Abstract
In response to inflammatory stimuli, microvascular endothelial cells become activated, initiating the capture and exit of neutrophils from the blood vessel and into the extravascular extracellular matrix (ECM). In the extravascular space, neutrophils bind to ECM proteins, regulating cellular functions via signaling through adhesion molecules known as integrins. The αVβ3 integrin is an important mediator of neutrophil adhesion to ECM proteins containing the Arg-Gly-Asp (RGD) peptide sequence, including fibrinogen and fibronectin. Despite the abundance of RGD sequence in the ECM, adhesion molecule-mediated neutrophil activity has been focused on the β2 (Mac-1, CD11b/CD18) and β1 integrin response to matrix proteins. Here we investigated αVβ3 integrin-mediated reactive oxidant suppression as a consequence of human neutrophil adhesion to RGD containing proteins. Using integrin ligand-modified (poly)ethylene glycol hydrogels and reactive oxygen species (ROS) sensitive fluorescent probes (dihydrotetramethylrhosamine, H2TMRos), we evaluated integrin–peptide interactions that effectively regulate ROS generation. This study demonstrates that neutrophil adhesion suppresses ROS production in an αVβ3-dependent manner. Additionally, we determine that p38 mitogen-activated protein kinase in the respiratory burst signaling pathway is interrupted by integrin-mediated adhesion. These data indicate that ECM/integrin interactions can induce αVβ3-mediated adhesion dependent downstream signaling of ROS regulation via a Mac-1 independent mechanism.
Keywords: Adhesion, Reactive oxygen species (ROS), Polyethylene glycol (PEG), Extracellular matrix (ECM)
Introduction
Neutrophils are major effector cells in innate immunity and host defense. During the inflammatory response, neutrophils are the first immune cells to migrate to sites of infection and release reactive oxygen species (ROS) and proteolytic enzymes to kill microbial pathogens.6 Neutrophil firm adhesion, a necessary precursor to migration, is mediated by integrins that play functional roles in cytoskeletal reorganization, though their role in ROS production through intracellular signaling is more poorly understood.34 Oxygen metabolite production results in neutrophil respiratory burst, releasing oxidants that contribute to remodeling of the extracellular matrix (ECM) proteins as neutrophils migrate through tissue.4 When adherent to ECM proteins, including fibrinogen (Fg), fibronectin (Fn), and laminin, neutrophils demonstrate an initial delay and subsequent burst of ROS production under inflammatory conditions.30 This initial integrin adhesion-mediated suppression of ROS is understood to be a tissue-protective mechanism during migration of neutrophils through ECM to sites of inflammation.23,39 However, uninhibited and persistent ROS released through neutrophil bursts is a critical cause of tissue damage during a neutrophil response to chronic inflammatory signals, ultimately contributing to severe pathologies and organ failure. Therefore, an improved understanding of regulatory and temporal factors in integrin-mediated ROS suppression will advance the identification of anti-inflammatory therapeutic targets.
Studies of integrin-mediated ROS generation have been primarily focused on the αMβ2 (CD11b/CD18, Mac-1) integrin signaling pathways in response to whole ECM proteins.36 Fg and Fn have been used to examine adhesion-mediated cell responses through β2 (e.g. Mac-1) or β1 integrin binding, respectively. Neutrophils become attached to Fg and Fn due to the presentation of multiple integrin binding domains within each of these proteins. Fg has three RGD (Arg-Gly-Asp) and one P2 (Asn-Arg-Leu-Thr-Ile-Gly-Gly) sequence, and Fn contains PHSRN (Pro-His-Ser-Arg-Asn), RGD, LDV (Leu-Asp-Val), and REDV (Arg-Glu-Asp-Val) peptide sequences.19,36 The accumulated data of many studies show that Fn and Fg participate in regulation of hydrogen peroxide (H2O2) and superoxide (O2−) of adhesive neutrophils.16 Further, such studies indicate that adhesion can regulate signaling processes including mitogen activated protein kinases (MAPKs) activation. Specifically, p38 MAP kinase has been shown to both regulate superoxide production while conversely, becoming regulated by ROS activation.1,12 However, the detailed molecular mechanisms by which integrin regulation of oxidant generation and release occur remain unclear. In addition, the presentation of multiple binding sites in whole ECM proteins hampers identification of the unique functional role of individual integrins. The experimental maneuver of inhibiting integrin binding sites by antibody treatment does not eliminate the possibility of integrin–ligand interactions with Fn and Fg in which a multi-integrin response is expected. Therefore, the use of a polymer-based biomaterial for presenting integrin-specific ligands is a more suitable system to elucidate independent functional roles of individual integrins. Here, we evaluate integrin–ligand interactions that regulate neutrophil respiratory burst through adhesion. In this study, inert (poly)ethylene glycol (PEG) chains were functionalized via chemical coupling with integrin ligands that enabled immobilization of the free ligands on the resulting hydrogel surface. PEG hydrogels have been used as artificial ECM in a wide range of research applications in which PEG provides a more physiologically relevant microenvironment for cell interaction than do commonly used surfaces, including glass or plastic.8,14,32 PEG provides a blank background that can be modified with peptide conjugation to investigate the functional results of integrin binding to isolated integrin binding domains. This bioengineered and highly tunable substrate was used to investigate adhesion-mediated neutrophil respiratory burst and subsequent activation of p38 MAPK.
Materials and Methods
Statement of Ethics
The use and attainment of human cells were approved by Yale University Human Investigation Committee (HIC) of the Internal Review Board (IRB) as part of the Human Research Protection Program. This research was conducted in accordance with approved protocols and in line with the standards set by the Helsinki Declaration. All advertisements for volunteers and written informed consent were approved by the Yale University HIC IRB and obtained from all human volunteers prior to blood collection. Data collection and analyses were performed on anonymized samples.
Preparation of PEG and Peptide Conjugates
All general chemical solvents and reagents were purchased from Sigma–Aldrich and used without further purification. Acrylated PEG and peptide–PEG conjugates were prepared as described previously.10 Briefly, PEG (10 kDa) was dissolved in anhydrous methylene chloride under Argon. Triethyl amine and acryloyl chloride were combined and stirred overnight at room temperature. The resulting PEG-diacrylate (PEG-DA) was precipitated with diethyl ether, filtered, and dialyzed. Crystalline PEG-DA was obtained after lyophilization. Peptides (RGDS, Arg-Gly-Asp-Ser, American Peptides; P2, NRLTIGG, Asn-Arg-Leu-Thr-Ile-Gly-Gly, GenScript Corp.) were conjugated with acryloyl-PEG-N-hydroxysuccinimide (acryloyl-PEG-NHS, 3,400 Da, Laysan Bio. Inc.) in aqueous sodium bicarbonate (pH 8.5), and 2-molar equivalent of peptides were used for complete conjugation at room temperature for 4 h. The PEG-peptide conjugates were dialyzed to remove unbound peptides and lyophilized. The synthesized PEG-DA and all peptide conjugates were confirmed by 1 H NMR (D2O, Bruker, 400 MHz) and MALDI-TOF (ABI Voyager). Peptide presenting hydrogels contained 2.6 μmol/mL RGD in RGD(1X) hydrogels, 5.2 μmol/mL RGD in RGD(2X) hydrogels, 4.0 μmol/mL NRLTIGG in P2 hydrogels, or 2.6 μmol/mL RGD and 2.0 μmol/mL P2 in RGD+P2 hydrogels.
Hydrogel Formation
Hydrogels were prepared by combining 0.1 g/mL PEG diacrylate and 0.01 g/mL (2.6 μmol/mL) acryloyl-PEG-RGDS, 0.02 g/mL (5.2 μmol/mL) acryloyl-PEG-RGDS, 0.02 g/mL (4.0 μmol/mL) acryloyl-PEG-P2, or a combination of 0.01 g/mL acryloyl-PEG-RGDS and 0.01 g/mL acryloyl-PEG-P2 in PBS (pH 7.2) containing 0.1 g/L glucose. The solution was sterilized by filtration (0.2 μm with 0.8 μm prefilter, Pall Corp., Ann Arbor, MI, USA). A photoinitiating crosslinker, 2,2-dimethoxy-2-phenylacetophenone (10 μL/mL) in n-vinylpyrrolidone (300 mg/mL) was added to the PEG solution. The resulting solution was aliquoted into 96 well plates (50 μL/well) for oxidation measurements or onto 25 mm coverslips (100 μL/coverslip) for adhesion assays, and subsequently exposed to UV light (365 nm, 10 mW/cm2) for approximately 30 s, converting the liquid polymer solution to a solid hydrogel. The polymerized gels were then incubated for 24 h in PBS to allow them to reach their equilibrium swelling. Swelling ratio after overnight incubation was calculated as previously described by Okay and Sariisik.25 q=mass of hydrogel after preparation mass of dry hydrogel. The resulting swelling ratios of PEG gels (2.9 ± 0.4) vs. RGD(1X) gels (3.1 ± 0.0.9), RGD(2X) gels (2.8 ± 0.5), or P2 gels (2.7 ± 0.2) were not significantly different from one another.
