Abstract
Objective
Self-rated health (SRH) predicts mortality above and beyond objective health risks and as such comprises an important aspect of health. Established contributors to self-rated health include affect, age, and disease, but neither their dynamic nor their synergistic contributions to SRH have been comprehensively tested.
Methods
The present study employed older adults (N = 150; M age = 75 years) and a longitudinal design with 6-month waves over up to 5 years. Positive and negative affect (PA, NA), chronic disease, and SRH were assessed at each wave.
Results
In multilevel models with single predictors, older age, more chronic disease, and higher NA predicted worse SRH, whereas higher PA predicted better SRH. Affect predicted SRH both between and within people. In multilevel models with interactions between affect and age or disease, individual differences in NA predicted worse SRH primarily in older people. Within people, changes in NA were associated with changes in SRH, but more so in younger than in older people. Within people, changes in PA were associated with changes in SRH, but only when health was better than usual.
Conclusions
There were both dynamic and synergistic relationships between affect and SRH that could only emerge in a multilevel, multivariable design. In the case of NA, between-person, trait NA had the opposite relationship to SRH and age compared with within-person, state NA. Which component of this relationship predicts mortality is an important question for future research.
Keywords: self-rated health, affect, age, disease, longitudinal
Whether one considers one’s overall health to be excellent, poor, or somewhere in between is an important facet of health. Self-rated health (SRH) is a prospective predictor of mortality above and beyond other risk factors (e.g., Haring et al., 2011; Idler & Benyamini, 1997; Idler & Kasl, 1991; Lyyra, Heikkinen, Lyyra, & Jylhä, 2006; Strawbridge & Wallhagen, 1999). Although there are occasional exceptions with regard to SRH’s incremental prediction over physical fitness or objective health (Deeg, van Zonneveld, van der Maas, & Habbema, 1989; Gander, Lee, Sui, Hébert, Hooker, & Blair, 2011; Lyrra et al., 2006), meta-analytic findings indicate an up to two-fold risk of mortality in those reporting “poor” versus “excellent” health, even after controlling for comorbidity, depression, functional status, socioeconomic status, and cognitive function (DeSalvo, Bloser, Reynolds, He, & Muntner, 2005). Although this relationship tends to be stronger in younger adults, the relationship holds in older adulthood (Benyamini, Blumstein, Lusky, & Modan, 2003; Giltay, Vollaard, & Kromhout, 2012; Kaplan, Barell, & Lusky, 1988; Strawbridge & Wallhagen, 1999).
A new generation of SRH research has focused on the dynamics of SRH and its relationship to mortality. Changes in SRH are important because more recent assessments of SRH are better predictors of mortality than more remote assessments (Benyamini et al., 2003; Bopp et al., 2012; Singh-Manoux, Gueguen, Marikainen, Ferrie, Marmot, & Shipley, 2007), so a person’s state SRH may have more predictive power than trait SRH. SRH typically declines with increasing chronological age, but there are individual differences in its slope (Miller & Wolinsky, 2007; Sargent-Cox, Anstey, & Luszcz, 2010). A faster-declining SRH slope predicts mortality through its terminus at a lower state SRH (Miller & Wolinsky, 2007). Both initial SRH and premorbid decline in SRH predicted mortality and sentinel health events, including stroke, heart disease, cancer, and hip fracture, in older adults (Diehr, Williamson, Patrick, Bild, & Burke, 2001; Perera, Studenski, Chandler, & Guralnik, 2005). Therefore, a dynamic picture of SRH and its predictors is both more nuanced and powerful than a static picture.
