Abstract
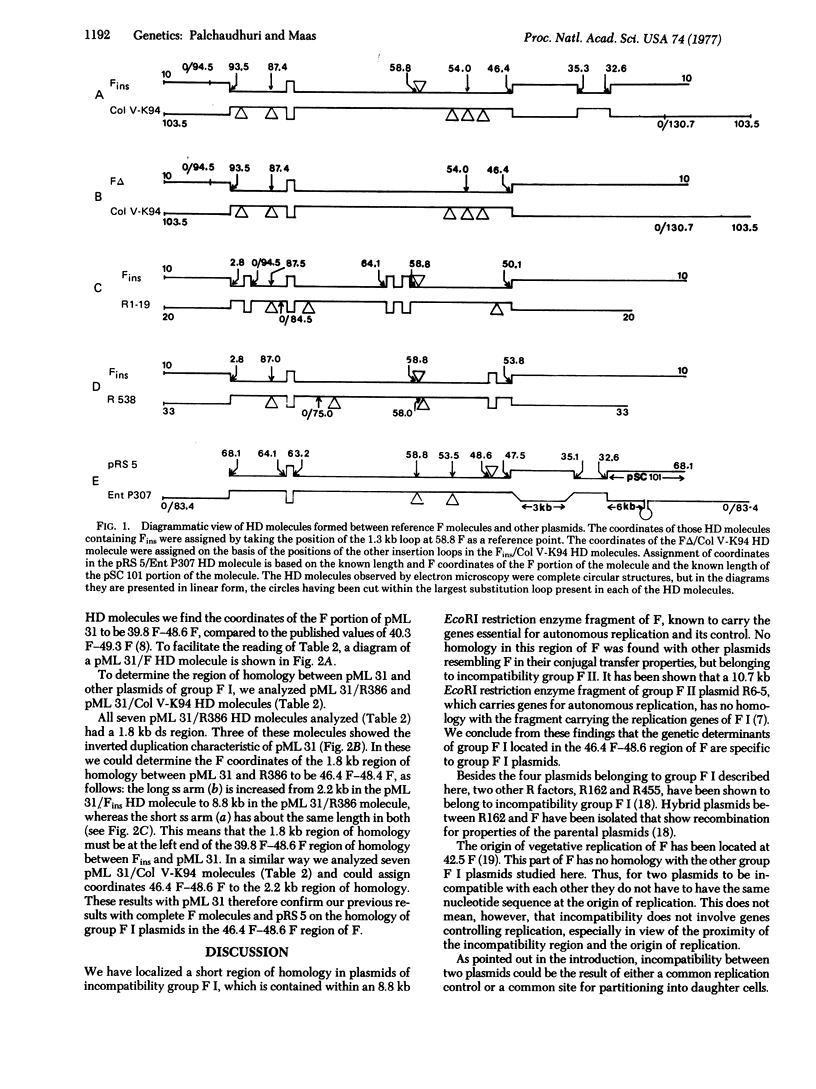
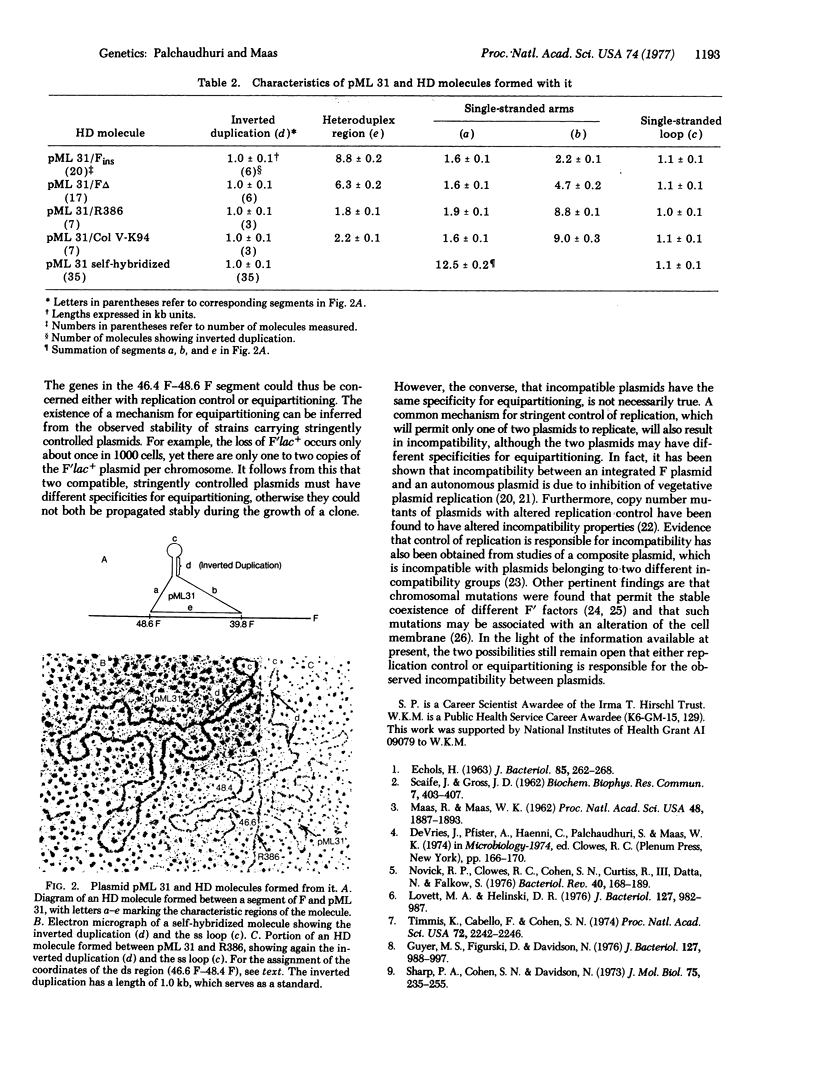
We have mapped a region of homology within a 2.2 kilobase segment of plasmids of incompatibility group F I, WHICH IS CONTAINED IN AN EcoRI restriction enzyme fragment bearing genes for autonomous replication; The coordinates of the 2.2 kilobase segment are 46.4F-48.6F on the physical map of the F plasmid. The orgin of vegetative replication has been mapped at 42.5F, at which site F has no sequence homology with other group F I plasmids. Plasmids belonging to other incompatibility groups have no homology with the 46.4-48.6 F segment. Possible mechanisms underlying incompatibility are discussed. We think that incompatibility is due to either a limitation in a common equipartitioning mechanism or a limitation in a common replication control. In either case, the common mechanism is specific for plasmids belonging to a given incompatibility group.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cabello F., Timmis K., Cohen S. N. Replication control in a composite plasmid constructed by in vitro linkage of two distinct replicons. Nature. 1976 Jan 29;259(5541):285–290. doi: 10.1038/259285a0. [DOI] [PubMed] [Google Scholar]
- Dennison S. Naturally occurring R factor, derepressed for pilus synthesis, belonging to the same compatibility group as the sex factor F of Escherichia coli K-12. J Bacteriol. 1972 Jan;109(1):416–422. doi: 10.1128/jb.109.1.416-422.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubnau E., Maas W. K. Inhibition of replication of an F'lac episome in Hfr cells of Escherichia coli. J Bacteriol. 1968 Feb;95(2):531–539. doi: 10.1128/jb.95.2.531-539.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echols H. PROPERTIES OF F' STRAINS OF ESCHERICHIA COLI SUPERINFECTED WITH F-LACTOSE AND F-GALATOSE EPISOMES. J Bacteriol. 1963 Feb;85(2):262–268. doi: 10.1128/jb.85.2.262-268.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenlaub R., Figurski D., Helinski D. R. Bidirection replication from a unique origin in a mini-F plasmid. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1138–1141. doi: 10.1073/pnas.74.3.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald T. J., Miller J. N., Sykes J. A. Treponema pallidum (Nichols strain) in tissue cultures: cellular attachment, entry, and survival. Infect Immun. 