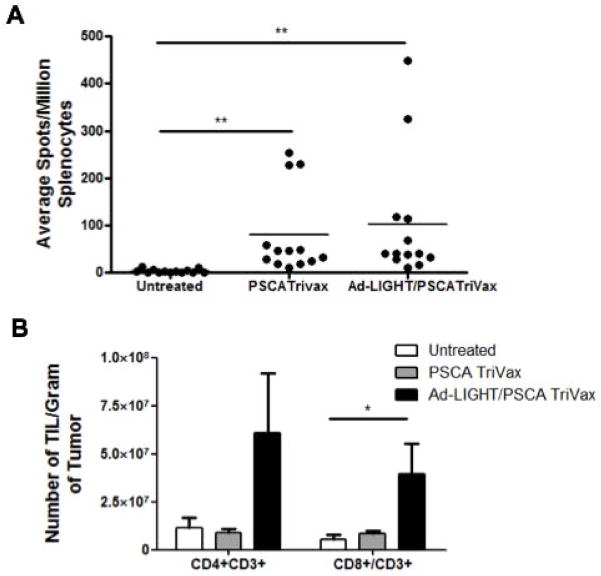
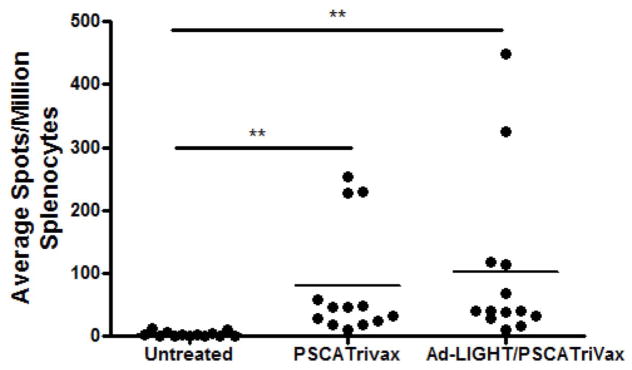
We hypothesized that the observed synergy between LIGHT treatment and PSCA TriVax vaccination in reducing tumor burden could be the result of two possible mechanisms of action. The first is that Ad-LIGHT may directly induce more TAA specific lymphocytes (as previously observed in a different tumor model (21)). The second is that LIGHT expression in the tumor recruits more TILs into the microenvironment; when combined with vaccination a greater proportion of TILs will be recruited. To investigate this, three groups of C57BL/6 mice challenged subcutaneously with TRAMP-C2 cells were treated with either PSCA TriVax, or Ad-LIGHT with PSCA TriVax, or were left untreated. Spleens were isolated one week after the last treatment and splenocytes were tested in an IFN-γ ELISpot assay with peptide PSCA83-91 to enumerate PSCA-specific T cells. Combination treatment with Ad-LIGHT and PSCA TriVax showed a non-statistical increase in the number of PSCA-specific IFN-γ secreting T cells induced compared to PSCA TriVax alone (Fig. 7a). This suggests that expression of LIGHT in the tumor enhances vaccine-induced TAA-specific T cells. Ad-LIGHT treatment alone did not induce TAA specific T cells (Supplemental Fig. 1C).
Fig. 7.
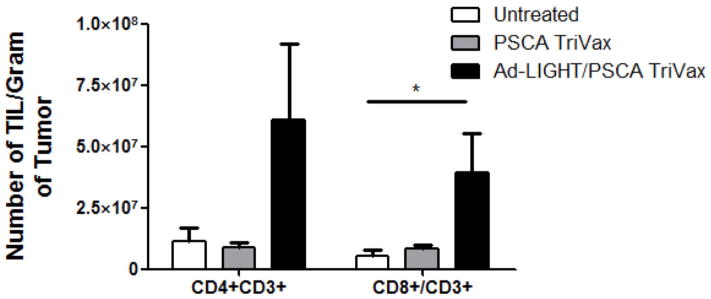
Intratumoral LIGHT treatment in combination with PSCA TriVax shows an increased number of CD4+ and CD8+ in a prostate cancer tumor model. (A) Combined Ad-LIGHT and PSCA TriVax vaccination shows an increased trend in TAA specific T cells as compared to PSCA TriVax alone but was not significantly different. (B) Expression of LIGHT in the tumor microenvironment recruited CD4+/CD3+ and CD8+/CD3+ T cells into the tumor microenvironment of PSCA TriVax vaccinated mice. Representative data are shown from two experiments. (*p<0.05, **p<0.01, one-way ANOVA).