Neutrophil Isolation
Neutrophils were isolated from the whole blood of healthy volunteers under a protocol approved by the Yale Human Investigation Committee.11 Red blood cells were removed by sedimentation with 6% Dextran followed by Ficoll-Histopaque (Sigma) gradient centrifugation to isolate purified neutrophils. Neutrophils were suspended in PBS containing 6% glucose, and cells were used at 1 × 106 cells/mL.
Static Adhesion Assay
Neutrophil adhesion was measured and quantified by a static adhesion assay as previously described.9,10 Briefly, biomimetic hydrogels were washed with PBS and placed on a glass coverslip within a static adhesion chamber (Sykes-Moore chamber, Bellco Glass Inc.) Neutrophil adhesion to fibrinogen (Fg, Calbiochem) was performed using 0.1 mg of Fg-coated coverslips. Neutrophils were seeded on the surface of the hydrogel or Fg-coated coverslip and allowed to adhere for 500 s. The 500 s time point is typically used for cells in the static adhesion chamber assays and is sufficient time for integrin-mediated cell adhesion to occur.10 The hydrogel in the chamber was then inverted, and the cells were allowed to fall away from the surface of the gel. Adherent neutrophils remaining on the hydrogel or Fg-coated coverslip surface were counted; the fraction of adherent cells was calculated. To evaluate integrin specificity, antibody inhibition was conducted using neutrophils pre-incubated with PMA (100 nM, phorbol-12-myristate-13-acetate, Sigma–Aldrich), mouse anti-human integrin αVβ3 mAb (LM609, 10 μg/mL, Chemicon International) or anti-β2 antibody (R15.7, 10 μg/mL, Boehringer Ingleheim) for 15 min at 37 °C.
Measurement of Oxygen Intermediates 7,18,28
96-well plates were coated with 200 μL Fg in PBS (100 μg/mL). After overnight incubation at 4 °C, wells were washed with PBS. For ROS sensitive hydrogels, PEG solution (50 μL) with and without peptide conjugates, containing photoinitiator was added to 96-well plates, and cup-shape hydrogel was prepared by UV photopolymerization. Gels were washed repeatedly with PBS prior to cell seeding. Neutrophils were pre-incubated with PMA (100 nM, phorbol-12-myristate-13-acetate, Sigma–Aldrich), mouse anti-human integrin αVβ3 mAb (LM609, 10 μg/mL, Chemicon International), anti-β2 antibody (R15.7, 10 μg/mL, Boehringer Ingleheim), or RGD (50 μg/mL) for 15 min at 37 °C. 100 μL of cell suspension (1 × 105 cells per well) was seeded into wells, and 50 μL of H2TMRos solution (1 mM, Molecular Probes) was added to each well. Plates were incubated at 37 °C throughout the experiment, and the extracellular ROS production was detected periodically over 2 h. The 2 h period allows for continuous integrin-mediated neutrophil attachment to the substrate, while remaining at the early time points of ex vivo neutrophil apoptosis. Fluorescent oxidation product from ROS generation was measured by microplate reader (λem/λex = 544/590 nm, VICTOR 2 Multilabel Counter, PerkinElmer). Curves presented represent microplate readings at each timepoint. A representative curve of absolute raw data is presented, and normalized curves are shown to demonstrate relative ROS production under varying conditions of neutrophil adhesion. The total ROS was obtained from the area under the curve (AUC) across the entire 2 h time course of ROS measurement. The totals represented are averages of the AUC across multiple experiments for each condition. Fold increase was calculated as an increase of ROS production when compared to cells on PEG alone.
Western Blot Analysis of Phosphorylated p38 MAPK
Isolated neutrophils suspended in PBS solution containing Ca2+, Mg2+, and 6% glucose were seeded at a density of 2 × 106 cells on the surface of a hydrogel containing RGD, P2, and PEG alone, or fibrinogen (100 μg/mL) coated coverslips contained within a static adhesion sealed chamber. Neutrophil suspension was allowed to adhere for 0, 30, 60, 90 or 120 min while incubated at 37 °C. The adherent cells were collected using trypsin (Trypsin–EDTA Solution, Sigma–Aldrich), and protein lysates were prepared via incubation of cells with RIPA-buffer and analyzed by Western blot. Membranes were blotted with p38-MAPK antibody (1:250) followed by goat anti-rabbit-HRP antibody (1:2500). Antibodies were detected using enhanced chemiluminescence. Equal loading of protein was determined by blotting the membranes with anti-β-actin antibody (1:5000) followed by goat anti-mouse IgG-HRP antibody (1:2000). Protein blot analysis was conducted using Image Studio Lite Ver 3.1 (LiCor). Intensity measurements were adjusted for β-actin protein levels.
Data Analysis
Statistical analysis was carried out using a paired Student’s t test for adhesion data and total ROS AUC comparisons. Two-way ANOVA with Tukey post-tests were conducted for individual timepoint ROS measurements. Samples were collected from >3 human volunteers and conducted in triplicate.
Results
Integrin-Specific Neutrophil Adhesion on Peptide Modified Hydrogel Surfaces
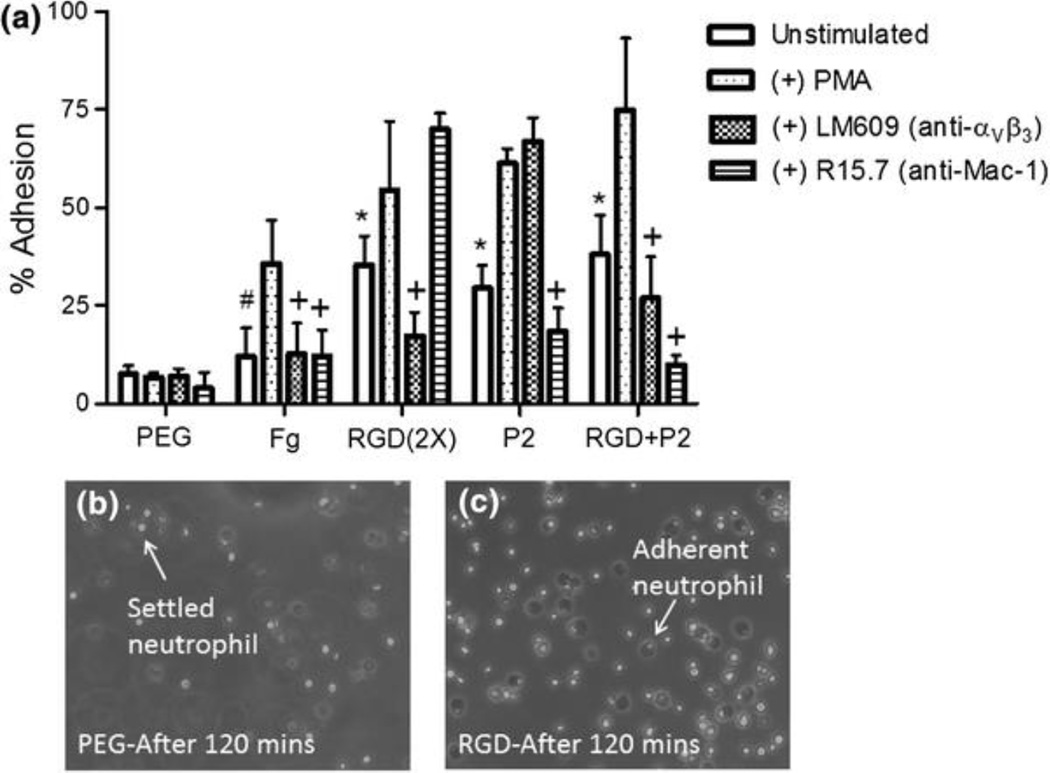
To determine the adhesive properties of αVβ3 and Mac-1 integrins in response to ECM protein derived peptides, isolated human neutrophils were seeded on peptide inclusive hydrogels in a closed static adhesion chamber.9,10 In this study we use phorbol 12-myristate 13-acetate (PMA) to induce maximum integrin upregulation and activation, mimicking a highly inflamed physiological state. Levels of unstimulated and PMA-stimulated neutrophil adhesion were evaluated on αVβ3 and Mac-1 integrin-specific ligand (RGD and P2, respectively) modified PEG gels following 500 s incubation (Fig. 1). The biologically inert PEG hydrogel without bioactive peptides supported non-specific cell attachment at levels <7.2%. The Fg-coated surface serving as a positive adhesive ligand, supported a 3.7-fold increase in neutrophil adhesion (26.5%), when compared to cells on PEG. The RGD tri-peptide, a ubiquitous adhesive ligand present in most ECM proteins, including Fg, Fn, vitronectin, and von Willebrand factor, was used as an αVβ3 specific ligand. RGD(1X) (2.6 μmol/mL of RGD) containing hydrogels supported 27.3% cell adhesion (compared in later figures), not significantly different from levels of adhesion on Fg. A twofold increase in RGD(2X) (5.2 μmol/mL of RGD) containing hydrogels resulted in adhesion levels of approximately 35.4% (Fig. 1). Even after 120 min, neutrophil attachment to PEG is minimal, whereas neutrophil integrin-mediated attachment to RGD is active, as indicated by cell spreading (Figs. 1b and 1c). The P2 peptide, a Mac-1 integrin-specific binding peptide, induced levels of neutrophil adhesion 29.5% when presented at a concentration (4.0 μmol/mL) matching RGD(2X). Combined RGD+P2 peptide presentation resulted in higher levels of adhesion (34.9%) than either peptide alone. When neutrophils were pretreated with PMA, a protein kinase C activator that induces an increase in surface expression of integrins, there was a universal increase in cell adhesion on all bioactive hydrogel surfaces (Fig. 1). Confirming the biologically inert nature of PEG, adhesion to unmodified PEG alone remained low and unchanged when neutrophils were PMA-stimulated.