It has long been established that SRH and other subjective measures of health “assess at least two distinct sources of variance, one that is clearly health-relevant and another that is more subjective and psychological” (Watson & Pennebaker, 1989, p. 245). Although the mortality studies indicate that the “subjective and psychological” source is also health-relevant, this quote introduces the two main contributors to SRH: objective health status and subjective experience of one’s health. SRH strongly correlates with objective health. This is true of dynamic SRH as well as static SRH: In a longitudinal study of older adults, those who experienced a decline in SRH at any point in the study were also more likely to have had both new and worsening health conditions (Rodin & McAvay, 1992). Subjective experience of one’s health, on the other hand, is part of what Watson and Pennebaker (1989) call “somatopsychic distress” (p. 248), because people who are more prone to negative emotional states (i.e., are high in negative affectivity) are also more prone to subjective health complaints (Cohen et al., 1995; Costa & McCrae, 1987; Watson, 1988; Watson & Pennebaker, 1989). In daily diary studies, both trait negative affectivity and state negative affect contributed to subjective health complaints and worse SRH (Gijsbers van Wijk, Huisman, & Kolk, 1999; Watson, 1988; Winter, Lawton, Langston, Ruckdescel, & Sando, 2007). In longer-term longitudinal studies, older adults were more likely to have declining SRH when they also had increases in NA (Han & Jylha, 2006; Rodin & McAvay, 1992; Schöllgen, Huxhold, & Schmiedek, 2011). Early reports indicated that proneness to positive emotional states (i.e., positive affectivity) was unrelated to subjective health (Watson & Pennebaker, 1989). However, subsequent reports found that positive affect (PA) contributed to subjective health complaints and SRH above and beyond negative affect (Andreasson, Szulkin, Unden, von Essen, Nilsson, & Lekander, in press; Gijsbers van Wijk et al., 1999; Pettit, Kline, Gencoz, Gencoz, & Joiner, 2001; Watson, 1988; Winter, Lawton, Langston, Ruckdescel, & Sando, 2007). Therefore, “somatopsychic distress” could be better called “somatopsychic affect”, as its influence on SRH is not due solely to distress.
The two sources of variance in SRH – objective health and affect – have generally been treated as additive, and there have been few tests of interactions among objective health and affective influences on SRH. Studies that have tested interactions between health and demographic predictors of SRH tend to suggest stronger relationships in younger and healthier adults (Rodin & McAvay, 1992; Strawbridge & Wallhagen, 1999). With regard to affect, improvement in depressive symptoms was more likely to correlate with improvement in SRH in healthier older adults (Han & Jylha, 2006). As objective influences on physical health such as aging and disease exert increasing pressure on SRH, they potentially limit the degree to which affect influences SRH. This possibility has been raised in the related context of discrete symptoms, where obvious injury provides “distinctive and unambiguous” symptoms that are less open to perceptual and cognitive influence (Suls & Howren, 2012, p. 131). The signals that aging and disease provide about physical health may account for more of the inputs to global SRH, and subjective influences such as affect may account for relatively less input. On the other hand, the symptom perception model (Watson & Pennebaker, 1989) provides a competing hypothesis that posits that when more objective health signals are present, affect should have an amplifying effect and therefore have greater input to SRH. In this model, people with high negative or low positive affect have low thresholds for perceiving physical symptoms and sensations as problematic and distressing. In a related model (Diehl & Wahl, 2010), the combination of attentional biases associated with affect (e.g., Williams, Mathews, & MacLeod, 1996) and cues about physical aging may increase “awareness of age-related change” (Diehl & Wahl, 2010, p. 340) and result in changes in SRH. These models would therefore predict that the influence of affect on SRH should be greater when more health signals are present to be perceived and interpreted. In one study, changes in self-efficacy were more strongly associated with SRH for older adults above age 75 than those below, although changes in depression did not interact with age (Rodin & McAvay, 1992). Therefore, there is some evidence for both the zero-sum and the amplification hypotheses (Han & Jylha, 2007; Rodin & McAvay, 1992), but these studies differed in measures (depression vs. self-efficacy) and moderators (health vs. chronological age).
The present study tested these competing hypotheses in the context of dynamic change in SRH, providing a comprehensive and systematic test of chronological age, disease, PA, and NA as they relate to SRH between and within people. Multi-level modeling was used in a 5-year, 11-wave longitudinal study of older adults to characterize the correlates of SRH. Older age, more chronic disease, more NA, and less PA were hypothesized to associate with poorer SRH, consistent with existing evidence. However, unlike most existing evidence, these hypothesized relationships were tested both between and within people over a period of years. Furthermore, two competing hypotheses that posit that affect should have interactive effects with regard to age and chronic disease were tested. One hypothesis frames the relationship as zero-sum: as objective inputs to SRH increase, subjective inputs decrease, and affect should have a lesser effect with older age or higher chronic disease. The other hypothesis frames the relationship as amplification: as objective inputs to SRH increase, the effect of interpreting those signals negatively or positively should also increase, and affect should have a greater effect with older age or higher chronic disease.
