Abstract
Background
Aspiration of gastroesophageal refluxate has been implicated in the pathogenesis of idiopathic pulmonary fibrosis (IPF) and the progression of bronchiolitis obliterans syndrome after lung transplantation. The goals of the present study were to identify lung transplant patients at the greatest risk of aspiration and to investigate the causative factors.
Materials and methods
From September 2009 to November 2011, 252 bronchoalveolar lavage fluid (BALF) samples were collected from 100 lung transplant patients. The BALF pepsin concentrations and the results of transbronchial biopsy, esophageal function testing, barium swallow, and gastric emptying scan were compared among those with the most common end-stage lung diseases requiring lung transplantation: IPF, chronic obstructive pulmonary disease, cystic fibrosis, and α1-antitrypsin deficiency.
Results
Patients with IPF had higher BALF pepsin concentrations and a greater frequency of acute rejection than those with α1-antitrypsin deficiency, cystic fibrosis, or chronic obstructive pulmonary disease (P = 0.037). Moreover, the BALF pepsin concentrations correlated negatively with a lower esophageal sphincter pressure and distal esophageal amplitude; negatively with distal esophageal amplitude and positively with total esophageal acid time, longest reflux episode, and DeMeester score in those with chronic obstructive pulmonary disease; and negatively with the upright acid clearance time in those with IPF.
Conclusions
Our results suggest that patients with IPF after lung transplantation are at increased risk of aspiration and a greater frequency of acute rejection episodes, and that the risk factors for aspiration might be different among those with the most common end-stage lung diseases who have undergone lung transplantation. These results support the role of evaluating the BALF for markers of aspiration in assessing lung transplant patients as candidates for antireflux surgery.
Keywords: Gastroesophageal reflux disease, GERD, Aspiration, Lung transplantation, Bronchiolitis obliterans syndrome, BOS
1. Introduction
Recent evidence has been increasingly convincing that aspiration of gastric contents is among the potential causative factors for the development of bronchiolitis obliterans syndrome (BOS) after lung transplantation [1–9]. The findings that support this hypothesis include that gastroesophageal reflux disease (GERD) is exceedingly common in lung transplant patients [2,3,10,11], that aspiration induces immunologic and inflammatory changes within the pulmonary allograft [9,12,13], and that lung transplant patients with GERD who undergo antireflux surgery have at least a stabilization of their pulmonary function, if not an improvement in their freedom from BOS [3–6].
Although we are beginning to unravel the biologic methods by which aspiration contributes to BOS, it remains unclear which lung transplant patients are at greatest risk of aspiration, and who, among them, might benefit most from antireflux surgery. Patients with idiopathic pulmonary fibrosis (IPF) have been thought to have the greatest risk of GERD and aspiration after lung transplantation. Even before transplantation, those with IPF have an increased prevalence of GERD [14–21] and seemingly benefit from antireflux surgery when it can be safely performed in the pretransplant stage [22]. Nevertheless, we still lack a clear understanding of the risk factors for aspiration after lung transplantation, especially in those with IPF. Therefore, we aimed to identify the lung transplant patients at greatest risk of aspiration and to investigate the causative factors. We hypothesized that patients with IPF would be at the greatest risk of aspiration after lung transplantation. To test this hypothesis, we measured the concentration of pepsin in the bronchoalveolar lavage fluid (BALF) of a large cohort of lung transplant patients and correlated these findings with the results of esophageal function testing, barium swallow, and gastric emptying to identify the risk factors for GERD. Our findings have indicated that lung transplant patients with IPF have greater levels of pepsin in their BALF and that the risk factors for GERD and aspiration might differ among patients with different end-stage lung diseases (ESLDs) after lung transplantation.
2. Materials and methods
We prospectively collected 252 BALF samples from 100 consecutive lung transplant patients from September 2009 to November 2011. The samples were obtained at routine surveillance bronchoscopy or when otherwise clinically indicated by a decline in pulmonary function. The concentration of pepsin in the BALF was compared among patients who were grouped by the four most common ESLDs requiring lung transplantation: IPF, chronic obstructive pulmonary disease (COPD), cystic fibrosis (CF), and α1-antitrypsin deficiency (AAT). Likewise, the results of esophageal manometry, ambulatory pH monitoring, barium swallow, and gastric emptying scans were compared between the groups. Finally, the findings of these tests were correlated with the BALF pepsin concentrations. All study subjects provided informed consent, and the Loyola University Medical Center institutional review board approved the present study (LU202400).
2.1. BALF collection, storage, and sample processing
BALF was collected from the right middle lobe for unilateral right and bilateral lung transplants and from the lingula for unilateral left lung transplants [6]. The BALF was placed on ice and immediately transferred to the research laboratory, where it was centrifuged at 1500 rpm for 10 min, separated into aliquots, and snap frozen at −80°C [6]. The BALF pepsin concentrations were then measured using an enzyme-linked immunosorbent assay, as previously described [6].
2.2. Transbronchial biopsy assessment
Transbronchial biopsy specimens were obtained from the right upper and lower lobes for bilateral lung transplants and the upper and lower lobes for unilateral lung transplants. The transbronchial biopsy specimens were assessed for acute cellular rejection and airway inflammation according to the “Revision of the 1996 Working Formulation for the Standardization of Nomenclature in the Diagnosis of Lung Rejection” [23]. Evidence of aspiration was determined by a pathologist who assessed the presence of exogenous material with foreign body giant cell reaction, large lipid droplets, and/or macrophages with large vacuoles.