Although the expression of intratumoral LIGHT and PSCA TriVax contributed a slight increase in frequency of PSCA-specific T cells in the periphery, we investigated the frequency of TIL in each treatment group. C57BL/6 mice were challenged and treated with PSCA TriVax or Ad-LIGHT with PSCA TriVax or left untreated to determine the frequency of TIL. The combination of Ad-LIGHT with PSCA TriVax resulted in an influx of TIL as compared to PSCA TriVax alone (Fig. 7b). The number of CD8+ T cells per gram of tumor was significantly higher in the Ad-LIGHT with PSCA TriVax group, while there was a trend towards increased numbers of infiltrating CD4+CD3+ T cells. Therefore, it appears that the mechanism of action underlying the synergy between intratumoral LIGHT expression with PSCA TriVax vaccination is based upon LIGHT’s ability to recruit TIL into the tumor microenvironment.
Discussion
Although immunotherapeutic vaccines against cancers are promising, these treatments often fail to elicit an effective response that results in tumor regression due to active immunosuppression within the prostate tumor microenvironment, stressing the need for concomitant treatment with agents that can overcome immunosuppression. The identification of such agents to utilize in therapeutic modalities is crucial for increasing TAA specific T cell responses, while simultaneously reducing tumor immunosuppression so as to reduce tumor burden and ultimately improve prostate cancer patient survival. With that goal in mind, we evaluated the role of LIGHT as a single agent treatment and concomitantly with immunotherapeutic vaccine by performing intratumoral injections of Ad-LIGHT in TRAMP-C2 challenged mice to recapitulate LIGHT lymphogenesis in the tumor microenvironment. Intratumoral LIGHT expression altered the suppressive tumor milieu and supported our hypothesis that LIGHT synergizes with PSCA TriVax vaccination to improve therapeutic efficacy by converting the tumor microenvironment from immunosuppressive to immunostimulatory.
In this study, LIGHT displayed a two pronged effect that highlighted its role as a possible adjuvant. LIGHT expression recruited a significantly higher frequency of TIL into the tumor microenvironment than untreated tumors. As supported by the literature, LIGHT protein, a 29 KDa homotrimer, has been shown to recruit lymphocytes through chemokine signaling via CXCR4/CCL21 in stromal cells and acts as a co-stimulatory molecule when engaging the HVEM receptor on T cells (25–27), a potential mechanism in our prostate cancer model system. Forced LIGHT expression increased the Teff:Treg ratio, indicative of a good prognosis since infiltrating lymphocytes are necessary for tumor regression. LIGHT’s value lies in its ability to recruit CD8+ T cells. In contrast to the HPV 16 tumor model where tumor antigen specific T lymphocytes were induced by intratumoral Ad-LIGHT injections, the TRAMP-C2 challenged model did not generate antigen specific T lymphocytes in the periphery with Ad-LIGHT. HPV 16 tumors have foreign tumor associated antigens for which there is no tolerance; this provides an advantage in immunotherapy where higher frequencies of T cell responses can be generated. This phenomenon suggests that Ad-LIGHT alone may not break tolerance to self antigens but acts to remodel the tumor microenvironment through alterations in cytokines and chemokines and patterns of infiltrating lymphocytes.
