Abstract
The Lyme disease agent, Borrelia burgdorferi, is maintained in a tick–mouse cycle1,2.Here we show that B. burgdorferi usurps a tick salivary protein, Salp15 (ref. 3), to facilitate the infection of mice. The level of salp15 expression was selectively enhanced by the presence of B. burgdorferi in Ixodes scapularis, first indicating that spirochaetes might use Salp15 during transmission. Salp15 was then shown to adhere to the spirochaete, both in vitro and in vivo, and specifically interacted with B. burgdorferi outer surface protein C. The binding of Salp15 protected B. burgdorferi from antibody-mediated killing in vitro and provided spirochaetes with a marked advantage when they were inoculated into naive mice or animals previously infected with B. burgdorferi. Moreover, RNA interference-mediated repression of salp15 in I. scapularis drastically reduced the capacity of tick-borne spirochaetes to infect mice. These results show the capacity of a pathogen to use a secreted arthropod protein to help it colonize the mammalian host.
Lyme borreliosis serves as a model to examine how microbe– vector interactions influence pathogen transmission to the mammalian host. B. burgdorferi, the spirochetal agent of Lyme disease, is primarily maintained in the USA in I. scapularis ticks and Peromyscus leucopus mice. Spirochaetes preferentially express specific genes to survive in a complex enzootic cycle4. For example, B. burgdorferi cells entering I. scapularis express outer surface protein (Osp)A to colonize the vector5. When the arthropod engorges on a host, the spirochaetes then downregulate OspA and upregulate OspC, a lipoprotein that facilitates the migration of B. burgdorferi from the I. scapularis gut to the tick salivary glands and can independently participate in the establishment of vertebrate infection6,7. While B. burgdorferi are being transmitted during tick feeding, the arthropod is also secreting saliva to aid in engorgement8. I. scapularis saliva possesses antigens with immunosuppressive, anticomplement and antihaemostatic activity, among other functions, which enable the vector to take an effective blood meal9,10. We now explore the hypothesis that B. burgdorferi in transit through the tick might use components of I. scapularis saliva to enhance spirochaete transmission to, and survival within, the vertebrate host.
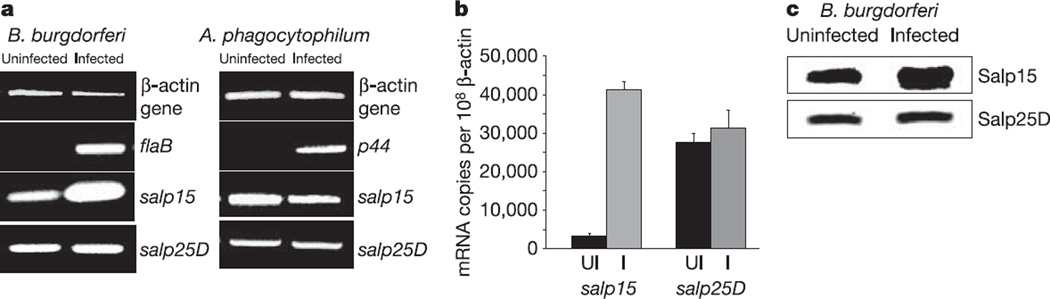
To determine first whether B. burgdorferi influenced the expression of tick genes, we examined the profile of the genes encoding 14 antigenic I. scapularis salivary proteins that elicit strong humoral responses in the host upon tick feeding11, in uninfected and B. burgdorferi-infected ticks. We found that the expression of one gene, salp15, which encodes an I. scapularis protein known to inhibit T-cell activation3, was selectively increased in B. burgdorferi-infected tick salivary glands during engorgement (Fig. 1a). As an example of one control, the expression of the gene encoding another salivary antigen, salp25D, remained the same regardless of infection. Quantitative polymerase chain reaction (PCR) further showed that salp15 mRNA levels were 13-fold higher (P < 0.001) in B. burgdorferi-infected engorged ticks than in engorged I. scapularis without B. burgdorferi (Fig. 1b). In contrast, the amount of salp25D mRNA was similar in both groups of ticks (Fig. 1b). The enhancement in salp15 expression was specific to B. burgdorferi, because the levels of salp15 mRNA in the salivary glands of engorged ticks infected with Anaplasma phagocytophilum, another pathogen borne by I. scapularis, remained unchanged (Fig. 1a). Salp15 protein levels were 1.6-fold higher in B. burgdorferi-infected salivary glands than in uninfected glands, as demonstrated by immunoblotting (Fig. 1c). The selective upregulation of a tick salivary antigen in the presence of B. burgdorferi raises the possibility that Salp15 might be used by the pathogen, either during its interim stay in the arthropod salivary gland or during its transit into the mammalian host.
