Abstract
Background
There is evidence that calcium (Ca2+) increases the proliferation of human advanced prostate cancer (PCa) cells but the ion channels involved are not fully understood. Here, we investigated the correlation between alpha1D-adrenergic receptor (alpha1D-AR) and the transient receptor potential vanilloid type 1 (TRPV1) expression levels in human PCa tissues and evaluated the ability of alpha1D-AR to cross-talk with TRPV1 in PCa cell lines.
Methods
The expression of alpha1D-AR and TRPV1 was examined in human PCa tissues by quantitative RT-PCR and in PCa cell lines (DU145, PC3 and LNCaP) by cytofluorimetry. Moreover, alpha1D-AR and TRPV1 colocalization was investigated by confocal microscopy in PCa cell lines and by fluorescence microscopy in benign prostate hyperplasia (BPH) and PCa tissues. Cell proliferation was assessed by BrdU incorporation. Alpha1D-AR/TRPV1 knockdown was obtained using siRNA transfection. Signalling pathways were evaluated by measurement of extracellular acidification rate, Ca2+ flux, IP3 production, western blot and MTT assay.
Results
The levels of the alpha1D-AR and TRPV1 mRNAs are increased in PCa compared to BPH specimens and a high correlation between alpha1D-AR and TRPV1 expression levels was found. Moreover, alpha1D-AR and TRPV1 are co-expressed in prostate cancer cell lines and specimens. Noradrenaline (NA) induced an alpha1D-AR- and TRPV1-dependent protons release and Ca2+ flux in PC3 cell lines; NA by triggering the activation of phospholipase C (PLC), protein kinase C (PKC) and extracellular signal-regulated kinase 1/2 (ERK1/2) pathways stimulated PC3 cell proliferation, that was completely inhibited by clopenphendioxan (WS433) and capsazepine (CPZ) combination or by alpha1D-AR/TRPV1 double knockdown.
Conclusions
We demonstrate a cross-talk between alpha1D-AR and TRPV1, that is involved in the control of PC3 cell proliferation. These data strongly support for a putative novel pharmacological approach in the treatment of PCa by targeting both alpha1D-AR and TRPV1 channels.
Electronic supplementary material
The online version of this article (doi:10.1186/1471-2407-14-921) contains supplementary material, which is available to authorized users.
Keywords: α1D-adrenoceptors, Transient receptor potential vanilloid type 1, Noradrenaline, Proliferation, PC3 cell line, Prostate cancer
Background
Calcium (Ca2+) signalling is essential for regulating physiological functions such as cell proliferation and differentiation [1]. Prostate cancer is the second most lethal tumour among men, and Ca2+ has been shown to be essential for increased cell proliferation in advanced prostate cancer (PCa) cells [2–4]. However, the ion channel(s) involved in Ca2+ entry are not fully understood and the mechanism that leads to alteration of Ca2+ handling in PCa is still poorly defined. Understanding the factors that drive PCa towards increased cell proliferation is crucial for the development of new therapies that can prevent and/or inhibit the initiation and/or progression of PCa.
Among the transient receptor potential (TRP) channel proteins, human TRP vanilloid type 1 (TRPV1) is expressed in normal prostate epithelial cells, PCa tissues and in PC3 as well as LNCaP cells [5]. The expression of TRPV1 has been found to be significantly up-regulated in PCa compared with benign prostate hyperplasia (BPH) tissues, and the increased expression of TRPV1 correlates with increasing PCa tumour grade [6].
Alpha1-Adrenoceptors (α1-ARs) mediate actions of the endogenous adrenaline and noradrenaline (NA) in several target cells. On the basis of pharmacological and binding studies α1-ARs have been subdivided into three subtypes, namely α1A (α1a), α1B (α1b) and α1D (α1d) [7]. α1-AR subtypes show different cellular localization: α1B-AR is mainly expressed on cell surface [8, 9], α1A-AR is evidenced on the cell surface and intracellularly [10, 11] and α1D-AR appears to be localized primarily perinuclearly [8, 9]. Moreover, α1-ARs are expressed in a variety of human tissues, including liver, kidneys, blood vessels, heart and prostate. In the human prostate α1A-AR and α1B-AR subtypes are expressed in BHP [12, 13], PCa tissues as well as in PC3 and DU145 PCa cell lines [14, 15] whereas the α1D-AR subtype in the PC3 cell line [16, 17].
The α1-ARs are G-protein-coupled receptors (GPCRs) that are linked to the heterotrimeric G-protein containing the Gαq/11/14/16 subunits. The Gαq subunit is a primary activator of phospholipase C (PLC), which promotes the cleavage of inositol 4,5-bisphosphate (PIP2) to yield diacylglycerol (DAG) and inositol 1,4,5-triphosphate (IP3). DAG and IP3 then promote the activation of protein kinase C (PKC). Growing evidence supports the role for α1-ARs in the direct mitogenic effect of catecholamines on prostate growth [18]. We previously reported the expression of the α1B- and α1D-AR subtypes in PC3 cells and the ability of NA to stimulate PC3 cell proliferation in a α1D-AR-dependent manner [17]. Therefore, the aim of the present study was to evaluate the correlation between α1D-AR and TRPV1 expression levels in patients with PCa and to demonstrate the role of TRPV1 in the regulation of NA-induced α1D-AR-dependent PC3 cell proliferation.
Methods
Prostate cancer cell line
Human prostate cancer cell lines PC3, and DU145 were purchased from American Type Culture Collection (ATCC, Rockville, MD). Cell lines were maintained in DMEM and RPMI medium (Lonza Group Ltd, Basel Switzerland), respectively, supplemented with 10% heat-inactivated fetal bovine serum (FBS, Lonza), 100 IU/ml of penicillin, 100 μg/ml of streptomycin at 37°C, 5% CO2 and 95% of humidity. The androgen sensitive cell line LNCaP was purchased from Istituto Zooprofilattico Sperimentale della Lombardia e dell’Emilia Romagna (IZSLER, Brescia, Italy) and maintained in RPMI supplemented as above described at 37°C, 5% CO2 and 95% of humidity.
PCa tissues
Specimens (n = 37), from adult patients with high-risk clinically localized adenocarcinoma of the prostate underwent radical prostectomy at the Urology Operative Unit, ASUR 9 Macerata, were collected for qRT-PCR analysis (Additional file 1: Table S1). As control, 5 samples of BPH tissues removed by transurethral resection were used. All the specimens were embedded in paraffin and 5–7 μm-thick sections were collected on slides. Patients, giving their informed written consent, that covered the use of their tissues for research purposes, were included in the prostate cancer database of the Pathology Unit, ASUR 9. The study was approved by the Ethics committee Ospedale Civile Macerata and ASUR 9 and all procedures were conducted in accordance with the Declaration of Helsinki.
Antibodies and reagents
The following anti-human polyclonal antibodies (Abs) were used: goat anti-TRPV1, rabbit anti-α1D-AR from Santa Cruz Biotechnology (Heidelberg, Germany), rabbit anti-ERK, rabbit anti-phospho p38 (anti-pp38), rabbit anti-p(Ser)-PKC substrate and rabbit anti-p38 from Cell Technology (Danvers, MA). The following anti-human mouse monoclonal Abs (mAbs) were used: anti-GAPDH (Sigma Aldrich, St. Louis, MO), anti-pERK (Cell) and anti-Bromodeoxyuridine (BrdU) fluorescein isothiocyanate (FITC)-conjugated (Prodotti Gianni, Italy). The horseradish peroxidise (HRP)-conjugated donkey anti-goat and donkey anti-mouse from Santa Cruz Biotechnology, HRP-conjugated goat anti-rabbit Ab from Cell Signaling. Purified FITC-conjugated rabbit anti-goat (RAG)IgG (EMD Chemicals, Inc. San Diego, CA), phycoerythrin (PE)-conjugated goat anti-rabbit (GARB) IgG (BD Biosciences, San Jose, CA), Alexa Fluor 488-conjugated rabbit anti-goat and Alexa Fluor 594-conjugated goat anti-rabbit Abs (Invitrogen, Carlsbad, CA) were used as secondary Abs. {2-[2-(4-Chlorobenzyloxy)phenoxy]ethyl}-[2-(2,6-dimethoxyphenoxy)ethyl]amine (clopenphendioxan, WS433, α1D-AR antagonist) was provided by Prof. Wilma Quaglia, School of Pharmacy, University of Camerino [17]. NA, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), BrdU and dimethyl sulfoxide (DMSO, used as vehicle) from Sigma Aldrich. Chelerythrine chloride (PKC inhibitor), U73122 (PLC inhibitor), PD98059 (MEK inhibitor) and the TRPV1 antagonist, capsazepine (CPZ) [19] were purchased from Tocris Bioscience (Bristol, UK).
