Abstract
Harvesting lymph nodes (LNs) after gastrectomy is essential for accurate staging. This trial evaluated the efficiency and quality of a conventional method and a methylene blue–assisted method in a randomized manner. The key eligibility criteria were as follows: (i) histologically proven adenocarcinoma of the stomach; (ii) clinical stage I-III; (iii) R0 resection planned by gastrectomy with D1+ or D2 lymphadenectomy. The primary endpoint was the ratio of the pathologic number of harvested LNs per time (minutes) as an efficacy measure. The secondary endpoint was the number of harvested LNs, as a quality measure. Between August 2012 and December 2012, 60 patients were assigned to undergo treatment using the conventional method (n=29) and the methylene blue dye method (n=31). The baseline demographics were mostly well balanced between the 2 groups. The number of harvested LNs (mean±SD) was 33.6±11.9 in the conventional arm and 43.4±13.9 in the methylene blue arm (P=0.005). The ratio of the number of the harvested LNs per time was 1.12±0.46 LNs/min in the conventional arm and 1.49±0.59 LNs/min in the methylene blue arm (P=0.010). In the subgroup analyses, the quality and efficacy were both superior for the methylene blue dye method compared with the conventional method. The methylene blue technique is recommended for harvesting LNs during gastric cancer surgery on the basis of both the quality and efficacy.
Key Words: gastric cancer, methylene blue, lymph node dissection
Gastric cancer is the second most common cause of cancer-related death, after lung cancer.1 D2 gastrectomy is the standard treatment for localized gastric cancer.2–4 After surgery, the progression of the tumor is determined by the depth of tumor invasion and the presence of lymph node (LN) metastasis. The patients who have positive LNs may be candidates for adjuvant treatment.5–8 The LN status is also considered to be the most important prognostic factor.9
The nodal status is determined by the number of positive LNs among the harvested LNs. The Union for International Cancer Control and the third edition of the Gastric Cancer Treatment Guideline recommend that at least 16 LNs be harvested for reliable staging.10,11
Although the harvesting of LNs after gastrectomy is essential for accurate staging of gastric cancer, the technique has not been standardized. The LN sampling from the specimens can be affected by the physician’s experience and persistence, the extent of dissection, type of gastrectomy, and the method used to harvest the LNs. Surgeons harvest LNs from fresh specimens immediately after surgery in Japan, whereas the pathologists generally harvest the nodes from specimens fixed with formalin after surgery in other countries. Although surgeons may be enthusiastic about harvesting LNs, it is often difficult to do after performing an extensive surgery. In contrast, nodal sampling may be difficult for pathologists, who are not familiar with the surgical anatomy. Moreover, after tissues are fixed with formalin, it is often difficult to separate and identify the LNs, which makes nodal sampling time-consuming work for most pathologists.
The methylene blue–assisted technique is another approach used for harvesting LNs.12 In this approach, the specimens are fixed with formalin-containing methylene blue after surgery. The physicians can easily pick up blue LNs, regardless of the their level of knowledge or skill. However, only a few Japanese surgeons have reported efficacy of this method in single-arm studies.13,14 It therefore remains unclear whether the methylene blue–assisted technique is superior to the conventional technique using fresh samples from gastric cancer patients.
We hypothesized that harvesting LNs by the methylene blue–assisted technique would be more efficient and provide better quality LN than the conventional technique. More accurate staging would lead to appropriate postoperative treatment, which could affect the patient’s survival. To confirm our hypothesis, we conducted a single-center, randomized, controlled study to evaluate the efficiency and quality of harvesting LNs between the conventional technique and the methylene blue technique.
MATERIALS AND METHODS
Inclusion and Exclusion Criteria
The inclusion criteria were as follows: (i) histologically proven adenocarcinoma of the stomach; (ii) clinical stage I, II, or III disease, as determined by the Japanese Classification of Gastric Carcinoma, third English edition11; and (iii) R0 resection achieved by gastrectomy with D1+ or D2 lymphadenectomy, according to the Japanese gastric cancer treatment guidelines 2010 (ver. 3).2 We excluded the patients who received preoperative chemotherapy and/or radiation therapy.