Figure 1.
Neutrophil adhesion on PEG, fibrinogen (Fg), and integrin ligand modified hydrogels. (a) Cells were pre-incubated with PMA (100 nM), anti-αVβ3 integrin antibody (LM609, 10 μg/mL) or anti-β2 integrin antibody (R15.7, 10 μg/mL) and standard 500 s adhesion assays were performed. Values represent means taken from a minimum of three experiments with a minimum of three blood donors ± SE. #Difference compared to PEG (unstimulated cells), p < 0.001; *Difference compared to PEG (unstimulated cells), p < 0.01, +Difference when compared with PMA stimulated cells on peptide without antibody inhibition, p < 0.05. (b) Brightfield images of neutrophils settled on the surface of the PEG hydrogel after 120 min and (c) neutrophils firmly attached to RGD hydrogels after 120 min
To verify that adhesion was integrin-mediated, neutrophils were pre-incubated with blocking antibody against αVβ3 integrin (LM609) or Mac-1 integrin (R15.7) (Fig. 1). Integrin-specific antibodies inhibited neutrophil adhesion on peptide modified gels: anti-αVβ3 and Mac-1 antibodies reduced cell adhesion to the corresponding RGD and P2 containing gels, 41.1 and 36.9% respectively. This adhesion inhibitory effect was additionally observed in RGD+P2 peptide combined PEG hydrogels with 29.2 and 73.8% decrease in response to anti-αVβ3 and anti-Mac-1 antibody treatments, respectively. Collectively, the results demonstrate integrin–ligand specific cell adhesion on peptide-modified PEG hydrogels, with enhanced adhesion under neutrophil stimulatory conditions. The significant inhibition of αVβ3 integrin-mediated adhesion via anti-αVβ3 blocking antibody pretreatment further supports the specificity of this integrin in adhesion to RGD ligand.
Integrin-Mediated ROS Suppression
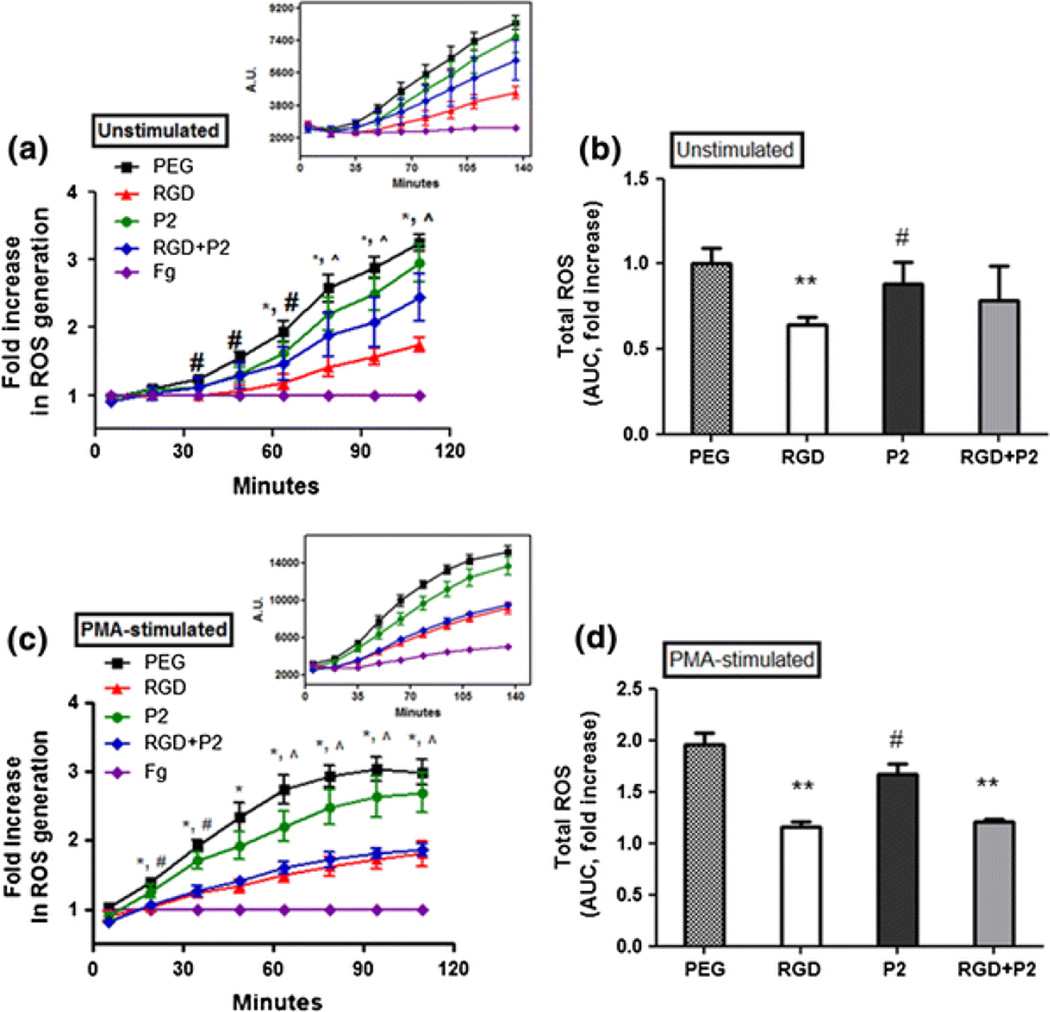
During respiratory burst, neutrophils convert large quantities of oxygen to ROS such as superoxide, hydrogen peroxide, and hydroxyl radicals that act as antimicrobial agents.3,29 To determine the relationship between neutrophil αVβ3 and Mac-1 integrin-dependent adhesion and subsequent oxidant production, ROS was measured over a 2 h period of cell engagement with integrin binding ligands. The total ROS production was measured by an oxygen radical responsive fluorescent probe (H2TMRos) that fluoresces at a distinctive emission wavelength upon oxidation.18 Unstimulated neutrophils were seeded on Fg, unmodified PEG, and peptide-modified PEG hydrogels to determine the influence of integrin-specific adhesion on ROS release. Neutrophils adhering to Fg consistently demonstrated a sustained and significant reduction in ROS generation (Fig. 2a). Consequent measurements of ROS released from neutrophils on Fg, PEG, RGD, P2 and RGD+P2 hydrogels were normalized to ROS measurements on Fg that showed low ROS generation throughout the incubation (Fig. 2a). The highest level ROS generation was consistently observed from non-adherent cells on PEG (Fig. 2a). Integrin adhesion-dependent inhibition of ROS production occured when neutrophils were adherent to RGD containing hydrogels, with statistically significant levels of suppression (*p < 0.01) observed throughout later incubation periods (65, 80, 95 and 110 min), when compared to non-adherent cells on PEG alone (Fig. 2a). Over a 2 h incubation, providing extended time for integrin binding to the substrate, a collective 35.8% ROS suppression was observed on RGD-containing hydrogels compared to total ROS production by neutrophils on PEG (Fig. 2b).
