Abstract
Background
Neurodegeneration plays an important role in permanent disability in multiple sclerosis (MS).
Objective
To determine whether progressive neurodegeneration occurs in MS eyes without clinically-evident inflammation.
Methods
Retinal nerve fiver layer thickness (RNFLT) and ganglion cell-inner plexiform layer thickness (GCIPT) were measured using Cirrus optical coherence tomography (OCT) in 133 relapsing-remitting MS (RRMS) patients (149 no-ON, 97 ON eyes, last optic neuritis (ON) ≥6 months). 93 patients were scanned at two visits. Percents of abnormal GCIPT vs RNFLT (<5% of machine norms) in cross-sectional data were compared. Relations between RNFLT/GCIPT and MS duration (cross-sectional) and follow-up time (longitudinal) were assessed.
Results
GCIPT was abnormal in more eyes than RNFLT (27% vs 16% p=0.004 in no-ON, 82% vs 72% p=0.007 in ON). RNFLT and GCIPT decreased with MS duration by −0.49 µm/yr (p=0.0001) and −0.36 (p=0.005) for no-ON; −0.52 (p=0.003) and −0.41 (p=0.007) for ON. RNFLT and GCIPT decreased with follow-up time by −1.49 µm/yr (p<0.0001) and −0.53 (p=0.004) for no-ON, −1.27 (p=0.002) and −0.49 (p=0.04) for ON.
Conclusions
In RRMS eyes without clinically-evident inflammation, progressive loss of RNFLT and GCIPT occurred, supporting the need for neuroprotection in addition to suppression of auto-immune responses and inflammation.
Introduction
Multiple sclerosis (MS), a chronic demyelinating and degenerative disease of the central nervous system (CNS), is the leading cause of non-traumatic neurological disability in young adults. The natural history of MS suggests that about 85% of patients initially experience a relapsing-remitting course (RRMS) and within 25 years of onset a high percentage transition into a secondary progressive phase (SPMS)1, 2 with continuous neurological decline leading to permanent disability.3 Currently available immuno-modulatory disease modifying therapies (DMT) have been successful in reducing inflammation, relapses4 and in slowing disease progression in RRMS.5 However, a recent study of MS patients with 15 years continuous use of an immunemodulatory drug reduced, but did not eliminate progression to SPMS.6 While it is generally agreed that permanent disability in MS is a consequence of irreversible axonal loss,3 the underlying causes of MS and progression of the disease remain unclear.
MS eyes provide a unique opportunity to study axonal degeneration. The retinal ganglion cells (RGC) and their naturally unmyelinated axons in the eye can be evaluated in vivo using spectral domain optical coherence tomography (OCT), a highly reproducible imaging technique. Inflammation of the optic nerve, i.e., optic neuritis (ON), is typically detectable with signs and symptoms such as eye pain, loss of vision, reduced color vision, swelling and presence of relative afferent pupillary defects.7 In this study, retinal nerve fiber layer thickness (RNFLT) and retinal ganglion cell-inner plexiform thickness (GCIPT) were measured in two groups of “clinically-silent” RRMS eyes: eyes without a history of ON (no-ON group) and those with a previous history of ON but the inflammatory event was at least 6 months prior to the onset of the present study (ON group). Separating the no-ON and ON groups allowed us to tease apart neurodegenerative effects that could be due to previous overt inflammatory episodes in ON eyes from those in no-ON eyes lacking a history of clinically-evident inflammation. The change of RNFLT and GCIPT over time was analyzed cross-sectionally and longitudinally.
Methods
Subjects
One hundred thirty-one RRMS patients8 from the University of Houston MS Eye CARE Clinic were included in the study. All patients underwent comprehensive eye examination by an experienced neuro-ophthalmologist. ON was diagnosed based on clinical signs and symptoms.7 To minimize the effect of edema and other sequelae of acute inflammation, eyes with last ON attack within 6 months of the OCT measurement or between the baseline and follow-up measurements were excluded. Patients with ocular or systemic conditions other than ON/MS that could potentially influence OCT measures were excluded.
Two hundred forty-seven eyes of 131 RRMS patients (85% on DMT) were included for cross-sectional analysis (Table 1). Seven eyes with acute ON, 3 with unclear ON history, 3 with other ocular abnormalities and 2 with OCT signal strength <7 were excluded. Twelve (7 no-ON and 5 ON) eyes did not have GCIPT. Among the 247 eyes, 241 had spherical equivalent refractive error (RE) less than −6.0D, 13 worse than −6.0D (range −6 to −15 D, median −7.5 D) and 6 eyes with unknown RE. Excluding these 19 eyes did not change the results reported below.
Table 1.
Demographic and clinical characteristics of RRMS patients in the cross-sectional analysis (n=131)
| Age (years, mean±SD, range) | 43.4±11.1, 20.7 to 69.9 | |
| F:M | 4.3:1 | |
| MS duration (years, mean±SD, range) | 8.5±8.0, <1 month to 32 | |
| No-ON eyes (n=149) | ON eyes (n= 98) | |
| VA 20/20 or better (%) | 140 (94%) | 62 (63%) |
| HVF * MD±SE (dB) | −1.3±0.2 | −3.2±0.5 |
| Mean RNFLT ** (µm) | 88.6±0.9 | 75.0±1.4 |
| Superior RNFLT ** (µm) | 112.1±1.5 | 93.4±2.0 |
| Nasal RNFLT ** (µm) | 67.2±0.9 | 61.2±1.1 |
| Inferior RNFLT ** (µm) | 117.9±1.6 | 99.1±2.5 |
| Temporal RNFLT ** (µm) | 56.9±1.1 | 47.1±1.4 |
| Mean GCIPT ** (µm) | 76.5±0.8 | 65.5±1.2 |
| 1 ON attack (%) | NA | 73 (75%) |
| >1 ON attack (%) | NA | 25 (25%) |
| Time since last ON (years, ±SD) | NA | 8.5 ± 8.0 |
HVF refers to Humphery visual field 30-2 or 24-2 SITA standard or SITA fast threshold tests with fixation loss, false positives and false negatives <33%.
mean±SE
Ninety-two RRMS patients (164 eyes) from the cross-sectional cohort had OCT at two different visits and were included for longitudinal analysis (Table 2). The time interval between the baseline and the follow-up visit ranged from 2 months to 3.8 years (median 1.0 years). For all eyes in the longitudinal cohort, OCT data from the second visit was reported in the cross-sectional analysis in order to include more eyes with longer duration of MS. Seven eyes with acute ON at the baseline, 5 with ON attack between two visits, 4 eyes with unclear ON history, 1 with other ocular abnormalities and 3 eyes with signal strength <7 in OCT in either of the visits were excluded. Fourteen (9 no-ON and 5 ON) eyes did not have GCIPT measured in one or both visits.