In contrast to other adjuvants that merely enhance antigen-specific immune responses, our data suggest that LIGHT targets the Treg mediated immunosuppressive pathway. Clinical outcome of immunotherapeutic treatments is tightly controlled by the balance between co-stimulatory and inhibitory signals. Traditional therapeutic vaccines induce T cells with antigen specificity but fail to target the inhibitory pathways in cancers, leading to an immune imbalance that favors tumor growth. As shown in this study, LIGHT has the ability to prevent the maturation of naïve T cells to Tregs and infiltration of Tregs into the tumor microenvironment, thereby derailing the suppressive effects of Tregs that promote tumor growth. Although TRAMP-C2 cells alone in the Treg induction assay reduced the frequency of maturated Tregs to similar extent as the negative control, TRAMP-C2 tumor cells express TGF-β receptors that are known to sequester TGF-β. This occurrence likely reduced the free TGF-β in culture that is used to drive Treg maturation, therefore a lower frequency of Tregs was detected in TRAMP-C2 cultures. The addition of LIGHT expressing TRAMP-C2 cells demonstrated a more pronounced effect in Treg induction, indicating that the effects of LIGHT may directly counteract Treg inducing factors with positive co-stimulation, explaining the lower frequency of Tregs. Mechanisms described in the literature support a role for LIGHT in compromising Treg function (17). LIGHT expression has been shown to play a dominating role in preventing the immunosuppressive interaction between HVEM and BTLA, which has been shown to inhibit T cell activation and enhance Treg mediated immunosuppression (28). Studies have indicated the interaction between LIGHT-HVEM and HVEM-BTLA play opposing roles in the tumor microenvironment. This phenomenon has been coined as the “molecular switch” of T cells, where LIGHT functions to activate T cells whereas BTLA inhibits this activation mechanism (29). The HVEM receptor has 3 cysteine rich domains (CRD); LIGHT has been shown to occupy CRD 2 and 3 while BTLA occupies CRD 1 (30,31). Due to a higher binding avidity and affinity, LIGHT is capable of dislodging the inhibitor interaction between HVEM-BLTA (20,32), indicating LIGHT’s potential in providing a positive effect of costimulation and T cell recruitment when bound to HVEM. As seen in our results, intratumoral forced LIGHT expression recruited T cells and reduced Treg mediated immunosuppression by reducing the Treg frequency and tumor mediated immunosuppression.
In contrast to our study, a previous group examining the effects of LIGHT on Tregs by Wang et al. showed that LIGHT does not affect the suppressive properties of Tregs in inflammatory bowel disease (IBD) (33). Wang et al. demonstrated that LIGHT expression in IBD propagated Treg expansion but had no effect on their suppressive capacity. Quite interestingly, IBD and tumors have opposing microenvironments; classically IBD shows heighted immune activation while tumors have immunosuppressed microenvironments and reduced immune activation (33,34). Therefore, differences in the disease model, as well as the inflammatory milieu versus the suppressive microenvironment, suggest that LIGHT expression may either activate or inhibit Treg immunosuppression based on the pathophysiology of the disease. In our prostate cancer model, intratumoral LIGHT counteracts the suppressive milieu by reducing suppressive function of Tregs and maturation of naïve T cells into Tregs.
The effects of intratumoral LIGHT in recruiting immune cells and overcoming immune suppression were striking; however, the adenovirus vector alone also produced intriguing results. Tumors treated with Ad-LIGHT displayed an increase in NOS expression as compared to vector control or untreated, and NOS has been shown to be cytotoxic to tumor cells at increased levels (35–38). It was also observed that Arg-2 and IDO, immunosuppressive and anti-tumoral factors associated with poor prognosis (39,40), were reduced in both Ad-LIGHT and Ad-Control treated tumors. IDO has become a gene of interest because expansion of Tregs has previously been shown to be induced in the presence of IDO, leading to immune tolerance against TAA (40). Arg-2 is known to be highly elevated in cancer patients with aggressive tumors (39,41,42); therefore, reduction in both IDO and Arg-2 expression can potentiate a better prognosis. Although we saw a reduction in Arg-2 and IDO in the Ad-LIGHT treated group, we conclude this is an adenovirus-mediated effect because vector controls also displayed a decreased expression of Arg-2 and IDO. Despite this adjuvant-like effect of adenovirus alone, our results show that LIGHT expression increases anti-tumor immunity, particularly with respect to lymphocyte infiltration.
Similar to a previous study in an HPV 16 induced-cervical cancer model (21), we show that LIGHT expression in prostate tumors resulted in an increased trend in immunostimulatory chemokines, MIP-1α and MIP-1α. MIP’s are directly involved with migration and activation of lymphocytes (43,44), potentially explaining the increased trafficking of T lymphocytes to the LIGHT expressing prostate tumors. Remarkably, VEGF, a pro-tumoral factor shows a trend to be increased in LIGHT-expressing prostate tumors. This coincides with the HPV 16 induced-cervical cancer model (21) where VEGF was also increased after LIGHT treatment. Although VEGF is known to associate with a poor prognosis in patients due to tumor angiogenesis (45), we have yet to determine whether VEGF is a negative regulator in LIGHT-expressing tumors. In a wound healing study, LIGHT has been demonstrated to promote macrophage apoptosis through VEGF expression, a process that is crucial for the resolution of inflammation (46). Likewise in our study, VEGF may have been up-regulated to resolve inflammation and further control tumor homeostasis.