Figure 2.
Integrin-mediated ROS suppression of neutrophils. Integrin-mediated ROS generations were evaluated by measuring fluorescent oxidation product of H2TMRos (λex/λem = 544/590 nm). ROS production from (a) unstimulated and (c) PMA-stimulated neutrophils on fibrinogen, PEG, RGD, P2, and RGD+P2 modified hydrogels. ±SE of >three independent experiments. *Difference between cells on RGD and PEG, p < 0.05, #Difference between cells on P2 and PEG, p < 0.05, ^Difference between cells on RGD+P2 and PEG, p < 0.05. (b) and (d) reflect total ROS suppression from the area AUC relative to the total ROS detected from neutrophils on PEG. Values represent the means of triplicates. **Difference when compared with cells on PEG, p < 0.01, #Difference when compared with cells on RGD, p < 0.05
Unlike the effects of neutrophil adhesion to RGD, a statistically significant (#p < 0.05) suppression of ROS generation was observed at earlier time points (35, 50, and 65 min) from the P2 adherent neutrophils when compared to non-adherent cells on PEG. Beyond the 65 min timepoint, the P2 adherent cells were no longer significantly reduced in their ROS generation. Relative to the maximum ROS release on PEG across all timepoints, neutrophil adhesion to P2 resulted in 12.0% suppression, peaking with 15.29% suppression at the 35 min timepoint. The total ROS suppression of P2-adherent unstimulated cells was 27% lower than the level of suppression experienced by RGD adherent cells (Fig. 2b), suggesting that αVβ3 engagement is a much more robust suppressor of ROS generation than Mac-1 dependent adhesion. Combined, RGD+P2 peptides showed an intermediate effect on suppression of ROS generation, significant at the latest timepoints (80, 95 and 110 min) (Fig. 2a). Adhesion to the combined RGD+P2 peptide sequences resulted in 21.3% suppression (Fig. 2b), with the maximum ROS suppression of 26.5% occurring at 95 min. Throughout the incubation, the level of suppression seen on the combined RGD+P2 peptides was 1.2-fold less than that observed on RGD alone in unstimulated cells (Fig. 2b).
Significantly higher amounts of reactive oxygen metabolites were observed from the PMA-activated neutrophils when compared to unstimulated neutrophils seeded upon all substrates (Fig. 2c). However, PMA-stimulated cells showed trends similar to those of unstimulated cells on the same substrates (Figs. 2a and 2c), indicating that, while primed to produce ROS, neutrophil integrin engagement remains active as an ROS inhibitory mechanism. Interestingly, RGD-mediated suppression was significant at all timepoints, resulting in 40.9% suppression in ROS throughout the incubation period (Fig. 2d). The highest suppression on RGD occurred at 65 min with 45.1% suppression. As with unstimulated neutrophils, PMA-stimulated neutrophils experienced significant (#p < 0.05) reduction in ROS generation when adherent to P2 peptide at early timepoints, 20 and 35 min (Fig. 2b). Collectively across all timepoints, P2 surface-adherent cells showed 14.4% total ROS suppression (Fig. 2d). Through the duration of incubation, the collective suppression of PMA-stimulated neutrophil ROS generation in RGD+P2 was 38.4% which was a 1.8-fold enhancement of ROS suppression when compared to unstimulated neutrophils on the same substrate, and not different from the effect of PMA-stimulated neutrophil adhesion to RGD alone (Fig. 2d).
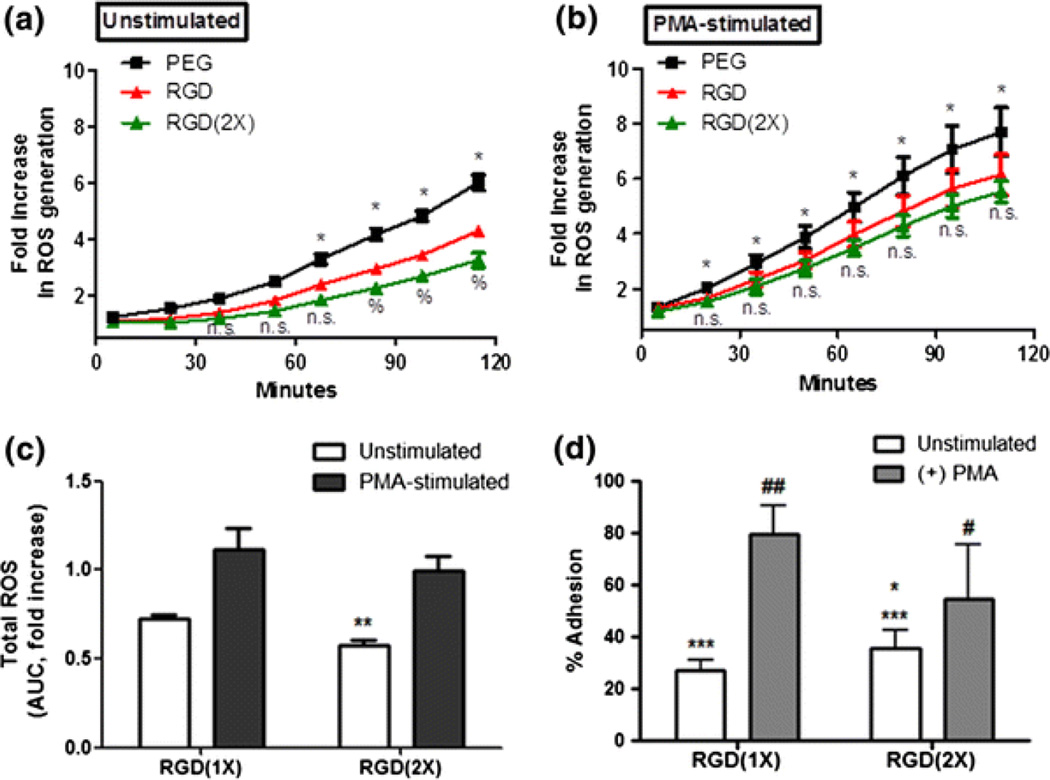
To determine the effects of RGD surface concentration in ROS suppression, either unstimulated or PMA-stimulated neutrophils were allowed to adhere to hydrogels containing 2.6 μmol/mL of RGD(1X) or 5.2 μmol/mL of RGD(2X) (Fig. 3). We confirm no reduction in ROS suppressive effect with the inclusion of higher concentrations of RGD. In fact, the use of RGD(2X) was a more effective inhibitor of ROS generation across all timepoints, (*p < 0.01 significance at ≥65 min for unstimulated cells and significant at ≥20 min for PMA-stimulated cells) when compared to PEG alone. Incubation on RGD(2X) significantly increased the level of ROS suppression when compared to neutrophils incubated on RGD at late time points (≥80 min) (%p < 0.01, RGD(1X) vs. RGD(2X)), though no significance was detected between these conditions in early incubation. Throughout the incubation, 42.6% ROS suppression was obtained from the RGD(2X) containing hydrogels compared to the RGD(1X) (27.7%), indicating that RGD-mediated suppression was increased with increased availability of RGD on the substrate (Fig. 3c). Unstimulated neutrophils were adherent to RGD inclusive gels at a level of 27.3% adhesion, which is approximately 8% lower than unstimulated neutrophil adhesion to RGD(2X) (Fig. 3d). The increase in adherent neutrophils could potentially account for an enhanced ROS suppression. Though, to determine the effect of early integrin activation in conjunction with increased RGD concentration, PMA-stimulated neutrophils on RGD(1X) and RGD(2X) were compared (Figs. 2b and 2c). The difference between RGD(1X) and RGD (2X) effective ROS inhibition when unstimulated neutrophils were evaluated at late time points did not hold true for PMA-stimulated neutrophils (Fig. 3b). However, across all timepoints the total ROS suppression of PMA-stimulated neutrophil on RGD(2X) was 28.6%, an improvement upon the lower effective suppression (19.9%) seen on RGD(1X) (Fig. 3c).