Figure 1. Salp15 levels are specifically enhanced in Borrelia burgdorferi-infected tick salivary glands.
a, RT–PCR profile of fed Ixodes scapularis salivary glands that were uninfected or infected with either B. burgdorferi or Anaplasma phagocytophilum. Expression of the β-actin gene from I. scapularis was used as a control. flaB and p44 expression are indicative of B. burgdorferi and A. phagocytophilum infection, respectively. b, salp15 was upregulated in B. burgdorferi-infected I. scapularis salivary glands. The difference between salp15 mRNA levels in infected and uninfected nymphs was significant, in contrast to salp25D expression (Student’s t-test). Results are means + s.e.m. from three quantitative PCR experiments. c, Salp15 protein levels were 1.6-fold higher in B. burgdorferi-infected salivary glands, as quantified by ImageJ (NIH). Salp25D served as a control.
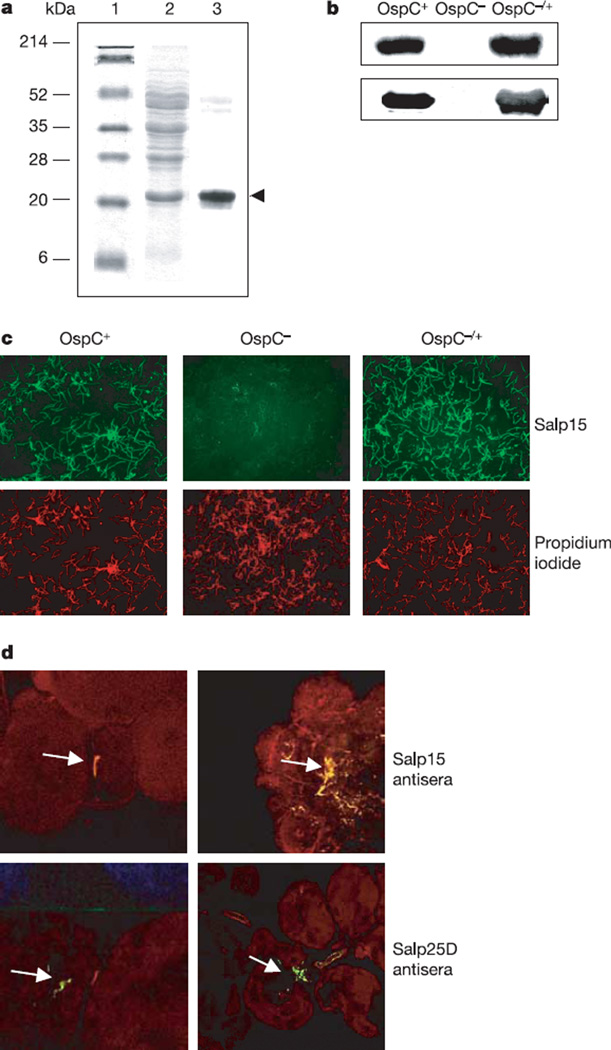
To assess whether Salp15 interacts with a B. burgdorferi antigen, a gel overlay assay, using recombinant Salp15, was performed. Salp15 bound a 22-kDa B. burgdorferi antigen that was identified as OspC (Fig. 2a) when subjected to matrix-assisted laser desorption ionization mass spectrometry peptide analysis (Supplementary Table S1). Consistent with this was our observation that Salp15 did not bind to lysates of OspC-deficient B. burgdorferi but did adhere to OspC-deficient B. burgdorferi that were genetically complemented to produce OspC (Fig. 2b). In addition to binding the spirochaete lysates, we also observed that Salp15 interacted with intact wild-type B. burgdorferi, but not with OspC-deficient B. burgdorferi, in vitro (Fig. 2c). These observations further confirmed that the Salp15– OspC interaction was specific. Moreover, B. burgdorferi in infected tick salivary glands were copiously covered with native Salp15, as detected with an antibody directed against recombinant Salp15 (Fig. 2d). As a control, an antibody against another tick salivary protein, Salp25D, failed to bind to B. burgdorferi in the salivary gland. Uninfected salivary glands stained diffusely for both proteins (data not shown). Salp15 therefore directly associates with B. burgdorferi within the vector.
Figure 2. Salp15 interacts with outer surface protein (Osp)C of Borrelia burgdorferi.
a, Salp15 bound specifically to OspC. Lanes 1 and 2, Ponceau S stain of molecular mass markers (lane 1) and B. burgdorferi lysate (lane 2); lane 3, Salp15 overlay. The protein band bound by Salp15 (marked by an arrowhead) was identified as OspC. b, Salp15 binding to OspC was confirmed by using wild-type (OspC+), OspC-deficient (OspC−) and OspC-complemented (OspC−/+) B. burgdorferi. Bacterial lysates of the isolates were probed with anti-Salp15 (top) and anti-OspC (bottom) antibody. c, Salp15 binds to the surface of intact B. burgdorferi. Unfixed wild-type (OspC+), OspC-deficient (OspC−) and OspC-complemented (OspC−/+) B. burgdorferi were probed with FITC-conjugated Salp15 (green) and propidium iodide (red). Original magnification × 40. d, Salp15 binds B. burgdorferi within the tick salivary gland. Top: salivary glands from B. burgdorferi-infected nymphs were probed with Salp15 antibody (red). The spirochaetes (as indicated by arrows) were stained with an FITC-labelled anti-B. burgdorferi antibody (green). Bottom: anti-Salp25D served as a control. Co-localization (yellow) was observed with Salp15 antisera, in contrast to Salp25D. Left, salivary gland with a single spirochaete; right, a cluster of B. burgdorferi is harboured. Images are representative of ten independent experiments. Original magnification × 40.