Table 2.
Demographic and clinical characteristics of RRMS patients in the longitudinal analysis (n=92)
| Baseline | Follow-up | |||
|---|---|---|---|---|
| Age (years, ±SD) | 42.5±11.8 | 43.9±11.9 | ||
| F:M | 4.4:1 | 4.4:1 | ||
| MS duration (years, ±SD) | 7.2±7.6 | 8.6±7.7 | ||
| No-ON eyes (n=96) | ON eyes (n=68) | |||
| Baseline | Follow-up | Baseline | Follow-up | |
| VA 20/20 or better (%) | 94 (98%) | 94 (98%) | 49 (72%) | 49 (72%) |
| HVF * MD±SE (dB) | −1.3±0.2 | −1.0±0.2 | −2.9±0.4 | −2.7±0.5 |
| Mean RNFLT ** (µm) | 90.7±1.2 | 89.4±1.2 | 75.1±2.3 | 74.4±1.8 |
| Superior RNFLT ** (µm) | 115.2±1.9 | 112.3±1.8 | 94.3±2.7 | 92.0±2.6 |
| Nasal RNFLT ** (µm) | 69.9±1.4 | 68.7±1.3 | 62.8±1.6 | 61.8±1.5 |
| Inferior RNFLT ** (µm) | 120.5±2.1 | 119.3±2.1 | 99.2±3.0 | 97.7±3.0 |
| Temporal RNFLT ** (µm) | 57.3±1.3 | 56.1±1.3 | 46.7±1.6 | 46.1±1.7 |
| Mean GCIPT ** (µm) | 77.5±1.0 | 76.8±1.0 | 65.1±1.6 | 64.9±1.4 |
| 1 ON attack (%) | NA | NA | 54 (79%) | 54 (79%) |
| >1 ON attack (%) | NA | NA | 14 (21%) | 14 (21%) |
| Time since last ON (years, ±SD) | NA | NA | 7.4±7.7 | 8.9±7.9 |
HVF refers to Humphery visual field 30-2 or 24-2 SITA standard or SITA fast threshold tests with fixation loss, false positives and false negatives <33%.
mean±SE
Procedures adhered to the tenets of Declaration of Helsinki, and the protocol was approved by the University of Houston Committee for the Protection of Human Subjects.
Optical Coherence Tomography
OCT was performed using Cirrus-HD OCT 4000 version 6.5 (Carl Zeiss Meditec, Inc., Dublin, CA) by a trained ophthalmic technician. Peripapillary RNFLT was acquired with the Optic Disc Cube 200 × 200 protocol that images the optic disc in a 6 mm × 6 mm region. The mean RNFLT and those from individual quadrants were obtained. Macular GCIPT was obtained using the Macular Cube 512 × 128 protocol that images a 6 mm × 6 mm area centered at the fovea. The GCIPT was derived automatically by the machine software over an elliptical annulus (2 mm × 2.4 mm radius), excluding the central foveal region (0.5 mm × 0.6 mm radius). In this paper, RNFLT and GCIPT refer to the overall mean unless a quadrant is specified. Only images with signal strength ≥7 and good centration were included. No OCT outputs had erroneous segmentation upon visual inspection.
Statistics
Statistical analyses were performed in SAS 9.2 (SAS Institute Inc., Cary, NC, USA). For the cross-sectional data, RNFLT and GCIPT were each defined as abnormal if the values were below 5% of their respective age-matched machine norms. The percent of eyes with abnormal RNFLT was compared to that of GCIPT using McNemar test. The relation between RNFLT/GCIPT and MS duration (cross-sectional data), RNFLT/GCIPT change and follow-up time (longitudinal data) were analyzed using the GEMOND procedure with generalized estimating equation (GEE) to account for age and intra-subject inter-eye correlation. The probability of an abnormal RNFLT and GCIPT as a function of MS duration was modeled using GEE logistic regression. P values less than 0.05 were considered statistically significant.
Results
Cross-sectional Analysis
Reduced RNFLT and GCIPT in no-ON and ON eyes
One hundred thirty-one RRMS patients (149 no-ON, 98 ON eyes) with MS duration ranging from <1 month to 32 years were included for cross-sectional analysis (Table 1). The mean (±SD, µm) RNFLT (75.0±14.0) and GCIPT (65.5±11.9) in ON were significantly thinner than in no-ON eyes (RNFLT: 88.6±11.5, GCIPT: 76.5±9.2, p<0.0001 for both). Both no-ON and ON eyes had significantly thinner measures compared to normative RNFLT (92.8±9.4 µm) and GCIPT (82.1±6.2 µm) for Cirrus OCT9 (p<0.0001 for all comparisons). Twenty-five percent of ON eyes (n=25) had more than one ON episode in the same eye (recurrent ON) and the mean RNFLT and GCIPT (µm) in these eyes were significantly thinner (67.1±10.9 and 59.4±11.9) than in eyes with single ON attacks (77.3±14.1 and 67.3±11.2, p < 0.001 for both).