Our next goal was to explore LIGHT in combination with an established therapeutic vaccine, PSCA-TriVax. PSCA-TriVax treatment is a three-component cocktail that elicits cytotoxic CD8+ T cell response against PSCA, a known prostate TAA. This cocktail contains the TAA peptide and two antigen presenting cell stimulators, anti-CD40 mAb and Poly-ICLC (47). In this study, the PSCA TriVax vaccine consisted of mouse PSCA83-91 peptide, anti-CD40 mAb and Poly-ICLC, a combination that has been demonstrated by our lab to result in tumor-free survival when used as a vaccine 20 days post TRAMP-C2 tumor challenge (unpublished data). We explored Ad-LIGHT with PSCA TriVax in vivo and demonstrated a reduced tumor burden throughout day 50 when compared to untreated or PSCA TriVax alone. Accurate survival statistics (e.g. log-rank survival curves) were hindered by the consistent occurrence of ulcerations, necessitating euthanasia prior to the endpoint of maximum tumor volume. Nevertheless, the tumor growth curves by themselves demonstrated reduced tumor burden, and can be used to establish the efficacy of LIGHT in combination with PSCA TriVax. Although LIGHT in combination with PSCA TriVax did not increase the frequency of TAA specific T cells in the periphery above that induced by PSCA TriVax alone, our data show that the significant increase of tumor-infiltrating cytotoxic CD8+T cells is mediated by forced expression of LIGHT in the tumors. Taken together, our data showing that the frequency of effector T cells that are recruited into the tumor is increased and that simultaneously Treg function is reduced upon expression of intratumoral LIGHT provides support for treating prostate cancer with a LIGHT and PSCA TriVax combination in order to increase anti-tumor efficacy. A recent study by Perret et al. demonstrate a similar increase in Teff:Treg ratio when poly-ICLC was used to influence tumor immunity (48). With the addition of LIGHT in our study, we have further enhanced the Teff:Treg ratio to favor anti-tumor immunity. LIGHT has previously been demonstrated in other settings to synergize with anti-CD40 ligand, a component in the PSCA Trivax, to enhance dendritic cells activation of cytotoxic T lymphocytes (49). Along those lines, LIGHT may potentially synergize with anti-CD40 mAb by enhancing PSCA TriVax mediated induction of TAA specific T cells.
Conclusion
In summary, our results provide evidence that the use of LIGHT is beneficial in directing the tumor microenvironment in becoming an immunostimulatory setting with increased chemokine signaling and infiltrating effector T lymphocytes. LIGHT treatment alone reduced Treg mediated immune suppression. In addition, LIGHT contributed to the effectiveness of a therapeutic vaccine by recruiting a vast number of effector T lymphocytes to the tumor microenvironment and subsequently reducing tumor burden. We show here that for the prostate cancer model, LIGHT makes an excellent adjuvant for a good therapeutic vaccine. Therefore, the use of LIGHT may be advantageous for the successful application of future therapeutic vaccinations in prostate and other cancers.
Supplementary Material
(A) Proliferation of Tresp co-cultured with Tregs isolated from untreated and naïve C57BL/6 mice show an increased proliferation with increased ratio of Tresp:Treg. (B) Tumor growth curve of untreated, Ad-Control and Ad-LIGHT treated tumor bearing mice. Tumor volumes were not significantly different from each treatment group, suggesting that Ad-LIGHT as a standalone treatment is not powerful enough in a non-immunogenic prostate cancer setting to induce tumor regression. (C) IFN-g ELISpot assay show that untreated, Ad-Control, and Ad-LIGHT do not induce TAA specific T cells against PSCA83-91 peptide.
