Abstract
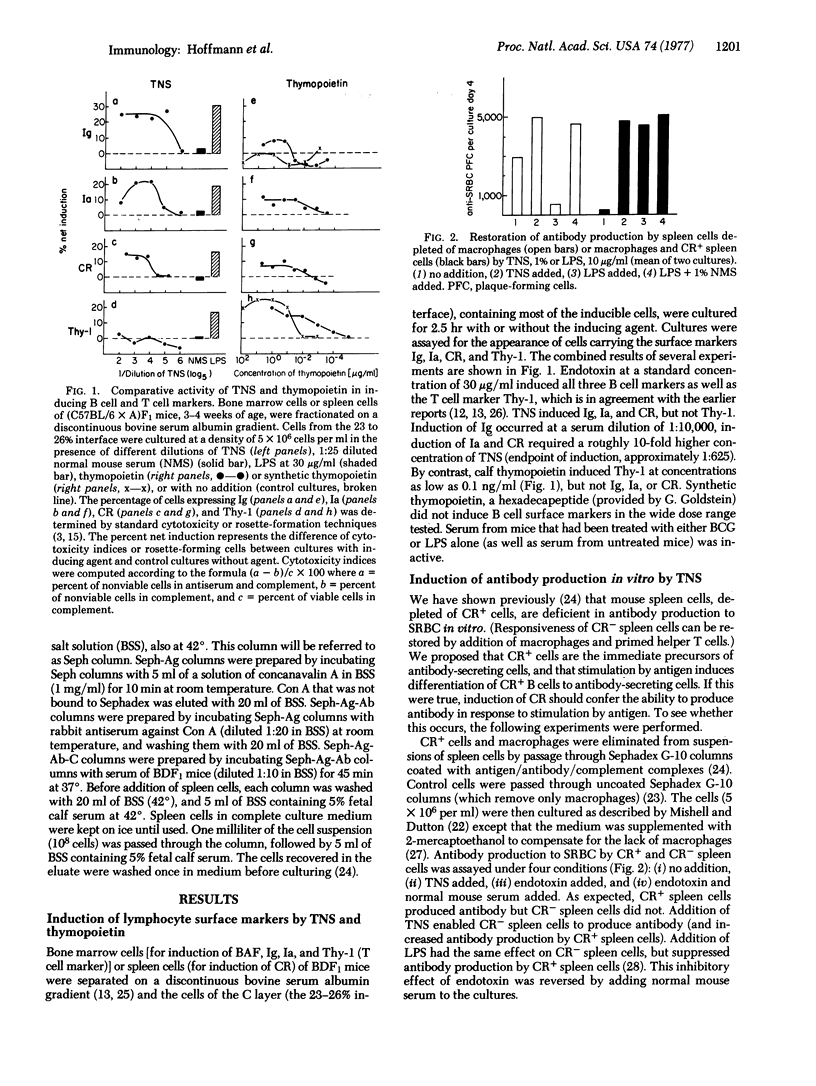
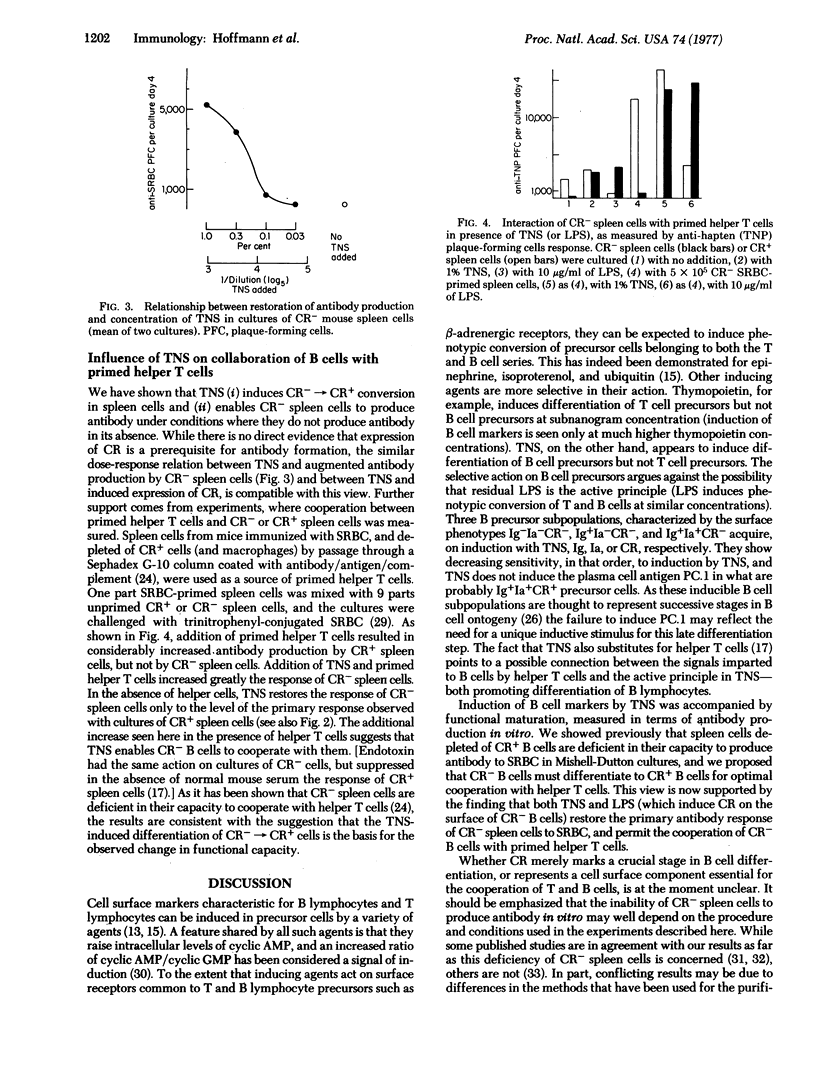
Serum from bacillus Calmette-Guerin-infected mice injected with endotoxin induces the appearance of surface immunoglobulin, Ia antigen, and complement receptor on the surface of precursor bone-marrow-derived (B) cells. While endotoxin itself causes phenotypic conversion of both thymus-derived (T) cells and B cells in vitro, the endotoxin-induced serum factor was found to be a selective inducer of B cell differentiation. Spleen cells rendered immunodeficient by removal of B cells bearing the complement receptor regained the capacity to cooperate with helper T cells and to produce antibody against red cell antigens in vitro upon upon addition of the serum factor to the culture medium. Thus, a factor that controls selective phenotypic and functional differentiation of B cells has been identified and can now be characterized,
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bianco C., Patrick R., Nussenzweig V. A population of lymphocytes bearing a membrane receptor for antigen-antibody-complement complexes. I. Separation and characterization. J Exp Med. 1970 Oct 1;132(4):702–720. doi: 10.1084/jem.132.4.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyse E. A., Miyazawa M., Aoki T., Old L. J. Ly-A and Ly-B: two systems of lymphocyte isoantigens in the mouse. Proc R Soc Lond B Biol Sci. 1968 Jun 11;170(1019):175–193. doi: 10.1098/rspb.1968.0032. [DOI] [PubMed] [Google Scholar]
- Brand A., Gilmour D. G., Goldstein G. Lymphocyte-differentiating hormone of bursa of fabricius. Science. 1976 Jul 23;193(4250):319–321. doi: 10.1126/science.180600. [DOI] [PubMed] [Google Scholar]
- Cantor H., Boyse E. A. Functional subclasses of T-lymphocytes bearing different Ly antigens. I. The generation of functionally distinct T-cell subclasses is a differentiative process independent of antigen. J Exp Med. 1975 Jun 1;141(6):1376–1389. doi: 10.1084/jem.141.6.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carswell E. A., Old L. J., Kassel R. L., Green S., Fiore N., Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3666–3670. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Hirsch J. G. Restoration of antibody-forming capacity in cultures of nonadherent spleen cells by mercaptoethanol. Science. 1972 Apr 7;176(4030):60–61. doi: 10.1126/science.176.4030.60. [DOI] [PubMed] [Google Scholar]
