Abstract
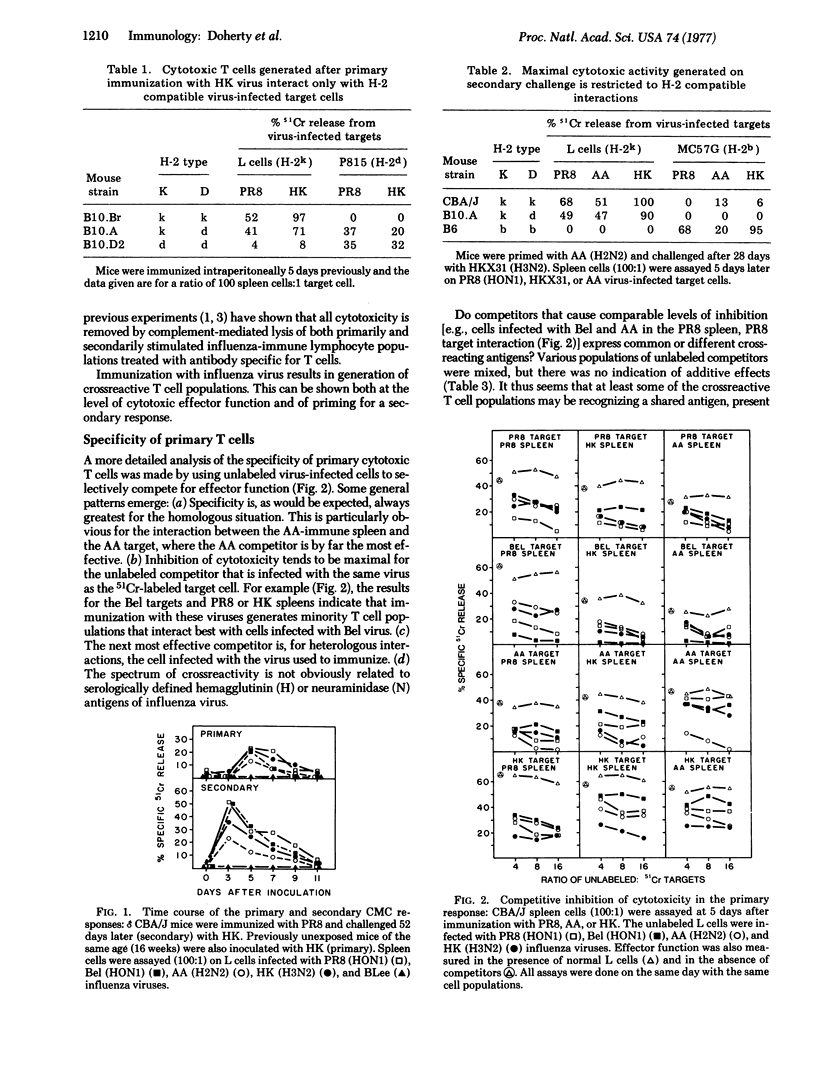
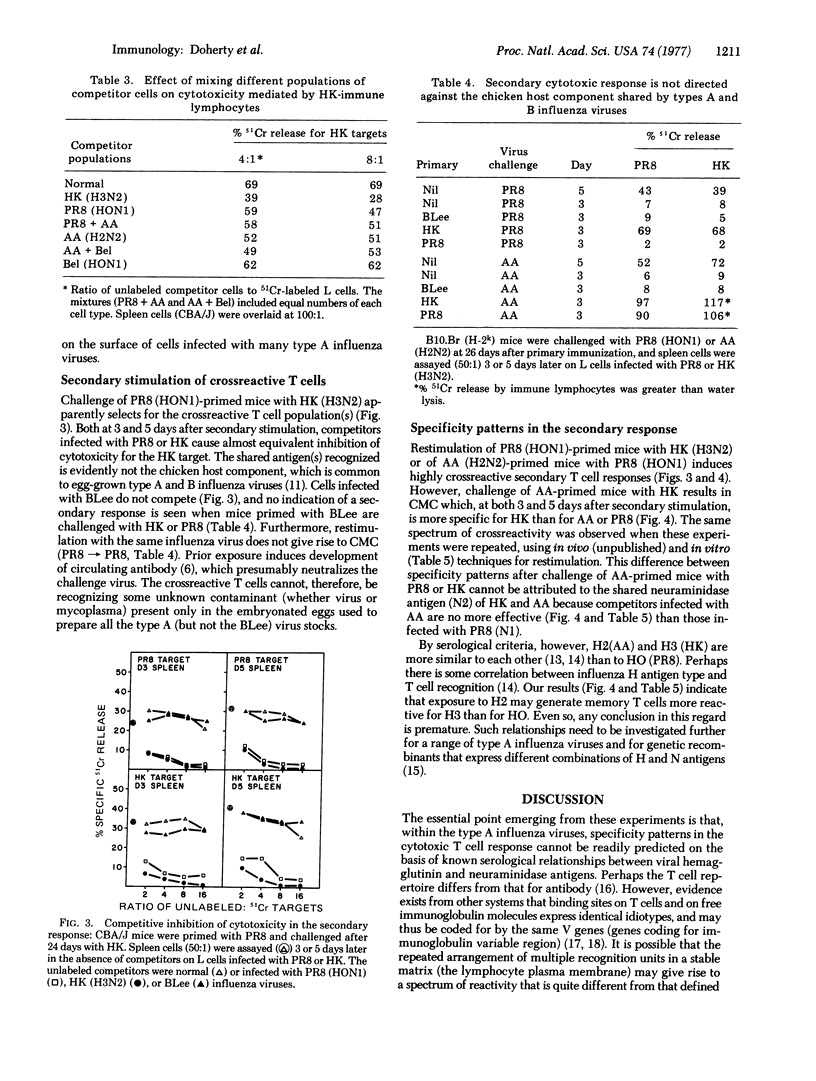
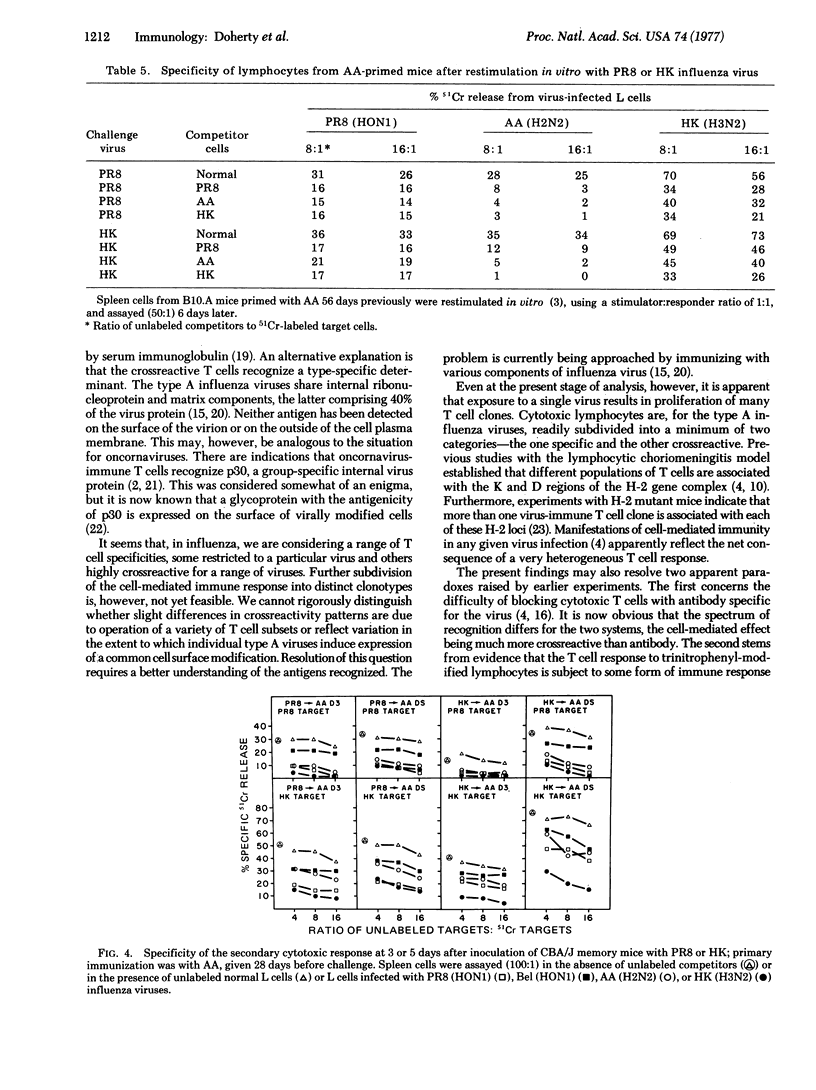
Immunization of mice with serologically distinct type A influenza viruses results in development of highly crossreactive populations of cytotoxic thymus-derived lymphocytes (T cells). This can be shown both at the level of effector function and of priming for an enhanced response after challenge with another type A virus. Cytotoxic activity is demonstrable, in both the primary and secondary situations, only for H-2 compatible interactions. Further analysis by competitive inhibition experiments indicates that some of the T cell clones generated are specific for the virus used to immunize, while others are much less restricted. Secondary stimulation may result in preferential stimulation of the crossreactive T cells if the type A viruses used are very different serologically. When more closely related viruses are used, however, some degree of specificity is seen for the challenge virus. Even so, the patterns of crossreactivity observed are complex, and cannot be readily predicted on the basis of known serological relationships between surface hemagglutinin and neuraminidase antigens of type A influenza viruses.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Binz H., Wigzell H. Shared idiotypic determinants on B and T lymphocytes reactive against the same antigenic determinants. II. Determination of frequency and characteristics of idiotypic T and B lymphocytes in normal rats using direct visualization. J Exp Med. 1975 Nov 1;142(5):1218–1230. doi: 10.1084/jem.142.5.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanden R. V., Hapel A. J., Jackson D. C. Mode of action of Ir genes and the nature of T cell receptors for antigen. Immunochemistry. 1976 Feb;13(2):179–191. doi: 10.1016/0019-2791(76)90287-1. [DOI] [PubMed] [Google Scholar]