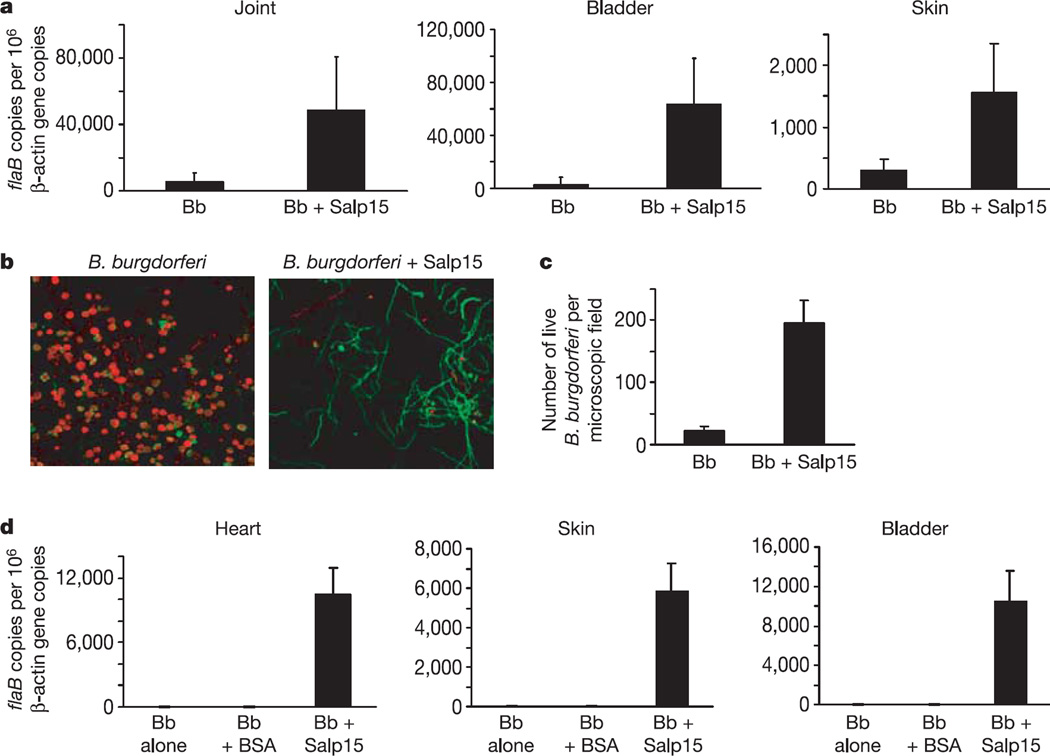
The enhanced expression of Salp15 in the presence of B. burgdorferi within ticks, and the specific adherence of Salp15 to OspC on the surface of B. burgdorferi, suggest a critical role for Salp15 in establishing spirochaete infection. To determine whether Salp15 influenced the ability of B. burgdorferi to colonize the mammalian host, spirochaetes were preincubated with Salp15 and injected into naive C3H mice. At 25 days the spirochaete load in animals that received B. burgdorferi and Salp15 was markedly elevated in the joints (ninefold; P < 0.001), skin (fivefold; P < 0.001) and bladder (25-fold; P < 0.001) as measured by quantitative PCR (Fig. 3a). The levels were higher than in mice that received B. burgdorferi alone (Fig. 3a) or in mice in which Salp15 was injected at a distal site from the B. burgdorferi inoculum (Supplementary Fig. S1). The antibody titres to B. burgdorferi was similar in all groups of mice. Spirochaete levels were also increased to a similar degree at earlier time points (8 and 15 days) in mice that received B. burgdorferi preincubated with Salp15 (data not shown). These results are consistent with previous reports that inoculation of B. burgdorferi together with tick salivary gland lysates enhanced the spirochaete load in mice12.