Linear regression between RNFLT/GCIPT and MS duration
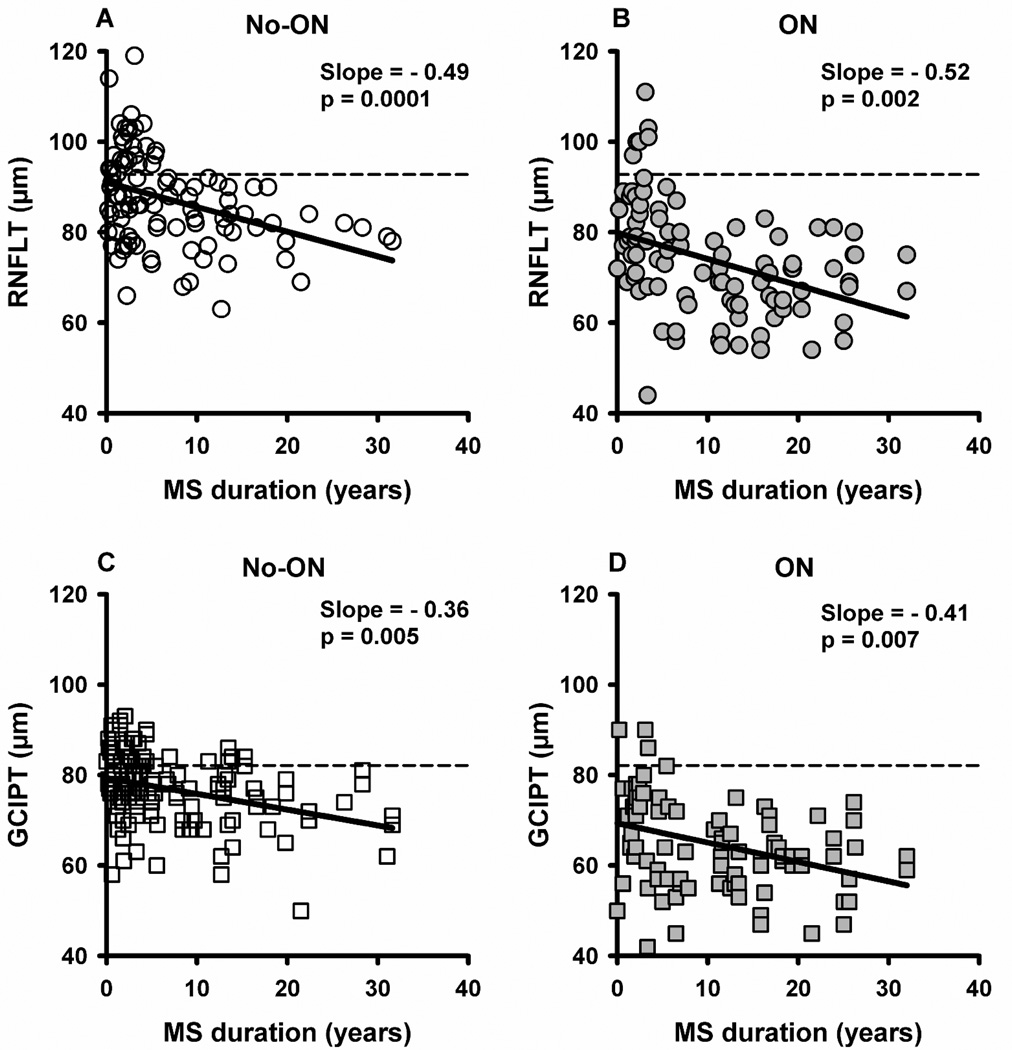
To determine the effects of long term MS on neuronal loss, we performed linear regression between RNFLT/GCIPT and MS duration using GEE model to correct for age and intra-subject inter-eye correlation (Figure 1). RNFLT decreased at a mean rate (µm/yr) of −0.49 (p=0.0001, Figure 1A) in no-ON and −0.52 (p=0.002, Figure 1B) in ON, and these two slopes were not significantly different (p=0.90). To examine whether RNFLT loss in no-ON eyes was influenced by an ON history in the fellow eye, we analyzed bilateral no-ON eyes (n=95) separately; which showed a slope of −0.37 µm/yr, not different from that for all no-ON eyes (p=0.56). In ON eyes, thinner RNFLT with longer MS might be partially attributed to more recurrent ON events over time. Interestingly, eyes with only one episode of ON (n=73) showed a slightly steeper slope (−0.88 µm/yr) than that of the whole ON group (−0.52 µm/yr) although they were not significantly different (p=0.17). RNFLT in individual quadrants also decreased with MS duration in no-ON and ON eyes (Table 3, cross-sectional data on the left). GCIPT decreased with MS duration at a mean rate (µm/yr) of −0.36 (p=0.005, Figure 1C) in no-ON and −0.41 (p=0.007, Figure 1D) in ON, and these two slopes were not different (p=0.81). GCIPT slope (µm/yr) for bilateral no-ON eyes was −0.33 (p=0.02) and single ON eyes was −0.67 (p<0.0001).
Figure 1.
Scatter plot showing RNFLT vs MS duration (A and B) and GCIPT vs MS duration (C and D) for individual no-ON and ON eyes. Each symbol (circles for RNFLT and squares for GCIPT) represents an individual eye of a patient. Open symbols represent no-ON and filled symbols represent ON eyes. The solid lines are the fitted linear regression lines (GEE models). The dashed lines are the average reported normative values for a cohort between 18 and 84 years of age.9
Table 3.