2.3. Esophageal manometry, ambulatory pH testing, barium swallow, and gastric emptying studies
Lung transplant patients were referred to the swallowing center at our institution for esophageal function testing, which was performed as previously described [24]. These patients were referred for esophageal function testing when GERD was clinically suspected and, most commonly, as early as possible after lung transplantation. In brief, proton pump inhibitors were stopped for 14 d and histamine H2 receptor antagonists were stopped for 3 d before pH monitoring. A dual-sensor 24-h esophageal pH catheter (Sleuth system with BioVIEW software, Sandhill Scientific, Denver, CO) was placed with the distal pH sensor positioned 5 cm from the manometrically determined upper border of the lower esophageal sphincter (LES). The diagnosis of GERD was determined by a DeMeester score > 14.7, as calculated from the distal pH recordings. Proximal reflux was defined as > 1% total time of pH < 4 recorded at the proximal sensor, located 20 cm above the LES [24]. Esophageal manometry was performed according to our previously published technique [25]. The presence and size of a hiatal hernia was assessed by measuring the axial length of the hernia, relative to the diaphragm, with the patient in the upright position on a posteroanterior barium esophagram, using eFilm Lite software (Merge Healthcare, Milwaukee, WI). Nuclear medicine gastric emptying studies were performed by obtaining dynamic scintigraphic images through the abdomen for 90 min after oral administration of 0.4 mCi technetium-99m-labeled sulfur colloid in ovalbumin. Gastric emptying was considered delayed if < 30% of the gastric contents had emptied into the small bowel within 90 min [24].
2.4. Pulmonary function testing and BOS staging
The diagnosis of BOS and its grade were determined according to the guidelines from the International Society of Heart and Lung Transplantation and as we have previously published [6,25].
2.5. Statistical analysis
All data were assessed for normality, and parametric or nonparametric tests were applied as appropriate. Dichotomous variables are reported as percentages and numbers, continuous nonparametric variables as the median and interquartile range, and continuous parametric variables as the mean ± standard deviation. Correlations were performed with Spearman’s rank correlation coefficient. Statistical analyses were calculated with Statistical Analysis System, version 9.1 (SAS Institute, Cary, NC), with corresponding graphs created using GraphPad Prism, version 5, for Windows (GraphPad Software, La Jolla, CA). A difference between the observed variables was considered statistically significant at P < 0.05.
3. Results
3.1. Demographics
The indications for lung transplantation among the 100 patients considered in the present study were COPD in 38, IPF in 24, CF in 14, AAT in 7, sarcoidosis in 4, pulmonary artery hypertension in 2, polymyositis in 2, and bronchiolitis obliterans organizing pneumonia, Jo-1 syndrome, lymphangioleiomyomatosis, pulmonary veno-occlusive disease, scleroderma, pulmonary fibrosis from work exposure, rheumatoid arthritis, dermatomyositis, and pneumoconiosis in 1 each. Of the cohort, 46% of the patients were women. At study enrollment, the median age and interval since lung transplantation was 59 y (range 50–62) and 5.8 mo (range 1.2–14.4), respectively. The median duration of follow-up since transplantation was 19 mo (range 12–31.5). The incidence of BOS was 23%, with a median interval to BOS of 19.5 mo (range 12.8–55), after excluding three patients who had transferred out of state and/or whose forced expiratory volume in 1 s data were incomplete. The mortality rate was 9% among 99 patients whose follow-up was sufficient to determine survival, with a median interval to death after lung transplantation of 23 mo (range 9–63.5).
3.2. BALF pepsin concentrations
Figure 1 demonstrates the BALF pepsin concentrations among the entire cohort of study subjects, subdivided by the indication for lung transplantation (in alphabetical order). Of the entire cohort, those with IPF had the highest BALF concentrations of pepsin. When grouped according to the most common indications for lung transplantation, those with IPF had significantly greater concentrations of pepsin in their BALF than did those with AAT, CF, and COPD (P < 0.05 versus each group; Fig. 2). Patients with IPF were also more likely to have pepsin levels ≥ 1 ng/mL detected in their BALF than those with AAT, CF, or COPD (68% versus 31%, 44%, and 47%, respectively; P < 0.05).
Fig. 1.
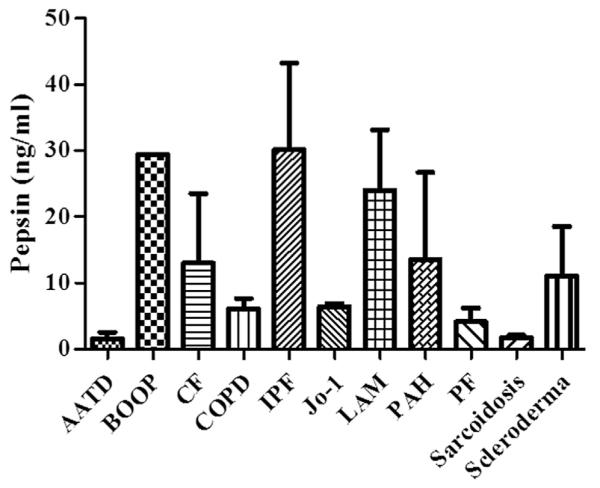
BALF pepsin concentrations among 100 lung transplant patients: AAT disease (AATD) (n = 7), bronchiolitis obliterans organizing pneumonia (BOOP) (n = 1), CF (n = 14), COPD (n = 38), IPF (n = 24), Jo-1 syndrome (n = 1), lymphangioleiomyomatosis (LAM) (n = 1), pulmonary artery hypertension (PAH) (n = 2), pulmonary veno-occlusive disease (n = 1), sarcoidosis (n = 4), scleroderma (n = 1), pulmonary fibrosis (PF) from work exposure (n = 1), rheumatoid arthritis RA (n = 1), dermatomyositis (n = 1), polymyositis (n = 2), and pneumoconiosis (n = 1). The BALF pepsin concentrations were highest in those with IPF.
Fig. 2.