- Dicke K. A., Lina P. H., van Bekkum D. W. Adaptation of albumin density gradient centrifugation to human bone marrow fractionation. Rev Eur Etud Clin Biol. 1970 Mar;15(3):305–309. [PubMed] [Google Scholar]
- Dickler H. B., Kunkel H. G. Interaction of aggregated -globulin with B lymphocytes. J Exp Med. 1972 Jul 1;136(1):191–196. doi: 10.1084/jem.136.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein G. Isolation of bovine thymin: a polypeptide hormone of the thymus. Nature. 1974 Jan 4;247(5435):11–14. doi: 10.1038/247011a0. [DOI] [PubMed] [Google Scholar]
- Green S., Dobrjansky A., Carswell E. A., Kassel R. L., Old L. J., Fiore N., Schwartz M. K. Partial purification of a serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1976 Feb;73(2):381–385. doi: 10.1073/pnas.73.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerling U., Chin A. F., Abbott J., Scheid M. P. The ontogeny of murine B lymphocytes. I. Induction of phenotypic conversion of Ia-to Ia+ lymphocytes. J Immunol. 1975 Nov;115(5):1425–1431. [PubMed] [Google Scholar]
- Hoffmann M. K., Green S., Old L. J., Oettgen H. F. Serum containing endotoxin-induced tumour necrosis factor substitutes for helper T cells. Nature. 1976 Sep 30;263(5576):416–417. doi: 10.1038/263416a0. [DOI] [PubMed] [Google Scholar]
- Hoffmann M. K., Hämmerling U., Simon M., Oettgen H. F. Macrophage requirements of CR- and CR+ B lymphocytes for antibody production in vitro. J Immunol. 1976 May;116(5):1447–1451. [PubMed] [Google Scholar]
- Hoffmann M. K., Weiss O., Koenig S., Hirst J. A., Oettgen H. F. Suppression and enhancement of the T cell-dependent production of antibody to SRBC in vitro by bacterial lipopolysaccharide. J Immunol. 1975 Feb;114(2 Pt 2):738–741. [PubMed] [Google Scholar]
- Hoffmann M., Kappler J. W. Regulation of the immune response. II. Qualitative and quantitative differences between thymus- and bone marrow-derived lymphocytes in the recognition of antigen. J Exp Med. 1973 Mar 1;137(3):721–739. doi: 10.1084/jem.137.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hämmerling U., Chin A. F., Abbott J. Ontogeny of murine B lymphocytes: sequence of B-cell differentiation from surface-immunoglobulin-negative precursors to plasma cells. Proc Natl Acad Sci U S A. 1976 Jun;73(6):2008–2012. doi: 10.1073/pnas.73.6.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisielow P., Hirst J. A., Shiku H., Beverley P. C., Hoffman M. K., Boyse E. A., Oettgen H. F. Ly antigens as markers for functionally distinct subpopulations of thymus-derived lymphocytes of the mouse. Nature. 1975 Jan 17;253(5488):219–220. doi: 10.1038/253219a0. [DOI] [PubMed] [Google Scholar]