Slope and p values from linear regression for RNFLT by quadrant and GCIPT in the cross-sectional and longitudinal analysis (corrected for age and intrasubject inter eye correlation, GEE models)
| Cross-sectional | Longitudinal | |||
|---|---|---|---|---|
| Slope, µm/yr* | (p value) | Slope, µm/yr** | (p value) | |
| No-ON | ON | No-ON | ON | |
| Mean RNFLT | −0.49 (0.001) | −0.52 (0.002) | −1.49 (<0.001) | −1.27 (0.002) |
| Superior RNFLT | −0.62 (0.01) | −0.63 (0.01) | −2.18 (<0.001) | −2.47 (0.0006) |
| Nasal RNFLT | −0.24 (0.02) | −0.29 (0.01) | −1.58 (0.007) | −0.83 (0.23) |
| Inferior RNFLT | −0.54 (0.05) | −0.58 (0.05) | −1.41 (0.01) | −1.36 (0.01) |
| Temporal RNFLT | −0.58 (0.0001) | −0.54 (0.0008) | −1.54 (0.03) | −0.59 (0.15) |
| Mean GCIPT | −0.36 (0.005) | −0.41 (0.007) | −0.53 (0.004) | −0.49 (0.04) |
analysis of RNFLT/GCIPT vs MS duration
analysis of RNFLT/GCIPT vs Follow-up time
Abnormalities in GCIPT/RNFLT and logistic regression
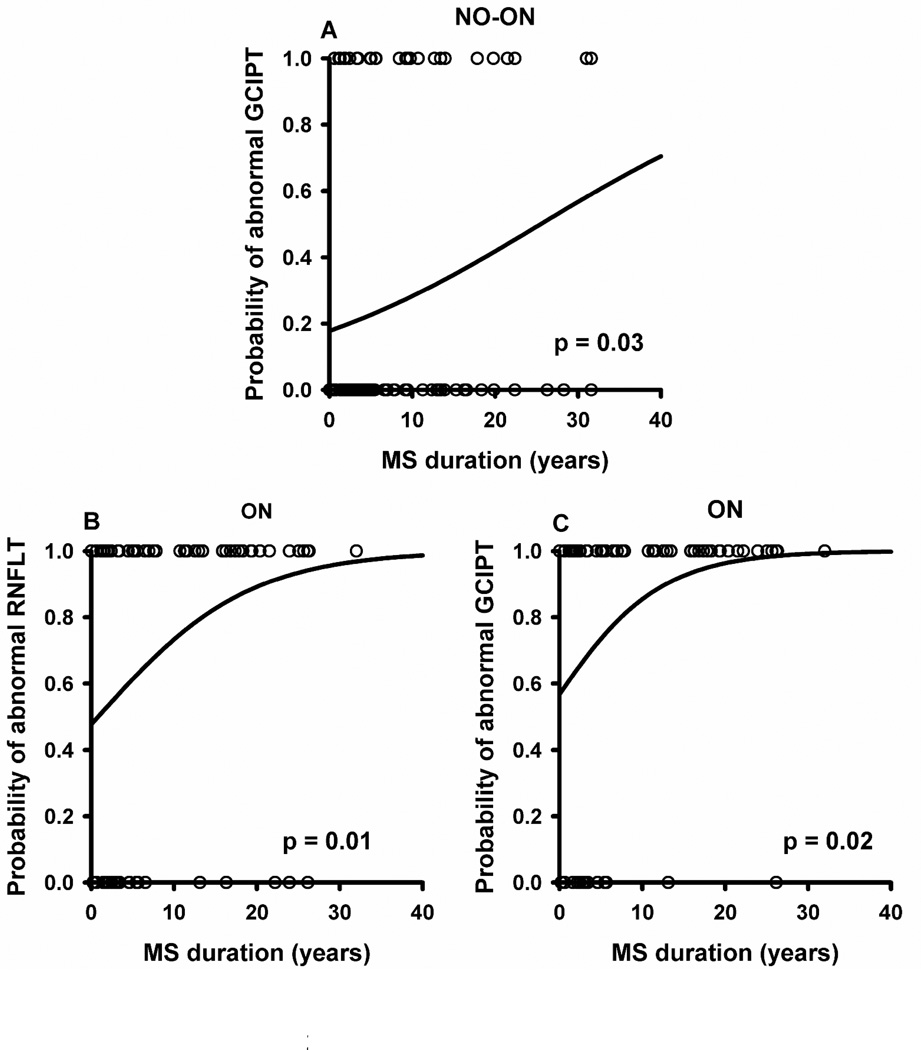
It is of clinical interest to detect neuronal loss on an individual basis; and to account for the effects of normal aging we examined RNFLT and GCIPT from individual eyes and classified them as abnormal if the values were below 5% of their respective age-matched machine norms. Notably, more eyes showed abnormal GCIPT than RNFLT (Table 4). Percent of eyes showing abnormal GCIPT vs RNFLT was 27% vs 16% (p=0.004) in no-ON and 82% vs 72% (p=0.007) in ON. GCIPT also was abnormal in more eyes than RNFLT when only the temporal quadrant of RNFLT, the receiving region for the macular fibers, was considered, 27% vs 15% (p=0.008) in no-ON and 82% vs 66% (p=0.003) in ON eyes. To examine whether more abnormal RNFLT/GCIPT existed in eyes with longer MS duration, we performed a logistic regression using MS duration as a continuous independent variable and the status of RNFLT/GCIPT (normal or abnormal) as a categorical dependent variable. In no-ON eyes, the probability of abnormal GCIPT increased significantly with MS duration (p=0.03, Figure 2A), however, for RNFLT the relation failed to reach statistical significance (p=0.56). In ON eyes, the probability of abnormal RNFLT (Figure 2B) and GCIPT (Figure 2C) both increased significantly with MS duration (p=0.01 for RNFLT, p=0.02 for GCIPT).
Table 4.
Percents of eyes with abnormal GCIPT and RNFLT and p values for comparing GCIPT vs mean RNFLT, and GCIPT vs temporal RNFLT (McNemar tests).
| Mean GCIPT |
Mean RNFLT (p value |
Temporal RNFLT (p value) |
|
|---|---|---|---|
| No-ON | 27% | 16% (0.004) | 15% (0.008) |
| ON | 82% | 72% (0.007) | 66% (0.003) |
Figure 2.
Logistic regression analysis showing increase in the probability of abnormalities with MS duration for GCIPT no-ON (A), RNFLT ON (B) and GCIPT ON (C). Circles represent the raw data (0 for normal, 1 for abnormal). The line is the fitted logistic regression curve (GEE model).