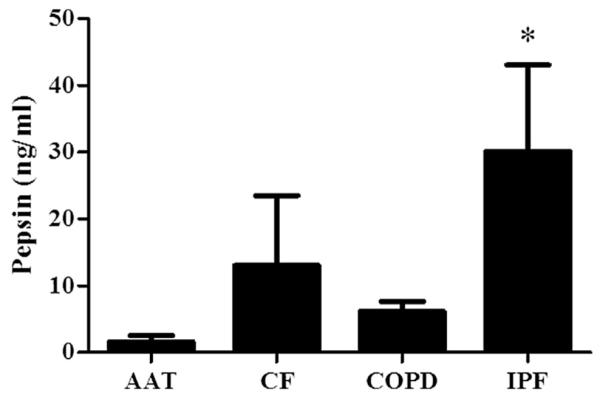
Bronchoalveolar lavage fluid pepsin concentrations among the most common indications for lung transplantation: AAT (n = 7), CF (n = 14), COPD (n = 38), and IPF (n = 24). *P < 0.05 versus all other groups (Kruskal-Wallis post-hoc analysis).
3.3. Demographics, reflux profile, and outcomes among the four most common indications for lung transplantation
As shown in Table 1, patients with AAT, CF, COPD, and IPF differed according to age, gender, transplant type, and frequency of acute cellular rejection at BALF sample collection. Specifically, patients with CF were younger (P < 0.05) and exclusively had undergone bilateral or re-do transplantation (P < 0.05). Those with IPF were predominantly men and those with AAT or IPF more frequently had acute cellular rejection identified on their transbronchial biopsy (P < 0.05). Among those who underwent ambulatory pH monitoring, the prevalence of GERD was high among all groups (ranging from 61%–88%); those with AAT and CF had the highest rates of proximal reflux (75% and 60%, respectively). Among those who underwent gastric emptying scans, the frequency of delayed gastric emptying was also high among all groups (ranging from 40%–86%). The length of follow-up, frequency of BOS, and mortality rates were not different among the patients with AAT, CF, COPD, and IPF (Table 1).
Table 1.
Demographics, GERD, and outcomes among lung transplant patients with the four most common indications for lung transplantation.
| AAT (n = 7) | CF (n = 14) | COPD (n = 38) | IPF (n = 24) | P value | |
|---|---|---|---|---|---|
| Age | 60 (52–69) | 28 (22–35) | 60 (55–62) | 61 (51–65) | <0.0001* |
| Gender | 0.009* | ||||
| Male | 3 (43) | 7 (50) | 16 (42) | 20 (83) | |
| Female | 4 (57) | 7 (50) | 22 (58) | 4 (17) | |
| Transplant type | 0.002* | ||||
| Right single | 2 (29) | 0 | 12 (32) | 8 (33) | |
| Left single | 2 (29) | 0 | 13 (34) | 6 (25) | |
| Bilateral | 3 (43) | 13 (93) | 9 (24) | 9 (38) | |
| Repeat transplant | 0 | 1 (7) | 4 (11) | 1 (4) | |
| GERD | |||||
| Prevalence | 3 (75) | 7 (88) | 14 (61) | 9 (69) | 0.634 |
| Proximal reflux | 3 (75) | 3 (60) | 5 (23) | 4 (33) | 0.127 |
| Hiatal hernia | 0 | 1 (33) | 2 (20) | 1 (14) | 1.000 |
| Delayed gastric emptying | 1 (50) | 6 (86) | 4 (40) | 5 (50) | 0.288 |
| Acute rejection events | 0.037* | ||||
| A0 | 8 (62) | 29 (81) | 70 (74) | 37 (62) | |
| A1 | 5 (38) | 2 (6) | 14 (15) | 17 (28) | |
| ≥A2 | 0 | 5 (14) | 11 (12) | 6 (10) | |
| Aspiration on biopsy | 1 (7) | 3 (8) | 17 (18) | 7 (12) | 0.387 |
| Follow-up (mo) | 14 (3–35) | 27 (17–51) | 22 (13–28) | 26 (14–41) | 0.596 |
| Incidence of BOS | 1 (17) | 3 (21) | 12 (32) | 4 (17) | 0.580 |
| Mortality rate | 2 (29) | 2 (14) | 1 (3) | 3 (13) | 0.079 |
Continuous data are presented as the median (interquartile range) and remaining data as n (%).
Statistically significant.
3.4. Manometric and pH-metric profile among the among the four most common indications for lung transplantation
The manometric and pH-metric profiles among the four most common indications for lung transplantation, irrespective of reflux status, are listed in Table 2. By and large, the groups did not differ in terms of esophageal anatomy, physiology, and acid contact, although patients with CF had a greater total episodes of reflux than the other groups (P = 0.04). Those with CF also tended to have more proximal esophageal acid exposure, and those with IPF tended to have a longer abdominal LES length, although these differences did not reach statistical significance.
Table 2.
Manometric and pH-metric profile among lung transplant patients with the four most common indications for lung transplantation.