Surgical Procedure
All patients underwent distal or total gastrectomy with nodal dissection for gastric cancer. In principle, a D1 or a D1+ lymphadenectomy was indicated for cT1N0 tumors, and D2 was indicated for cN+ or cT2-T4 tumors, regardless of the approach.2 Spleen-preserving D2 total gastrectomy was permitted in this study. The omentum was preserved except where resection was necessary for LN dissection along the right gastroepiploic artery.
LN Harvesting Methods and the Pathologic Diagnosis
Surgeons registered the information in the data center after confirming the eligibility criteria during the surgery. Then, the patients were randomized and assigned to the conventional method or the methylene blue–assisted method by a centralized dynamic method using the following factors: lymphadenectomy (D1+/D2), type of gastrectomy (subtotal/total), and surgical experience (<15/15+ y).
During surgery, the stomach and the perigastric tissues containing the LNs, fat, and vessels were removed in 1 block (by so called “en-bloc dissection”). Then, the perigastric tissues were separated from the stomach and divided into (1) the area that contained the LNs located along the proper hepatic artery, common hepatic artery, celiac artery, and splenic artery, (2) the area that had been attached to the lesser curvature and contained the LNs along the right and left gastric arteries, and (3) the area that had been attached to the greater curvature and contained LNs along the right and left gastroepiploic arteries.
In the conventional arm, the LNs were harvested from each area immediately after surgery. First, all palpable LNs were removed from each area. Usually, most LNs could be harvested from the area along the major branched arteries by this method because of the limited fat tissues in this area, whereas those in the lesser and greater curvatures were buried in fatty tissues. Thereafter, the remaining tissues were sliced and stretched to detect visible LNs. After that, the resected stomach and all harvested LNs were fixed with 10% buffered formalin for at least 48 hours. In the methylene blue arm, each separated tissue that contained LNs was fixed with 10% buffered formalin with methylene blue for 2 nights. Figure 1 shows a photograph of a representative tissue sample containing LNs in the area that had been attached to the greater curvature. The LNs are stained with methylene blue dye. After that, the LNs stained with the blue dye were harvested from each area. The concentration of the formalin was standardized throughout the study.
FIGURE 1.
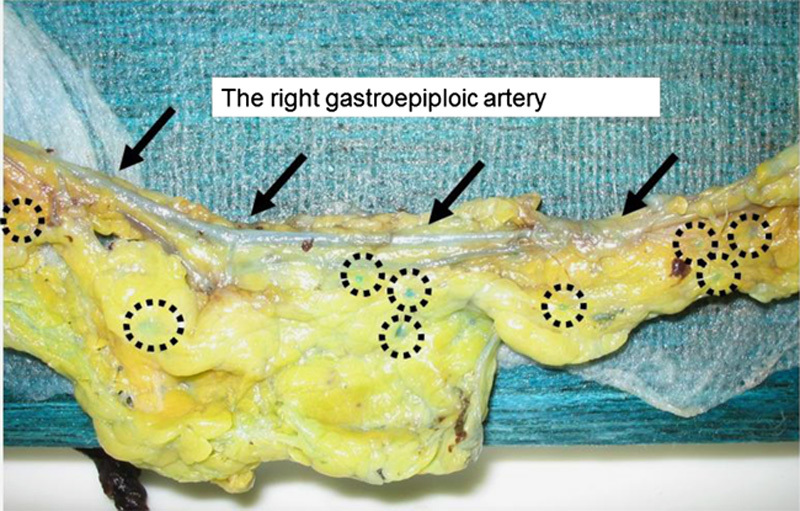
The arrows point the right gastroepiploic artery. The circle points LNs stained blue by the methylene blue–assisted technique.
After standard histologic processing of the tissue, and paraffin embedding, 2-step sections were cut from each block and stained with hematoxylin and eosin. The methylene blue stain vanished completely during the histologic processing and did not influence the evaluation of the slides. All slides were screened by experienced pathologists for LN metastases.