Longitudinal Analysis
RNFLT and GCIPT Change as a Function of Follow-up Time
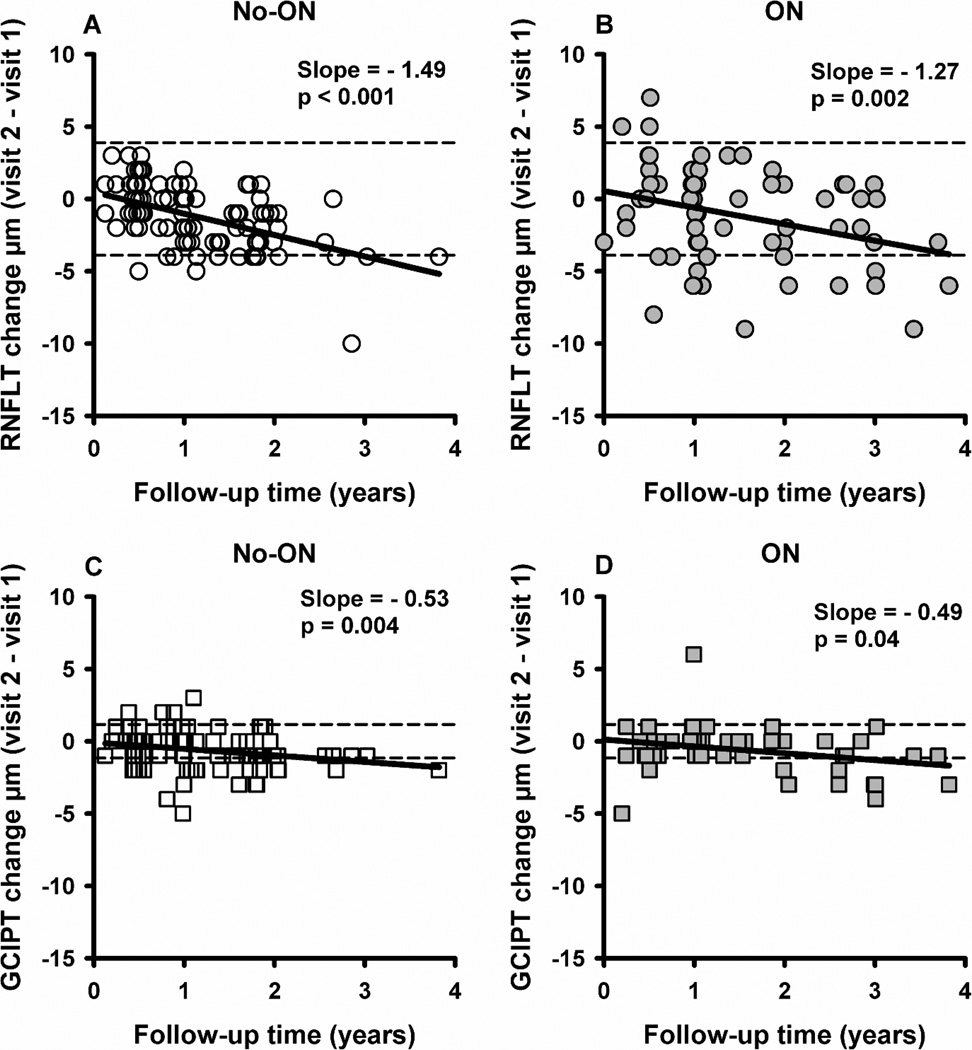
Ninety-two RRMS patients (96 no-ON, 68 ON eyes) had two OCT measurements separated by a follow-up time up to 3.8 years (median 1 year) (Table 2). The rate of change was obtained by performing linear regression between the inter-visit change in RNFLT/GCIPT and follow-up time for no-ON and ON groups. RNFLT decreased with increase in follow-up time at a rate (µm/yr) of −1.49 (p<0.001) in no-ON (Figure 3A) and −1.27 (p=0.002) in ON (Figure 3B) and the slopes were not different (p=0.64). To eliminate any possible effect of having ON history in the fellow eye, we analyzed bilateral no-ON eyes (n=53) separately. The rate of RNFL loss was −1.6, not different from that (−1.49) for all no-ON eyes (p=0.83). RNFLT in individual quadrants also decreased with follow-up time in no-ON or ON eyes (nasal and temporal quadrant in ON did not reach statistical significance) (Table 3, longitudinal data on the right). GCIPT decreased with follow-up time at a rate (µm/yr) of −0.53 (p=0.004) in no-ON (Figure 3C) and −0.49 (p=0.04) in ON (Figure 3D) and the slopes were not different (p=0.90). The rate of GCIPT loss in bilateral no-ON eyes was −0.69, not different from that (−0.53) for all no-ON eyes (p=0.56). The association between RNFLT/GCIPT reduction and follow-up time remained significant for no-ON and ON eyes even after accounting for other co-variables such as age, MS duration, baseline RNFLT and time from last ON event (for ON eyes only) (Table 5).
Figure 3.
Scatter plot showing relationship between the RNFLT change (A and B) and GCIPT change (C and D) with follow-up time for no-ON and ON eyes. Each symbol (circles for RNFLT and squares for GCIPT) represents an individual eye of a patient. Open symbols represent no-ON eyes and filled symbols represent ON eyes. The solid lines are the fitted linear regression lines (GEE models). The dashed lines are the testretest variability limits reported for Cirrus OCT.27, 28
Table 5.
Multivariate linear regression between RNFLT/GCIPT change and different co-variables in longitudinal analysis (GEE models).