| AAT (n = 4) | CF (n = 8) | COPD (n = 23) | IPF (n = 13) | P value | |
|---|---|---|---|---|---|
| LES pressure (mm Hg) | 21 (7.4–39.8) | 33.6 (16.8–48.5) | 23.6 (14.0–39.1) | 28.2 (15.7–33.5) | 0.810 |
| LES total length (cm) | 2.0 (2.0–3.0) | 3.0 (1.5–3.0) | 3.5 (2.0–5.0) | 3.0 (2.8–4.0) | 0.214 |
| LES abdominal length (cm) | 1.0 (0.0–1.0) | 1.0 (0.5–1.5) | 1.0 (1.0–2.8) | 2.0 (1.0–2.0) | 0.184 |
| DEA (mm Hg) | 93 (60–200) | 79 (53–83) | 114 (60–152) | 81 (39–113) | 0.323 |
| Total time pH < 4 (%) | 8.6 (0.5–18.0) | 8.8 (6.4–20.0) | 4.3 (1.5–12.9) | 3.6 (0.2–8.6) | 0.346 |
| Upright | 13.3 (0.8–17.1) | 6.0 (5.4–14.7) | 3.8 (2.4–10.2) | 3.9 (0.3–10.1) | 0.349 |
| Supine | 0.6 (0.0–20.8) | 10.4 (7.7–32.6) | 0.6 (0.0–14.5) | 1.1 (0.0–6.1) | 0.388 |
| Episodes > 5 min | 8.5 (0.0–15.9) | 6.3 (4.0–14.6) | 3.4 (1.0–8.1) | 2.3 (0.0–6.4) | 0.305 |
| Longest episode (min) | 17.0 (1.4–30.1) | 19.8 (14.1–54.2) | 14.9(6.0–63.9) | 14.5 (2.7–18.7) | 0.602 |
| Total episodes | 46.7 (32.1–86.9) | 68.9 (60.0–80.6) | 25.2 (11.7–54.3) | 32.3 (6.3–49.7) | 0.040* |
| DeMeester score (normal < 14.7) | 25.9 (3.6–65.7) | 33.0 (25.5–78.5) | 17.5 (5.8–46.5) | 15.3 (1.3–28.3) | 0.306 |
| Total mean acid clearance time (s) | 159 (14–179) | 111 (84–229) | 127 (80–247) | 127 (53–164) | 0.980 |
| Upright | 158 (14–171) | 62 (56–162) | 116 (74–167) | 109 (53–150) | 0.772 |
| Supine | 193 (7–205) | 182 (141–390) | 120 (0–311) | 71 (0–257) | 0.646 |
| Proximal pH sensor | |||||
| Total time pH < 4 (normal < 1%) | 0.3 (0.1–1.6) | 1.1 (0.2–4.9) | 0.5 (0.2–1.0) | 0.0 (0.0–2.1) | 0.311 |
| Upright | 0.1 (0.4–2.6) | 1.5 (0.2–3.9) | 0.6 (0.3–0.9) | 0.0 (0.0–1.9) | 0.360 |
| Supine | 0.0 (0.0–0.0) | 0.5 (0.2–6.1) | 0.0 (0.0–0.7) | 0.0 (0.0–0.5) | 0.150 |
DEA = distal esophageal amplitude.
Data presented as median (interquartile range).
Statistically significant.
The manometric and pH-metric profiles for the four most common indications for lung transplantation among those with GERD found with pH monitoring are listed in Table 3. Those with COPD had the greatest total LES length (P = 0.038), although all other comparisons were short of significance, despite the trend for a greater abdominal LES length among those with IPF and a considerably shorter mean upright acid clearance time among those with CF.
Table 3.
Manometric and pH-metric profile among GERD-positive lung transplant patients with the four most common indications for lung transplantation.
| AAT (n = 3) | CF (n = 7) | COPD (n = 14) | IPF (n = 9) | P value | |
|---|---|---|---|---|---|
| LES pressure (mm Hg) | 30.5 (21.1–39.8) | 33.6 (16.8–48.5) | 18.5 (13.5–35.4) | 23.8 (12.7–33.6) | 0.631 |
| LES total length (cm) | 2.0 (2.0–2.0) | 3.0 (1.5–3.0) | 4.0 (3.3–5.0) | 3.0 (2.0–4.0) | 0.038* |
| LES abdominal length (cm) | 0.5 (0.0–1.0) | 1.0 (0.5–1.5) | 1.0 (1.0–2.0) | 2.0 (1.0–2.0) | 0.145 |
| DEA (mm Hg) | 130 (60–120) | 79 (53–83) | 64 (33–114) | 37 (25–81) | 0.503 |
| Total time pH < 4 (%) | 13.3 (8.6–18.0) | 8.8 (6.4–20.0) | 4.3 (1.5–12.9) | 3.6 (0.2–8.6) | 0.346 |
| Upright | 15.2 (13.3–17.1) | 6.0 (5.4–14.7) | 6.4 (5.5–17.6) | 8.0 (1.8–12.9) | 0.628 |
| Supine | 10.7 (0.6–20.8) | 10.4 (7.7–32.6) | 13.9 (8.5–44.7) | 5.0 (2.6-24.0) | 0.441 |
| Episodes > 5 min | 12.2 (8.5–15.9) | 6.3 (4.0–14.6) | 7.4 (5.3–15.5) | 5.3 (3.0–8.3) | 0.344 |
| Longest episode (min) | 24.0 (17.0–30.1) | 19.8 (14.1–54.2) | 58.3 (18.9–94.0) | 16.2 (13.9–32.3) | 0.382 |
| Total episodes | 66.8 (46.7–86.9) | 68.9 (60.0–80.6) | 49.6 (29.5–75.0) | 43.9 (31.1–56.3) | 0.219 |
| DeMeester score (normal < 14.7) | 45.8 (25.9–65.7) | 33.0 (25.5–78.5) | 36.6 (27.0–102.3) | 27.2 (16.3–48.7) | 0.412 |
| Total mean acid clearance time (s) | 169 (159–179) | 111 (84–229) | 205 (138–362) | 136 (113–217) | 0.298 |
| Upright | 165 (158–171) | 62 (56–162) | 128 (104–262) | 112 (61–129) | 0.112 |
| Supine | 199 (193–205) | 182 (141–390) | 306 (152–426) | 252 (117–400) | 0.777 |
| Proximal pH sensor | |||||
| Total time pH < 4 (normal < 1%) | 1.0 (0.3–1.6) | 1.1 (0.2–4.9) | 0.8 (0.4–2.1) | 1.5 (0.0–2.7) | 0.993 |
| Upright | 1.5 (0.4–2.6) | 1.5 (0.2–3.9) | 0.9 (0.3–2.3) | 1.1 (0.0–4.4) | 0.983 |
| Supine | 0.0 (0.0–0.0) | 0.5 (0.2–6.1) | 0.6 (0.0–2.1) | 0.3 (0.0–1.9) | 0.388 |
DEA = distal esophageal amplitude.