1975 May;11(5):1141–1146. doi: 10.1128/iai.11.5.1141-1146.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer M. S., Figurski D., Davidson N. Electron microscope study of a plasmid chimera containing the replication region of the Escherichia coli F plasmid. J Bacteriol. 1976 Aug;127(2):988–997. doi: 10.1128/jb.127.2.988-997.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horodniceanu T., Bouanchaud D. H. Genetic and physical studies of recombinant plasmids formed between an R plasmid of compatibility group FI and sex factor F of HfrH. J Bacteriol. 1976 Jan;125(1):58–67. doi: 10.1128/jb.125.1.58-67.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer V. N., Iyer R. V., Palchoudhury S. R., Becker S., Stevenson I. An aspect of the physiology of strains carrying a dnaB mutation. Impairment in F piliation and its phenotypic reversal. Mol Gen Genet. 1974;133(2):111–122. doi: 10.1007/BF00264832. [DOI] [PubMed] [Google Scholar]
- Lovett M. A., Helinski D. R. Method for the isolation of the replication region of a bacterial replicon: construction of a mini-F'kn plasmid. J Bacteriol. 1976 Aug;127(2):982–987. doi: 10.1128/jb.127.2.982-987.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAAS R., MAAS W. K. Introduction of a gene from Escherichia coli B into HFR and F-strains of Escherichia coli K-12. Proc Natl Acad Sci U S A. 1962 Nov 15;48:1887–1893. doi: 10.1073/pnas.48.11.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. P., Clowes R. C., Cohen S. N., Curtiss R., 3rd, Datta N., Falkow S. Uniform nomenclature for bacterial plasmids: a proposal. Bacteriol Rev. 1976 Mar;40(1):168–189. doi: 10.1128/br.40.1.168-189.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palchaudhuri S., Chakrabarty A. Isolation of plasmid deoxyribonucleic acid from Pseudomonas putida. J Bacteriol. 1976 Apr;126(1):410–416. doi: 10.1128/jb.126.1.410-416.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palchaudhuri S., Maas W. K. Fusion of two F-prime factors in Escherichia coli studied by electron microscope heteroduplex analysis. Mol Gen Genet. 1976 Aug 2;146(3):215–231. doi: 10.1007/BF00701244. [DOI] [PubMed] [Google Scholar]
- Palchoudhury S. R., Iyer V. N. Compatibility between two F' factors in an Escherichia coli strain bearing a chromosomal mutation affecting DNA synthesis. J Mol Biol. 1971 Apr 28;57(2):319–333. doi: 10.1016/0022-2836(71)90349-4. [DOI] [PubMed] [Google Scholar]