Acknowledgments
This research was supported by Department of Defense grant PC100519 (to W.M.K). Contributions from the Karl H. and Ruth M. Balz Trust are also gratefully acknowledged. Poly-ICLC was a generous gift from Dr. Andres Salazar (Oncovir). W. Martin Kast holds the Walter A. Richter cancer researcher chair. Lisa Yan is a TL1 scholar and supported by SC CTSI (NIH/NCCR/CATS) grant #TL1TR000132. Bhavna Verma is supported by a Roche post-doctoral fellowship. We thank ASM Scientific Writing and Publishing Institute for critical reading of the manuscript. Elispot assays were run with the assistance of the USC Norris Comprehensive Cancer Center Beckman Center for Immune Monitoring. The project described was supported in part by award number P30CA014089 from the National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. International journal of cancer Journal international du cancer. 2010;127(12):2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Sivarajan G, Prabhu V, Taksler GB, Laze J, Lepor HL. Ten-year Outcomes of Sexual Function After Radical Prostatectomy: Results of a Prospective Longitudinal Study. European urology. 2013 doi: 10.1016/j.eururo.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 3.Ratcliff CG, Cohen L, Pettaway CA, Parker PA. Treatment regret and quality of life following radical prostatectomy. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2013 doi: 10.1007/s00520-013-1906-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Azzouni F, Mohler J. Biology of castration-recurrent prostate cancer. The Urologic clinics of North America. 2012;39(4):435–452. doi: 10.1016/j.ucl.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 5.Zhang S, Wang Y, Chen Z, Kim S, Iqbal S, Chi A, Ritenour C, Wang YA, Kucuk O, Wu D. Genistein enhances the efficacy of cabazitaxel chemotherapy in metastatic castration-resistant prostate cancer cells. The Prostate. 2013 doi: 10.1002/pros.22705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yin LN, Hu QZ, Hartmann RW. Recent Progress in Pharmaceutical Therapies for Castration-Resistant Prostate Cancer. Int J Mol Sci. 2013;14(7):13958–13978. doi: 10.3390/ijms140713958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.NCCN Guidelines(R) Updates. Journal of the National Comprehensive Cancer Network : JNCCN. 2013;11(8):xxix–xxxiv. doi: 10.6004/jnccn.2013.0113. [DOI] [PubMed] [Google Scholar]
- 8.Snoeks LL, Ogilvie AC, van Haarst EP, Siegert CE. New treatment options for patients with metastatic prostate cancer. The Netherlands journal of medicine. 2013;71(6):290–294. [PubMed] [Google Scholar]
- 9.Schellhammer PF, Chodak G, Whitmore JB, Sims R, Frohlich MW, Kantoff PW. Lower Baseline Prostate-specific Antigen Is Associated With a Greater Overall Survival Benefit From Sipuleucel-T in the Immunotherapy for Prostate Adenocarcinoma Treatment (IMPACT) Trial. Urology. 2013;81(6):1297–1302. doi: 10.1016/j.urology.2013.01.061. [DOI] [PubMed] [Google Scholar]
- 10.O’Lorcain P, Comber H. Prostate cancer mortality predictions for Ireland up to 2015. Eur J Cancer Prev. 2007;16(4):328–333. doi: 10.1097/01.cej.0000236248.63489.4c. [DOI] [PubMed] [Google Scholar]
- 11.Center MM, Jemal A, Lortet-Tieulent J, Ward E, Ferlay J, Brawley O, Bray F. International variation in prostate cancer incidence and mortality rates. European urology. 2012;61(6):1079–1092. doi: 10.1016/j.eururo.2012.02.054. [DOI] [PubMed] [Google Scholar]