Figure 3. Salp15 markedly enhances the Borrelia burgdorferi load in the murine host.
a, Naive mice were inoculated with B. burgdorferi in the presence (Bb + Salp15) and absence (Bb) of recombinant Salp15. B. burgdorferi flaB was measured in the different tissues. Results are means + s.e.m. from three independent experiments. b, Salp15 protects B. burgdorferi from antibody-mediated destruction in vitro. B. burgdorferi antiserum was incubated with wild-type spirochaetes for 18 h. Live bacteria stained green (Syto 9 stain); dead bacteria stained red (propidium iodide). Left, untreated B. burgdorferi; right, B. burgdorferi preincubated with Salp15. Original magnification × 63. c, Quantitative assessment of the data shown in b. After exposure to antibody, B. burgdorferi preincubated with Salp15 were 8.5-fold more viable than the untreated B. burgdorferi.Numbers are averages of 20 random microscopic fields. Results are means + s.e.m. from one representative experiment. d, Protection from in vivo killing of B. burgdorferi by Salp15 in immune mice. Salp15 markedly enhanced the B. burgdorferi load in a previously infected murine host. Mice were treated as described in Methods. The different groups of mice received phosphate-buffered saline (Bb), bovine serum albumin (Bb + BSA) as control, or Salp15 (Bb + Salp15). B. burgdorferi flaB levels were quantified from the different tissues. Results are means + s.e.m. from three quantitative PCR experiments.
Innate and adaptive immune responses contribute to controlling the levels of B. burgdorferi during infection13,14. In particular, humoral immunity has repeatedly been shown to destroy spirochaetes, both in vitro and in vivo15–17. Antibody-mediated killing assays were therefore performed to determine whether Salp15 could protect spirochaetes from the borreliacidal effects of B. burgdorferi antisera. Cultured wild-type B. burgdorferi were killed by a monoclonal antibody against OspA, sera from B. burgdorferi-infected mice (data not shown) or rabbit B. burgdorferi antisera within 18 h, whereas spirochaetes preincubated with Salp15 were significantly protected (Fig. 3b, c). This effect was not noted with OspC-deficient spirochaetes but was evident with OspC-deficient B. burgdorferi that were genetically complemented to produce OspC (data not shown). Microscopic observation revealed an 8.5-fold higher level of viable spirochaetes. The B. burgdorferi remained viable until 24 h after exposure to the antisera (Fig. 3b, c). Beyond this time, the Salp15- treated spirochaetes began dying, perhaps because dividing spirochaetes lacking the protective interaction with Salp15 might have been targeted by the borreliacidal antibodies.
In areas where Lyme disease is endemic, mice are repeatedly exposed to ticks with B. burgdorferi, and often have evidence of a humoral response to the spirochaete18. Therefore, in nature, B. burgdorferi transmitted by tick bites are frequently exposed to B. burgdorferi-specific antibodies19. This is the environment—during the natural infection of mice—in which the influence of Salp15 is likely to be most important. In laboratory experiments, tick-borne spirochaetes are more resistant than cultured spirochaetes to being killed in vivo by immune sera20. It was therefore important to examine whether the interaction between Salp15 and B. burgdorferi could enable spirochaetes to colonize mice that had previously developed an immune response to B. burgdorferi. To mimic this situation, C3H mice were infected with B. burgdorferi for 14 days, a period during which a protective anti-B. burgdorferi humoral response to infection has been noted20, and the mice were then treated with ceftriaxone, an antibiotic that eradicates spirochaete infection21. As expected, these animals were resistant to infection with in vitro cultured B. burgdorferi administered alone or in the presence of bovine serum albumin (Fig. 3d). In contrast, these mice were fully susceptible to infection with spirochaetes that had been preincubated with Salp15 (Fig. 3d). Quantitative PCR performed 25 days after infection detected B. burgdorferi only in mice that received spirochaetes with Salp15, and not in animals that received B. burgdorferi alone. Moreover, the spirochaete burdens were comparable to those in the non-immune mice infected with B. burgdorferi in the presence of Salp15. Taken together, these data indicate that Salp15 allows B. burgdorferi to successfully colonize mice that had been previously exposed to spirochaetes and also indicate that Salp15 might be important in spirochaete colonization and dissemination.