Method
Participants
Participants were 150 community-dwelling, married older adults over the age of 60 (mean = 75; range = 60 – 93 at study entry). Although only married individuals were included in the parent study to prospectively assess dyadic stressors of older age (e.g., caregiving, bereavement), no dyads were included in the sample. Consistent with the gender ratio in older age, 42% of the sample was male and 58%, female. The majority of the sample was white (96%) and the remainder, African-American (4%). Median annual household income was $57,000 (range = $12,000 – $400,000), and median education was 16 years (range = 7 – 22). Because the parent study also involved vaccination and measurement of immune parameters, exclusion criteria at enrollment included diseases or disorders affecting the immune system, chemotherapy or radiation treatment within the past 5 years, unwillingness to undergo vaccination or venipuncture, immunomodulatory medications including opiates and steroids, and more than two of the following classes of medications: psychotropics, antihypertensives, hormone replacement, or thyroid supplements. No participants were cognitively impaired.
Procedure
Participants were recruited from a volunteer subject pool maintained by the Sanders-Brown Center on Aging at the University of Kentucky. Prospective participants were contacted and screened by phone. Those who were interested and eligible were enrolled and completed questionnaire measures verbally with the assistance of a research assistant and response cards. These interviews took place at 6-month intervals over up to 11 waves (5 years). Participants received a $20 gift card at each completed wave. Of the 150 participants who completed Wave 1, 135 completed Wave 2; 124, Wave 3; 117, Wave 4; 111, Wave 5; 110, Wave 6; 100, Wave 7; 97, Wave 8; 80, Wave 9; 51, Wave 10; and 29, Wave 11. Data were missing at later waves due to dropout (n = 64; 408 missing waves) and death (n = 6; 49 missing waves). In addition, because some participants were recruited later than others, those participants were able to complete fewer waves before the end of the study (89 missing waves). Overall, 1,104 waves of data were available for analysis.
Measures
Demographics
Demographic information was collected at the first interview. Date of birth and interview date were used to calculate exact chronological age at each interview.
Self-rated health
SRH was measured using a single item from the Medical Outcomes Study (MOS) Health-Related Quality of Life scale (Stewart & Ware, 1992). The item reads, “In general, would you say your health is … ”, with possible responses excellent, very good, good, fair, and poor. The variable was coded for analysis so that higher values represent better SRH.
Affect
Positive and negative affect were measured with the Geriatric Depression Scale (Yesavage et al., 1983). The GDS is appropriate for administration to older adults, who may have inflated scores on other depression measures due to somatic complaints associated with aging. There are 20 yes-no items that reflect negative affect (e.g., “Do you often feel downhearted and blue?”, “Do you frequently get upset over little things?”) and 10 items that reflect positive affect (e.g., “Are you hopeful about the future?”, “Do you feel happy most of the time?”). Because the present study was concerned with both individual differences and change over time, the reliability of these two sets of items was calculated both between and within people (Cranford, Shrout, Iida, Rafaeli, Yip, & Bolger, 2006). Both sets showed excellent between-person reliability (.96 – .97; Cranford et al., 2006, Equation 4) and adequate within-person reliability (.58–.60; Cranford et al., 2006, Equation 5). These reliability coefficients are consistent with other measures of affect (Cranford et al., 2006).