Data presented as median (interquartile range).
Statistically significant.
3.5. Correlations of BALF pepsin concentrations with manometric and pH-metric findings
The correlation of BALF pepsin concentrations and manometric findings among the four most common indications for lung transplantation is presented in Table 4. The comparisons for those with AAT were limited by the few patients in this group who had undergone esophageal function testing. Nonetheless, the BALF pepsin concentrations correlated negatively with the LES pressures in the entire cohort (r = −0.35, P = 0.03). In addition, the BALF pepsin concentrations correlated negatively with the distal esophageal amplitude among those with COPD (r = −0.50, P = 0.03).
Table 4.
Spearman correlations of pepsin concentrations in the BALF and manometric profile.
| LES pressure |
LES total length |
LES abdominal length |
DEA | |
|---|---|---|---|---|
| AAT | ||||
| r | ID | 0.87 | 0.00 | −0.50 |
| P value | ID | 0.33 | 1.00 | 1.00 |
| CF | ||||
| r | −0.15 | 0.86 | −0.34 | 0.31 |
| P value | 0.78 | 0.08 | 0.52 | 0.78 |
| COPD | ||||
| r | −0.43 | 0.05 | −0.15 | −0.50 |
| P value | 0.08 | 0.85 | 0.59 | 0.03 |
| IPF | ||||
| r | −0.48 | −0.08 | 0.07 | −0.30 |
| P value | 0.13 | 0.83 | 0.85 | 0.38 |
DEA = distal esophageal amplitude; ID = insufficient data available for statistical analysis.
The correlations of BALF pepsin concentrations and pH-metric findings among the four most common indications for lung transplantation are listed in Table 5. Again, the comparisons for those with AAT were limited by the few patients in this group who had undergone esophageal function testing. Regardless, the BALF pepsin concentrations correlated positively with the total time that the pH was < 4 among those with COPD (r = 0.54, P = 0.03); correlated positively with the longest episode of reflux among those with COPD (r = 0.53, P = 0.03); correlated positively with the DeMeester score among those with COPD (r = 0.52, P = 0.03); correlated negatively with the upright mean acid clearance time among those with IPF (r = −0.68, P = 0.02); and correlated positively with the supine mean acid clearance time among those with COPD (r = 0.48, P = 0.04).
Table 5.
Spearman correlations of pepsin concentrations in the BALF and pH-metric profile.
| pH < 4% (min) |
Reflux episodes |
DeMeester score (normal < 14.7) |
Mean acid clearance time |
Proximal pH < 4 (normal < 1%) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total time | Time upright | Time supine | > 5 min | Longest (min) | Total | Total | Upright | Supine | Interval | Upright | Supine | ||
| AAT | |||||||||||||
| r | — | — | — | — | — | — | — | — | — | — | −0.50 | −0.50 | — |
| P value | — | — | — | — | — | — | — | — | — | — | 1.00 | 1.00 | — |
| CF | |||||||||||||
| r | −0.41 | 0.21 | −0.41 | −0.36 | −0.05 | −0.21 | −0.41 | −0.41 | −0.21 | 0.10 | −0.26 | −0.15 | −0.21 |
| P value | 0.52 | 0.52 | 0.52 | 0.52 | 0.95 | 0.78 | 0.52 | 0.52 | 0.78 | 0.95 | 0.68 | 0.78 | 0.78 |
| COPD | |||||||||||||
| r | 0.54 | 0.43 | 0.40 | 0.38 | 0.53 | 0.44 | 0.52 | 0.42 | 0.20 | 0.48 | 0.28 | 0.32 | 0.35 |
| P value | 0.03* | 0.09 | 0.11 | 0.13 | 0.03 | 0.08 | 0.03* | 0.09 | 0.44 | 0.04* | 0.27 | 0.21 | 0.16 |
| IPF | |||||||||||||
| r | 0.22 | 0.01 | 0.18 | 0.07 | −0.20 | 0.24 | 0.24 | −0.43 | −0.68 | 0.23 | −0.18 | −0.16 | −0.40 |
| P value | 0.52 | 0.98 | 0.59 | 0.84 | 0.56 | 0.48 | 0.81 | 0.19 | 0.02* | 0.50 | 0.60 | 0.64 | 0.22 |
ID = insufficient data for statistical analysis.
Statistically significant.
4. Discussion
Patients with ESLD, in particular, the IPF population, are known to have elevated rates of GERD [2,4,10,11,16,26–28]. These rates will continue to increase after lung transplantation [15,17,18,20,21,29–31]. In previous studies, GERD has been used as a surrogate marker for aspiration. Our study aimed to ascertain the real prevalence of aspiration in lung transplant patients by directly measuring pepsin, a gastric refluxate agent, within the bronchoalveolar samples. In addition, we sought to identify the risk factors for the development of aspiration and subsequent allograft rejection. The primary outcome of our study was that lung transplant patients with IPF appear to be at the greatest risk of aspiration compared with other indications for lung transplantation; however, the risk factors for aspiration seem different among the indications for lung transplant, and the underlying etiology for aspiration is likely multifactorial.