Double immunofluorescence and flow cytometry
To determine the co-expression of TRPV1 and α1D-AR, 3×105 PC3, DU145 and LNCaP cells were fixed with 4% paraformaldheyde in PBS for 10 min at room temperature, washed with permeabilizing solution (1% FBS, 0.1% saponin and 0.1% sodium azide in PBS) and stained for 30 min at 4°C first with anti-TRPV1 Ab (1:25) followed by FITC-RAG (1:40) and then with anti-α1D-AR (1:25) followed by PE-GARB (1:40). Normal goat and rabbit serum were used as negative control. In some experiments double immunofluorescence and FACS analysis were performed in PC3 cells double silenced for α1D-AR and TRPV1 genes. The percentage of positive cells determined over 10,000 events was analyzed on a FACScan cytofluorimeter (BD Bioscience) and fluorescent intensity was expressed in arbitrary units on a logarithmic scale.
Confocal laser scanning microscopy analysis
-
2
× 105/mL PC3 cells grown for 24 h at 37°C in poly-L-lysine coated slides, were permeabilized using 2% of paraformaldehyde with 0.5% of Triton X-100 in PBS and fixed by 4% of paraformaldehyde in PBS. After washes in PBS, cells were incubated with 3% of bovine serum albumin (BSA) and 0.1% of Tween-20 in PBS for 1 h at room temperature and then double stained with anti-TRPV1 (1:25) and anti-α1D-AR (1:25) Abs overnight at 4°C. Finally, samples were washed with 0.3% of Triton X-100 in PBS, incubated with Alexa Fluor 488-conjugated and Alexa Fluor 594-conjugated secondary Abs (1:100) for 1 h at 37°C and analysed with MRC600 confocal laser scanning microscope (BioRad, Hercules, CA) equipped with a Nikon (Diaphot-TMD) inverted microscope. Fluorochrome was excited with the 600 line of an argon-kripton laser. Serial optical sections were taken at 1-μm intervals through the cells. Images were processed using Jacs Paint Shop Pro (Jacs Sotfware Inc).
Fluorescence microscopy analysis
The co-expression of α1D-AR and TRPV1 in tissue specimens from patients with adenocarcinoma or BHP, used as control, was evaluated by double immunofluorescence. Briefly fixed paraffin-embedded tissue slices were deparaffinized, rehydrated and washed with 0.3% Triton X-100 in PBS. After incubation with 3% of BSA and 0.3% of Triton X-100 in PBS for 1 h at room temperature, sections were first stained with anti-α1D-AR (1:25) Ab overnight at 4°C, washed with 0.3% of Triton X-100 in PBS and then labelled with Alexa Fluor 594-conjugated secondary Ab (1:100) for 1 h at 37°C. Subsequently, samples were washed with 0.3% of Triton X-100 in PBS and incubated with anti-TRPV1 (1:25) overnight at 4°C followed by Alexa Fluor 488-conjugated Ab (1:100) for 1 h at 37°C. Sections were analyzed using a BX51 fluorescence microscope at 10× magnification (Olympus, Milan, Italy). Merge images were obtained by using the DP controller software (Olympus).
Western blot analysis
PC3 cells were lysed in lysis buffer (10 mM Tris, pH 7.4, 100 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM NaF, 20 mM Na4P2O7, 2 mM Na3VO4, 1% Triton X-100, 10% glycerol, 0.1% SDS, 0.5% deoxycholate, 1 mM phenylmethylsulfonylfluoride) containing protease inhibitor cocktail (SigmaAldrich) by using the Mixer Mill MM300 (Qiagen GmbH, Hilden, Germany). Lysates from PC3 cells treated for different times with NA (100 μM), WS433 (1 μM) and CPZ (1 μM) alone or in combination, were separated on 8 and 12% SDS-polyacrylamide gels, transferred and blotted with anti-pERK (1:1000) mAb followed by HRP-conjugated anti-mouse (1:1000) Ab, anti-ERK (1:1000), anti-p(Ser)-PKC substrate (1:1000), anti-pp38 (1:2000) and anti-p38 (1:2000) Abs followed by HRP-conjugated anti-rabbit (1:2000) Ab. Anti-GAPDH mAb was used as protein loading control. To verify silencing efficiency, lysates from siTRPV1 or siα1D-AR PC3 cells were immunoblotted with anti-TRPV1 (1:100) or anti-α1D-AR (1:1000) Abs followed by HRP-conjugated anti-goat (1:1000) and anti-rabbit (1:2000) Abs respectively. Immunoreactivity was detected using the LiteAblot ®PLUS (EuroClone, Milan, Italy) kits and densitometric analysis was carried out by evaluating three independent experiments by a Chemidoc using the Quantity One software (BioRad, Hèrcules, CA).
Measurement of extracellular acidification rate (ECAR)
The eight-channel CytosensorTM microphysiometer (Molecular Devices Corp., Sunnyvale, CA, USA) was used as previously described [20] to evaluate small changes in the extracellular release of protons induced by NA in the culture medium surrounding PC3 cells. Briefly, 3 × 105 PC3 cells were seeded into 12-mm capsule cups and cultured for 24 h. Then the capsule cups were loaded into the sensor chambers and the chambers were perfused with running medium (bicarbonate-free DMEM with 0.584 g/L glutamine and 2.59 g/L NaCl) at a flow rate of 100 μL /min. PC3 cells were stimulated for 30 sec with vehicle, NA (100 μM), WS433 (1 μM), CPZ (1 μM), alone or in combination, diluted into running medium and perfused through either fluid path. ECAR rate data were expressed as percentages of response respect to the baseline value.
Gene silencing
siGENOME SMARTpools for TRPV1 (siTRPV1) and for α1D-AR (siα1D-AR) consisting of four RNA duplex targeting respectively TRPV1 and α1D-AR genes, and a siCONTROL non-targeting small interfering RNA (siGLO) with at least four mismatches to any human gene used as negative control were purchased from Dharmacon (Lafayette, CO). Briefly, 4×104/ml PC3 cells were plated and after an overnight incubation, 20 nM of siTRPV1, siα1D-AR or siGLO was added to the wells, following METAFECTENE SI (Biontex Laboratories GmbH, Martinsried/Planegg, Germany) transfection protocol. Cells were harvested at day 2 post-transfection for analysis.
Total RNA extraction and complementary DNA synthesis
Total RNA from siGLO, siTRPV1 and siα1D-AR PC3 cells was extracted with the RNeasy Mini Kit (Qiagen GmbH, Hilden, Germany). Total RNA from fixed paraffin-embedded tissue slices (5–7 μm-thick) was isolated by Absolutely RNA® formalin-fixed, paraffin-embedded (FFPE) kit (Stratagene, Austin, TX, USA). Five hundred ng of extracted RNA were subjected to reverse transcription using the High-Capacity cDNA Archive Kit (Life Technologies Corporation, Carlsbad, CA). One μL of the resulting cDNA products was used as template for qRT-PCR quantification.
qRT-PCR analysis
qRT-PCR was performed using the iQ5 Multicolor Real-Time PCR Detection System (Bio-Rad). The reaction mixture contained the Syber Green Master Mix (Bio-Rad) and primer sets. Human β-actin, TRPV1 and α1D-AR primers designed with Primer Premier 5 (Bio-Rad, Hèrcules, CA) and purchased from SigmaAldrich. Primers sequences were: β-actin: forward 5′-ATCAGCAAGCAGGAGTATGACG -3′; reverse 5′-AAAGCCATGCCAATCTCATCTG-3′; TRPV1: forward 5′ -CTGATGGCAAGGACGACTACC-3′; reverse: 5′ -TTGACCGCAGGGAGAAGCTC-3′; α1D-AR: forward 5′ -GGTCGTAGCCCTGGTGGTG -3′; reverse: 5′ -CGGAGGAGAAGACAGCGTAGC -3′. Each amplification consisted of heat activation for 15 min at 95°C followed by 40 cycles at 95°C for 10 sec and 60°C for 50 sec. All samples were assayed in triplicate in the same plate and in three different experiments. Measurement of β-actin levels was used to normalize mRNA contents. TRPV1 and α1D-AR levels were calculated by the 2-ΔΔCt method and expressed as relative fold respect to control levels.
[Ca2+]i measurement
3×106/mL PC3 cells were washed in calcium and magnesium free PBS supplemented with 4.5 g/L of glucose used as experimental medium. Cells were resuspended in the medium supplemented with 7 μmol/L FLUO 3-AM and 1 μg/mL Pluronic F-127 (Life Technologies Corporation, Carlsbad, CA) and incubated in the dark for 30 min at 37°C, 5% CO2. After washing, cells were resuspended in the medium containing or not 2 mmol/L Ca2+ and stimulated with vehicle, NA (100 μM), WS433 (1 μM), CPZ (1 μM), alone or in combination. Fluorescence was measured by FACScan; not stimulated cells were analyzed for 2 min to establish baseline fluorescence levels.