| RNFLT | GCIPT | |||
|---|---|---|---|---|
| Slope, µm/yr | (p value) | Slope, µm/yr | (p value) | |
| Co-variables | No-ON | ON | No-ON | ON |
| Follow-up time | −1.42 (<0.001) | −1.0 (0.02) | −0.59 (0.01) | −0.57 (0.004) |
| Age at follow-up | 0.02 (0.45) | 0.03 (0.49) | 0.02 (0.30) | 0.05 (0.02) |
| MS duration at follow-up | −0.003 (0.93) | 0.07 (0.12) | −0.02 (0.42) | −0.06 (0.20) |
| Baseline RNFLT | −0.03 (0.04) | −0.06 (0.06) | −0.03 (0.04) | 0.001 (0.98) |
| Time from last ON event | NA | −0.08 (0.11) | NA | 0.02 (0.71) |
Humphrey Visual Field (HVF) in Cross-sectional and Longitudinal Analyses
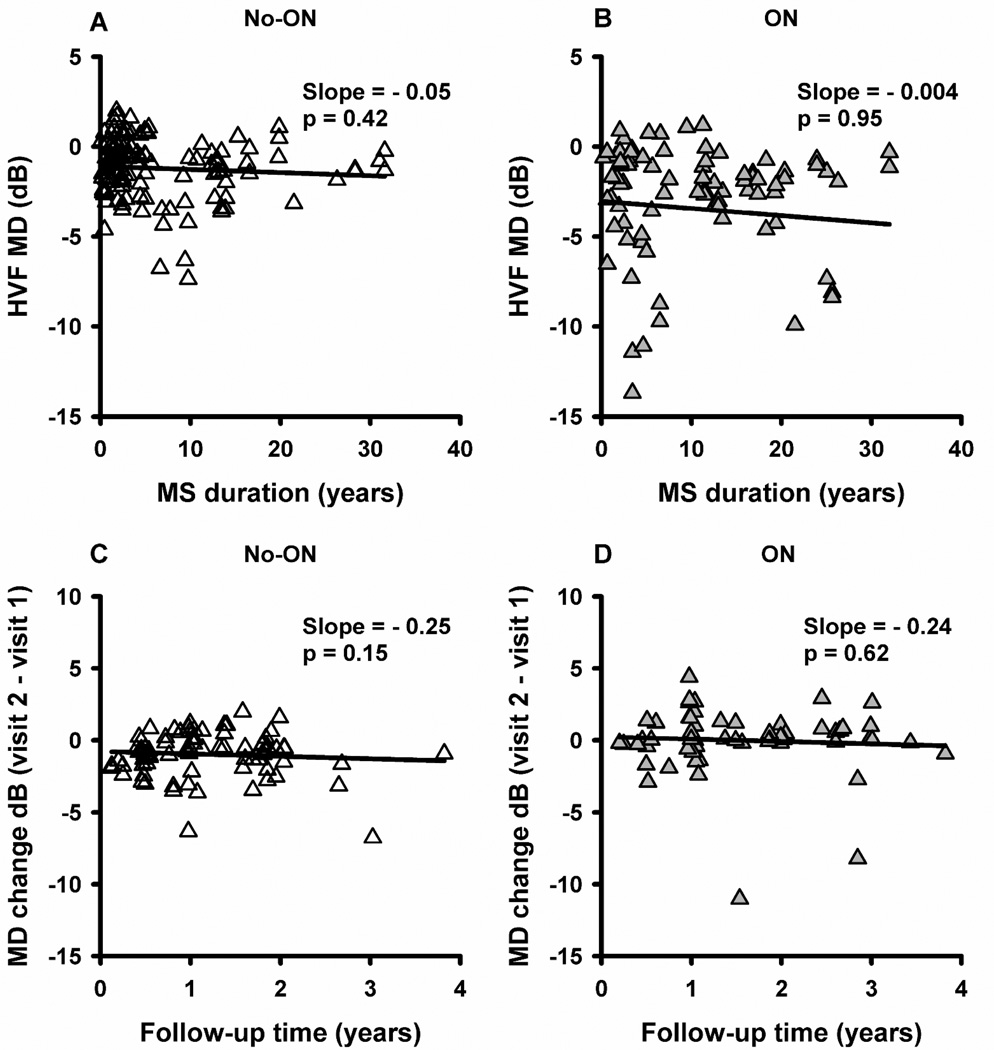
No association was found between HVF mean deviation (MD) and MS duration in cross-sectional analysis (Figure 4A and B: slope=−0.05 dB/yr, p=0.42 in no-ON; slope=−0.004 dB/yr, p=0.95 in ON), or inter-vist change in MD and follow-up time in longitudinal analysis (Figure 4C and D: slope=−0.25 dB/yr, p=0.15 in no-ON; slope=−0.24 dB/yr, p=0.62 in ON). In the longitudinal cohort, the mean MD was similar during baseline and follow-up visits for no-ON (−1.3 dB vs −1.0 dB, p=0.26) and ON (−2.9 dB vs −2.7 dB, p=0.97) (Table 2). When the slope of MD vs follow-up time was calculated for individual eyes, the mean (±SE) of individual slopes was −0.12±0.20 dB/yr for no-ON and −0.13±0.23 for ON. HVF MD probably did not show a significant association with duration of disease or follow-up time because of the large inter-subject variability in our study (Figure 4A and B) and the previously documented poor repeatability in ON eyes.10
Figure 4.
Scatter plot of MD vs MS duration (A and B) and MD change vs follow-up time (C and D) for no-ON and ON eyes. Each symbol represents an individual eye of a patient. Open symbols represent no-ON eyes and filled symbols represent ON eyes. The solid lines are the fitted linear regression lines (GEE models).
Discussion
Our results clearly indicate that progressive loss of RNFLT and GCIPT occurs in the absence of clinically-evident inflammation in RRMS eyes. Progressive neuronal loss observed in no-ON eyes was not due to effects from ON in the contralateral eye as indicated by similar results in bilateral no-ON eyes. In the current study, the rates of change in RNFLT in cross-sectional (around −0.5 µm/yr for both no-ON and ON eyes) and longitudinal data (−1.49 µm/yr in no-ON and −1.27 µm/yr in ON eyes) are both greater that of the normal age-related loss reported for Cirrus HD OCT: −0.19 µm/yr in one cross-sectional study,11 −0.37 µm/yr in another,12 and −0.52 µm/yr in a longitudinal study.13 It is interesting to note that the rate of change in RNFLT obtained from cross-sectional analysis is less than that from longitudinal analysis for both normal and MS subjects. This emphasizes that rate comparison is only reasonable among studies with similar designs. The rate of change in RNFLT may not be a constant throughout the entire disease course therefore caution should be exercised in extrapolating results from a short time interval to long term effect. In our previous unpublished study using Stratus OCT, we obtained similar findings.
The rate of GCIPT loss in our cross-sectional data was about −0.4 µm/yr for both no-ON and ON eyes, about 2.8 times the rate of normal aging (−0.14 µm/yr) as reported in Mwanza’s cross-sectional study of normal subjects between 18 and 84 years of age, their Figure 6A.9 The rate of GCIPT loss in our longitudinal analysis (−0.49 µm/yr in no-ON and −0.53 µm/yr in ON eyes), was about 60% greater than −0.32 µm/yr reported by Leung et al13 in normal subjects. Similarly Ratchford et al14 reported GCIPT loss of −0.37 µm/yr in MS and −0.20 µm/yr in normals. As previously reported,15 the rate of neuronal loss was similar for no-ON and ON eyes, indicating that documented acute inflammatory episodes had little impact on the rate of progression six months beyond the event.