Our results have shown that patients with IPF after lung transplantation are at an increased risk of aspiration and have a greater frequency of acute rejection episodes than patients with other common indications for lung transplantation. Regardless of whether pepsin itself plays a pathogenic role in allograft dysfunction, GERD and aspiration have both been associated with lung transplant rejection. Our studies [6] and the study by Ward et al. [32] have demonstrated that aspiration in lung transplant patients, as evidenced by the presence of pepsin in the BALF, has been associated with more episodes of acute rejection and a quicker progression to BOS. However, the mechanism by which aspiration might cause allograft dysfunction is largely unknown. Studies by D’Ovidio et al. [33,34] and research from our center [1,13,35] have seemed to suggest that a proinflammatory and profibrotic state, mediated by various cytokines, chemokines, and growth factors in the pulmonary parenchyma, is promoted by aspiration and responsible for the development of BOS.
Our study has also shown that although the patients with IPF had the highest pepsin concentrations and rates of acute rejection, they did not have a significantly greater incidence of BOS compared with the patients with other indications for lung transplantation. This might imply that pepsin serves more as a sensitive marker of aspiration and might not be the refluxate agent responsible for the pulmonary damage. We have previously reported that pepsin is absent in the BALF of healthy controls without lung transplantation or GERD [6]. Despite pepsin being a protease, its enzymatic activity requires a low pH to function optimally, and it is unclear whether the pulmonary microenvironment of the allograft is sufficiently acidic to activate pepsin [36]. The idea that pepsin might simply serve as a sensitive marker has been supported by the findings from Blondeau et al. [37], who demonstrated that pepsin was present in the BALF of all postlung transplant patients they studied, although they did not find a significant correlation between its presence and the development of BOS. However, this lack of a correlation in our study and that by Blondeau et al. can be explained by the short follow-up period of both studies. Our cohort, in particular, had a median follow-up duration of < 2 y (19.0 mo); however, the progression to BOS usually becomes more predominant at 2 y after transplantation and continues to increase with time. For example, Christie et al. [38] estimated the occurrence of BOS to be 27% by 2.5 y after transplant and 51% by 5.6 y. Thus, we intend to track this cohort in our patient database for a longer period to identify the effect our findings might have on the development of BOS.
Our study has also shown that the etiology and risk factors for GERD and aspiration seem to differ among the various indications for lung transplantation. In general, the pathophysiologic characteristics that might predispose patients with ESLD to GERD have been poorly studied. However, with regard to patient with IPF, many investigators have demonstrated greater rates of esophageal dysmotility, hypotensive LES, delayed gastric emptying, and hiatal hernia compared with controls with GERD [19,20,26,27,39]. However, these findings have been controversial, because other groups have found no such difference in the rates of gastroesophageal pathologic features. Bandeira et al. [40], in their prospective study of 28 patients with IPF, showed no significant differences with controls regarding the demographic characteristics, pulmonary function, clinical presentation, or manometric findings. Our study was in agreement with these findings with regard to showing no differences in the manometric, barium swallow, or gastric emptying study findings in patients with IPF compared with those with other etiologies of ESLD. Even with the sample size limitation, the lack of differences in risk factors supports the concept that not all patients with GERD aspirate and that, therefore, the risk factors for GERD and aspiration could be different among patients with different ESLDs. The corollary that follows is that the detection of pepsin in the BALF might be a better diagnostic tool, especially in patients with IPF. Instead of the more commonly used esophageal function studies, BALF pepsin measurements might be the more accurate diagnostic test in assessing lung transplant patients as candidates for antireflux surgery.
One limitation of our study was the sample size of some of the groups, which might have rendered our analysis less powerful. Future studies with a larger cohort are needed to address unanswered questions and to yield a more powerful study. Additionally, not all lung transplant patients underwent each type of gastroesophageal function test. We could not justify performing studies such as the barium swallow or gastric emptying scan on patients with negative findings for GERD using ambulatory pH testing, because no clinical benefit would have been realized by doing so. Finally, our manometric findings do not directly explain why the BALF pepsin levels were highest in those with IPF, in particular, because the results of the manometric studies did not differ among the groups. However, as stated in our “Results” section, among the entire cohort, the BALF pepsin concentrations correlated negatively with the LES pressures. This association was also nearly significant specifically for the COPD and IPF groups, which were also the groups with the lower LES pressures, although this was not a statistically significant difference. Although our data are suggestive of manometric and pH-metric findings that could explain the differences in BALF pepsin levels among the groups, we failed to show a definitive association. Therefore, additional research is warranted to identify the pathophysiology behind aspiration and the increased levels of pepsin in the BALF of lung transplant patients, particularly, those with IPF.
In conclusion, our results suggest that patients with IPF after lung transplantation are at an increased risk of aspiration and a greater frequency of acute rejection episodes. In addition, the risk factors for aspiration might be different among the patients with different types of ESLD who have undergone transplantation. These results support the role of evaluating the BALF for markers of aspiration when evaluating lung transplant patients as candidates for laparoscopic antireflux surgery.
Acknowledgment
We especially thank the research nurses, clinical nurses, and respiratory therapists at Loyola University Medical Center. Without their dedicated assistance, the present study would not have been possible.
This study was supported by the Dr. Ralph and Marian C. Falk Medical Research Trust and by funding from Loyola University Chicago.
Footnotes
Presented at the 32nd Annual Meeting and Scientific Sessions of the International Society of Heart and Lung Transplantation, Prague, Czech Republic, April 2012.