- Bruce J., Mitchison N. A., Shellam G. R. Studies on a gross-virus-induced lymphoma in the rat. III. Optimisation, specificity and applications of the in vitro immune response. Int J Cancer. 1976 Mar 15;17(3):342–350. doi: 10.1002/ijc.2910170310. [DOI] [PubMed] [Google Scholar]
- Cambridge G., Mackenzie J. S., Keast D. Cell-mediated immune response to influenza virus infections in mice. Infect Immun. 1976 Jan;13(1):36–43. doi: 10.1128/iai.13.1.36-43.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty P. C., Blanden R. V., Zinkernagel R. M. Specificity of virus-immune effector T cells for H-2K or H-2D compatible interactions: implications for H-antigen diversity. Transplant Rev. 1976;29:89–124. doi: 10.1111/j.1600-065x.1976.tb00198.x. [DOI] [PubMed] [Google Scholar]
- Doherty P. C., Zinkernagel R. M. Specific immune lysis of paramyxovirus-infected cells by H-2-compatible thymus-derived lymphocytes. Immunology. 1976 Jul;31(1):27–32. [PMC free article] [PubMed] [Google Scholar]
- Dowdle W. R., Coleman M. T., Gregg M. B. Natural history of influenza type A in the United States, 1957-1972. Prog Med Virol. 1974;17(0):91–135. [PubMed] [Google Scholar]
- Fazekas de St Groth, Webster R. G. Disquisitions of Original Antigenic Sin. I. Evidence in man. J Exp Med. 1966 Sep 1;124(3):331–345. doi: 10.1084/jem.124.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARBOE A. The influenza virus haemagglutinnation inhibition by antibody to host material. Acta Pathol Microbiol Scand. 1963;57:317–330. doi: 10.1111/j.1699-0463.1963.tb05101.x. [DOI] [PubMed] [Google Scholar]
- Hämmerling G. J., Black S. J., Berek C., Eichmann K., Rajewsky K. Idiotypic analysis of lymphocytes in vitro. II. Genetic control of T-helper cell responsiveness to anti-idiotypic antibody. J Exp Med. 1976 Apr 1;143(4):861–869. doi: 10.1084/jem.143.4.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilbourne E. D. Future influenza vaccines and the use of genetic recombinants. Bull World Health Organ. 1969;41(3):643–645. [PMC free article] [PubMed] [Google Scholar]