Chronic disease
At each visit, participants provided a list of all prescription medications, which were category-coded by a graduate-level nurse. These medication categories were used to derive the Chronic Disease Score (CDS), which quantifies chronic disease burden. This score was empirically derived in a large (N > 250,000) sample of adults to predict health care utilization, hospitalization, and death. For example, the odds ratio for hospitalization in the top 10% of scores vs. the bottom 10% was 22.7; the odds ratio for death was 500.1 (Clark, von Korff, Saunders, Baluch, & Simon, 1995). This score therefore directly represents the chronic diseases for which a participant was being treated, weighted by the likelihood that the disease would result in hospitalization or death. While this measure does not directly measure those outcomes (e.g., as opposed to a chart review), it has the advantages of being an objective measure of chronic disease that does not share method variance with SRH; providing a reasonable, validated indirect measure; and maximizing feasibility in studies such as this one in which there are multiple providers and medical records both between and within participants.
The CDS is a weighted sum of medication categories (e.g., antihypertensive agents); the original included psychiatric medications, but these medications were not included in the CDS for the present study so as to avoid contamination with regard to affect. The most prevalent chronic diseases in the sample were hypertension (71% of person-waves), hyperlipidemia (50%), heart disease (39%), Parkinson’s disease (19%), thyroid disease (13%), vascular disease (12%), respiratory disease (12%), inflammatory pain (11%), and diabetes (10%). Because of the nature of the study, all participants had a primary care physician. They were Medicare-eligible at 97% of visits. Therefore, access to prescription medications was likely to be high across the sample.
Personality
At Wave 2, participants completed the NEO-FFI (Costa & McCrae, 1992). Scores for neuroticism (α = .79) and extraversion (α = .75) provided validity evidence for the affectivity scores from the GDS.
Data analysis
The data were analyzed in multi-level models with people at the higher level and waves at the lower level in SAS PROC MIXED using REML estimation (Singer, 2002). These models utilize all available data without the need for either list-wise deletion or data imputation (Singer & Willett, 2003). Null models with no predictors were used to estimate the intraclass correlations and intercepts (i.e., sample means) for model variables. Note that the null model intercept is a more valid estimate of a sample mean when there are dependencies in the data than a simple mean across all people and waves because it avoids biases due to aggregation across different cluster sizes (Singer & Willett, 2003; Snijders & Bosker, 1999).
Subsequent models added age, CDS, NA, or PA as univariate predictors. With regard to centering of these predictors, Enders and Tofighi (2007); Kreft, de Leeuw, and Aiken (1995); and Singer and Willett (2003) provide extensive discussion of the implications of different centering strategies, briefly described here. Age was centered around the youngest age in the study (60 years) to estimate the intercept at this age and slopes from this age onward. CDS, NA, and PA were Z score transformed before being individual-mean-centered. Each of these three variables therefore generated two terms: an average score for each person across all waves in Z score units, which captured between-person variance, and a deviation from each person’s average score at each wave, also in Z score units, which captured within-person variance. When entered together, these predictors are orthogonal and quantify the effect at each level (between- and within-person). The difference between within- and between-person coefficients was tested by substituting the total scores for the deviation scores. If the magnitude of predictive power is the same at both levels, when entered together, the coefficient for the average score provides no additional explanatory power. Therefore, the significance test of this coefficient can be interpreted as a test of the difference between the within- and between-person effects (Enders & Tofighi, 2007; see Kreft et al., 1995, for the mathematical proof).
Additional trimmed models recentering the appropriate terms were used to generate tests of the significance of simple slopes at chosen levels of the moderator (Aiken & West, 1991). Results are reported as gamma weights with their standard errors and associated t tests. Gamma weights are similar to unstandardized beta weights in regression. In the reported models, the gamma weight can be interpreted as the change in SRH on a scale of 1 to 5 associated with change of 1 Z unit in the affect and disease predictors and 1 year in the age predictor. Variance of the intercept (τ2, i.e., between-person variance not accounted for by predictors) and residual (ε2, i.e., within-person variance not accounted for by predictors) are also reported for each model.