- Komuro K., Boyse E. A. In-vitro demonstration of thymic hormone in the mouse by conversion of precursor cells into lymphocytes. Lancet. 1973 Apr 7;1(7806):740–743. doi: 10.1016/s0140-6736(73)92127-2. [DOI] [PubMed] [Google Scholar]
- Ly I. A., Mishell R. I. Separation of mouse spleen cells by passage through columns of sephadex G-10. J Immunol Methods. 1974 Aug;5(3):239–247. doi: 10.1016/0022-1759(74)90108-2. [DOI] [PubMed] [Google Scholar]
- Mason D. W. The requirement for C3 receptors on the precursors of 19S and 7S antibody-forming cells. J Exp Med. 1976 May 1;143(5):1111–1121. doi: 10.1084/jem.143.5.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishell R. I., Dutton R. W. Immunization of dissociated spleen cell cultures from normal mice. J Exp Med. 1967 Sep 1;126(3):423–442. doi: 10.1084/jem.126.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parish C. R. Separation and functional analysis of subpopulations of lymphocytes bearing complement and Fc receptors. Transplant Rev. 1975;25:98–120. doi: 10.1111/j.1600-065x.1975.tb00727.x. [DOI] [PubMed] [Google Scholar]
- REIF A. E., ALLEN J. M. THE AKR THYMIC ANTIGEN AND ITS DISTRIBUTION IN LEUKEMIAS AND NERVOUS TISSUES. J Exp Med. 1964 Sep 1;120:413–433. doi: 10.1084/jem.120.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff M. C., Sternberg M., Taylor R. B. Immunoglobulin determinants on the surface of mouse lymphoid cells. Nature. 1970 Feb 7;225(5232):553–554. doi: 10.1038/225553a0. [DOI] [PubMed] [Google Scholar]
- Scheid M. P., Goldstein G., Hammerling U., Boyse E. A. Lymphocyte differentiation from precursor cells in vitro. Ann N Y Acad Sci. 1975 Feb 28;249:531–540. doi: 10.1111/j.1749-6632.1975.tb29102.x. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Old L. J., Boyse E. A. Surface alloantigens of plasma cells. J Exp Med. 1970 Jun 1;131(6):1325–1341. doi: 10.1084/jem.131.6.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T., Old L. J., McIntire K. R., Boyse E. A. Immunoglobulin and other surface antigens of cells of the immune system. J Exp Med. 1971 Oct 1;134(4):815–832. doi: 10.1084/jem.134.4.815. [DOI] [PMC free article] [PubMed] [Google Scholar]