Proliferation assay
BrdU incorporation was determined in PC3 cells treated for 24 h with NA (100 μM), WS433 (1 μM) and CPZ (1 μM), alone or in combination and in siGLO, siTRPV1 and siα1D-AR PC3 cells treated with NA (100 μM) for 24 h. Cells were labelled by adding 20 μL/well of BrdU. After trypsinization and washing in PBS supplemented with 0.5% BSA and 2 mM ethylenediaminetetraacetic acid, cells were fixed for 30 min in PBS containing 30% methanol and 0.4% paraformaldheyde, permealized with PBS containing 1% paraformaldheide and 0.01% Tween-20, and then incubated for 15 min in DNAse buffer containing 500 KU/mL of DNAse (SigmaAldrich). Thereafter, cells were stained with anti-BrdU FITC-conjugated Ab (1:10) incubated for l h at room temperature and washed in PBS containing 0.5% BSA and 2 mM ethylenediaminetetraacetic acid. Samples were analyzed by a FACScan cytofluorimeter as above described.
MTT assay
Cell growth was measured by MTT assay. 4 × 104 PC3 cells/mL were plated in a 96-well microtiter plate, treated for 24 h with NA (100 μM), WS433 (1 μM) and CPZ (1 μM), alone or in combination and then incubated with 0.8 mg/ml of MTT for the last 3 h. Four replicates were used for each treatment. The supernatants were discarded and colored formazan crystals, dissolved with 100 μL/well of DMSO, were read at 570 nm wavelength by an ELISA reader (BioTek Instruments, Bad Friedrichshall, Germany). In some experiments, MTT assay was performed in PC3 cells treated for 24 h with NA (100 μM), chelerythrine (0.5 μM), U73122 (5 μM) and PD98059 (50 μM), alone or in combination. Dose response curves have been performed after 24 h treatments for chelerythrine, U73122 and PD98059 compounds (Additional file 2: Figure S1F). The highest doses that did not affect cell viability were used.
Inositol-1,4,5-trisphosphate (IP3) measurement
IP3 was measured using the inositol-1,4,5-trisphosphate [3H] radioreceptor assay kit (PerkinElmer Life Sciences, Inc., Waltham, MA) [21]. Briefly, 1.5×105/mL PC3 cells were treated with NA (100 μM), WS433 (1 μM) and CPZ (1 μM), alone or in combination for different times in DMEM supplemented with 1% FBS. After treatment IP3 was read by β scintillation counter in 5 ml of Atomlight scintillation cocktail (PerkinElmer).
Statistical analysis
The statistical significance was determined by one-way Anova or by 2-way Anova with Bonferroni post-test. Unpaired t tests and Spearman’s rank correlation tests were performed with GraphPad Prism version 5.0 (GraphPad Software, San Diego, CA, USA). No differences were found comparing vehicle-treated with untreated PC3 cells (control).
Results
α1D-AR and TRPV1 are co-expressed in the prostate cancer cell lines
Previously reports have evidenced the expression of the vanilloid receptor, TRPV1 [6] and the α1D-AR [17] in the PC3 cell line; however, data on correlation between these receptors in PCa has not been provided so far. Therefore, by double immunofluorescence and flow cytometric analysis, we found that the α1D-AR and TRPV1 proteins were co-expressed in the androgens-resistant PC3 and DU145 cell lines, although at different levels. Approximately 78.9% and 64.3% of PC3 cells and 71.6% and 58.1% of DU145 expressed TRPV1 and α1D-AR, respectively, with 52.4% and 40.7% of them co-expressing both receptors (Figure 1A). In addition double immunofluorescence, performed in the androgens sensitive cell line LNCaP, showed that about 66.9% and 50.3% of this cell line expressed TRPV1 and α1D-AR, with 39.6% co-expressing both receptors, suggesting that the co-expression of α1D-AR and TRPV1 does not depend on the androgen sensitivity. Moreover, by confocal microscopy, we observed the co-localization of α1D-AR and TRPV1 primarily in the plasma membrane and in intracellular perinuclear vesicles of PC3 cells (Figure 1C).
Figure 1.
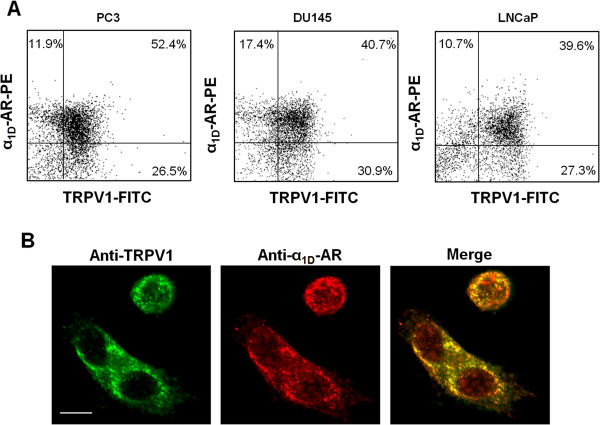
α 1D -AR and TRPV1 are co-expressed and co-localized in prostate cancer cells. (A) FACS analysis was performed in PC3, DU145 and LNCaP cells double-stained with anti-TRPV1 and anti-α1D-AR Abs followed by respective secondary Abs. (B) Confocal microscopy analysis was performed using PC3 cells grown for 24 h on poly-L-lysine coated slides, permeabilized and double-stained with anti-TRPV1 and anti-α1D-AR Abs, followed by Alexa Fluor 488- and Alexa Fluor 594-conjugated secondary Abs, respectively. Bar: 20 μm. Data shown are representative of one out of three separate experiments.
Co-expression of α1D-AR and TRPV1 in PCa tissues
The results obtained in prostate cancer cell lines were confirmed at mRNA levels in PCa specimens. Thus, we assessed the expression of α1D-AR mRNA in tissues from prostatectomised patients (n = 37) with advanced PCa (Additional file 1: Table S1). Using quantitative RT-PCR (qRT-PCR), we found that α1D-AR was over-expressed (2.44 ± 0.20) in PCa compared with BPHs (1.0 ± 0.05) (Figure 2A). As previously reported [6], we observed that TRPV1 mRNA expression significantly increased in PCa tissues (1.84 ± 0.12) compared with BPH tissues (0.61 ± 0.01) (Figure 2B); interestingly we found that TRPV1 levels highly correlated with α1D-AR expression (correlation index: r = 0.88; p < 0.0001) (Figure 2C). Dramatic reduction in the α1D-AR and TRPV1 mRNA levels was observed in PCa specimens (n = 5, pathological staging: pT3b) from patients that had received neoadjuvant androgen deprivation therapy compared with tissues from untreated PCa patients (Figure 2D and E). Moreover, by using double immunofluorescence and fluorescence microscopy analysis, we also showed that α1D-AR and TRPV1 proteins are co-expressed in PCa tissues and that their expression is increased in PCa compared with BPH tissues (Figure 2F).
Figure 2.
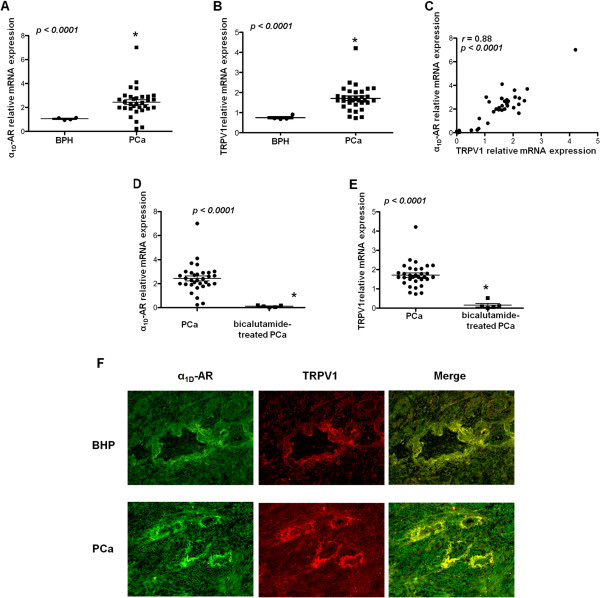
Co-expression of α 1D -AR and TRPV1 in PCa specimens. (A-B) Fold expression levels of α1D-AR and TRPV1 in PCa and BPH specimens analysed by qRT-PCR. Each point represents the expression level in a single subject. (C) Correlation between the α1D-AR and TRPV1 expression levels. Spearman’s rank correlation coefficient (r) is indicated in the graph. (D-E) Fold expression levels of α1D-AR and TRPV1 in PCa and bicalutamide-treated PCa specimens analysed by qRT-PCR. Each point represents the expression level in a single subject. The horizontal bars within the plots indicate the median percentage for each group. Significant differences between groups were identified by unpaired t tests and are indicated above the groups: *p < 0.0001. (F) Fluorescence microscopy analysis was performed in PCa and BHP tissues double-stained with anti-TRPV1 and anti-α1D-AR Abs, followed by Alexa Fluor 488- and Alexa Fluor 594-conjugated secondary Abs, respectively. Magnification: 10×. Data shown are representative of one out of three separate experiments.