Figure 3.
ROS production as an effect of RGD concentration. Time-course ROS monitoring from (a) untreated and (b) PMA-stimulated neutrophils seeded on hydrogels containing PEG alone, RGD and RGD(2X). *Difference between RGD(2X) and PEG, p < 0.01, %Difference between RGD(1X) and RGD(2X), p < 0.01. (c) Total ROS suppression from adherent neutrophils to the RGD using AUC. Values represent the means of triplicates ± SE of >three independent experiments. **Difference between RGD(1X) and RGD(2X), p < 0.01. (d) % adhesion of unstimulated and PMA-stimulated cells on RGD(1X) and RGD(2X). ***Difference between the PEG and RGD containing gels, p < 0.0001, *Difference between RGD(1X) and RGD(2X), p < 0.05, #Difference between unstimulated and PMA-stimulated cells, ###p < 0.0001, #p < 0.05
αVβ3 Antibody and Peptide Adhesion Inhibitory Effects on ROS Generation
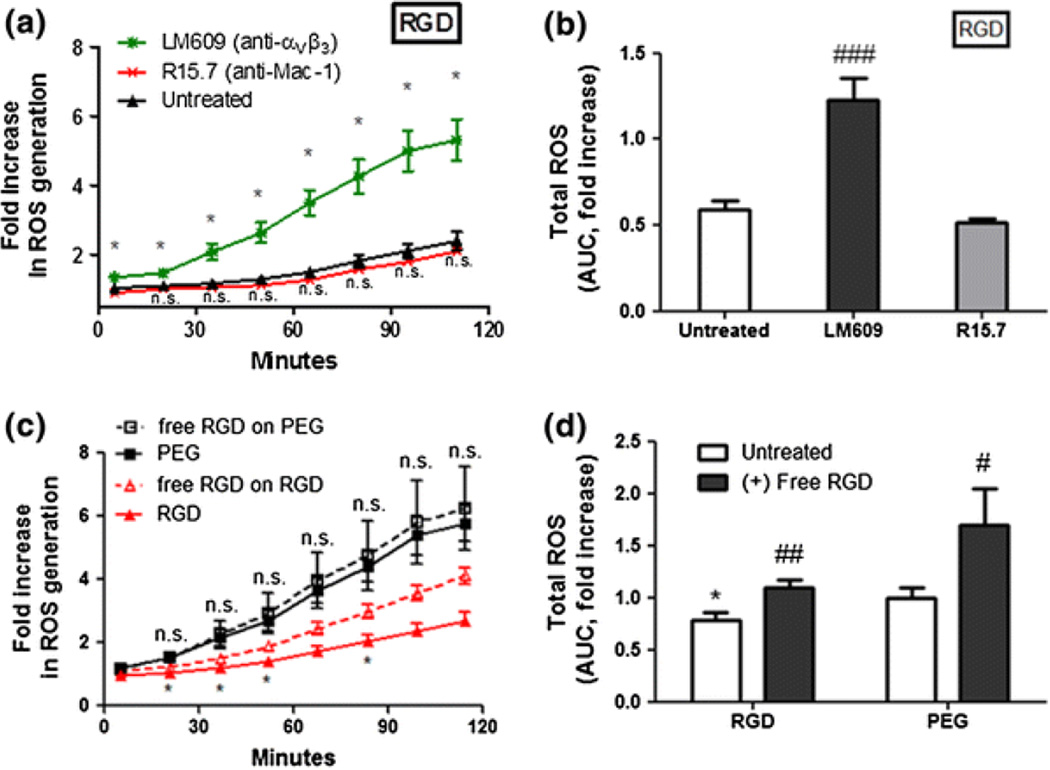
The results of neutrophil adhesion assays using integrin ligand-modified hydrogels verified unique integrin specificity in this study (Fig. 1). We conducted integrin antibody blocking experiments to validate the effects of integrin-dependent adhesion on ROS production. Neutrophils were pre-incubated with antibodies against αVβ3 and Mac-1 integrins prior to seeding on hydrogels. The ROS secretion was evaluated over a 2 h period. When αVβ3 integrin was blocked with antibody (LM609) and integrin blocked neutrophils were seeded on RGD-presenting substrates, continuous ROS production was observed throughout the incubation (*p < 0.05 when compared to untreated neutrophils on RGD, Fig. 4a), eliminating the suppressive effect of RGD. Use of blocking antibody against Mac-1 (R15.7) on neutrophils seeded on RGD had a negligible effect on ROS at individual timepoints (Fig. 4a), compared to unstimulated cells on RGD.
Figure 4.
Effects of anti-integrin antibody blocking and free RGD blocking of adhesion on ROS secretion. Neutrophils were pre-incubated with 10 μg/mL of αVβ3 integrin antibody (LM609) or β2 integrin antibody (R15.7) and seeded on the gels. ROS production from PEG hydrogels containing (a) RGD. *Difference when compared to untreated neutrophils on RGD p < 0.05; (b) Total ROS suppression (AUC) when normalized to PEG values represent the means of triplicates ± SE of >three independent experiments. *Difference compared to untreated cells, p < 0.001, ###Difference when compared with untreated cells, p < 0.0001. (c) Unstimulated neutrophils were pre-incubated with free RGD peptides (50 μg/mL) and seeded on RGD containing hydrogels or PEG alone. *Difference when compared with untreated cells (without free RGD) on RGD, p < 0.001. (d) Total ROS (AUC) induced by free RGD ligation. *Difference compared with PEG, p < 0.05, #Difference when compared with untreated cells (without free RGD), p < 0.05, ##p < 0.01
In order to verify that ROS suppression was an effect of αVβ3 integrin-dependent adhesion 22 and not integrin receptor occupation, cells were pre-incubated with soluble free RGD peptide prior to seeding on PEG and RGD containing gels (Fig. 4c). The use of soluble free RGD, at saturating concentrations,2,38 as an inhibitor of neutrophil adhesion to immobilized RGD on the hydrogel would also eliminate the use of LM609 as an αVβ3-blocking moiety with the potential for neutrophil activation.15,40 For a 2 h measurement, the highest level of ROS was detected to a similar degree from both free RGD primed cells and untreated cells on the biologically inert PEG gel, indicating that free RGD occupation of integrins alone did not suppress or increase the incidence of oxidative burst (Fig. 4c). On RGD hydrogel, pre-incubation with free RGD peptide partially inhibited the adhesion-mediated ROS suppression, producing a significantly increased amount of ROS than the untreated cells on RGD at 20, 35, 50 and 80 min. Across all timepoints, the pre-incubation with free RGD had no statistically significant effect in neutrophil ROS suppression when compared to the PEG. However, a significant reduction in the suppressive effect (1.4-fold) was observed from neutrophils adherent to RGD after pre-incubated with free RGD, when compared to untreated cells adherent to RGD (Fig. 4d). Our results further clarify the role of ECM–receptor interactions, suggesting that αVβ3 integrin occupation by RGD plays a crucial role in inhibiting ROS generation through functional adhesion, and not solely through receptor binding.