The time required for harvesting the LNs was defined as that from the initiation to the termination. The ratio of the number of harvested LNs per time (in minutes) was calculated. The tumors were staged according to the third edition of the Gastric Cancer Treatment Guideline published by the Japanese Gastric Cancer Association.2
Qualification of the Participating Surgeons
The protocol prespecified that the examiners were surgeons who had previous experience with harvesting LNs from >50 specimens from gastric cancer surgery.
Study Design and Statistical Analyses
The primary endpoint was the ratio of the number of harvested LNs per time (in minute) as an efficacy measure. The secondary endpoint was the number of harvested LNs as a quality measure. We expected that there would be a 25% reduction in the ratio of harvested LNs/time (in minutes) for this test treatment, considering the balance between the cost and benefit. On the basis of the retrospective data at our institution and described in previous reports, we estimated that the ratio of the number of harvested LNs per time (minutes) would be 40/30 minutes in the conventional arm. To achieve 25% risk reduction, a ratio of 40/22.5 minutes in the methylene blue arm was expected. On the basis of this assumption, a sample size of 26 per arm (52 total patients) was needed to ensure a statistical power of 80% at a 2-sided α of 5%. Considering the likelihood of enrolling ineligible patients, the number of patients to be accrued was set at 60 in total. The primary endpoint of the study was analyzed on an intent-to-treat principle, and the data were compared using t tests. The secondary endpoints, the number of harvest LNs and the time required to harvest the LNs, were similarly analyzed using t tests. A subgroup analysis was also performed to explore the effects of the type of gastrectomy, type of lymphadenectomy, sex, and body mass index (BMI) on the endpoints. This study was approved by the Ethics Review Board of Kanagawa Cancer Center. Trial registration: UMIN000008624.
RESULTS
Patients
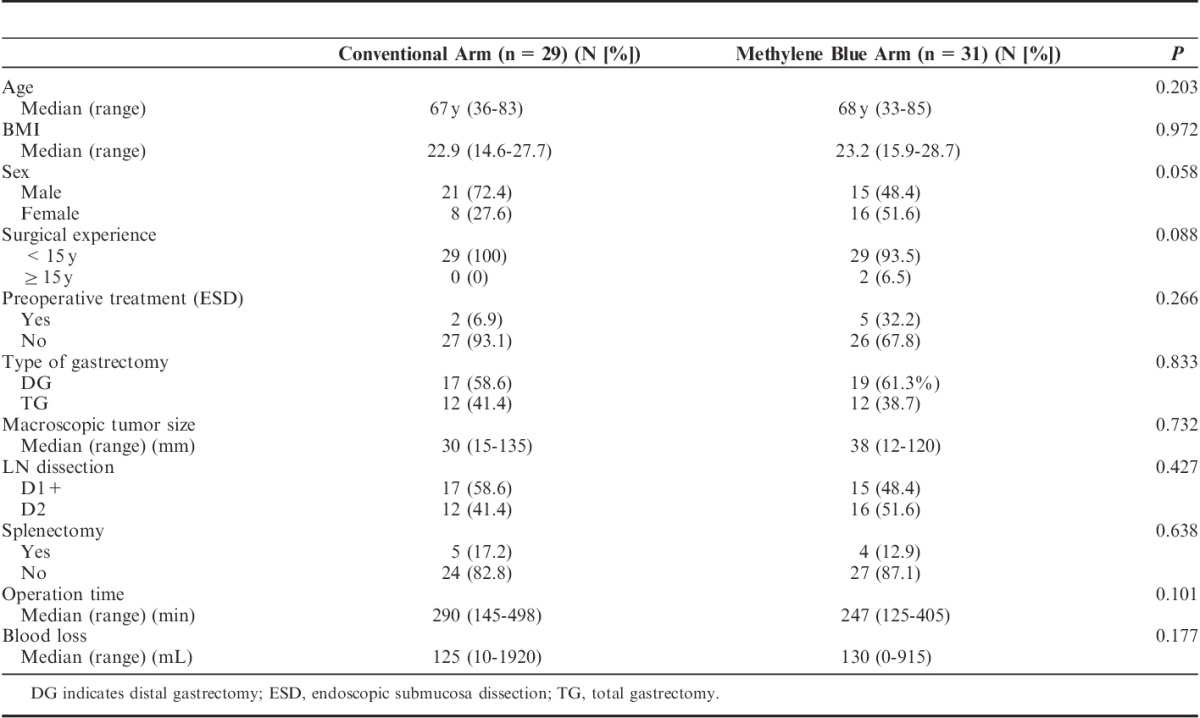
Between August 2012 and December 2012, 60 patients were registered in this study, and were randomly allocated to either the conventional arm (n=29) or the methylene blue arm (n=31). Figure 2 shows the CONSORT diagram. All patients were judged to be eligible. All patients were treated following the protocol of the arm to which they were allocated. The background characteristics of both arms are shown in Table 1. The age, BMI, preoperative endoscopic submucosa dissection, type of gastrectomy, macroscopic tumor size, lymphadenectomy, splenectomy, length of operation, and blood loss were well balanced between the 2 arms except for sex and surgical experience. Male individuals were dominant in the conventional arm, whereas female individuals were dominant in the methylene blue arm. No surgeons had >15 years of experience in the conventional arm, but 2 had >15 years of experience in the methylene blue arm. However, the differences in sex and surgical experience were not statistically significant.