The cross-talk between α1D-AR and TRPV1 receptors
The ability of NA to sensitise TRPV1 was studied in PC3 cells. First, we used a CytosensorTM microphysiometer to evaluate the μV/sec response induced by NA stimulation (100 μM) with or without α1D-AR antagonist, WS433, (1 μM) and TRPV1 antagonists, CPZ (1 μM). We found that protons release following NA administration, was not only α1D-AR- but also TRPV1-dependent as shown by the inhibitory effects induced by both WS433 (60%) and CPZ (35%). In addition, the combination of WS433 and CPZ completely inhibited the NA-induced acid activation (Figure 3A). No activation was found when using WS433 and CPZ alone respect to control PC3 cells (Additional file 2: Figure S1A). Thereafter, we followed NA-induced Ca2+ flux by FACS analysis in PC3 cells loaded with Fluo-3 AM. NA stimulation in Ca2+-containing PBS/glucose medium induced a rapid response that peaked at 40 sec after treatment with an increase in [Ca2+]i from 300 to 650 nM (Figure 3B). Next, we addressed the contribution of both α1D-AR and TRPV1 components by using WS433 and CPZ. Complete abrogation of the NA-induced increase in [Ca2+]i was observed with all the antagonists (Figure 3B). No Ca2+ flux was found when using WS433 and CPZ alone respect to control PC3 cells (Additional file 2: Figure S1B).
Figure 3.
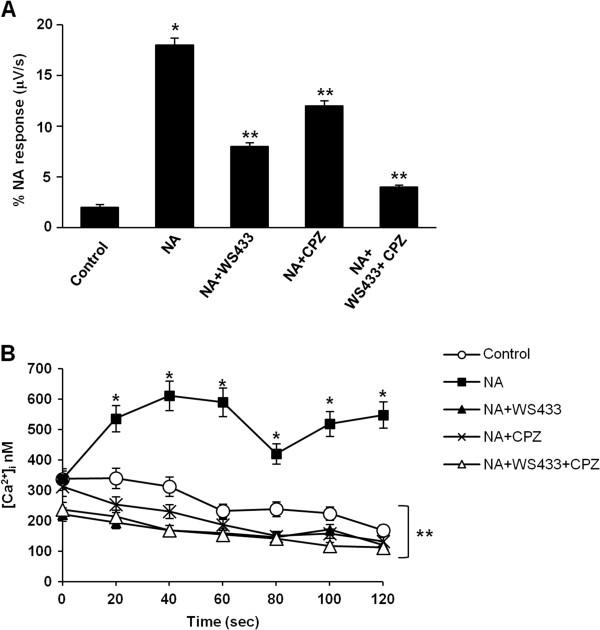
The α1D -AR and TRPV1 cross-talk in PC3 cells. (A) The measurement of the extracellular acidification rate using an eight-channel Cytosensor TM microphysiometer was performed with PC3 cells stimulated for 30 sec with vehicle (control) or NA (100 μM) alone or in combination with WS433 (1 μM) or/and CPZ (1 μM). Data shown are the mean ± SD of three separate experiments. Statistical analysis was performed by comparing NA-treated cells with control cells (*) and NA + WS433-, NA + CPZ- and NA + WS433 + CPZ- with NA-treated cells (**), p ≤ 0.01. (B) The time course of [Ca2+]i increase in Fluo-3-loaded PC3 cells vehicle-treated (control) or treated with NA alone or in combination with WS433 or/and CPZ was evaluated by FACS analysis. Data shown are the mean ± SD of three separate experiments. Statistical analysis was performed by comparing NA-treated cells with control cells (*) and NA + WS433-, NA + CPZ- and NA + WS433 + CPZ- with NA-treated cells (**), p ≤ 0.01.
The α1D-AR/TRPV1 cross-talk in response to NA activates specific signalling pathways
Several reports have examined the role of the PLC-PKC-MAPK signalling pathways in the catecholamine-induced α1D-AR-mediated proliferation of prostate cancer cells [22, 23]. Thus, we studied the effect of α1D-AR and TRPV1 cross-talk in the NA-induced activation of PLC, PKC and ERK signalling pathways. Time-course analysis of NA-induced PLC activation, evaluated as IP3 production, evidenced that NA stimulates IP3 production 5 min after NA treatment, remaining sustained at 30 min and declining thereafter (Figure 4A). Moreover, we found that ERK1/2 is phosphorylated at basal level and NA induces a rapid and transient increase of its phosphorylation at 3 min after treatment (Figure 4B); NA also increased the (Ser)-PKC substrate phosphorylation at 3–5 min and then return to basal level (Figure 4C); p38 was basally phosphorylated, and NA stimulation did not affect its phosphorylation state (Figure 4B).
Figure 4.
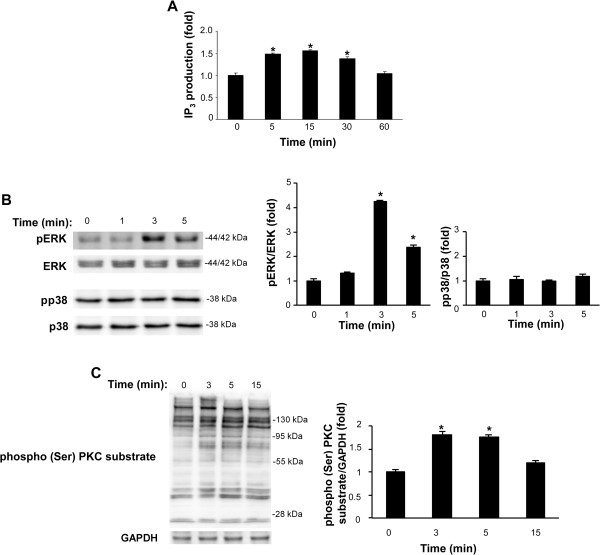
NA induces PLC, PKC and ERK activation in PC3 cells. (A) The measurement of IP3 levels using the IP3 [3H] Radioreceptor assay kit was performed in PC3 cells vehicle-treated (control) or treated for different times with NA (100 μM). Data shown are the mean ± SD of three separate experiments. Statistical analysis was performed by comparing NA-treated with control cells, *p ≤ 0.01. (B-C) Lysates from PC3 cells vehicle-treated (control) or treated for different times with NA (100 μM) were separated on SDS-polyacrylamide gels, transferred and then blotted with anti-pERK, anti-ERK, anti-p38, anti-pp38 and anti-phospho-(Ser) PKC substrate Abs. The GAPDH protein level was evaluated as a loading control. Data shown are representative of one out of three separate experiments. Densitometric analysis was performed evaluating three different experiments and statistical analysis was carried out comparing NA-treated with control cells, *p ≤ 0.01.
Then the ability of WS433 or CPZ to inhibit PLC activation, ERK1/2 phosphorylation and the production of phospho-(Ser) PKC substrates was assessed. In regard to PLC activation, when used in combination with NA CPZ, but not WS433, partially inhibited IP3 production; however, NA administered in combination with WS433 and CPZ induced a IP3 production, but at a lower rate than NA in combination with CPZ (Figure 5A). No IP3 production was found when using WS433 and CPZ alone respect to control PC3 cells (Additional file 2: Figure S1C). The NA-induced ERK1/2 phosphorylation was markedly affected by both the α1D-AR and TRPV1 antagonists or by their combination (Figure 5B), whereas the NA-induced increase of (Ser)-PKC substrate phosphorylation was reduced only by CPZ and WS433 used in combination (Figure 5C). No ERK1/2 and (Ser)-PKC substrate phosphorilation was found when using WS433 and CPZ alone respect to control PC3 cells (Additional file 2: Figure S1D, E). Next, the ability of pharmacological inhibitors of PLC, PKC and ERK to inhibit the NA-induced PC3 cell growth was evaluated. We found that U73122, chelerythrine chloride and PD98059, used at the highest doses that did not affect cell viability (Additional file 2: Figure S1F), markedly inhibited the cell growth of NA-stimulated PC3 cells; in addition, when all the three pharmacological inhibitors were used in combination, they completely reverted the NA-mediated effects (Figure 6). These results confirmed that the PLC/PKC/ERK axis plays a major role in the control of α1D-AR- and TRPV1-dependent PC3 cell growth.
Figure 5.