Results
Means and zero-order relationships
Table 1 shows the bivariate relationships among the variables. On a between-subjects level, SRH had the expected negative relationships with negative affectivity, chronic disease, age, and neuroticism and positive relationships with positive affectivity and extraversion. Although some reports have found women to have worse SRH than men (McCullough & Laurenceau, 2004; Singh-Manoux et al., 2007), gender was unrelated to SRH in the present sample. All of the repeated measures had intraclass correlations indicating substantial stability across the study period. However, approximately a third of the variance was due to change over time within subjects (except for chronic disease, which was somewhat more stable). Perhaps as a result of there being more between-person than within-person variance, the within-subjects relationships (i.e., correlations of deviation scores) were of smaller magnitude, although in the same directions. Higher levels of chronic disease across the entire study period were associated with poorer SRH, but changes in degree of disease were not associated with changes in SRH at the level of the zero-order correlation. Finally, NA and PA were strongly associated with each other (and with neuroticism and extraversion) between people, but changes in NA and PA were less strongly associated with each other within people. Therefore, subsequent analyses employed NA and PA as separate predictors.
Table 1.
Null model intercepts (i.e., estimated means), ICCs, and correlations among study variables.
| Variables with between- and within-person variance | |||||||
|---|---|---|---|---|---|---|---|
| Intercept | ICC | 1 | 2 | 3 | 4 | Wave | |
|
|
|||||||
| 1. SRH | 3.61 | .68 | - | −.17 | .16 | −.03 | −.08 |
| 2. Negative affect | 2.50 | .68 | −.48* | - | −.39 | .01 | .05 |
| 3. Positive affect | 8.19 | .67 | .56* | −.82* | - | −.02 | −.08 |
| 4. Chronic disease | 1104 | .78 | −.40* | .09 | −.16 | - | .23 |
| Variables with between-person variance only | |||||||
| Mean | SD | ||||||
|
|
|||||||
| Age at entry (years) | 74.6 | 6.19 | −.24* | .06 | −.19* | .21* | |
| Gender (1 = female) | .03 | .14 | −.14 | −.05 | |||
| Neuroticism (mean item) | 1.85 | 0.52 | −.31* | .56* | −.40* | .06 | |
| Extraversion (mean item) | 3.16 | 0.47 | .29* | −.45* | .47* | .00 | |
| Number of waves completed | 7.39 | 3.44 | .15 | −.21* | .19* | −.14 | |
Note: N and E correlated with each other −.31. N = 135 for correlations with N and E, otherwise N = 150. In the upper block, between-person correlations are shown below the diagonal and within-person, above the diagonal. No statistical significance indication for within-person correlations was provided, as these may be biased when the data are not independent observations; correlations are shown for the purpose of illustrating effect sizes only.
Univariate models
The first set of models examined the univariate effects of age, chronic disease, and affect on SRH (see Table 2). In a preliminary model not shown in Table 2, there was a linear effect of age (γ = −.026 (.009), p < .005); change in the model −2 log likelihood indicated that including age as a random effect significantly improved the fit of the model, and so all subsequent models including age include it as a random effect. This linear effect was moderated by a significant quadratic effect of age in the model shown in Table 2. This quadratic effect indicated that the negative effect of age on SRH accelerated at older ages.
Table 2.