FIGURE 2.
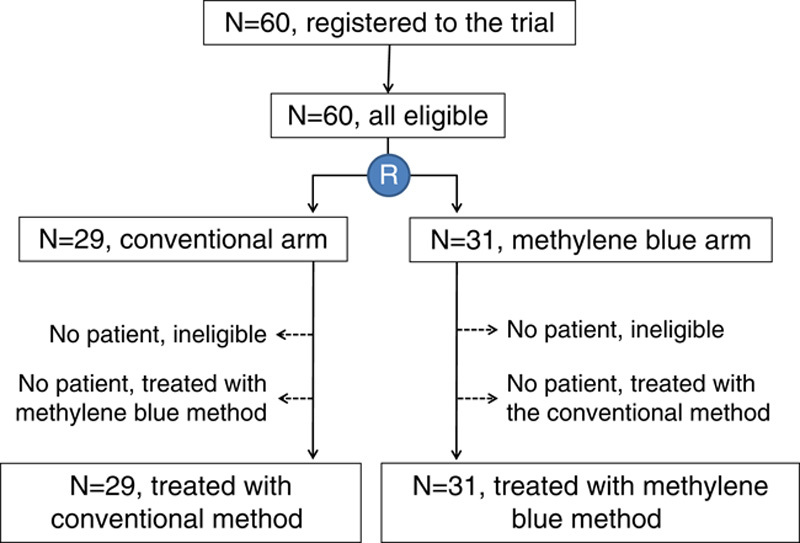
The CONSORT diagram of this study.
TABLE 1.
Patient’s Background in the Conventional and the Methylene Blue Arms
The Harvested LNs and Pathologic Findings
The number of harvested LNs (mean±SD), which was the secondary endpoint, was 33.6±11.9 in the conventional arm and 43.4±13.9 in the methylene blue arm (P=0.005, difference of the means [95% confidence interval], 9.8 [3.1-16.5]). The time per case for harvesting LN was 32.5±11.7 min/case in the conventional arm and 33.9±19.0 min/case in the methylene blue arm (P=0.743). The ratio of the number of the harvested LNs per time, which was the primary endpoint, was 1.12±0.46 LNs/min in the conventional arm and 1.49±0.59 LNs/min in the methylene blue arm (P=0.010, difference of the means, 0.36 [0.09-0.64]).
Waterfall plots of the number of harvested LNs in both arms are shown in Figure 3. One patient (3.4%) in the conventional arm had <16 LNs harvested, whereas at least 16 LNs were harvested from each patient in the methylene blue arm. Figure 4 demonstrates the waterfall plots of the ratios of the harvested LNs/min in both arms.
FIGURE 3.

A waterfall plot of the numbers of harvested LNs in the conventional and methylene blue arms. The orange line indicates the 16 LNs recommended for accurate staging. One patient in the conventional arm had <16 LNs harvested.
FIGURE 4.