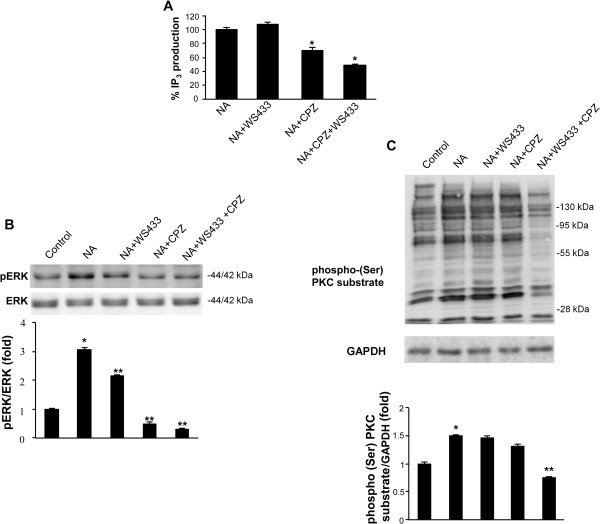
Role of TRPV1 and α1D -AR in NA-induced PLC, PKC and ERK activation. (A) The IP3 levels, measured as above described, were evaluated in PC3 cells treated for 5 min with NA (100 μM), either alone or in combination with CPZ (1 μM), WS433 (1 μM) or CPZ + WS433. Data shown are the mean ± SD of three separate experiments. Statistical analysis was performed by comparing NA + WS433-, NA + CPZ- or NA + CPZ + WS433- with NA-treated cells, *p ≤ 0.01. (B-C) Lysates from PC3 cells vehicle-treated (control) or treated for 3 min with NA (100 μM) alone or in combination with WS433 (1 μM) and/or CPZ (1 μM), were separated on SDS-polyacrylamide gels, transferred and then blotted with anti-pERK, ERK and anti-phospho-(Ser) PKC substrate Abs. The GAPDH protein level was evaluated as a loading control. Data shown are representative of one out of three separate experiments. Densitometric analysis was performed evaluating three different experiments and statistical analysis was performed by comparing NA-treated cells with control cells (*) and NA + WS433-, NA + CPZ- and NA + WS433 + CPZ- with NA-treated cells (**), p ≤ 0.01.
Figure 6.
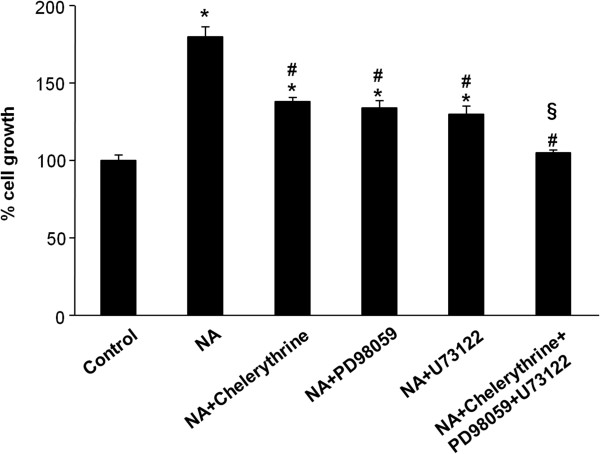
PLC, PKC and MAPK inhibitors block the NA-induced PC3 cell survival. Cell growth was assessed by MTT assay in PC3 cells treated for 24 h with vehicle (control) or NA (100 μM), alone or in combination with chelerythrine (0.5 μM), U73122 (5 μM) and PD98059 (50 μM). Data shown are the mean ± SD of three separate experiments. Statistical analysis was performed by comparing NA-treated with control cells (*), NA + chelerythrine-, NA + PD98059-, NA + U73122- and NA + chelerythrine + PD98059 + U73122- with NA-treated cells (#) and NA + chelerythrine + PD98059 + U73122- with NA + chelerythrine-, NA + PD98059- and NA + U73122-treated cells (§), p ≤ 0.01.
NA stimulates the proliferation of PC3 cells in a α1D-AR- and TRPV1-dependent manner
We previously reported the role of α1D-AR in the NA-induced proliferation of PC3 cells [17]. Thus, we evaluated the potential contribution of TRPV1 to the NA-induced α1D-AR-mediated proliferation of PC3 cells. We found that WS433 and CPZ (Figure 7A) inhibited NA-induced proliferation. No change in proliferation rate was observed when using WS433 and CPZ alone respect to control PC3 cells (Additional file 2: Figure S1G). Then, to strengthen these findings, we performed cell proliferation in single (α1D-AR or TRPV1) and double (α1D-AR/TRPV1) silenced PC3 cells (Additional file 3: Figure S2A, B, C). A marked decrease in NA-induced proliferation was already evident in siα1D-AR and siTRPV1 cells even if a stronger inhibition (< 80%) was observed in double siα1D-AR/siTRPV1 PC3 cells (Figure 7B).
Figure 7.
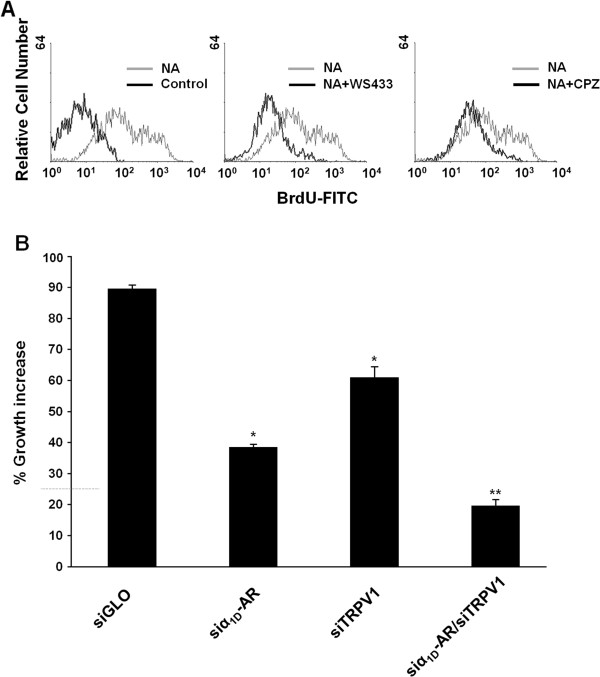
NA stimulates the proliferation of PC3 cells in an α1D -AR and TRPV1 dependent manner. (A) The proliferation of PC3 cells was analysed by labelling with BrdU. The cells were treated with vehicle (control) or NA (100 μM) for 24 h, alone or in combination with WS433 (1 μM) and CPZ (1 μM). Cells were stained with an anti-BrdU FITC-conjugated Ab and analysed using a FACScan flow cytometer. Data shown are representative of one of three separate experiments. (B) siGLO-, siα1D-AR-, siTRPV1- and siα1D-AR/siTRPV1-transfected PC3 cells were treated for 24 h with NA (100 μM), and cell proliferation was assessed by BrdU labelling. The percentage increase of cell proliferation was evaluated with respect to vehicle-treated cells. The data shown are the mean ± SD of three separate experiments. Statistical analysis was performed by comparing siα1D-AR-, siTRPV1- with siGLO-transfected cells (*), and siα1D-AR/siTRPV1- with siα1D-AR- or siTRPV1-transfected cells (**), p ≤ 0.01.
Discussion
TRP channels may participate in Ca2+ homeostasis in PCa cells [24]. It has been hypothesised that abnormal Ca2+ signalling may be an essential step in the α1-AR-mediated increased proliferation in PCa cells [3].
Herein, we firstly demonstrated that prostate cancer cell lines co-expressed α1D-AR and TRPV1 proteins and these receptors co-localized mainly in the plasma membrane, perinuclear region and intracellular vesicles. Therefore, we investigated whether these receptors functionally interact each other. Previously, cross-sensitisation between TRPV1 and other members of the GPCR family, such as P2X3 and CCR1 in DRG neurons [25] and CCR1/TRPV1-transfected human embryonic kidney (HEK293) cells, respectively, have been demonstrated [26].
We also evaluated the α1D-AR and TRPV1 co-expression at mRNA and protein levels in advanced PCa tissues. As previously reported [6], TRPV1 mRNA expression is higher in PCa than in BPH tissues. Our data demonstrated that α1D-AR mRNA and protein levels were markedly increased in PCa samples compared with BPH specimens and, more importantly, that there is a great correlation between α1D-AR and TRPV1 expression levels. Moreover, a strong reduction of the α1D-AR and TRPV1 mRNAs was observed in 5/37 PCa patients who received neoadjuvant androgen deprivation therapy compared with untreated PCa patients. This result suggests that the transcriptional activity of TRPV1 and α1D-AR may be androgen dependent. Chromatin immunoprecipitation analysis revealed that TRPV1 is a novel androgen receptor target gene in castration-resistant C4-2 PCa cells [27]. Similarly, decreased TRPV6 levels were detected in androgen-insensitive tumours after androgen deprivation therapy [2]. No data on the relationship between α1D-AR and androgen receptors has been published to date; however, the expression of β2-AR, a well-known activator of the androgen receptors, was transiently down-regulated in hormone-sensitive LNCaP cells treated with the anti-androgen compound bicalutamide [28, 29]. Thus, there may be a similar effect on α1D-AR in PCa patients treated with anti-androgen drugs.