Consistent with linear regression, our logistic regression analysis showed that proportion of abnormal eyes with respect to GCIPT significantly increased with MS duration. By 30 years of MS, more than 50% of the no-ON eyes and 100% of the ON eyes would have significantly thinner GCIPT compared to age-matched normal individuals.
MS is traditionally viewed as a primary autoimmune disease in which inflammatory T cells cross the blood brain barrier and attack the central nervous system (CNS), causing demyelination and axonal degeneration.16 Recently, Stys et al 17 proposed that cytodegeneration of oligodendrocyte-myelin complex and underlying axons could be the primary event, and antigenic debris released as a consequence promotes a secondary inflammatory immune response in a susceptible host.17 Our finding that in the absence of clinically-evident inflammation, neurodegeneration progresses over time, supports the possibility of primary neurodegenerative process in MS. However, we could also explain our results based on the primary autoimmune model, considering that there might be undetectable subclinical episodes of ON and/or chronic low-level inflammation in optic nerve. Though there is evidence of subclinical demyelination as demonstrated by delayed latency of visual evoked potentials in no-ON MS eyes,18 the presence of subclinical demyelination per se does not prove that the initiating trigger is autoimmune in nature, as it could also be interpreted as primary degeneration of myelin and underlying axons without sufficient antigenic myelin debris to initiate a secondary inflammatory immune response.17 RGC axonal loss could also result from retrograde degeneration from lesions in optic tract and/or posterior visual pathway. In our study, none of the subjects showed homonymous visual field defects characteristic of optic tract lesion, although optic tract lesions could also be asymptomatic.19 Lesions in the optic radiations could potentially lead to loss of RGC axons/neurons via retrograde transynaptic degeneration. Although there is some recent evidence of transynaptic degeneration in patients with acquired occipital lobe/optic radiation damage due to stroke,20 this is yet to be demonstrated in MS.
In our study, more eyes showed abnormal GCIPT than mean or temporal RNFLT. GCIPT is likely more sensitive for detecting abnormal eyes (values outside of the norms) because it has less normal variation than RNFLT. In the normal population, the distribution of GCIPT had smaller coefficient of variation (CoV) (7.6%) than mean RNFLT (10%) and temporal RNFLT (16%) (calculated from Mwanza et al 20119 and Knight et al 201211). Anatomically, the intersubject variability for RGC counts is lowest in the parafoveal region, but highest at far superior and inferior retina, predicting larger variability in peripapillary axons than central RGC counts.21 For OCT, differences in optic nerve head size, blood vessel patterns and glial content across normal subjects may also contribute to higher variability in RNFLT. In fact in MS eyes it has been shown that GCIPT is less confounded by axonal edema and gliosis 22, 23 and therefore correlates better with visual dysfunction,24 clinical and radiological markers of disease activity14 than RNFLT. Another possibility is that RGC somas atrophy before the axons even though in retrograde degeneration, axonal pathology/dysfunction precedes that of ganglion cell body. Fairless et al25 demonstrated in a rat model of MS, that at the time of significant RGC soma loss, the axons appeared to be intact in numbers but showed ultrastructural signs of degeneration. Recent studies in glaucoma demonstrated the value of measuring macular RGC.26 Compared to temporal RNFLT, GCIPT measurements are more reproducible27, 28 and might be a better measure to reflect macular damage. Therefore we believe that GCIPT is a great addition to tests for detecting and tracking neuronal changes in MS eyes.14, 24 In fact, in our longitudinal data, more no-ON eyes showed worsening based on GCIPT than RNFLT (see data points below the lower test-retest variability limits in Figure 3 A, C).
In summary our data showed increased loss of RNFLT and GCIPT with increase in MS duration and follow-up time. There were significantly more abnormal eyes in the GCIPT measurements than in the RNFLT measurements for both no-ON and ON eyes. Progressive neuronal loss, in the absence of clinically-evident inflammation, suggests a significant role for neurodegeneration in RRMS, traditionally viewed as an autoimmune disease. New therapeutic options should focus on remyelination and neuroprotection in addition to reducing inflammation and relapses.
Acknowledgements
The authors would like to thank Drs. Ying Sheng Hu and Siva Tian for their help on statistical analysis.
Funding
This study was supported by NIH P30 EY07551, NIH T35 007088, Fight for Sight summer student fellowship and the Minnie Flaura Turner memorial fund for impaired vision research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors declare that they have no conflicts of interest.