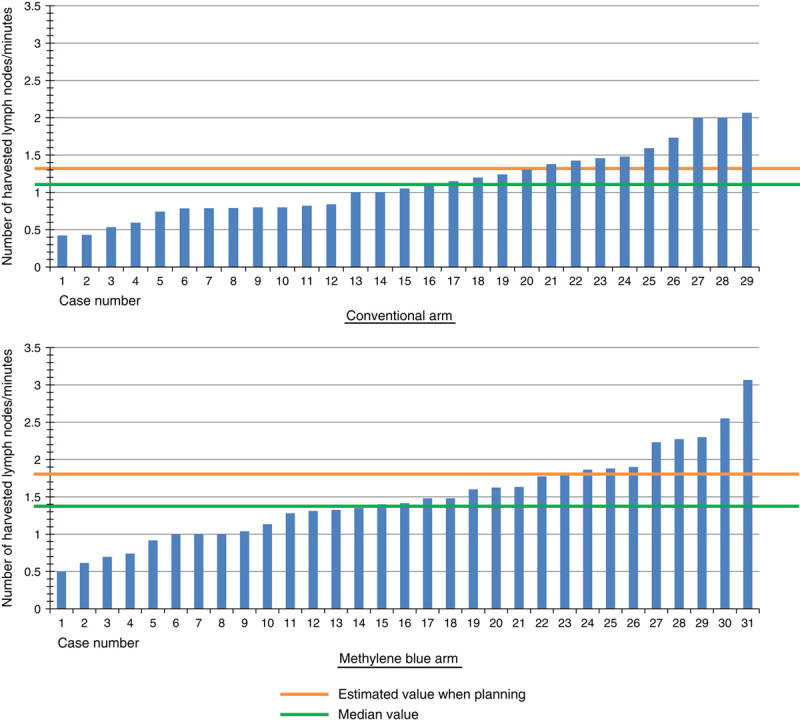
A waterfall plot of the number of harvested LNs per time (in minutes) in the conventional and methylene blue arms. The orange line indicates the estimated value determined when planning the study. The green line indicates the median value.
The pathologic findings are shown in Table 2. None of the pathologic factors were significantly different between the groups.
TABLE 2.
Pathologic Findings Between the Conventional and Methylene Blue Arms

Subgroup Analyses
The subgroup analyses were performed according to the type of gastrectomy, the extent of LN dissection, sex, and BMI. The difference in the mean between arms and its 95% confidence interval in the number of the harvested LNs and the ratios of the number of the harvested LNs per time were plotted in Figures 5 and 6, respectively. The P values for the interaction were not statistically significant in any of the categories, although the BMI tended to have interaction with the number of LNs harvested (P=0.136). The methylene blue arm showed better results than the conventional arm in terms of both the number and the ratio in all subgroups of each category.
FIGURE 5.
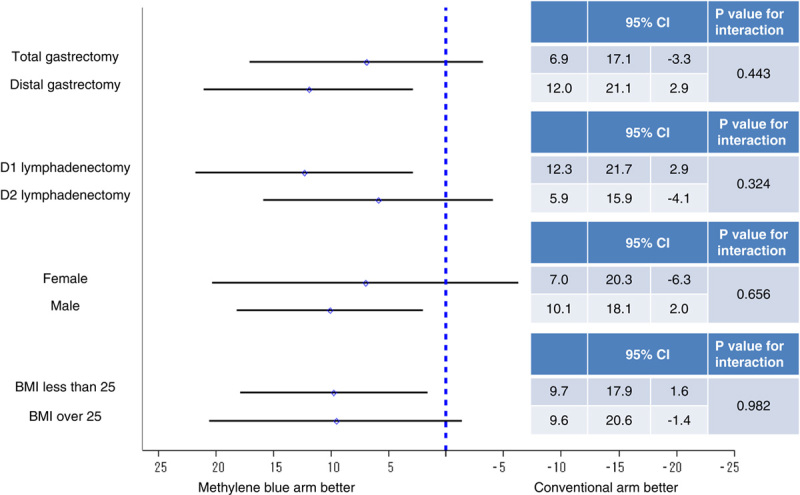
The results of the subgroup analysis of the differences in the mean number of harvested LNs for the eligible population.