α1D-AR has a 10- to 100-fold higher affinity for endogenous catecholamines than the α1A- and α1B-AR subtypes [30]. Therefore, we studied whether the binding of NA to α1D-AR sensitises TRPV1 in PC3 cells. By protons release and Ca2+ flux analysis NA resulted in a rapid response, which was inhibited by WS433 and CPZ. Moreover, NA stimulates a cross-talk between α1D-AR and TRPV1 in PC3 cells that involves the PLC-PKC-ERK pathways. In particular, NA sensitises TRPV1, but not α1D-AR, to activate the PLC pathway. This result is in agreement with previous findings demonstrating a weaker coupling between PLC and α1D-AR than between PLC and other α1-AR subtypes [9, 31]. Calcium flowing through TRPV1 activates PLC, and the resulting depletion of PIP2 plays a role in PKC-dependent TRPV1 sensitisation [32]. Moreover, recently TRPV1 has been demonstrated to be either inhibited or activated by PIP2 [33]. In this regard, we found that the IP3 production is a TRPV1-dependent event; WS433 in combination with CPZ significantly inhibited the IP3 production to a greater extent than CPZ alone, suggesting a co-stimulatory effect of α1D-AR signalling in sustained TRPV1-dependent PLC activation. Moreover, the CPZ and WS433 drug combination significantly reduced the NA-induced phospho-(Ser) PKC substrate phosphorylation. Finally, both CPZ and WS433 antagonists alone markedly inhibited the ERK1/2 phosphorylation, although the maximal effect was evidenced by their use in combination, suggesting that ERK1/2 represents a downstream component of NA-induced TRPV1 and α1D-AR signalling pathway. In this regard, in human HEK293 cell line transfected with α1D-AR, a constitutive ERK1/2 phosphorylation, which was reduced by incubation with the selective α1D-AR antagonist BMY7378, was evidenced; in addition a rapid and transient ERK1/2 phosphorylation, that was not inhibited by the PKC inhibitor, Ro-8425, following α1D-AR activation was demonstrated [34]. Moreover, α1D-AR activation stimulated the ERK pathway in CHO cells [35] and in lacrimal gland epithelial cells [36].
Previous reports have indicated the contribute of α1D-AR in NA-induced proliferation of PC3 cells [17], however no data on the potential effects of TRPV channels in PCa proliferation were reported. Herein we found that complete abrogation of NA-induced increase in PC3 cell proliferation was reached only in double-silenced α1D-AR/TRPV1 PC3 cells. The silencing of the α1D-AR or TRPV1 gene or the use of WS433 or CPZ alone, partially but not completely, inhibited the NA-induced effects suggesting that these receptors act cooperatively.
Coupling of α1-AR to Ca2+-permeable TRPCs channels has been reported in LNCaP cells [24, 37, 38] and WB4101 and the TRP channel blockers 2-ABP and SK&F 96365 [24]. Furthermore, naftopidil, an α1D-AR selective antagonist, has been also reported to affect the proliferation of human prostate epithelial cells [39], and labedipinedilol-A, which shows high selectivity for α1A- and α1D-AR, to inhibit the NA-stimulated proliferation and ERK phosphorylation in LNCaP and PC3 cells [40]. Thus, because of the lack of potent and selective subtype-specific α1-AR antagonists, experimental or clinical trials using these compounds are few [41, 42].
Conclusion
Overall, our findings, demonstrating a functional cooperative role played by the α1D-AR/TRPV1 cross-talk in NA-induced proliferation of PCa cells, strongly support for a new pharmacological approach in the care of PCa by targeting both the α1D-AR and TRPV1 receptors.
Electronic supplementary material
Additional file 1: Table S1: Patient demographics. IQR = interquartile range; PSA = prostate-specific antigen. (XLSX 31 KB)
Additional file 2: Figure S1: Effects of WS433 and CPZ antagonists and chelerythrine, PD98059, U73122 inhibitors in PC3 cells. (A) The measurement of the extracellular acidification rate was performed as above described with PC3 cells treated for 30 sec with vehicle (control) or WS433 (1 μM) or/and CPZ (1 μM). Data shown are the mean ± SD of three separate experiments. (B) The time course of [Ca2+]i increase in Fluo-3-loaded PC3 cells vehicle-treated (control) or treated with WS433 or/and CPZ was evaluated by FACS analysis. Data shown are the mean ± SD of three separate experiments. (C) The measurement of IP3 levels performed as above described in PC3 cells vehicle-treated (control) or treated for different times with WS433 (1 μM) or/and CPZ (1 μM). Data shown are the mean ± SD of three separate experiments. (D-E) Lysates from PC3 cells vehicle-treated (control) or treated for different times with WS433 (1 μM) or/and CPZ (1 μM) were separated on SDS-polyacrylamide gels, transferred and then blotted with anti-pERK, ERK and anti-phospho-(Ser) PKC substrate Abs. The GAPDH protein level was evaluated as a loading control. Data shown are representative of one out of three separate experiments. (F) Cell growth was assessed by MTT assay in PC3 cells treated for 24 h with vehicle (control) or with chelerythrine (0.05-5 μM), PD98059 (1–100 μM) and U73122 (0.5-50 μM). Data shown are the mean ± SD of three separate experiments. Statistical analysis was performed by comparing chelerythrine, PD98059 and U73122 treated cells with control (*), p ≤ 0.01. (G) The proliferation of PC3 cells was analysed by labelling with BrdU. The cells treated with WS433 (1 μM) or CPZ (1 μM) were stained with an anti-BrdU FITC-conjugated Ab and analysed using a FACScan flow cytometer. Data shown are representative of one of three separate experiments. (TIFF 13 MB)
Additional file 3: Figure S2: Silencing of the α1D-AR and TRPV1 genes in PC3 cells. (A) The α1D-AR and TRPV1 mRNA levels were evaluated by qRT-PCR in siGLO-, siα1D-AR- and siTRPV1-transfected PC3 cells. The relative α1D-AR and TRPV1 expression levels, normalised to the β-actin mRNA level, were calculated using siGLO as a calibrator. (B) Lysates from siGLO-, siα1D-AR- and siTRPV1-transfected PC3 cells were separated by SDS-polyacrylamide gel electrophoresis and probed with anti-α1D-AR or anti-TRPV1 Abs. The GAPDH protein level was evaluated as a loading control. Representative immunoblots are shown. (C) FACS analysis was performed in PC3 cells double silenced for α1D-AR and TRPV1 genes. Silenced cells were double-stained with anti-TRPV1 and anti-α1D-AR Abs followed by respective secondary Abs. Data shown are representative of one of three separate experiments. (TIFF 3 MB)
Acknowledgements
This research was supported by Fondazione Italiana Ricerca sul Cancro (11095 to M.B.M.) and by University of Camerino (Fondo di Ateneo per la Ricerca 2011–2012). The authors would like to thank Andrea Fabiani and Matteo Piergallina, the Urology Operative Unit ASUR 9, Macerata for their technical assistance in sample collection.
Abbreviations
- α1-AR
Alpha1-adrenergic receptor
- PCa
Advanced prostate cancer
- Ab
Antibody
- mAb
Mouse monoclonal Ab
- BPH
Benign prostate hyperplasia
- BrdU
Bromodeoxyuridine
- CPZ
Capsazepine
- WS433
Clopenphendioxan
- [Ca2+]i
Calcium influx
- DAG
Diacylglycerol
- DMSO
Dimethyl sulfoxide
- qRT-PCR
Quantitative real time-PCR
- ERK1/2
Extracellular signal-regulated kinase 1/2
- ECAR
Extracellular acidification rate
- FBS
Fetal bovine serum
- FITC
Fluorescein isothiocyanate
- GPCRs
G-protein-coupled receptors
- HRP
Horseradish peroxidise
- HEK293
Human embryonic kidney
- PIP2
Inositol 4,5-bisphosphate
- IP3
Inositol 1,4,5-triphosphate
- I-RTX
Iodoresinferatoxin
- NA
Noradrenaline
- PE
Phycoerythrin
- PLC
Phospholipase C
- PKC
Protein kinase C
- PSA
Prostate specific antigen
- siTRPV1
siGENOME SMARTpools for TRPV1
- siα1D-AR
α1D-AR
- siGLO
siCONTROL non-targeting small interfering RNA
- TRPV1
Transient receptor potential vanilloid type 1
- TRPC
Transient receptor potential channel.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
MBM and CA carried-out all experiments, and analyzed data with the assistance of MN, SL, CC, VF, DT, AF, AG. GS conceived the study, participated in its design with all authors, coordinated and helped to draft the manuscript with the assistance of all authors. Critical revision was performed by WQ, AP, AB, FDB, LS, MS and GM. All authors read and approved the final manuscript.
Contributor Information
Maria Beatrice Morelli, Email: beatricemorelli@hotmail.com.
Consuelo Amantini, Email: consuelo.amantini@unicam.it.
Massimo Nabissi, Email: massimo.nabissi@unicam.it.
Sonia Liberati, Email: sonia.liberati@studenti.unicam.it.
Claudio Cardinali, Email: claudio.cardinali@uniroma1.it.
Valerio Farfariello, Email: valerio.farfariello@inserm.fr.
Daniele Tomassoni, Email: daniele.tomassoni@unicam.it.
Wilma Quaglia, Email: wilma.quaglia@unicam.it.
Alessandro Piergentili, Email: alessandro.piergentili@unicam.it.
Alessandro Bonifazi, Email: alessandro.bonifazi@unicam.it.
Fabio Del Bello, Email: fabio.delbello@unicam.it.
Matteo Santoni, Email: mattymo@alice.it.
Gabriele Mammana, Email: gabriele.mammana@sanita.marche.it.