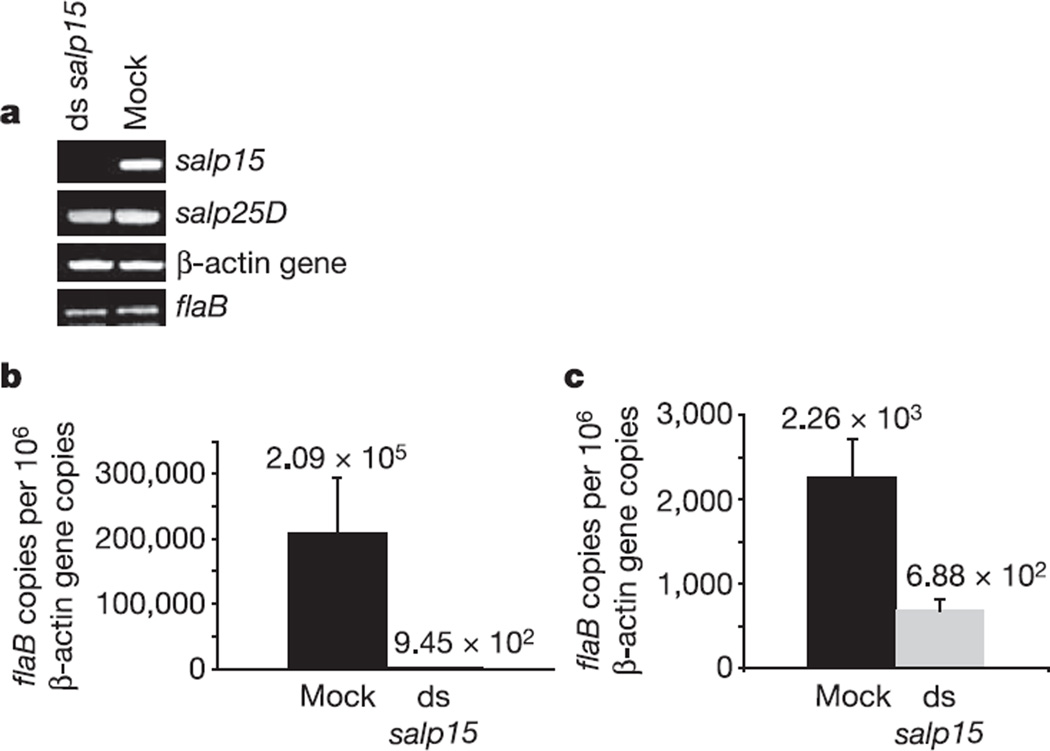
To further examine the role of Salp15 in B. burgdorferi survival in vivo, salp15-deficient I. scapularis nymphs were generated by RNA interference (RNAi). The successful downregulation of salp15 expression in tick salivary glands was determined by PCR with reverse transcription (RT–PCR) (Fig. 4a). The unchanged levels of salp25D and tick β-actin gene confirmed the specificity of RNAi. Buffer-injected (mock) and salp15 double-stranded RNA (dsRNA)- injected B. burgdorferi-infected nymphs were then fed on naive laboratory mice and on mice that had previously developed an immune response to B. burgdorferi. Quantitative PCR revealed a significant decrease in spirochaete levels in the skin of mice that were fed on by the salp15-deficient ticks in comparison with mock-injected ticks, both in naive mice (Fig. 4b; P < 0.01) and in mice that had an immune response (Fig. 4c; P < 0.001). In contrast, spirochaete levels in the salivary glands of engorged salp15-deficient and control tick salivary glands (2.9 ± 1.3) × 102 and (3.1 ± 2.1) × 102 flaB copies per 106 β-actin gene copies, respectively, (P > 0.5) were similar. Spirochaete numbers in the host were unaffected for salp25D-deficient nymphs (data not shown). Studies were also performed with P. leucopus, a natural reservoir of B. burgdorferi, and similar results were obtained when salp15 dsRNA injected B. burgdorferi-infected nymphs were fed on naive P. leucopus mice and on mice that had an immune response to B. burgdorferi (Supplementary Fig. S2a, b). The salp15-deficient ticks showed no alterations in engorgement, ruling out the possibility of reduced transmission as a result of decreased feeding. The lack of complete abrogation of pathogen transmission in salp15-deficient I. scapularis might be due to the inability of RNAi to suppress gene expression totally or due to the spirochaetes’ ability to compensate for the absence of salp15. These in vivo data show conclusively that Salp15 facilitates tick-borne B. burgdorferi infection in the host.
Figure 4. Gene silencing of salp15 expression by RNA interference.
a, Borrelia burgdorferi-infected nymphal ticks were microinjected with salp15 dsRNA (ds salp15) or buffer alone (mock), and fed on naive mice. Levels of salp15, salp25D and β-actin gene were assessed in the salivary gland by RT–PCR. Levels of flaB were also measured. Data are representative of three independent experiments. b, c, salp15 dsRNA reduced the transmission of B. burgdorferi to the host. Nymphal ticks were injected with salp15 dsRNA (ds salp15) or buffer alone (mock), fed on naive mice or on mice previously infected with B. burgdorferi and then treated as described in Methods. Quantification of flaB revealed lower levels of spirochaete in the skin of mice that were fed upon by salp15-deficient ticks in both the naive mice (b) and the preimmune mice (c) in comparison with mock-injected ticks (Student’s t-test). Results are means + s.e.m. and data are representative of three independent experiments.