Multi-level model parameters from univariate models predicting self-rated health
| Predictor | Model parameter | Estimate (SE) | p < |
|---|---|---|---|
| Null model (no predictors) | Variance components | ||
| Intercept (τ2) | 0.57 (0.08) | .0001 | |
| Residual (ε2) | 0.27 (0.01) | .0001 | |
| Age | Random effects | ||
| Intercept | 3.56 (0.24) | .0001 | |
| Age (years > 60) | 0.04 (0.03) | .15 | |
| Fixed effects | |||
| Quadratic age (years > 60 2) | −.002 (.0009) | .02 | |
| Variance components | |||
| Intercept (τ2) | 0.58 (0.32) | .04 | |
| Linear age slope | 0.002 (0.001) | .06 | |
| Intercept-slope covariance | −0.02 (0.02) | .34 | |
| Residual (ε2) | 0.26 (0.01) | .0001 | |
| Change in -2LL from null model | 7.3 (df = 4) | .12 | |
| Chronic disease | Random effects | ||
| Intercept | 3.62 (0.06) | .0001 | |
| Fixed effects | |||
| Within-person | −0.03 (0.04) | .44 | |
| Between-person | −0.32 (0.06) | .0001 | |
| Variance components | |||
| Intercept (τ2) | 0.47 (0.06) | .0001 | |
| Residual (ε2) | 0.27 (0.01) | .0001 | |
| Change in -2LL from null model | 17.0 (df = 2) | .0002 | |
| Negative affect | Fixed effects | ||
| Intercept | 3.64 (0.06) | .0001 | |
| Within-person | −0.14 (0.03) | .0001 | |
| Between-person | −0.37 (0.06) | .0001 | |
| Variance components | |||
| Intercept (τ2) | 0.44 (0.06) | .0001 | |
| Residual (ε2) | 0.26 (0.01) | .0001 | |
| Change in -2LL from null model | 54.6 (df = 2) | .0001 | |
| Positive affect | Fixed effects | ||
| Intercept | 3.64 (0.06) | .0001 | |
| Within-person | 0.13 (0.03) | .0001 | |
| Between-person | 0.46 (0.06) | .0001 | |
| Variance components | |||
| Intercept (τ2) | 0.40 (0.05) | .0001 | |
| Residual (ε2) | 0.26 (0.01) | .0001 | |
| Change in -2LL from null model | 66.7 (df = 2) | .0001 |
There was also a linear effect of higher chronic disease score on lower SRH between but not within people, consistent with the correlations in Table 1. The difference between the between-person and within-person effects was statistically significant (t(148) = 4.43, p < .0001). Including CDS as a random effect did not significantly improve the fit of the model, and so all subsequent models including chronic disease include it as a fixed effect. The between-person relationship held after controlling for age (γ = −.320 (.062), p < .0001). Therefore, age and chronic disease score made independent contributions to SRH.
More negative affect was significantly associated with poorer SRH both between and within people; the between-person effect was significantly larger than the within-person effect (t(148) = 3.77, p < .0002). A similar pattern was observed for positive affect (difference: t(148) = 5.15, p < .0001). In a model in which PA and NA terms were entered together (model τ2= .40, ε2 = .26), within-person changes in PA and NA each made significant contributions to changes in SRH over time, above and beyond their shared variance (PA: γ = .088 (.029), p < .002; NA: γ = −.101 (.028), p < .0004). Between-person differences in PA made a significant contribution to differences in SRH above and beyond the substantial shared variance with NA (γ = .395 (.097), p < .0001). Between-person differences in NA, however, did not make a significant contribution above and beyond their shared variance with PA (p < .44).
Affect as a moderator
It was hypothesized that, in addition to having direct effects on SRH, affect would moderate the effects of age and chronic disease. Therefore, models were tested that included the main effects and interactions between affect and these other influences on SRH.
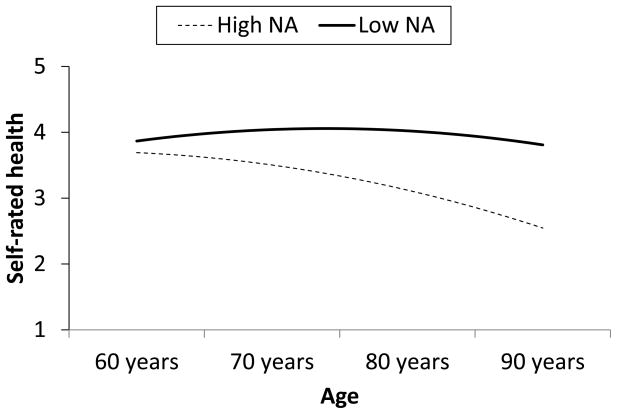
With regard to age, NA moderated the linear effect of age both within and between people, but in different directions (model τ2= .25, ε2 = .25). Between people, the negative effect of NA on SRH was stronger with increasing age (γ = −.018 (.007), p < .01). Figure 1 shows the effect of age for people who are typically high (+1 Z) versus low (−1 Z) in NA; with increasing age, the difference between people who are typically high versus low in NA becomes larger. The linear effect of age was negative and statistically significant at high levels of typical NA (γ = −.040 (.010), p < .0001) but not at low levels (γ = −.003 (.012), p > .05). After including this interaction, the random effect of age no longer made a significant contribution to the model. Therefore, negative affectivity accounted for individual differences in the relationship of age to SRH.