References
- 1.Weinshenker BG, Bass B, Rice GP, et al. The natural history of multiple sclerosis: a geographically based study. 2. Predictive value of the early clinical course. Brain. 1989;112(Pt 6):1419–1428. doi: 10.1093/brain/112.6.1419. [DOI] [PubMed] [Google Scholar]
- 2.Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis. N Engl J Med. 2000;343:938–952. doi: 10.1056/NEJM200009283431307. [DOI] [PubMed] [Google Scholar]
- 3.Dutta R, Trapp BD. Mechanisms of neuronal dysfunction and degeneration in multiple sclerosis. Prog Neurobiol. 2011;93:1–12. doi: 10.1016/j.pneurobio.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castro-Borrero W, Graves D, Frohman TC, et al. Current and emerging therapies in multiple sclerosis: a systematic review. Ther Adv Neurol Disord. 2012;5:205–220. doi: 10.1177/1756285612450936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rudick RA, Cutter GR, Baier M, et al. Estimating long-term effects of disease-modifying drug therapy in multiple sclerosis patients. Mult Scler. 2005;11:626–634. doi: 10.1191/1352458505ms1203oa. [DOI] [PubMed] [Google Scholar]
- 6.Ford C, Goodman AD, Johnson K, et al. Continuous long-term immunomodulatory therapy in relapsing multiple sclerosis: results from the 15-year analysis of the US prospective open-label study of glatiramer acetate. Mult Scler. 2010;16:342–350. doi: 10.1177/1352458509358088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The clinical profile of optic neuritis. Experience of the Optic Neuritis Treatment Trial. Optic Neuritis Study Group. Arch Ophthalmol. 1991;109:1673–1678. doi: 10.1001/archopht.1991.01080120057025. [DOI] [PubMed] [Google Scholar]
- 8.Polman CH, Reingold SC, Edan G, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the "McDonald Criteria". Ann Neurol. 2005;58:840–846. doi: 10.1002/ana.20703. [DOI] [PubMed] [Google Scholar]
- 9.Mwanza JC, Durbin MK, Budenz DL, et al. Profile and predictors of normal ganglion cell-inner plexiform layer thickness measured with frequency-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2011;52:7872–7879. doi: 10.1167/iovs.11-7896. [DOI] [PubMed] [Google Scholar]
- 10.Wall M, Johnson CA, Kutzko KE, Nguyen R, Brito C, Keltner JL. Long- and short-term variability of automated perimetry results in patients with optic neuritis and healthy subjects. Archives of ophthalmology. 1998;116:53–61. doi: 10.1001/archopht.116.1.53. [DOI] [PubMed] [Google Scholar]
- 11.Knight OJ, Girkin CA, Budenz DL, Durbin MK, Feuer WJ. Effect of race, age, axial length on optic nerve head parameters and retinal nerve fiber layer thickness measured by Cirrus HD-OCT. Arch Ophthalmol. 2012;130:312–318. doi: 10.1001/archopthalmol.2011.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Celebi AR, Mirza GE. Age-Related Change in Retinal Nerve Fiber Layer Thickness Measured with Spectral Domain Optical Coherence Tomography. Invest Ophthalmol Vis Sci. 2013 doi: 10.1167/iovs.13-12634. [DOI] [PubMed] [Google Scholar]
- 13.Leung CK, Yu M, Weinreb RN, et al. Retinal nerve fiber layer imaging with spectral-domain optical coherence tomography: a prospective analysis of age-related loss. Ophthalmology. 2012;119:731–737. doi: 10.1016/j.ophtha.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 14.Ratchford JN, Saidha S, Sotirchos ES, et al. Active MS is associated with accelerated retinal ganglion cell/inner plexiform layer thinning. Neurology. 2013;80:47–54. doi: 10.1212/WNL.0b013e31827b1a1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garcia-Martin E, Pueyo V, Almarcegui C, et al. Risk factors for progressive axonal degeneration of the retinal nerve fibre layer in multiple sclerosis patients. Br J Ophthalmol. 2011;95:1577–1582. doi: 10.1136/bjo.2010.199232. [DOI] [PubMed] [Google Scholar]
- 16.Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372:1502–1517. doi: 10.1016/S0140-6736(08)61620-7. [DOI] [PubMed] [Google Scholar]
- 17.Stys PK, Zamponi GW, van Minnen J, Geurts JJ. Will the real multiple sclerosis please stand up? Nat Rev Neurosci. 2012;13:507–514. doi: 10.1038/nrn3275. [DOI] [PubMed] [Google Scholar]
- 18.Laron M, Cheng H, Zhang B, Schiffman JS, Tang RA, Frishman LJ. Assessing visual pathway function in multiple sclerosis patients with multifocal visual evoked potentials. Mult Scler. 2009;15:1431–1441. doi: 10.1177/1352458509350470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davies MB, Williams R, Haq N, Pelosi L, Hawkins CP. MRI of optic nerve and postchiasmal visual pathways and visual evoked potentials in secondary progressive multiple sclerosis. Neuroradiology. 1998;40:765–770. doi: 10.1007/s002340050681. [DOI] [PubMed] [Google Scholar]
- 20.Jindahra P, Petrie A, Plant GT. The time course of retrograde trans-synaptic degeneration following occipital lobe damage in humans. Brain. 2012;135:534–541. doi: 10.1093/brain/awr324. [DOI] [PubMed] [Google Scholar]
- 21.Curcio CA, Allen KA. Topography of ganglion cells in human retina. J Comp Neurol. 1990;300:5–25. doi: 10.1002/cne.903000103. [DOI] [PubMed] [Google Scholar]
- 22.Syc SB, Saidha S, Newsome SD, et al. Optical coherence tomography segmentation reveals ganglion cell layer pathology after optic neuritis. Brain. 2012;135:521–533. doi: 10.1093/brain/awr264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Green AJ, McQuaid S, Hauser SL, Allen IV, Lyness R. Ocular pathology in multiple sclerosis: retinal atrophy and inflammation irrespective of disease duration. Brain. 2010;133:1591–1601. doi: 10.1093/brain/awq080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saidha S, Syc SB, Durbin MK, et al. Visual dysfunction in multiple sclerosis correlates better with optical coherence tomography derived estimates of macular ganglion cell layer thickness than peripapillary retinal nerve fiber layer thickness. Mult Scler. 2011;17:1449–1463. doi: 10.1177/1352458511418630. [DOI] [PubMed] [Google Scholar]
- 25.Fairless R, Williams SK, Hoffmann DB, et al. Preclinical retinal neurodegeneration in a model of multiple sclerosis. J Neurosci. 2012;32:5585–5597. doi: 10.1523/JNEUROSCI.5705-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hood DC, Raza AS, de Moraes CG, Liebmann JM, Ritch R. Glaucomatous damage of the macula. Prog Retin Eye Res. 2013;32:1–21. doi: 10.1016/j.preteyeres.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mwanza JC, Chang RT, Budenz DL, et al. Reproducibility of peripapillary retinal nerve fiber layer thickness and optic nerve head parameters measured with cirrus HD-OCT in glaucomatous eyes. Invest Ophthalmol Vis Sci. 2010;51:5724–5730. doi: 10.1167/iovs.10-5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mwanza JC, Oakley JD, Budenz DL, Chang RT, Knight OJ, Feuer WJ. Macular ganglion cell-inner plexiform layer: automated detection and thickness reproducibility with spectral domain-optical coherence tomography in glaucoma. Invest Ophthalmol Vis Sci. 2011;52:8323–8329. doi: 10.1167/iovs.11-7962. [DOI] [PMC free article] [PubMed] [Google Scholar]