Transmission of an arthropod-borne pathogen occurs at the complex interface of microbe, vector and vertebrate host. Although host–pathogen, vector–pathogen and vector–host interactions have been delineated22,23, we now describe a triangular relationship in which an infectious agent exploits an arthropod protein to facilitate infection of the mammalian host. The use of a specific I. scapularis salivary protein by B. burgdorferi to enhance infection in mice serves as a model for the other arthropod-borne infections. In particular, the presence of B. burgdorferi within the vector induces the expression of Salp15 in ticks, and this pathogen–vector interaction is needed for the establishment of B. burgdorferi infection in mice that have previously been exposed to the spirochaetes, the most common reservoir host environment that this microbe encounters naturally. Although these present data show that Salp15 is used by B. burgdorferi to augment infection, the increased Salp15 levels in ticks during spirochaete infection could also be advantageous to I. scapularis. Perhaps B. burgdorferi-induced enhancement of Salp15, with its immunosuppressive properties3, might enable ticks to more effectively engorge and/or avoid rejection by the host24, providing a preferential survival advantage for both I. scapularis and B. burgdorferi. As microbes and vectors have evolved together over millions of years, the most successful relationship between them will be mutual, rather than parasitic or commensal, as the interaction between B. burgdorferi and I. scapularis may well be. The vector and pathogen factors that influence successful microbial infection of the mammalian host might also serve as targets for vaccines and therapeutics to combat arthropod-borne diseases.
METHODS
Spirochaetes
A low-passage clonal isolate of Borrelia burgdorferi strain N40 that is infectious in mice25 was used throughout the study. Clonal isolates of OspC-deficient and OspC-complemented and wild-type B. burgdorferi 297 (ref. 6) were used for immunofluorescence and in vitro binding assays.
Mice
C3H/HeJ (C3H) mice were purchased from the Jackson Laboratory. Peromyscus leucopus were obtained from a colony at Yale University, maintained by D. Fish.
Ticks
Ixodes scapularis nymphs were obtained from The Connecticut Agricultural Experiment Station. Laboratory-infected I. scapularis nymphs were obtained as described previously26. The uninfected and infected nymphs were fed on pathogen-free C3H mice and were collected after 66 h of feeding for the dissection of salivary glands.
RNA extractions from tick salivary glands
RNA was extracted from the following tick pools: uninfected and infected nymphal tick salivary glands, in accordance with the kit manufacturer’s directions (AquaPure RNA isolation kit; Bio-Rad). The RNA samples purified from these groups were used in the qualitative RT–PCR and quantitative PCR assays.
RT–PCR and quantitative PCR
The primer pairs for each assayed gene are listed in Supplementary Table S2. Tick salivary gland complementary DNA was made with the iScript cDNA synthesis kit (Bio-Rad) in accordance with the manufacturer’s protocol. Quantitative PCR was performed in accordance with the manufacturer’s protocol with a Bio-Rad i-Cycler. The probes used contained a 5′ reporter, 6-carboxyfluorescein (FAM), and a 3′ quencher, 6-carboxy-N,N,N′,N′ -tetramathylrhodamine (TAMRA; Applied Biosystems). The tick salivary-gland cDNA levels were normalized to I. scapularis β-actin gene, and salp15, salp25D and B. burgdorferi N40 flagellin (flaB) were then quantified. In the murine infection studies, the mouse β-actin gene was used to normalize the amount of DNA in these samples and flaB was used to quantify the levels of spirochaete in the murine tissue samples.
Western blotting
Western blotting was performed as described previously11. The blots were probed with guinea pig Salp15 or Salp25D antisera.
Solid-phase overlay assay
B. burgdorferi lysates were separated by conventional SDS–PAGE and transferred by western blotting. The blots were then overlaid with purified recombinant Salp15 (ref. 3). The membranes were probed with horseradish peroxidase-conjugated monoclonal anti-V5 antibody (Invitrogen). Mouse anti-OspC monoclonal antibody was also used to probe the blots.
Confocal microscopy
Salivary glands from nymphal ticks were prepared for confocal microscopy as described previously27. In brief, acetone-fixed glands were incubated with the guinea pig antisera raised against Salp15 and Salp25D, followed by rhodamine-conjugated anti-guinea-pig IgG (Molecular Probes). The glands were counterstained with fluorescein isothiocyanate (FITC)- conjugated goat anti-B. burgdorferi antibody (Kirkegaard and Perry Laboratories), and viewed with a Zeiss LSM 510 scanning laser confocal microscope.
Immunofluorescence and in vitro binding assay
Wild-type B. burgdorferi 297, OspC-deficient and OspC-complemented isolates (107 spirochaetes ml−1) were placed on sialyated slides (PGC Scientific). The spirochaetes were then incubated with Salp15-FITC or BSA-FITC3, counterstained with propidium iodide and examined with a Zeiss Axioscope fluorescence microscope.
In vitro protection assay
B. burgdorferi N40, OspC-deficient and OspC-complemented isolates (108 spirochaetes ml−1) were used. Salp15 was incubated with the spirochaete for 1 h at 25 °C. The spirochaetes were then incubated with OspA monoclonal antibody or polyclonal mouse or rabbit B. burgdorferi antisera for 18, 24 or 48 h at 33 °C. The percentage of viable spirochaetes was quantified with the Live–Dead Bacterial viability kit (Molecular Probes).