FIGURE 6.

The results of the subgroup analysis of the differences in the mean ratio of the number of harvested LNs per time (in minutes) for the eligible population.
Accuracy of Clinically Harvested LNs
The number of the clinically harvested LNs was 44.2±16.3 and the number of pathologic LNs was 33.6±11.9 in the conventional arm, whereas the former was 47.1±17.0 and the latter was 43.4±13.9 in the methylene blue arm. The mean ratio of the former to the latter was 0.79 in the conventional arm and 0.95 in the methylene blue arm (P=0.004).
DISCUSSION
This is the first report that has evaluated different procedures for harvesting LNs and demonstrating that the methylene blue–assisted technique was superior to the conventional method in terms of the ratio of the number of harvested LNs per time as a quality measure and in the number of the harvest LNs as an efficacy measure, in a prospective randomized controlled study. Moreover, the similar efficacy and quality of the methylene blue–assisted technique were observed regardless of the patient’s sex, BMI, type of gastrectomy, and type of lymphadenectomy. Therefore, the methylene blue technique is recommended for harvesting LNs from the viewpoints of both quality and efficacy during gastric cancer surgery.
In this study, the primary endpoint was the ratio of the number of harvested LNs per time (in minutes). Theoretically, each patient would have a similar number of LNs. If physicians spend an unlimited amount of time harvesting LNs, they might accurately collect all LNs, even those buried in the specimens. Although harvesting LNs after gastrectomy is essential for accurate staging of gastric cancer, most physicians consider that harvesting LNs is time-consuming and relatively undesirable work, because their specialty is surgery or pathology. All physicians would like to harvest as many LNs as possible in a limited period of time. Considering these factors, the harvesting method must be evaluated for efficacy. Therefore, we set the number of harvested LNs per time (in minutes) as the primary endpoint in this study.
On the basis of the retrospective data at our institution and previous reports, we estimated that the ratio of the number of harvested LNs per time (in minutes) would be 40/30 minutes in the conventional arm and 40/22.5 minutes in the methylene blue arm. We hypothesized that an equal number of LNs could be harvested in a shorter time using the methylene blue method than the conventional method. However, whereas the number of harvested LNs was significantly higher in the methylene blue arm than in the conventional arm, the time for harvesting the LNs was almost equal between the 2 arms. This could be explained by the threshold and accuracy of detecting LNs. As shown in Figure 1, not only large LNs but also very small LNs, which would not be picked up by the conventional method, were stained with methylene blue, which allowed very small LNs to be harvested only in the methylene blue arm. The time required to harvest the LNs would be longer because of the harvesting of such small LNs in the methylene blue arm. This suggests that the accuracy of detecting LNs should also be taken into consideration. In the present study, the concordance rate was significantly higher in the methylene blue arm than in the conventional arm. These results suggested that pathologic LNs could be accurately picked up by the methylene blue method, regardless of the size of the LNs.
In the present study, the number of LNs harvested was >16 in all patients except 1 in the conventional arm, which suggested that the final N-staging was appropriate in most patients. This indicates that the examiners were all experts who could pick up the LNs without the assistance of the blue dye in this study, which may have decreased the benefit of the methylene blue technique in the randomized study. Nevertheless, the number and the ratio were superior in the methylene blue arm than in the conventional arm, and it is expected that more superior results would be obtained if the examiners were less experienced.
Although this study showed positive results, there were some limitations associated with this study. First, all surgeons had previous experience with harvesting LNs, and all had harvested LNs from >50 specimens. All surgeons already had sufficient skills and knowledge for harvesting LNs using the conventional method. Moreover, our hospital is a high-volume center, in which >200 gastric cancer surgeries are performed every year. It is therefore unclear whether our results can be generalized to less experienced surgeons in low-volume hospitals. Second, this was a single-center study. Our results must be validated in other hospitals or in the multicenter study. Third, we excluded the patients who received preoperative chemotherapy. On the basis of the results of the MAGIC trial, perioperative chemotherapy is now the standard treatment for gastric cancer in Europe.6 However, it is well known that preoperative chemo-radiotherapy in patients with rectal and breast cancer is associated with a reduced number of LNs.15–17 It should therefore be clarified whether the methylene blue technique is superior to the conventional method in terms of the efficacy and quality in cancers for which preoperative treatment is frequently selected.