- 12.Perez O, Batista-Duharte A, Gonzalez E, Zayas C, Balboa J, Cuello M, Cabrera O, Lastre M, Schijns VE. Human prophylactic vaccine adjuvants and their determinant role in new vaccine formulations. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas/Sociedade Brasileira de Biofisica [et al] 2012;45(8):681–692. doi: 10.1590/S0100-879X2012007500067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pardoll D. New strategies for active immunotherapy with genetically engineered tumor cells. Current opinion in immunology. 1992;4(5):619–623. doi: 10.1016/0952-7915(92)90037-f. [DOI] [PubMed] [Google Scholar]
- 14.Lasaro MO, Ertl HC. Targeting inhibitory pathways in cancer immunotherapy. Current opinion in immunology. 2010;22(3):385–390. doi: 10.1016/j.coi.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller AM, Pisa P. Tumor escape mechanisms in prostate cancer. Cancer immunology, immunotherapy : CII. 2007;56(1):81–87. doi: 10.1007/s00262-005-0110-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang J, Lo JC, Foster A, Yu P, Chen HM, Wang Y, Tamada K, Chen L, Fu YX. The regulation of T cell homeostasis and autoimmunity by T cell-derived LIGHT. The Journal of clinical investigation. 2001;108(12):1771–1780. doi: 10.1172/JCI13827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tao R, Wang L, Murphy KM, Fraser CC, Hancock WW. Regulatory T cell expression of herpesvirus entry mediator suppresses the function of B and T lymphocyte attenuator-positive effector T cells. J Immunol. 2008;180(10):6649–6655. doi: 10.4049/jimmunol.180.10.6649. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Wang J, Sun Y, Wu Q, Fu YX. Complementary effects of TNF and lymphotoxin on the formation of germinal center and follicular dendritic cells. J Immunol. 2001;166(1):330–337. doi: 10.4049/jimmunol.166.1.330. [DOI] [PubMed] [Google Scholar]
- 19.Rennert PD, James D, Mackay F, Browning JL, Hochman PS. Lymph node genesis is induced by signaling through the lymphotoxin beta receptor. Immunity. 1998;9(1):71–79. doi: 10.1016/s1074-7613(00)80589-0. [DOI] [PubMed] [Google Scholar]
- 20.Cheung TC, Humphreys IR, Potter KG, Norris PS, Shumway HM, Tran BR, Patterson G, Jean-Jacques R, Yoon M, Spear PG, Murphy KM, Lurain NS, Benedict CA, Ware CF. Evolutionarily divergent herpesviruses modulate T cell activation by targeting the herpesvirus entry mediator cosignaling pathway. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(37):13218–13223. doi: 10.1073/pnas.0506172102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanodia S, Da Silva DM, Karamanukyan T, Bogaert L, Fu YX, Kast WM. Expression of LIGHT/TNFSF14 combined with vaccination against human papillomavirus Type 16 E7 induces significant tumor regression. Cancer research. 2010;70(10):3955–3964. doi: 10.1158/0008-5472.CAN-09-3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lizee G, Cantu MA, Hwu P. Less yin, more yang: confronting the barriers to cancer immunotherapy. Clinical cancer research : an official journal of the American Association for Cancer Research. 2007;13(18 Pt 1):5250–5255. doi: 10.1158/1078-0432.CCR-07-1722. [DOI] [PubMed] [Google Scholar]
- 23.de Garcia-Hernandez ML, Gray A, Hubby B, Klinger OJ, Kast WM. Prostate stem cell antigen vaccination induces a long-term protective immune response against prostate cancer in the absence of autoimmunity. Cancer research. 2008;68(3):861–869. doi: 10.1158/0008-5472.CAN-07-0445. [DOI] [PubMed] [Google Scholar]
- 24.Yu P, Lee Y, Liu W, Chin RK, Wang J, Wang Y, Schietinger A, Philip M, Schreiber H, Fu Y-X. Priming of naive T cells inside tumors leads to eradication of established tumors. Nat Immunol. 2004;5(2):141–149. doi: 10.1038/ni1029. [DOI] [PubMed] [Google Scholar]
- 25.Mauri DN, Ebner R, Montgomery RI, Kochel KD, Cheung TC, Yu GL, Ruben S, Murphy M, Eisenberg RJ, Cohen GH, Spear PG, Ware CF. LIGHT, a new member of the TNF superfamily, and lymphotoxin alpha are ligands for herpesvirus entry mediator. Immunity. 1998;8(1):21–30. doi: 10.1016/s1074-7613(00)80455-0. [DOI] [PubMed] [Google Scholar]