Lucilla Servi, Email: lucilla.servi@libero.it.
Alessandra Filosa, Email: alessandrafilosa@yahoo.com.
Angela Gismondi, Email: angela.gismondi@uniroma1.it.
Giorgio Santoni, Email: giorgio.santoni@unicam.it.
References
- 1.Roderick HL, Cook SJ. Ca2+ signalling checkpoints in cancer: remodelling Ca2+ for cancer cell proliferation and survival. Nat Rev Cancer. 2008;8:361–375. doi: 10.1038/nrc2374. [DOI] [PubMed] [Google Scholar]
- 2.Fixemer T, Wissenbach U, Flockerzi V, Bonkhoff H. Expression of the Ca2+-selective cation channel TRPV6 in human prostate cancer: a novel prognostic marker for tumor progression. Oncogene. 2003;22:7858–7861. doi: 10.1038/sj.onc.1206895. [DOI] [PubMed] [Google Scholar]
- 3.Flourakis M, Prevarskaya N. Insights into Ca2+ homeostasis of advanced prostate cancer cells. Biochim Biophys Acta. 2009;1793:1105–1109. doi: 10.1016/j.bbamcr.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 4.Wissenbach U, Niemeyer B, Himmerkus N, Fixemer T, Bonkhoff H, Flockerzi V. TRPV6 and prostate cancer: cancer growth beyond the prostate correlates with increased TRPV6 Ca2+ channel expression. Biochem Biophys Res Commun. 2004;322:1359–1363. doi: 10.1016/j.bbrc.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 5.Sanchez MG, Sanchez AM, Collado B, Malagarie-Cazenave S, Olea N, Carmena MJ, Prieto JC, Diaz-Laviada II. Expression of the transient receptor potential vanilloid 1 (TRPV1) in LNCaP and PC3 prostate cancer cells and in human prostate tissue. Eur J Pharmacol. 2005;515:20–27. doi: 10.1016/j.ejphar.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 6.Czifra G, Varga A, Nyeste K, Marincsák R, Tóth BI, Kovács I, Kovács L, Bíró T. Increased expression of cannabinoid receptor-1 and transient receptor potential vanilloi-1 in human prostate carcinoma. J Cancer Res Clin Oncol. 2009;135:507–514. doi: 10.1007/s00432-008-0482-3. [DOI] [PubMed] [Google Scholar]
- 7.Bylund DB, Eikenberg DC, Hieble JP, Langer SZ, Lefkowitz RJ, Minneman KP, Molinoff PB, Ruffolo RR, Jr, Trendelenburg U. International union of pharmacology nomenclature of adrenoceptors. Pharmacol Rev. 1994;46:121–136. [PubMed] [Google Scholar]
- 8.Walden PD, Gerardi C, Lepor H. Localization and expression of the alpha1A-1, alpha1B and alpha1D-adrenoceptors in hyperplastic and non-hyperplastic human prostate. J Urol. 1999;161:635–640. doi: 10.1016/S0022-5347(01)61986-4. [DOI] [PubMed] [Google Scholar]
- 9.McCune DF, Edelmann SE, Olges JR, Post GR, Waldrop BA, Waugh DJ, Perez DM, Piascik MT. Regulation of the cellular localization and signaling properties of the alpha(1B)- and alpha(1D)-adrenoceptors by agonists and inverse agonists. Mol Pharmacol. 2000;57:59–666. doi: 10.1124/mol.57.4.659. [DOI] [PubMed] [Google Scholar]
- 10.Docherty JR. Subtypes of functional α1-adrenoceptor. Cell Mol Life Sci. 2010;67:405–417. doi: 10.1007/s00018-009-0174-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chalothorn D, McCune DF, Edelmann SE, García-Cazarín ML, Tsujimoto G, Piascik MT. Differences in the cellular localization and agonist-mediated internalization properties of the alpha(1)-adrenoceptor subtypes. Mol Pharmacol. 2002;61:1008–1016. doi: 10.1124/mol.61.5.1008. [DOI] [PubMed] [Google Scholar]
- 12.Milligan G, Canals M, Pediani JD, Ellis J, Lopez-Gimenez JF. The role of GPCR dimerisation/oligomerisation in receptor signalling. Ernst Schering Found Symp Proc. 2006;2:145–161. doi: 10.1007/2789_2006_007. [DOI] [PubMed] [Google Scholar]
- 13.Kojima Y, Sasaki S, Shinoura H, Hayase M, Kubota Y, Hayashi Y, Tsujimoto G, Kohri K. Change of expression levels of alpha1-adrenoceptor subtypes by administration of alpha1d-adrenoceptor-subtype-selective antagonist naftopidil in benign prostate hyperplasia patients. Prostate. 2007;67:1285–1292. doi: 10.1002/pros.20624. [DOI] [PubMed] [Google Scholar]
- 14.Terrillon S, Bouvier M. Roles of G-protein-coupled receptor dimerization. EMBO J. 2004;23:3950–3961. doi: 10.1038/sj.emboj.7600387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi T, Gaivin RJ, McCune DF, Gupta M, Perez DM. Dominance of the alpha1B-adrenergic receptor and its subcellular localization in human and TRAMP prostate cancer cell lines. J Recept Signal Transduct Res. 2007;27:27–45. doi: 10.1080/10799890601087487. [DOI] [PubMed] [Google Scholar]
- 16.Prinster SC, Hague C, Hall RA. Heterodimerization of G-protein coupled receptors: specificity and functional significance. Pharmacol Rev. 2005;57:289–298. doi: 10.1124/pr.57.3.1. [DOI] [PubMed] [Google Scholar]
- 17.Quaglia W, Santoni G, Pigini M, Piergentili A, Gentili F, Buccioni M, Mosca M, Lucciarini R, Amantini C, Nabissi MI, Ballarini P, Poggesi E, Leonardi A, Giannella M. Structure-activity relationships in 1,4-benzodioxan-related compounds. 8. (1) {2-[2-(4-chlorobenzyloxy)phenoxy]ethyl}-[2-(2,6-dimethoxyphenoxy)ethyl]amine (clopenphendioxan) as a tool to highlight the involvement of alpha1D- and alpha1B-adrenoreceptor subtypes in the regulation of human PC3 prostate cancer cell apoptosis and proliferation. J Med Chem. 2005;48:7750–7763. doi: 10.1021/jm0580398. [DOI] [PubMed] [Google Scholar]
- 18.McVary KT, McKenna KE, Lee C. Prostate innervation. Prostate Suppl. 1998;8:2–13. doi: 10.1002/(SICI)1097-0045(1998)8+<2::AID-PROS2>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 19.Huang JK1, Cheng HH, Huang CJ, Kuo CC, Chen WC, Liu SI, Hsu SS, Chang HT, Lu YC, Tseng LL, Chiang AJ, Chou CT, Jan CR. Effect of capsazepine on cytosolic Ca(2+) levels and proliferation of human prostate cancer cells. Toxicol In Vitro. 2006;20:567–574. doi: 10.1016/j.tiv.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 20.Pihlavisto M, Scheinin M. Functional assessment of recombinant human α2-adrenoceptor subtypes with cytosensor microphysiometry. Eur J Pharmacol. 1999;385:247–253. doi: 10.1016/S0014-2999(99)00715-3. [DOI] [PubMed] [Google Scholar]
- 21.Bredt DS, Mourey RJ, Snyder SH. A simple, sensitive, and specific radioreceptor assay for inositol 1,4,5-trisphosphate in biological tissues. Biochem Biophys Res Commun. 1989;159:976–982. doi: 10.1016/0006-291X(89)92204-3. [DOI] [PubMed] [Google Scholar]
- 22.Marshall I, Burt RP, Chapple CR. Signal transduction pathways associated with α1-adrenoceptor subtypes in cells and tissues including human prostate. Eur Urol. 1999;36(Suppl 1):42–47. doi: 10.1159/000052317. [DOI] [PubMed] [Google Scholar]
- 23.Hein P, Michel MC. Signal transduction and regulation: are all α1-adrenergic receptor subtypes created equal? Biochem Pharmacol. 2007;73:1097–1106. doi: 10.1016/j.bcp.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 24.Thebault S, Roudbaraki M, Sydorenko V, Shuba Y, Lemonnier L, Slomianny C, Dewailly E, Bonnal JL, Mauroy B, Skryma R, Prevarskaya N. α1-adrenergic receptors activate Ca(2+)-permeable cationic channels in prostate cancer epithelial cells. J Clin Invest. 2003;111:1691–1701. doi: 10.1172/JCI16293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stanchev D, Blosa M, Milius D, Gerevich Z, Rubini P, Schmalzing G, Eschrich K, Schaefer M, Wirkner K, Illes P. Cross-inhibition between native and recombinant TRPV1 and P2X3 receptors. Pain. 2009;143:26–36. doi: 10.1016/j.pain.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 26.Zhang N, Inan S, Cowan A, Sun R, Wang JM, Rogers TJ, Caterina M, Oppenheim JJ. A proinflammatory chemokine, CCL3, sensitizes the heat- and capsaicin-gated ion channel TRPV1. Proc Natl Acad Sci U S A. 2005;102:4536–4541. doi: 10.1073/pnas.0406030102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jariwala U, Prescott J, Jia L, Barski A, Pregizer S, Cogan JP, Arasheben A, Tilley WD, Scher HI, Gerald WL, Buchanan G, Coetzee GA, Frenkel B. Identification of novel androgen receptor target genes in prostate cancer. Mol Cancer. 2007;6:39. doi: 10.1186/1476-4598-6-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kasbohm EA, Guo R, Yowell CW, Bagchi G, Kelly P, Arora P, Casey PJ, Daaka Y. Androgen receptor activation by G(s) signaling in prostate cancer cells. J Biol Chem. 2005;280:11583–11589. doi: 10.1074/jbc.M414423200. [DOI] [PubMed] [Google Scholar]