In conclusion, the methylene blue–assisted technique is recommended for harvesting LNs in gastric cancer surgery.
ACKNOWLEDGMENTS
The authors express their sincere gratitude to Mrs Rika Takahashi for her excellent data management in this study.
Footnotes
T.A. and T.Y. contributed equally.
Conflicts of Interest and Source of Funding: Supported, in part, by the Non-Governmental Organizations Kanagawa Standard Anti-cancer Therapy Support System. The authors have disclosed that they have no significant relationships with, or financial interest in, any commercial companies pertaining to this article.
REFERENCES
- 1.Ohtsu A, Yoshida S, Saijo N. Disparities in gastric cancer chemotherapy between the East and West. J Clin Oncol. 2006;24:2188–2196. [DOI] [PubMed] [Google Scholar]
- 2.Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011;14:113–123. [DOI] [PubMed] [Google Scholar]
- 3.Okines A, Verheij M, Allum W, et al. The ESMO Guidelines Working Group. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21suppl 5v50–v54. [DOI] [PubMed] [Google Scholar]
- 4.NCCN. NCCN Clinical Practice Guidelines in Oncology. Gastric Cancer. Version 2.2011. 2011. Available at: http://www.nccn.org. Accessed December 5, 2011.
- 5.Macdonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345:725–730. [DOI] [PubMed] [Google Scholar]
- 6.Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20. [DOI] [PubMed] [Google Scholar]
- 7.Sakuramoto S, Sasako M, Yamaguchi T, et al. ACTS-GC Group. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007;357:1810–1820. [DOI] [PubMed] [Google Scholar]
- 8.Bang YJ, Kim YW, Yang HK, et al. CLASSIC trial investigators. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet. 2012;379:315–321. [DOI] [PubMed] [Google Scholar]
- 9.Siewert JR, Bottcher K, Stein HJ, et al. Relevant prognostic factors in gastric cancer: ten-year results of the German gastric cancer study. Ann Surg. 1998;228:449–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sobin LH, Gospodarowicz MK, Wittekind C. TNM Classification of Malignant Tumours. 2010West Sussex: Wiley. [Google Scholar]
- 11.Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101–112. [DOI] [PubMed] [Google Scholar]
- 12.Märkl B, Kerwel TG, Jähnig HG, et al. Methylene blue-assisted lymph node dissection in colon specimens: a prospective, randomized study. Am J Clin Pathol. 2008;130:913–919. [DOI] [PubMed] [Google Scholar]
- 13.Isozaki E, Okajima K, Fujiwara A, et al. A study of lymph node-metastases of gastric cancer using the methylene blue formalin fixing method [Article in Japanese]. Rinsho Shinkeigaku. 1986;26:710–716. [Google Scholar]
- 14.Kurosu Y, Isozumi M, Aoki N, et al. Study on lymph node metastasis of cancer using the methylene blue formalin fixing method [Article in Japanese]. J Nihon Univ Med Assoc. 1987;46:1057–1059. [Google Scholar]
- 15.Mekenkamp LJ, van Krieken JH, Marijnen CA, et al. Lymph node retrieval in rectal cancer is dependent on many factors: the role of the tumor, the patient, the surgeon, the radiotherapist, and the pathologist. Am J Surg Pathol. 2009;33:1547–1553. [DOI] [PubMed] [Google Scholar]
- 16.Neuman H, Carey LA, Ollila DW, et al. Axillary lymph node count is lower after neoadjuvant chemotherapy. Am J Surg. 2006;191:827–829. [DOI] [PubMed] [Google Scholar]
- 17.Rullier A, Laurent C, Capdepont M, et al. Lymph nodes after preoperative chemoradiotherapy for rectal carcinoma: number, status, and impact on survival. Am J Surg Pathol. 2008;32:45–50. [DOI] [PubMed] [Google Scholar]