In vivo infection of non-immune mice
Pathogen-free C3H mice were infected with B. burgdorferi (102 spirochaetes per mouse) with and without recombinant Salp15 or BSA (30 µg ml−1) intradermally. At 25 days after infection, tissue samples were collected for DNA isolation and quantitative PCR.
In vivo infection of immune mice
Pathogen-free C3H mice (five mice per group) were infectedwith B. burgdorferi (102 spirochaetes per mouse). At 14 days after infection, the mice were tested for antibody raised against B. burgdorferi by enzyme-linked immunosorbent assay. They were then treated with ceftriaxone (16 mg per kg body weight). Ear punches were taken to amplify flaB DNA to confirm the absence of spirochaete. The mice were then inoculated with B. burgdorferi (104 spirochaetes per mouse) with recombinant Salp15 (30 µg per mouse) intradermally. Control mice received B. burgdorferi with or without BSA. Tissue samples were collected, and spirochaete levels were quantified 25 days later.
RNA interference
cDNA from nymphs was prepared as described previously and salp15 was amplified with gene-specific primers 5′-GAGCTCGCATCAA CCGCTGACAAA-3′ and 5′-GGTACCCTAACATCCGGGAATGTG-3′ containing SacI and KpnI restriction sites. The salp15 fragment was cloned into the L440 double T7 Script vector, and the dsRNA was synthesized and purified with the Megascript RNAi kit in accordance with the manufacturer’s protocol (Ambion). salp15 dsRNA was injected as described28 into nymphal I. scapularis infected with B. burgdorferi. The ticks were then placed on naive or previously infected C3H mice or P. leucopus (as described above) and allowed to feed until 72 h. Ticks were collected, cDNA was made from salivary glands and the expression of salp15 was assessed. Seven days after tick feeding, skin samples from the site of a tick bite were excised and levels of flaB were measured with quantitative PCR.
Supplementary Material
Acknowledgements
We thank D. Beck for help with the in vivo experiments, K. DePonte and N. Marcantonio for assistance with the microinjection and RNA interference experiments, M. Vasil for the maintenance of ticks, and M. Papero and L. Rollend for guidance with the experiments using Peromyscus leucopus. This work was supported by grants from the American Heart Association, National Institutes of Health, and Centers for Disease Control and Prevention. E.F. is the recipient of a Burroughs Wellcome Clinical Scientist Award in Translational Research.
Footnotes
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.
The authors declare no competing financial interests.
References
- 1.Burgdorfer W, et al. Lyme disease—a tick-borne spirochetosis? Science. 1982;216:1317–1319. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- 2.Ribeiro JM, Mather TN, Piesman J, Spielman A. Dissemination and salivary delivery of Lyme disease spirochetes in vector ticks (Acari: Ixodidae) J. Med. Entomol. 1987;24:201–205. doi: 10.1093/jmedent/24.2.201. [DOI] [PubMed] [Google Scholar]
- 3.Anguita J, et al. Salp15, an Ixodes scapularis salivary protein, inhibits CD4+ T cell activation. Immunity. 2002;16:849–859. doi: 10.1016/s1074-7613(02)00325-4. [DOI] [PubMed] [Google Scholar]
- 4.Steere AC, Coburn J, Glickstein L. The emergence of Lyme disease. J. Clin. Invest. 2004;113:1093–1101. doi: 10.1172/JCI21681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwan TG, Piesman J. Temporal changes in outer surface proteins A and C of the Lyme disease-associated spirochete, Borrelia burgdorferi, during the chain of infection in ticks and mice. J. Clin. Microbiol. 2000;38:382–388. doi: 10.1128/jcm.38.1.382-388.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pal U, et al. OspC facilitates Borrelia burgdorferi invasion of Ixodes scapularis salivary glands. J. Clin. Invest. 2004;113:220–230. doi: 10.1172/JCI19894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grimm D, et al. Outer-surface protein C of the Lyme disease spirochete: a protein induced in ticks for infection of mammals. Proc. Natl Acad. Sci. USA. 2004;101:3142–3147. doi: 10.1073/pnas.0306845101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wikel SK. Host immunity to ticks. Annu. Rev. Entomol. 1996;41:1–22. doi: 10.1146/annurev.en.41.010196.000245. [DOI] [PubMed] [Google Scholar]
- 9.Sauer JR, McSwain JL, Bowman AS, Essenberg RC. Tick salivary gland physiology. Annu. Rev. Entomol. 1995;40:245–267. doi: 10.1146/annurev.en.40.010195.001333. [DOI] [PubMed] [Google Scholar]