REFERENCES
- [1].D’Ovidio F, Mura M, Tsang M, et al. Bile acid aspiration and the development of bronchiolitis obliterans after lung transplantation. Thorac Cardiovasc Surg. 2005;129:1144. doi: 10.1016/j.jtcvs.2004.10.035. [DOI] [PubMed] [Google Scholar]
- [2].Hadjiliadis D, Davis RD, Steele MP, et al. Gastroesophageal reflux disease in lung transplant recipients. Clin Transplant. 2003;17:363. doi: 10.1034/j.1399-0012.2003.00060.x. [DOI] [PubMed] [Google Scholar]
- [3].Cantu E, III, Appel JZ, III, Hartwig MG, et al. Maxwell Chamberlain Memorial Paper: early fundoplication prevents chronic allograft dysfunction in patients with gastroesophageal reflux disease. Ann Thorac Surg. 2004;78:1142. doi: 10.1016/j.athoracsur.2004.04.044. [DOI] [PubMed] [Google Scholar]
- [4].Davis RD, Jr, Lau CL, Eubanks S, et al. Improved lung allograft function after fundoplication in patients with gastroesophageal reflux disease undergoing lung transplantation. J Thorac Cardiovasc Surg. 2003;125:533. doi: 10.1067/mtc.2003.166. [DOI] [PubMed] [Google Scholar]
- [5].Lau CL, Palmer SM, Howell DN, et al. Laparoscopic antireflux surgery in the lung transplant population. Surg Endosc. 2002;16:1674. doi: 10.1007/s00464-001-8251-2. [DOI] [PubMed] [Google Scholar]
- [6].Fisichella PM, Davis CS, Lundberg PW, et al. The protective role of laparoscopic antireflux surgery against aspiration of pepsin after lung transplantation. Surgery. 2011;150:598. doi: 10.1016/j.surg.2011.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hartwig MG, Appel JZ, Li B, et al. Chronic aspiration of gastric fluid accelerates pulmonary allograft dysfunction in a rat model of lung transplantation. J Thorac Cardiovasc Surg. 2006;131:209. doi: 10.1016/j.jtcvs.2005.06.054. [DOI] [PubMed] [Google Scholar]
- [8].Li B, Hartwig MG, Appel JZ, et al. Chronic aspiration of gastric fluid induces the development of obliterative bronchiolitis in rat lung transplants. Am J Transplant. 2008;8:1614. doi: 10.1111/j.1600-6143.2008.02298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Meltzer AJ, Weiss MJ, Veillette GR, et al. Repetitive gastric aspiration leads to augmented indirect allorecognition after lung transplantation in the miniature swine. Transplantation. 2008;86:1824. doi: 10.1097/TP.0b013e318190afe6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Young LR, Hadjiliadis D, Davis RD, Palmer SM. Lung transplantation exacerbates gastroesophageal reflux disease. Chest. 2003;124:1689. doi: 10.1378/chest.124.5.1689. [DOI] [PubMed] [Google Scholar]
- [11].Davis CS, Shankaran V, Kovacs EJ, et al. Gastroesophageal reflux disease after lung transplantation: pathophysiology and implications for treatment. Surgery. 2010;148:737. doi: 10.1016/j.surg.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Neujahr DC, Mohammed A, Ulukpo O, et al. Surgical correction of gastroesophageal reflux in lung transplant patients is associated with decreased effector CD8 cells in lung lavages: a case series. Chest. 2010;138:937. doi: 10.1378/chest.09-2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Fisichella PM, Davis CS, Lowery E, et al. Pulmonary immune changes early after laparoscopic antireflux surgery in lung transplant patients with gastroesophageal reflux disease. J Surg Res. 2012;177:e65. doi: 10.1016/j.jss.2012.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Basseri B, Conklin JL, Pimentel M, et al. Esophageal motor dysfunction and gastroesophageal reflux are prevalent in lung transplant candidates. Ann Thorac Surg. 2010;90:1723. doi: 10.1016/j.athoracsur.2010.06.104. [DOI] [PubMed] [Google Scholar]
- [15].Tobin RW, Pope CE, II, Pellegrini CA, et al. Increased prevalence of gastroesophageal reflux disease in patients with idiopathic pulmonary fibrosis. Am J Respir Cri Care Med. 1998;158:1804. doi: 10.1164/ajrccm.158.6.9804105. [DOI] [PubMed] [Google Scholar]
- [16].Sweet MP, Patti MG, Leard LE, et al. Gastroesophageal reflux in patients with idiopathic pulmonary fibrosis referred for lung transplantation. J Thorac Cardiovasc Surg. 2007;133:1078. doi: 10.1016/j.jtcvs.2006.09.085. [DOI] [PubMed] [Google Scholar]
- [17].Salvioli B, Belmonte G, Stanghellini V, et al. Gastrooesophageal reflux and interstitial lung disease. Dig Liver Dis. 2006;38:879. doi: 10.1016/j.dld.2006.05.012. [DOI] [PubMed] [Google Scholar]
- [18].Raghu G, Freudenberger TD, Yang S, et al. High prevalence of abnormal acid gastro-oesophageal reflux in idiopathic pulmonary fibrosis. Eur Respir J. 2006;27:136. doi: 10.1183/09031936.06.00037005. [DOI] [PubMed] [Google Scholar]
- [19].Mays EE, Dubois JJ, Hamilton GB. Pulmonary fibrosis associated with tracheobronchial aspiration: a study of the frequency of hiatal hernia and gastroesophageal reflux in interstitial pulmonary fibrosis of obscure etiology. Chest. 1976;69:512. doi: 10.1378/chest.69.4.512. [DOI] [PubMed] [Google Scholar]
- [20].Patti MG, Tedesco P, Golden J, et al. Idiopathic pulmonary fibrosis: how often is it really idiopathic? J Gastrointest Surg. 2005;9:1053. doi: 10.1016/j.gassur.2005.06.027. [DOI] [PubMed] [Google Scholar]