- 26.Mauri D, Ebner R, Montgomery R, Kochel K, Cheung TC, Yu GL, Ruben S, Murphy M, Eisenberg RJ, Cohen GH, Spear PG, Ware CF. LIGHT, a new pro-apoptotic cytokine member of the TNF superfamily, and lymphotoxin-alpha are ligands for the herpesvirus entry mediator (HVEM) Faseb J. 1998;12(4):A301–A301. doi: 10.1016/s1074-7613(00)80455-0. [DOI] [PubMed] [Google Scholar]
- 27.Ware CF, Butrovich KD, Mauri DN, Tillman J, Rooney I. Light, a new lymphotoxin-related cytokine that engages the LT beta receptor and herpesvirus entry mediator (HVEM) Eur Cytokine Netw. 1998;9(3):358–358. [Google Scholar]
- 28.Sedy JR, Gavrieli M, Potter KG, Hurchla MA, Lindsley RC, Hildner K, Scheu S, Pfeffer K, Ware CF, Murphy TL, Murphy KM. B and T lymphocyte attenuator regulates T cell activation through interaction with herpesvirus entry mediator. Nat Immunol. 2005;6(1):90–98. doi: 10.1038/ni1144. [DOI] [PubMed] [Google Scholar]
- 29.Ware CF. Targeting the LIGHT-HVEM pathway. Advances in experimental medicine and biology. 2009;647:146–155. doi: 10.1007/978-0-387-89520-8_10. [DOI] [PubMed] [Google Scholar]
- 30.Sedy JR, Gavrieli M, Potter KG, Hurchla MA, Lindsley RC, Hildner K, Scheu S, Pfeffer K, Ware CF, Murphy TL, Murphy KM. B and T lymphocyte attenuator regulates T cell activation through interaction with herpesvirus entry mediator. Nature Immunology. 2005;6(1):90–98. doi: 10.1038/ni1144. [DOI] [PubMed] [Google Scholar]
- 31.Gonzalez LC, Loyet KM, Calemine-Fenaux J, Chauhan V, Wranik B, Ouyang W, Eaton DL. A coreceptor interaction between the CD28 and TNF receptor family members B and T lymphocyte attenuator and herpesvirus entry mediator. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(4):1116–1121. doi: 10.1073/pnas.0409071102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kinkade A, Ware CF. The DARC conspiracy--virus invasion tactics. Trends Immunol. 2006;27(8):362–367. doi: 10.1016/j.it.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 33.Wang Y, Zhu M, Yu P, Fu YX. Promoting immune responses by LIGHT in the face of abundant regulatory T cell inhibition. J Immunol. 2010;184(3):1589–1595. doi: 10.4049/jimmunol.0901582. [DOI] [PubMed] [Google Scholar]
- 34.Miller AM, Lundberg K, Ozenci V, Banham AH, Hellstrom M, Egevad L, Pisa P. CD4+CD25high T cells are enriched in the tumor and peripheral blood of prostate cancer patients. J Immunol. 2006;177(10):7398–7405. doi: 10.4049/jimmunol.177.10.7398. [DOI] [PubMed] [Google Scholar]
- 35.Xu W, Liu LZ, Loizidou M, Ahmed M, Charles IG. The role of nitric oxide in cancer. Cell research. 2002;12(5–6):311–320. doi: 10.1038/sj.cr.7290133. [DOI] [PubMed] [Google Scholar]
- 36.Juang SH, Xie KP, Xu L, Shi Q, Wang YF, Yoneda JY, Fidler IJ. Suppression of tumorigenicity and metastasis of human renal carcinoma cells by infection with retroviral vectors harboring the murine inducible nitric oxide synthase gene. Hum Gene Ther. 1998;9(6):845–854. doi: 10.1089/hum.1998.9.6-845. [DOI] [PubMed] [Google Scholar]
- 37.Juang SH, Xie KP, Xu L, Wang YF, Yoneda JY, Fidler IJ. Use of retroviral vectors encoding murine inducible nitric oxide synthase gene to suppress tumorigenicity and cancer metastasis of murine melanoma. Cancer Biother Radio. 1997;12(3):167–175. doi: 10.1089/cbr.1997.12.167. [DOI] [PubMed] [Google Scholar]