- Morita M., Suto T., Ishida N. Antigenic memory in man in response to sequential infections with influenza A viruses. J Infect Dis. 1972 Jul;126(1):61–68. doi: 10.1093/infdis/126.1.61. [DOI] [PubMed] [Google Scholar]
- Nowinski R. C., Watson A. Immune response of the mouse to the major core protein (p30) of ecotropic leukemia viruses. J Immunol. 1976 Aug;117(2):693–696. [PubMed] [Google Scholar]
- Schmitt-Verhulst A. M., Shearer G. M. Multiple H-2 linked immune response gene control of H-2 D-associated T-cell-mediated lympholysis to trinitrophenyl-modified autologous cells: Ir-like genes mapping to the left of I-A and within the I region. J Exp Med. 1976 Dec 1;144(6):1701–1706. doi: 10.1084/jem.144.6.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shellam G. R., Knight R. A., Mitchison N. A., Gorczynski R. M., Maoz A. The specificity of effector T cells activated by tumours induced by murine oncornaviruses. Transplant Rev. 1976;29:249–276. doi: 10.1111/j.1600-065x.1976.tb00204.x. [DOI] [PubMed] [Google Scholar]
- Starzinski-Powitz A., Pfizenmaier K., Koszinowski U., Röllinghoff M., Wagner H. Shared determinants between virus-infected and trinitrophenyl-conjugated H-2-identical target cells detected in cell-mediated lympholysis. Eur J Immunol. 1976 Sep;6(9):630–634. doi: 10.1002/eji.1830060907. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. H-2 compatability requirement for T-cell-mediated lysis of target cells infected with lymphocytic choriomeningitis virus. Different cytotoxic T-cell specificities are associated with structures coded for in H-2K or H-2D;. J Exp Med. 1975 Jun 1;141(6):1427–1436. doi: 10.1084/jem.141.6.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkernagel R. M., Dunlop M. B., Blanden R. V., Doherty P. C., Shreffler D. C. H-2 compatibility requirement for virus-specific T-cell-mediated cytolysis. Evaluation of the role of H-2I region and non-H-2 genes in regulating immune response. J Exp Med. 1976 Aug 1;144(2):519–532. doi: 10.1084/jem.144.2.519. [DOI] [PMC free article] [PubMed] [Google Scholar]