Inhibition of p38 MAPK Activation Through ROS
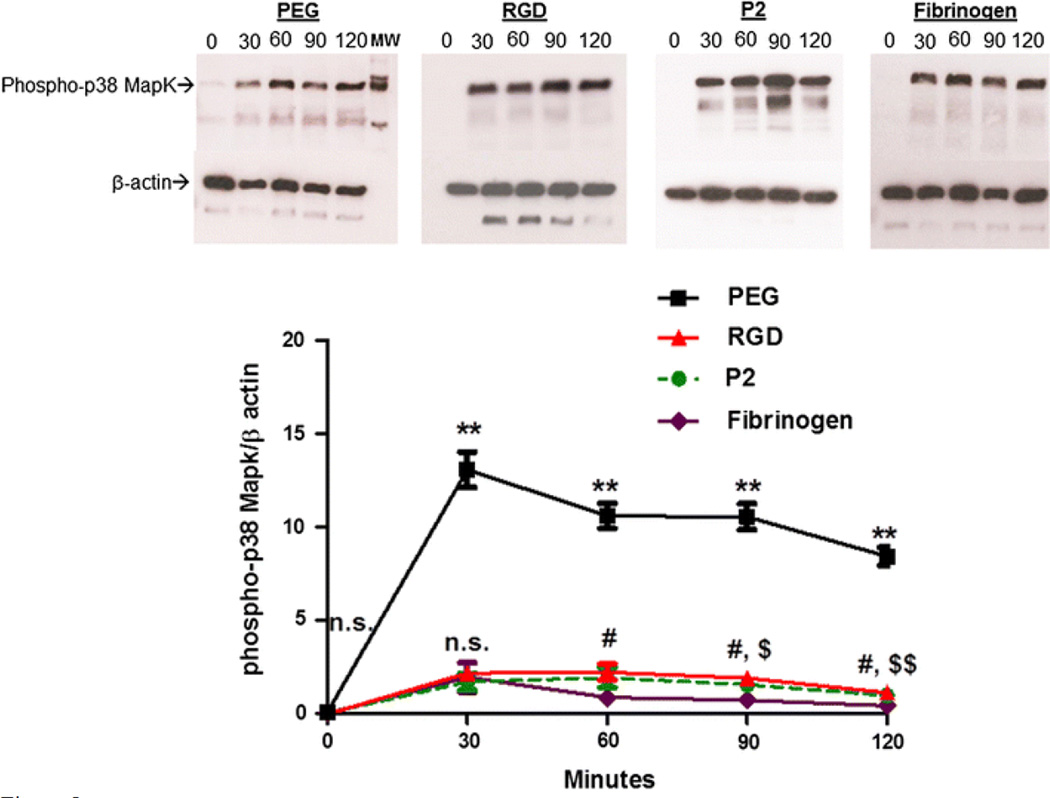
MAPKs, particularly p38 MAP kinases, have been shown to regulate superoxide production and, conversely, be regulated by ROS.1,12,13 Here, we investigated whether p38 MAPK was involved in the αVβ3 integrin binding to the peptide ligand RGD and subsequent oxidant suppression. Following incubation on PEG, RGD and P2 hydrogels over a 2 h period, adherent neutrophils were retrieved, and lysates were collected for immunoblotting activated p38 MAPK with a phospho-p38 MAPK antibody. The elevation of phospho-p38 MAPK was evaluated at 0, 30, 60, 90 and 120 min timepoints of neutrophil incubation on Fg, PEG, RGD and P2 hydrogels, corresponding to ROS detection in previous experiments. The expression levels of p38 MAPK were similar for RGD and P2 presenting gels at 30 min and gradually increased during incubation time (Figs. 5a and 5b). The highest phospho-p38 MAPK was obtained from the non-adherent cells seeded on PEG. Fibrinogen adherent cells showed lower p38 MAPK levels among the tested substrates. Interestingly, after the highest induction of p38 MAPK reached at 30 min in all four substrates, the expression remained at similar levels thereafter, particularly in PEG and Fg. The result indicates that the integrin-mediated adhesion through ECM substrates inhibit the phosphorylation of p38 MAPK, suggesting that neutrophil oxidant production is concurrent with non-adherent neutrophil ROS production at high levels on PEG, though attenuated by adhesion to Fg-derived peptides, RGD and P2.
Figure 5.
p38 MAPK activation from ECM peptide adherent neutrophils. (a) Western blots of p38 MAPK activity in isolated neutrophils seeded on Fg-coated coverslips, PEG, P2 and RGD peptide hydrogels following 0, 30, 60, 90, or 120 min incubation at 37 °C in sealed chambers. (b) Quantitative measurement of phospho-p38 MAPK. Western blots were performed in triplicate. Analysis conducted with Image Studio Lite software intensity measurements and compared to β-actin for each individual blot. **Difference when compared with cells on Fg, RGD and P2, p < 0.001, #Difference between RGD adherent cells and cells attached to Fg, p < 0.01, $Difference between P2 adherent cells and cells attached to Fg, p < 0.01, $$p < 0.001
Discussion
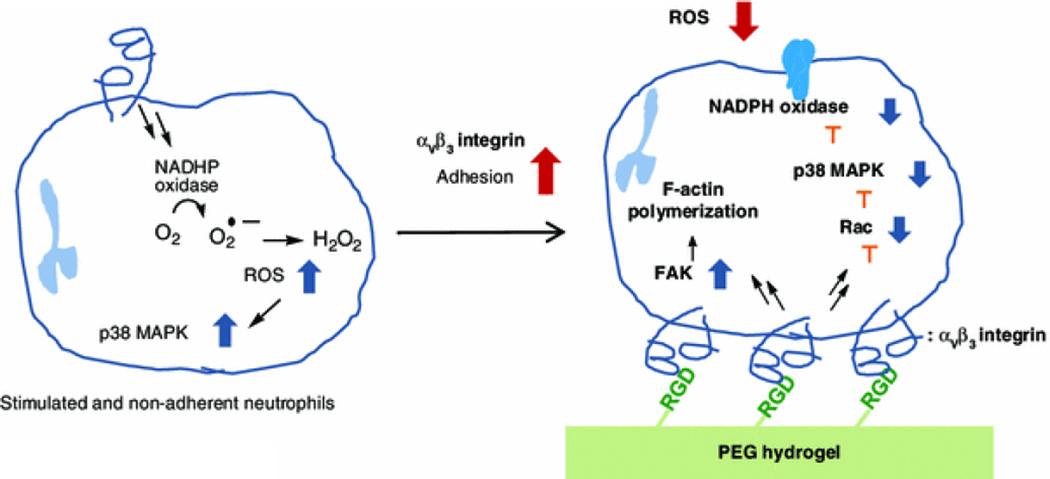
While viewed primarily as adhesion molecules, integrins play important roles in signaling neutrophil functional activities, including migration and phagocytosis. Through integrin-mediated “outside-in” signaling, integrin–ECM interactions initiate cellular functions that are critical to acute autoimmune responses. Neutrophil adhesions to ECM proteins, such as Fg and Fn, have resulted in suppression of oxidant production and release through a combination of integrin-dependent adhesion and resultant cytoskeletal reorganization.21 These functions have speculatively been attributed to β2 and β1 integrin binding to Fg and Fn, respectively.31,33 In this study, we focused on neutrophil responses from αVβ3 integrin and RGD interactions, a bioactive domain repeated in Fg and Fn, in addition to Mac-1 (αMβ2) response to P2, a bioactive domain found in the γ-chain of Fg. The significance of Fg and Fn in relation to neutrophil ROS generation is particularly important when considering the proinflammatory response elicited in fibrotic tissue containing a Fg and Fn enriched extravascular matrix. The abnormal neutrophil oxidant generation in the ECM of tissues has been considered important to models of tissue damage and dysfunction in tumor metastasis and pulmonary fibrosis.16,27 Interestingly, neutrophil infiltration, along with ROS production, has been determined a precursor to breast cancer metastasis into the lung, with fibroblast and neutrophil tissue remodeling of the lung detectable well in advance of primary tumor cell metastasis.26,35 While both Fg and Fn facilitate neutrophil interactions via integrins, the complex nature of a multi-motif whole protein prevents integrin-specific functional study of human neutrophils without the use of cross-reactive antibodies. Here, we avoid the complications of multi-domain interactions presented by whole protein by using PEG hydrogels that are conjugated to present only the bioactive domains of interest, RGD and P2. As our positive controls, Fg was adsorbed to glass coverslips to completely cover the glass surface and to avoid non-specific interactions with glass. During surface coating, the conformation of Fg is random, and the active binding sites presented are variable, controlled mostly by polarizing interactions with the substrate. This method does not facilitate evaluation of integrin specific cell function, as both multi-integrin–protein interactions are uncontrolled, as is the density of the investigated binding site. In this study, however, we do not attempt to replicate Fg in its binding density, but rather (1) attempt to determine which, if any, of the relevant binding domains could potentiate an ROS inhibitory response, and (2) if that response is integrin specific. We demonstrate both of those effects using the RGD-modified PEG gel. With the use of a highly integrin-specific bioactive hydrogels, we demonstrated that the neutrophil binding to RGD via αVβ3 integrin following firm adhesion is largely responsible for the functional suppression of ROS oxidant release (Fig. 6).
Figure 6.