- 38.Xie KP, Huang SY, Dong ZY, Juang SH, Gutman M, Xie QW, Nathan C, Fidler IJ. Transfection with the Inducible Nitric-Oxide Synthase Gene Suppresses Tumorigenicity and Abrogates Metastasis by K-1735 Murine Melanoma-Cells. J Exp Med. 1995;181(4):1333–1343. doi: 10.1084/jem.181.4.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sousa MSA, Latini FRM, Monteiro HP, Cerutti JM. Arginase 2 and nitric oxide synthase: Pathways associated with the pathogenesis of thyroid tumors. Free Radical Bio Med. 2010;49(6):997–1007. doi: 10.1016/j.freeradbiomed.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 40.Curran TA, Jalili RB, Farrokhi A, Ghahary A. IDO expressing fibroblasts promote the expansion of antigen specific regulatory T cells. Immunobiology. 2014;219(1):17–24. doi: 10.1016/j.imbio.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 41.Mumenthaler SM, Yu H, Tze S, Cederbaum SD, Pegg AE, Seligson DB, Grody WW. Expression of arginase II in prostate cancer. International journal of oncology. 2008;32(2):357–365. [PubMed] [Google Scholar]
- 42.Ino Y, Yamazaki-Itoh R, Oguro S, Shimada K, Kosuge T, Zavada J, Kanai Y, Hiraoka N. Arginase II Expressed in Cancer-Associated Fibroblasts Indicates Tissue Hypoxia and Predicts Poor Outcome in Patients with Pancreatic Cancer. PLoS ONE. 2013;8(2) doi: 10.1371/journal.pone.0055146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taub DD, Conlon K, Lloyd AR, Oppenheim JJ, Kelvin DJ. Preferential migration of activated CD4+ and CD8+ T cells in response to MIP-1 alpha and MIP-1 beta. Science. 1993;260(5106):355–358. doi: 10.1126/science.7682337. [DOI] [PubMed] [Google Scholar]
- 44.Taub DD, Lloyd AR, Wang JM, Oppenheim JJ, Kelvin DJ. The effects of human recombinant MIP-1 alpha, MIP-1 beta, and RANTES on the chemotaxis and adhesion of T cell subsets. Advances in experimental medicine and biology. 1993;351:139–146. doi: 10.1007/978-1-4615-2952-1_15. [DOI] [PubMed] [Google Scholar]
- 45.George DJ, Halabi S, Shepard TF, Vogelzang NJ, Hayes DF, Small EJ, Kantoff PW. Prognostic significance of plasma vascular endothelial growth factor levels in patients with hormone-refractory prostate cancer treated on Cancer and Leukemia Group B 9480. Clinical cancer research : an official journal of the American Association for Cancer Research. 2001;7(7):1932–1936. [PubMed] [Google Scholar]
- 46.Petreaca ML, Yao M, Ware C, Martins-Green MM. Vascular endothelial growth factor promotes macrophage apoptosis through stimulation of tumor necrosis factor superfamily member 14 (TNFSF14/LIGHT) Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2008;16(5):602–614. doi: 10.1111/j.1524-475X.2008.00411.x. [DOI] [PubMed] [Google Scholar]
- 47.Cho HI, Celis E. Optimized peptide vaccines eliciting extensive CD8 T-cell responses with therapeutic antitumor effects. Cancer research. 2009;69(23):9012–9019. doi: 10.1158/0008-5472.CAN-09-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Perret R, Sierro SR, Botelho NK, Corgnac S, Donda A, Romero P. Adjuvants that improve the ratio of antigen-specific effector to regulatory T cells enhance tumor immunity. Cancer research. 2013;73(22):6597–6608. doi: 10.1158/0008-5472.CAN-13-0875. [DOI] [PubMed] [Google Scholar]
- 49.Morel Y, Truneh A, Sweet RW, Olive D, Costello RT. The TNF superfamily members LIGHT and CD154 (CD40 ligand) costimulate induction of dendritic cell maturation and elicit specific CTL activity. J Immunol. 2001;167(5):2479–2486. doi: 10.4049/jimmunol.167.5.2479. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Proliferation of Tresp co-cultured with Tregs isolated from untreated and naïve C57BL/6 mice show an increased proliferation with increased ratio of Tresp:Treg. (B) Tumor growth curve of untreated, Ad-Control and Ad-LIGHT treated tumor bearing mice. Tumor volumes were not significantly different from each treatment group, suggesting that Ad-LIGHT as a standalone treatment is not powerful enough in a non-immunogenic prostate cancer setting to induce tumor regression. (C) IFN-g ELISpot assay show that untreated, Ad-Control, and Ad-LIGHT do not induce TAA specific T cells against PSCA83-91 peptide.