Figure 1.
Effects of between-person differences in negative affect (NA) and age on self-rated health. High NA is 1 Z score unit above the grand mean; low NA is 1 unit below.
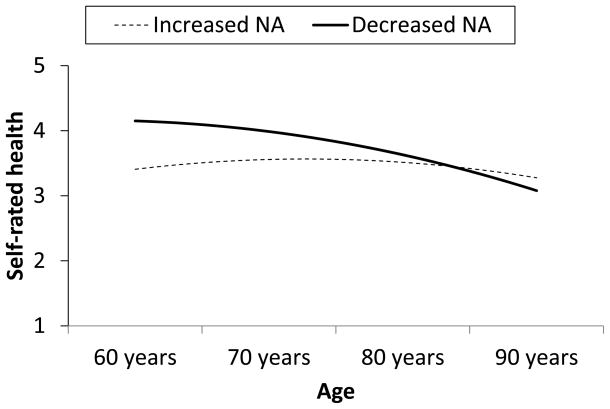
Within people, the negative effect of changes in NA on changes in SRH was weaker with increasing age (γ = .016 (.004), p < .0003). Figure 2 shows the effect of having more than usual NA (+1 Z) or less than usual NA (−1 Z) across a representative age range; with increasing age, the effect of these fluctuations in NA on SRH becomes smaller. The effect was largest at age 60 (γ = −.370 (.073), p < .0001), and progressively smaller at ages 70 (γ = −.219 (.036), p < .0001), 80 (γ = −.068 (.031), p < .03), and 90, when the effect of shifts in NA on SRH was no longer statistically significant (γ = .083 (.066), p > .05). PA did not moderate the effect of linear age (model τ2= .33, ε2 = .25), and neither NA nor PA moderated the quadratic effect of age.
Figure 2.
Effects of within-person changes in negative affect (NA) and age on self-rated health. Increased NA is 1 Z score unit above the individual’s mean; decreased NA is 1 unit below.
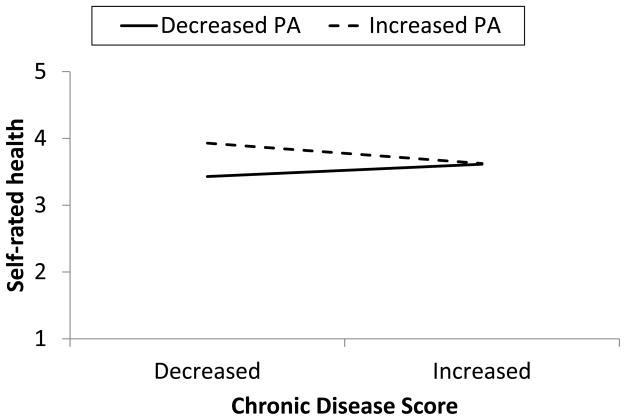
With regard to chronic disease score, NA did not moderate the effect of disease on SRH (model τ2= .36, ε2 = .26). However, there was a tendency for change in PA to interact with change in chronic disease score to predict SRH (γ = −.122 (.065), p = .06, model τ2= .34, ε2 = .26). Figure 3 shows the degree to which change in PA would affect SRH when chronic disease increased or decreased. With increases in chronic disease, there was little effect of accompanying change in PA on SRH (γ = .010 (.071), p > .05). However, when there were decreases in chronic disease burden, accompanying increases in PA resulted in significantly better SRH (γ = .226 (.072), p < .002).
Figure 3.
Effects of within-person changes in chronic disease score and positive affect (PA) on self-rated health. Decreased PA and CDS are 1 Z score unit below the individual’s means; increased PA and CDS are 1 unit above.