- SCAIFE J., GROSS J. D. Inhibition of multiplication of an Flac factor in Hfr cells of Escherichia coli K-12. Biochem Biophys Res Commun. 1962 May 11;7:403–407. doi: 10.1016/0006-291x(62)90324-8. [DOI] [PubMed] [Google Scholar]
- Saitoh T., Hiraga S. F deoxyribonucleic acid superinfected into phenocopies of donor strains. J Bacteriol. 1975 Mar;121(3):1007–1013. doi: 10.1128/jb.121.3.1007-1013.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- San Blas F., Thompson R., Broda P. An Escherichia coli K12 mutant apparently carrying two autonomous F-prime factors. Mol Gen Genet. 1974 May 21;130(2):153–163. doi: 10.1007/BF00269086. [DOI] [PubMed] [Google Scholar]
- Santos D. S., Palchaudhuri S., Maas W. K. Genetic and physical characteristics of an enterotoxin plasmid. J Bacteriol. 1975 Dec;124(3):1240–1247. doi: 10.1128/jb.124.3.1240-1247.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. A., Cohen S. N., Davidson N. Electron microscope heteroduplex studies of sequence relations among plasmids of Escherichia coli. II. Structure of drug resistance (R) factors and F factors. J Mol Biol. 1973 Apr 5;75(2):235–255. doi: 10.1016/0022-2836(73)90018-1. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Hsu M. T., Otsubo E., Davidson N. Electron microscope heteroduplex studies of sequence relations among plasmids of Escherichia coli. I. Structure of F-prime factors. J Mol Biol. 1972 Nov 14;71(2):471–497. doi: 10.1016/0022-2836(72)90363-4. [DOI] [PubMed] [Google Scholar]
- Skurray R. A., Nagaishi H., Clark A. J. Molecular cloning of DNA from F sex factor of Escherichia coli K-12. Proc Natl Acad Sci U S A. 1976 Jan;73(1):64–68. doi: 10.1073/pnas.73.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmis K., Cabello F., Cohen S. N. Cloning, isolation, and characterization of replication regions of complex plasmid genomes. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2242–2246. doi: 10.1073/pnas.72.6.2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlin B. E., Nordström K. Plasmid incompatibility and control of replication: copy mutants of the R-factor R1 in Escherichia coli K-12. J Bacteriol. 1975 Nov;124(2):641–649. doi: 10.1128/jb.124.2.641-649.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]