Schematic representation of αVβ3 integrin ligation and adhesion-mediated ROS suppression of neutrophils. The ROS suppression from adhesive neutrophils is regulated by multiple intracellular molecules through extracelluar integrin ligation. This inhibitory effect in ROS production was distinctively observed as an effect of αVβ3 integrin ligation
We first examined αVβ3 and Mac-1 integrin adhesion to peptide-modified PEG hydrogels, which allowed us to measure neutrophil adhesion solely as an effect of integrin-specific ligand binding (Fig. 1). Neutrophils were non-adherent to the biologically inert PEG gels, but firmly adherent to Fg, and Fg-derived peptides, RGD and P2 throughout incubation time (120 min). The adherent cells on RGD presenting hydrogels are distinctive from the cells settling on the surface of PEG alone, demonstrating the ability to firmly attach and spread, as shown in Figs. 1b and 1c. More specifically, we verified that neutrophil adhesion via RGD/αVβ3 is concentration dependent, indicating the importance of receptor–ligand availability for adhesion. Additionally, we confirmed that neutrophil adhesion to P2 peptide derived from Fg is Mac-1 specific by antibody treatment. These data provide a platform in support of chemically engineered biomimetic matrices for use in investigation of integrin-targeted cell function. The bioengineered matrix has eliminated many confounding issues related to quantitative neutrophil studies on substrates previously used, including glass, plastics, and whole protein matrices, in addition to providing ECM-like microenvironments.5,24
Extracellular oxygen radicals are released though the assembly of NADPH-oxidase that gives rise to intracellular production of oxygen radicals and plasma membrane reorganization.17 Studies have shown higher extracellular ROS production from nonspecifically adhered neutrophils on an IgG-coated surface than on plasma protein or Fg.20 The measurement of ROS as an effect of specific integrin–ligand interactions allows us to present the first report of ROS suppression in correlation with αVβ3-RGD ligation-mediated adhesion (Fig. 2). Interestingly, a distinctive inhibitory effect of integrins in ROS generation were found; αVβ3 integrin showed functional roles in both unstimulated and PMA-stimulated neutrophils while Mac-1 was more functional in resting state ROS production. ROS suppression by P2 appears most effective at early timepoints, whereas RGD-mediated suppression is most effective at late timepoints. Additionally, the highest adhesion was found in RGD+P2 gels, but the ROS suppression was more effective in RGD containing gels. This finding implicates complex regulatory mechanisms from multiple bindings in respiratory burst, concurrent with the adhesion. Specifically, we found that adhesion to RGD via the αVβ3 integrin delays and suppress the release of ROS, quite effectively when compared to the robust release of ROS of non-adherent cells on PEG alone. 1.3-fold higher ROS suppression was achieved from the RGD(2X) than the RGD(1X) (Fig. 3), which indicated that αVβ3-RGD mediated ROS inhibition is adhesion dependent. The correlation between adhesion and ROS production was also examined in Fig. 4, demonstrating that anti-αVβ3 antibody pre-incubated cells showed decreased adhesion level but significant increase in ROS generation, particularly in RGD containing hydrogels. Interestingly, free RGD peptide pretreatment did not suppress the induction of oxygen metabolites as effectively as that of untreated neutrophils (Fig. 4). An excess amount of free RGD occupied most αVβ3 integrins of the neutrophils, competitively inhibiting αVβ3 from accessing hydrogel presented RGD, confirming that integrin-mediated ROS suppression is adhesion dependent. It also explains the putative role of RGD containing ECM fragments released from tissue damage; free RGD sequence could potentially induce ROS through αVβ3 receptor engagement, inhibiting cell adhesion required for ROS suppression. The delay of oxygen radical metabolite generation through the αVβ3-RGD binding and firm adhesion suggests a protective role against inappropriate tissue damage during normal neutrophil migration to inflammatory sites.37
In conclusion, this study has illustrated the specificity of two distinct integrin receptors, αVβ3 and Mac-1 to their ligand-binding partners, RGD and P2. Using PEG hydrogels, physiologically relevant ECM mimetic gels, we have identified the role of these integrins in neutrophil respiratory burst. Neutrophil adhesion, ROS production, and p38MAPK expression were investigated by specific integrin ligand coupled PEG hydrogels to exclude interference with other integrins and ligands from ECM binding domains. The results are the first to demonstrate that the inhibition of ROS production was dependent upon αVβ3 integrin-mediated adhesion. Moreover, biocompatible and functional PEG hydrogels provide a variety of biological applications as a modular tool to investigate individual integrin and membrane receptor-regulated cellular responses. Further study of matrix dictated cellular function will lead to significant therapeutic developments for pathologies involving robust cell infiltration and cell mediated ECM remodeling, including autoimmune diseases and metastatic cancers.
Acknowledgments
The authors would like to thank Drs. Jordan Pober and Dan Wu for helpful discussion and advising. The authors would also like to thank Dr. Themis Kyriakides for critical review. This work was supported in part of Grant Support of T32 GM086287 (P.I. Laura E. Niklason).
Footnotes
Conflict of interest
Hye-Yeong Kim, Eleni A. Skokos, Deborah J. Myer, Perez Agaba, and Anjelica L. Gonzalez declare that they have no conflicts of interest.
Ethical Standards
All human subjects research was carried out in accordance with Yale University Human Investigation Committee (HIC) of the Internal Review Board (IRB) as part of the Human Research Protection Program guidelines and approved as HIC protocol #0902004786. No animal studies were carried out by the authors for this article.
References
- 1.Aoshiba K, Yasui S, Hayashi M, Tamaoki J, Nagai A. Role of p38-mitogen-activated protein kinase in spontaneous apoptosis of human neutrophils. J. Immunol. 1999;162:1692–1700. [PubMed] [Google Scholar]
- 2.Asman B, Strand V, Bylin G, Bergstrom K. Peripheral neutrophils after allergic asthmatic reactions. Int. J. Clin. Lab. Res. 1997;27:185–188. doi: 10.1007/BF02912455. [DOI] [PubMed] [Google Scholar]
- 3.Baldridge CW, Gerard RW. The extra respiration of phagocytosis. Am. J. Physiol. 1933;103:235–236. [Google Scholar]
- 4.Balliet RM, Capparelli C, Guido C, Pestell TG, Martinez-Outschoorn UE, Lin Z, Whitaker-Menezes D, Chiavarina B, Pestell RG, Howell A, Sotgia F, Lisanti MP. Mitochondrial oxidative stress in cancer-associated fibroblasts drives lactate production, promoting breast cancer tumor growth: understanding the aging and cancer connection. Cell Cycle. 2011;10:4065–4073. doi: 10.4161/cc.10.23.18254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berman DM, Bosenberg MW, Orwant RL, Thurberg BL, Draetta GF, Fletcher CDM, Loda M. Investigative pathology: leading the post-genomic revolution. Lab. Investig. 2012;92:4–8. doi: 10.1038/labinvest.2011.147. [DOI] [PubMed] [Google Scholar]
- 6.Borregaard N, Cowland JB. Granules of the human neutrophilic polymorphonuclear leukocyte. Blood. 1997;89:3503–3521. [PubMed] [Google Scholar]
- 7.Cai LL, Liu P, Li X, Huang X, Ye YQ, Chen FY, Yuan H, Hu FQ, Du YZ. Rgd peptide-mediated chitosan-based polymeric micelles targeting delivery for integrin-overexpressing tumor cells. Int. J. Nanomed. 2011;6:3499–3508. doi: 10.2147/IJN.S26670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen N, Zhang Z, Soontornworajit B, Zhou J, Wang Y. Cell adhesion on an artificial extracellular matrix using aptamer-functionalized PEG hydrogels. Biomaterials. 2012;33:1353–1362. doi: 10.1016/j.biomaterials.2011.10.062. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez AL, El-Bjeirami W, West JL, McIntire LV, Smith CW. Transendothelial migration enhances integrin-dependent human neutrophil chemokinesis. J. Leukoc. Biol. 2007;81:686–695. doi: 10.1189/jlb.0906553. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez AL, Gobin AS, West JL, McIntire LV, Smith CW. Integrin interactions with immobilized peptides in polyethylene glycol diacrylate hydrogels. Tissue Eng. 2004;10:1775–1786. doi: 10.1089/ten.2004.10.1775. [DOI] [PubMed] [Google Scholar]