- 10.Ribeiro JM, Francischetti IM. Role of arthropod saliva in blood feeding: sialome and post-sialome perspectives. Annu. Rev. Entomol. 2003;48:73–88. doi: 10.1146/annurev.ento.48.060402.102812. [DOI] [PubMed] [Google Scholar]
- 11.Das S, et al. Salp25D, an Ixodes scapularis antioxidant, is 1 of 14 immunodominant antigens in engorged tick salivary glands. J. Infect. Dis. 2001;184:1056–1064. doi: 10.1086/323351. [DOI] [PubMed] [Google Scholar]
- 12.Zeidner NS, Schneider BS, Nuncio MS, Gern L, Piesman J. Coinoculation of Borrelia spp. with tick salivary gland lysate enhances spirochete load in mice and is tick species-specific. J. Parasitol. 2002;88:1276–1278. doi: 10.1645/0022-3395(2002)088[1276:COBSWT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 13.Steere AC, Glickstein L. Elucidation of Lyme arthritis. Nature Rev. Immunol. 2004;4:143–152. doi: 10.1038/nri1267. [DOI] [PubMed] [Google Scholar]
- 14.Wooten RM, et al. Toll-like receptor 2 is required for innate, but not acquired, host defense to Borrelia burgdorferi. J. Immunol. 2002;168:348–355. doi: 10.4049/jimmunol.168.1.348. [DOI] [PubMed] [Google Scholar]
- 15.McKisic MD, Barthold SW. T-cell-independent responses to Borrelia burgdorferi are critical for protective immunity and resolution of Lyme disease. Infect. Immun. 2000;68:5190–5197. doi: 10.1128/iai.68.9.5190-5197.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fikrig E, Barthold SW, Chen M, Chang CH, Flavell RA. Protective antibodies develop, and murine Lyme arthritis regresses, in the absence of MHC class II and CD4+ T cells. J. Immunol. 1997;159:5682–5686. [PubMed] [Google Scholar]
- 17.Sadziene A, Thompson PA, Barbour AG. In vitro inhibition of Borrelia burgdorferi growth by antibodies. J. Infect. Dis. 1993;167:165–172. doi: 10.1093/infdis/167.1.165. [DOI] [PubMed] [Google Scholar]
- 18.Bunikis J, et al. Borrelia burgdorferi infection in a natural population of Peromyscus leucopus mice: a longitudinal study in an area where Lyme borreliosis is highly endemic. J. Infect. Dis. 2004;189:1515–1523. doi: 10.1086/382594. [DOI] [PubMed] [Google Scholar]
- 19.Brunet LR, Sellitto C, Spielman A, Telford SR. Antibody response of the mouse reservoir of Borrelia burgdorferi in nature. Infect. Immun. 1995;63:3030–3036. doi: 10.1128/iai.63.8.3030-3036.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Silva AM, et al. Immune evasion by tickborne and host-adapted Borrelia burgdorferi. J. Infect. Dis. 1998;177:395–400. doi: 10.1086/514200. [DOI] [PubMed] [Google Scholar]
- 21.Malawista SE, Barthold SW, Persing DH. Fate of Borrelia burgdorferi DNA in tissues of infected mice after antibiotic treatment. J. Infect. Dis. 1994;170:1312–1316. doi: 10.1093/infdis/170.5.1312. [DOI] [PubMed] [Google Scholar]
- 22.Coleman JL, et al. Plasminogen is required for efficient dissemination of Borrelia burgdorferi in ticks and for enhancement of spirochetemia in mice. Cell. 1997;89:1111–1119. doi: 10.1016/s0092-8674(00)80298-6. [DOI] [PubMed] [Google Scholar]
- 23.Nagamune K, et al. Surface sialic acids taken from the host allow trypanosome survival in tsetse fly vectors. J. Exp. Med. 2004;199:1445–1450. doi: 10.1084/jem.20030635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nazario S, et al. Prevention of Borrelia burgdorferi transmission in guinea pigs by tick immunity. Am. J. Trop. Med. Hyg. 1998;58:780–785. doi: 10.4269/ajtmh.1998.58.780. [DOI] [PubMed] [Google Scholar]
- 25.Barthold SW, Beck DS, Hansen GM, Terwilliger GA, Moody KD. Lyme borreliosis in selected strains and ages of laboratory mice. J. Infect. Dis. 1990;162:133–138. doi: 10.1093/infdis/162.1.133. [DOI] [PubMed] [Google Scholar]
- 26.Piesman J. Standard system for infecting ticks (Acari: Ixodidae) with the Lyme disease spirochete, Borrelia burgdorferi. J. Med. Entomol. 1993;30:199–203. doi: 10.1093/jmedent/30.1.199. [DOI] [PubMed] [Google Scholar]
- 27.Pal U, et al. Inhibition of Borrelia burgdorferi-tick interactions in vivo by outer surface protein A antibody. J. Immunol. 2001;166:7398–7403. doi: 10.4049/jimmunol.166.12.7398. [DOI] [PubMed] [Google Scholar]
- 28.Narasimhan S, et al. Disruption of Ixodes scapularis anticoagulation by using RNA interference. Proc. Natl Acad. Sci. USA. 2004;101:1141–1146. doi: 10.1073/pnas.0307669100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.