- 29.Ramberg H, Eide T, Krobert KA, Levy FO, Dizeyi N, Bjartell AS, Bjartell AS, Abrahamsson PA, Taskén KA. Hormonal regulation of β2-adrenergic receptor level in prostate cancer. Prostate. 2008;68:1133–1142. doi: 10.1002/pros.20778. [DOI] [PubMed] [Google Scholar]
- 30.Hampel C, Dolber PC, Smith MP, Savic SL, JW T r, Thor KB, Schwinn DA. Modulation of bladder alpha1-adrenergic receptor subtype expression by bladder outlet obstruction. J Urol. 2002;167:1513–1521. doi: 10.1016/S0022-5347(05)65355-4. [DOI] [PubMed] [Google Scholar]
- 31.Zhong H, Lee D, Robeva A, Minneman KP. Signaling pathways activated by alpha1-adrenergic receptor subtypes in PC12 cells. Life Sci. 2001;68:2269–2276. doi: 10.1016/S0024-3205(01)01015-3. [DOI] [PubMed] [Google Scholar]
- 32.Rohacs T, Thyagarajan B, Lukacs V. Phospholipase C mediated modulation of TRPV1 channels. Mol Neurobiol. 2008;37:153–163. doi: 10.1007/s12035-008-8027-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chuang HH, Prescott ED, Kong H, Shields S, Jordt SE, Basbaum AI, Chao MV, Julius D. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2-mediated inhibition. Nature. 2001;411:957–962. doi: 10.1038/35082088. [DOI] [PubMed] [Google Scholar]
- 34.Perez-Aso M, Segura V, Montó F, Barettino D, Noguera MA, Milligan G, D’Ocon P. The three α1-adrenoceptor subtypes show different spatio-temporal mechanisms of internalization and ERK1/2 phosphorylation. Biochim Biophys Acta. 2013;1833:2322–2333. doi: 10.1016/j.bbamcr.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 35.Keffel S, Alexandrov A, Goepel M, Michel MC. alpha(1)-adrenoceptor subtypes differentially couple to growth promotion and inhibition in Chinese hamster ovary cells. Biochem Biophys Res Commun. 2000;272:906–911. doi: 10.1006/bbrc.2000.2850. [DOI] [PubMed] [Google Scholar]
- 36.Chen L, Hodges RR, Funaki C, Zoukhri D, Gaivin RJ, Perez DM, Dartt DA. Effects of alpha1D-adrenergic receptors on shedding of biologically active EGF in freshly isolated lacrimal gland epithelial cells. Am J Physiol Cell Physiol. 2006;291:C946–C956. doi: 10.1152/ajpcell.00014.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sydorenko V, Shuba Y, Thebault S, Roudbaraki M, Lepage G, Prevarskaya N, Skryma R. Receptor-coupled, DAG-gated Ca2+-permeable cationic channels in LNCaP human prostate cancer epithelial cells. J Physiol. 2003;548:823–836. doi: 10.1113/jphysiol.2002.036772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thebault S, Flourakis M, Vanoverberghe K, Vandermoere F, Roudbaraki M, Lehen’kyi V, Slomianny C, Beck B, Mariot P, Bonnal JL, Mauroy B, Shuba Y, Capiod T, Skryma R, Prevarskaya N. Differential role of transient receptor potential channels in Ca2+ entry and proliferation of prostate cancer epithelial cells. Cancer Res. 2006;66:2038–2047. doi: 10.1158/0008-5472.CAN-05-0376. [DOI] [PubMed] [Google Scholar]
- 39.Kojima Y, Sasaki S, Oda N, Koshimizu TA, Hayashi Y, Kiniwa M, Tsujimoto G, Kohri K. Prostate growth inhibition by subtype-selective alpha(1)-adrenoceptor antagonist naftopidil in benign prostatic hyperplasia. Prostate. 2009;69:1521–1528. doi: 10.1002/pros.21003. [DOI] [PubMed] [Google Scholar]
- 40.Liou SF, Lin HH, Liang JC, Chen IJ, Yeh JL. Inhibition of human prostate cancer cells proliferation by a selective alpha1-adrenoceptor antagonist labedipinedilol-A involves cell cycle arrest and apoptosis. Toxicology. 2009;256:13–24. doi: 10.1016/j.tox.2008.10.025. [DOI] [PubMed] [Google Scholar]
- 41.Kyprianou N, Benning CM. Suppression of human prostate cancer cell growth by alpha1-adrenoceptor antagonists doxazosin and terazosin via induction of apoptosis. Cancer Res. 2000;60:4550–4555. [PubMed] [Google Scholar]
- 42.Liu CM, Lo YC, Tai MH, Wu BN, Wu WJ, Chou YH, Chai CY, Huang CH, Chen IJ. Piperazine-designed alpha 1A/alpha 1D-adrenoceptor blocker KMUP-1 and doxazosin provide down-regulation of androgen receptor and PSA in prostatic LNCaP cells growth and specifically in xenografts. Prostate. 2009;69:610–623. doi: 10.1002/pros.20919. [DOI] [PubMed] [Google Scholar]
Pre-publication history
- The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2407/14/921/prepub
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1: Patient demographics. IQR = interquartile range; PSA = prostate-specific antigen. (XLSX 31 KB)
Additional file 2: Figure S1: Effects of WS433 and CPZ antagonists and chelerythrine, PD98059, U73122 inhibitors in PC3 cells. (A) The measurement of the extracellular acidification rate was performed as above described with PC3 cells treated for 30 sec with vehicle (control) or WS433 (1 μM) or/and CPZ (1 μM). Data shown are the mean ± SD of three separate experiments. (B) The time course of [Ca2+]i increase in Fluo-3-loaded PC3 cells vehicle-treated (control) or treated with WS433 or/and CPZ was evaluated by FACS analysis. Data shown are the mean ± SD of three separate experiments. (C) The measurement of IP3 levels performed as above described in PC3 cells vehicle-treated (control) or treated for different times with WS433 (1 μM) or/and CPZ (1 μM). Data shown are the mean ± SD of three separate experiments. (D-E) Lysates from PC3 cells vehicle-treated (control) or treated for different times with WS433 (1 μM) or/and CPZ (1 μM) were separated on SDS-polyacrylamide gels, transferred and then blotted with anti-pERK, ERK and anti-phospho-(Ser) PKC substrate Abs. The GAPDH protein level was evaluated as a loading control. Data shown are representative of one out of three separate experiments. (F) Cell growth was assessed by MTT assay in PC3 cells treated for 24 h with vehicle (control) or with chelerythrine (0.05-5 μM), PD98059 (1–100 μM) and U73122 (0.5-50 μM). Data shown are the mean ± SD of three separate experiments. Statistical analysis was performed by comparing chelerythrine, PD98059 and U73122 treated cells with control (*), p ≤ 0.01. (G) The proliferation of PC3 cells was analysed by labelling with BrdU. The cells treated with WS433 (1 μM) or CPZ (1 μM) were stained with an anti-BrdU FITC-conjugated Ab and analysed using a FACScan flow cytometer. Data shown are representative of one of three separate experiments. (TIFF 13 MB)
Additional file 3: Figure S2: Silencing of the α1D-AR and TRPV1 genes in PC3 cells. (A) The α1D-AR and TRPV1 mRNA levels were evaluated by qRT-PCR in siGLO-, siα1D-AR- and siTRPV1-transfected PC3 cells. The relative α1D-AR and TRPV1 expression levels, normalised to the β-actin mRNA level, were calculated using siGLO as a calibrator. (B) Lysates from siGLO-, siα1D-AR- and siTRPV1-transfected PC3 cells were separated by SDS-polyacrylamide gel electrophoresis and probed with anti-α1D-AR or anti-TRPV1 Abs. The GAPDH protein level was evaluated as a loading control. Representative immunoblots are shown. (C) FACS analysis was performed in PC3 cells double silenced for α1D-AR and TRPV1 genes. Silenced cells were double-stained with anti-TRPV1 and anti-α1D-AR Abs followed by respective secondary Abs. Data shown are representative of one of three separate experiments. (TIFF 3 MB)