- 11.Gopalan PK, Burns AR, Simon SI, Sparks S, McIntire LV, Smith CW. Preferential sites for stationary adhesion of neutrophils to cytokine-stimulated huvec under flow conditions. J. Leukoc. Biol. 2000;68:47–57. [PubMed] [Google Scholar]
- 12.Hazan-Halevy I, Levy T, Wolak T, Lubarsky I, Levy R, Paran E. Stimulation of NADPH oxidase by angiotensin ii in human neutrophils is mediated by erk, p38 map-kinase and cytosolic phospholipase A2. J. Hypertens. 2005;23:1183–1190. doi: 10.1097/01.hjh.0000170381.53955.68. [DOI] [PubMed] [Google Scholar]
- 13.Herlaar E, Brown Z. P38 MAPK signalling cascades in inflammatory disease. Mol. Med. Today. 1999;5:439–447. doi: 10.1016/s1357-4310(99)01544-0. [DOI] [PubMed] [Google Scholar]
- 14.Hoffman AS. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2012;64(Supplement):18–23. doi: 10.1016/s0169-409x(01)00239-3. [DOI] [PubMed] [Google Scholar]
- 15.Jakus Z, Berton G, Ligeti E, Lowell CA, Mócsai A. Responses of neutrophils to anti-integrin antibodies depends on costimulation through low affinity fcγrs: full activation requires both integrin and nonintegrin signals. J. Immunol. 2004;173:2068–2077. doi: 10.4049/jimmunol.173.3.2068. [DOI] [PubMed] [Google Scholar]
- 16.Kanazawa H, Shiraishi S, Hirata K, Yoshikawa J. Imbalance between levels of nitrogen oxides and peroxynitrite inhibitory activity in chronic obstructive pulmonary disease. Thorax. 2003;58:106–109. doi: 10.1136/thorax.58.2.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karlsson A, Dahlgren C. Assembly and activation of the neutrophil NADPH oxidase in granule membranes. Antioxid Redox Signal. 2002;4:49–60. doi: 10.1089/152308602753625852. [DOI] [PubMed] [Google Scholar]
- 18.Kindzelskii AL, Zhou MJ, Haugland RP, Boxer LA, Petty HR. Oscillatory pericellular proteolysis and oxidant deposition during neutrophil locomotion. Biophys. J. 1998;74:90–97. doi: 10.1016/S0006-3495(98)77770-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lishko VK, Podolnikova NP, Yakubenko VP, Yakovlev S, Medved L, Yadav SP. Multiple binding sites in fibrinogen for integrin αmβ2 (mac-1) J. Biol. Chem. 2004;279:44897–44906. doi: 10.1074/jbc.M408012200. [DOI] [PubMed] [Google Scholar]
- 20.Liu L, Elwing H, Karlsson A, Nimeri G, Dahlgren C. Surface-related triggering of the neutrophil respiratory burst. Characterization of the response induced by igg adsorbed to hydrophilic and hydrophobic glass surfaces. Clin. Exp. Immunol. 1997;109:204–210. doi: 10.1046/j.1365-2249.1997.4311329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lowell CA, Berton G. Integrin signal transduction in myeloid leukocytes. J. Leukoc. Biol. 1999;65:313–320. doi: 10.1002/jlb.65.3.313. [DOI] [PubMed] [Google Scholar]
- 22.Maheshwari G, Brown G, Lauffenburger DA, Wells A, Griffith LG. Cell adhesion and motility depend on nanoscale RGD clustering. J. Cell Sci. 2000;113:1677–1686. doi: 10.1242/jcs.113.10.1677. [DOI] [PubMed] [Google Scholar]
- 23.Nathan CF. Neutrophil activation on biological surfaces. Massive secretion of hydrogen peroxide in response to products of macrophages and lymphocytes. J. Clin. Investig. 1987;80:1550–1560. doi: 10.1172/JCI113241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nathan CF. Respiratory burst in adherent human neutrophils: triggering by colony-stimulating factors CSF-GM and CSF-G. Blood. 1989;73:301–306. [PubMed] [Google Scholar]
- 25.Okay O, Sariisik SB. Swelling behavior of poly(acrylamide-co-sodium acrylate) hydrogels in aqueous salt solutions: theory versus experiments. Eur. Polym. J. 2000;36:393–399. [Google Scholar]
- 26.Orr FW, Warner DJ. Effects of systemic complement activation and neutrophil-mediated pulmonary injury on the retention and metastasis of circulating cancer cells in mouse lungs. Lab. Investig. 1990;62:331–338. [PubMed] [Google Scholar]
- 27.Padmanabhan J, Gonzalez AL. The effects of extracellular matrix proteins on neutrophil–endothelial interaction—a roadway to multiple therapeutic opportunities. Yale J. Biol. Med. 2012;85:167–185. [PMC free article] [PubMed] [Google Scholar]
- 28.Reumaux D, Kuijpers TW, Hordijk PL, Duthilleul P, Roos D. Involvement of fcgamma receptors and beta2 integrins in neutrophil activation by anti-proteinase-3 or anti-myeloperoxidase antibodies. Clin. Exp. Immunol. 2003;134:344–350. doi: 10.1046/j.1365-2249.2003.02280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sbarra AJ, Karnovsky ML. The biochemical basis of phagocytosis. I. Metabolic changes during the ingestion of particles by polymorphonuclear leukocytes. J. Biol. Chem. 1959;234:1355–1362. [PubMed] [Google Scholar]
- 30.Shappell SB, Toman C, Anderson DC, Taylor AA, Entman ML, Smith CW. Mac-1 (cd11b cd18) mediates adherence-dependent hydrogen-peroxide production by human and canine neutrophils. J. Immunol. 1990;144:2702–2711. [PubMed] [Google Scholar]
- 31.Sud’ina GF, Tatarintsev AV, Koshkin AA, Zaitsev SV, Fedorov NA, Varfolomeev SD. The role of adhesive interactions and extracellular matrix fibronectin from human polymorphonuclear leukocytes in the respiratory burst. Biochim. Biophys. Acta. 1991:257–260. doi: 10.1016/0167-4889(91)90187-3. 1091. [DOI] [PubMed] [Google Scholar]
- 32.Tibbitt MW, Anseth KS. Hydrogels as extracellular matrix mimics for 3d cell culture. Biotechnol. Bioeng. 2009;103:655–663. doi: 10.1002/bit.22361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Umanskiy K, Robinson C, Cave C, Williams MA, Lentsch AB, Cuschieri J, Solomkin JS. NADPH oxidase activation in fibronectin adherent human neutrophils: a potential role for beta1 integrin ligation. Surgery. 2003;134:378–383. doi: 10.1067/msy.2003.253. [DOI] [PubMed] [Google Scholar]
- 34.Williams MA, Solomkin JS. Integrin-mediated signaling in human neutrophil functioning. J. Leukoc. Biol. 1999;65:725–736. doi: 10.1002/jlb.65.6.725. [DOI] [PubMed] [Google Scholar]
- 35.Wu QD, Wang JH, Bouchier-Hayes D, Redmond HP. Neutrophil-induced transmigration of tumour cells treated with tumour-conditioned medium is facilitated by granulocyte-macrophage colony-stimulating factor. Eur. J. Surg. 2000;166:361–366. doi: 10.1080/110241500750008899. [DOI] [PubMed] [Google Scholar]
- 36.Yakubenko VP, Solovjov DA, Zhang L, Yee VC, Plow EF, Ugarova TP. Identification of the binding site for fibrinogen recognition peptide γ383–395 within the αmi-domain of integrin αmβ2. J. Biol. Chem. 2001;276:13995–14003. doi: 10.1074/jbc.M010174200. [DOI] [PubMed] [Google Scholar]
- 37.Yan SR, Novak MJ. Diverse effects of neutrophil integrin occupation on respiratory burst activation. Cell. Immunol. 1999;195:119–126. doi: 10.1006/cimm.1999.1524. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Z, Ni J, Chen L, Yu L, Xu JW, Ding JD. Encapsulation of cell-adhesive rgd peptides into a polymeric physical hydrogel to prevent postoperative tissue adhesion. J. Biomed. Mater. Res. Part B. 2012;100B:1599–1609. doi: 10.1002/jbm.b.32728. [DOI] [PubMed] [Google Scholar]
- 39.Zhao T, Benard V, Bohl BP, Bokoch GM. The molecular basis for adhesion-mediated suppression of reactive oxygen species generation by human neutrophils. J. Clin. Investig. 2003;112:1732–1740. doi: 10.1172/JCI19108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zheng L, Sjölander A, Eckerdal J, Andersson T. Antibody-induced engagement of beta 2 integrins on adherent human neutrophils triggers activation of p21ras through tyrosine phosphorylation of the protooncogene product vav. Proc. Natl. Acad. Sci. 1996;93:8431–8436. doi: 10.1073/pnas.93.16.8431. Over 8.5 million scientific documents at your fingertips. [DOI] [PMC free article] [PubMed] [Google Scholar]