- [21].Soares RV, Forsythe A, Hogarth K, et al. Interstitial lung disease and gastroesophageal reflux disease: key role of esophageal function tests in the diagnosis and management. Arq Gastroenterol. 2011;48:91. doi: 10.1590/s0004-28032011000200002. [DOI] [PubMed] [Google Scholar]
- [22].Linden PA, Gilbert RJ, Yeap BY, et al. Laparoscopic fundoplication in patients with end-stage lung disease awaiting transplantation. J Thorac Cardiovasc Surg. 2006;131:438. doi: 10.1016/j.jtcvs.2005.10.014. [DOI] [PubMed] [Google Scholar]
- [23].Stewart S, Fishbein MC, Snell GI, et al. Revision of the 1996 working formulation for the standardization of nomenclature in the diagnosis of lung rejection. J Heart Lung Transplant. 2007;26:1229. doi: 10.1016/j.healun.2007.10.017. [DOI] [PubMed] [Google Scholar]
- [24].Mendez B, Davis C, Weber C, et al. Gastroesophageal reflux disease in lung transplant patients with cystic fibrosis. Am J Surg. 2012;204:e21. doi: 10.1016/j.amjsurg.2012.07.019. [DOI] [PubMed] [Google Scholar]
- [25].Estenne M, Maurer JR, Boehler A, et al. Bronchiolitis obliterans syndrome 2001, an update of the diagnostic criteria. J Heart Lung Transplant. 2002;21:297. doi: 10.1016/s1053-2498(02)00398-4. [DOI] [PubMed] [Google Scholar]
- [26].Sweet MP, Patti MG, Hoopes C, et al. Gastro-oesophageal reflux and aspiration in patients with advanced lung disease. Thorax. 2009;64:167. doi: 10.1136/thx.2007.082719. [DOI] [PubMed] [Google Scholar]
- [27].Sweet MP, Herbella FA, Leard L, et al. The prevalence of distal and proximal gastroesophageal reflux in patients awaiting lung transplantation. Ann Surg. 2006;244:491. doi: 10.1097/01.sla.0000237757.49687.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hoppo T, Jarido V, Pennathur A, et al. Anti-reflux surgery preserves lung function in patients with gastroesophageal reflux disease and end-stage lung disease before and after lung transplantation. Arch Surg. 2011;146:1041. doi: 10.1001/archsurg.2011.216. [DOI] [PubMed] [Google Scholar]
- [29].Hershcovici T, Jha LK, Johnson T, et al. Systematic review: the relationship between interstitial lung diseases and gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2011;34:1295. doi: 10.1111/j.1365-2036.2011.04870.x. [DOI] [PubMed] [Google Scholar]
- [30].Raghu G, Yang ST, Spada C, et al. Sole treatment of acid gastroesophageal reflux in idiopathic pulmonary fibrosis: a case series. Chest. 2006;129:794. doi: 10.1378/chest.129.3.794. [DOI] [PubMed] [Google Scholar]
- [31].Scott M, Gelhot AR. Gastroesophageal reflux disease: diagnosis and management. Am Fam Physician. 1999;59:1161. [PubMed] [Google Scholar]
- [32].Ward C, Forrest IA, Brownlee IA, et al. Pepsin like activity in bronchoalveolar lavage fluid is suggestive of gastric aspiration in lung allografts. Thorax. 2005;60:872. doi: 10.1136/thx.2004.036426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].D’Ovidio F, Mura M, Ridsdale R, et al. The effect of reflux and bile acid aspiration on the lung allograft and its surfactant and innate immunity molecules SP-A and SP-D. Am J Transplant. 2006;6:1930. doi: 10.1111/j.1600-6143.2006.01357.x. [DOI] [PubMed] [Google Scholar]
- [34].D’Ovidio F, Mura M, Waddell TK, et al. Bile acids in bronchoalveolar lavage after lung transplantation as a marker of pulmonary aspiration associated with alveolar neutrophilia. J Heart Lung Transplant. 2004;23(2 Suppl 1):42S. [Google Scholar]
- [35].Fisichella PM, Davis CS, Lowery E, et al. Aspiration, localized pulmonary inflammation, and predictors of early-onset bronchiolitis obliterans syndrome after lung transplantation. J Am Coll Surg Epub. 2013 Apr 26; doi: 10.1016/j.jamcollsurg.2013.03.008. doi: pii: S1072–7515(13) 00226-3. http://dx.doi.org/10.1016/j.jamcollsurg.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Jaoude PA, Knight PR, Ohtake P, et al. Biomarkers in the diagnosis of aspiration syndrome. Expert Rev Mol Diagn. 2010;10:309. doi: 10.1586/erm.10.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Blondeau K, Mertens V, Vanaudenaerde BA, et al. Gastrooesophageal reflux and gastric aspiration in lung transplant patients with or without chronic rejection. Eur Respir J. 2008;31:707. doi: 10.1183/09031936.00064807. [DOI] [PubMed] [Google Scholar]
- [38].Christie JD, Edwards LB, Aurora P, et al. Registry for the International Society for Heart and Lung Transplantation: twenty-fifth official adult lung and heart/lung transplantation report. J Heart Lung Transplant. 2008;27:957. doi: 10.1016/j.healun.2008.07.018. [DOI] [PubMed] [Google Scholar]
- [39].D’Ovidio F, Singer LG, Hadjiliadis D, et al. Prevalence of gastroesophageal reflux in end-stage lung disease candidates for lung transplant. Ann Thorac Surg. 2005;80:1254. doi: 10.1016/j.athoracsur.2005.03.106. [DOI] [PubMed] [Google Scholar]
- [40].Bandeira CD, Rubin AS, Cardoso PF, et al. Prevalence of gastroesophageal reflux disease in patients with idiopathic pulmonary fibrosis. J Bras Pneumol. 2009;35:1182. doi: 10.1590/s1806-37132009001200004. [DOI] [PubMed] [Google Scholar]