Discussion
The present study tested two competing hypotheses regarding how objective indicators of health (chronological age and chronic disease) might interact with affect to associate with SRH. One hypothesis proposes a zero-sum dynamic in which more input from objective inputs crowds out subjective inputs (e.g., Suls & Howren, 2012), whereas the other proposes an amplification of objective inputs by subjective inputs (e.g., Diehl & Wahl, 2010; Watson & Pennebaker, 1989). The present study suggests that both models may be correct, depending on whether one considers stable individual differences in affectivity or within-person change in state affect. In this longitudinal study of older adults, those who were high in negative affectivity showed age-related differences in SRH, whereas those who were low did not show age-related differences, even across a range of over 30 years. In the present study, age is likely to serve as a proxy for health changes that do not fall under the rubric of chronic disease for which one is prescribed medication (i.e., those that contribute to the CDS). Such changes could include increased fatigue, decreased strength and flexibility, and health conditions that people often treat with over-the-counter medications, such as minor arthritic changes. These results are consistent with a model in which people with higher negative affectivity do in fact perceive and interpret such changes more negatively, resulting in poorer SRH.
However, affective changes showed a different pattern of results with regard to the relationships among age, chronic disease, and SRH. Whereas the effects of negative affectivity were greatest at older ages, the effects of changes in state NA were greatest at younger ages. The effect of fluctuations in NA on SRH declined over time and essentially vanished at the oldest ages in the study. Fluctuations in PA interacted with changes in objective, chronic disease. At the zero-order level, it appeared that these constructs would make independent contributions to SRH, as affect was unrelated to chronic disease (see Table 1). However, when objective disease improved, it made a difference to SRH whether positive state affect was higher than usual or lower than usual. When improvements in objective disease were accompanied by improvements in positive state affect, SRH also improved; without this accompaniment, SRH did not improve. Therefore, these results suggest that an improvement in objective disease was a necessary but not sufficient condition for better SRH. This pattern is consistent with the interaction between NA and age insofar as state changes were more likely to conform to the zero-sum model than the amplification model: worsening disease and older age were both associated with an absence of influence of changing affect on SRH.
In general, results with affectivity supported the amplification model, whereas results with state affect supported the zero-sum model. These two components may have different contributing factors: affectivity may arise more from stable influences such as temperament or cognitive styles, whereas state affect may arise more from unstable influences such as life events (Cole, Nolen-Hoeksema, Girgus, & Paul, 2006; Hankin, Fraley, & Abela, 2005). Therefore, with increasing age, SRH may be more influenced by trait factors and less influenced by stressors and life events that drive state affect. Likewise, with increasing disease burden, SRH may be less susceptible to these state influences. One possibility is that older and more ill individuals had less variability in their affect that could then predict their SRH. However, there was no correlation between age and affective variability in this sample; correlations with age and objective disease, respectively, partialling total number of visits, were .01 and .00 for the SD of positive affect and .04 and .17 for the SD of negative affect.
More generally, one important implication of this pattern is that cross-sectional studies may underestimate the role of affect. Affect at a particular point in time can be decomposed into one’s usual, trait affect (i.e., affectivity) and one’s current, state difference from that trait. If affectivity is influencing SRH in one particular way and state affect, in the opposite way (as in the interactions with age in the present study), important effects may be undetectable or the results may be misleading (Hamaker, 2012). In fact, in the present study, when NA was not decomposed into trait and state effects, there was a significant effect in the same direction as that for state affect (γ = .009 (.004), p < .01), but this result both obscured the effect of affectivity and generated a gamma weight that was about half the size of the separated affectivity (γ = −.018) and state affect (γ = .016) gamma weights.
One of the distinct strengths of this study, therefore, was the longitudinal design that allowed the examination of both stable individual differences and change over time. Multivariate multilevel models such as this one, in which multiple variables are measured and related to each other over multiple time points, are rare in health research (Ryu, West, & Sousa, 2012), but as this study demonstrated, they can be illuminating. Another strength was the large age range within older age (60–94 years). However, the study also had limitations. Negative and positive affect can be effectively derived from negatively and positively framed items in depression measures (e.g., the CES-D; Hertzog, Van Alstine, Usala, Hultsch, & Dixon, 1990), but such measures preclude the examination of specific affects such as anxiety, anger, and depression. The measure of chronic disease was derived from prescription medications. The advantage of such as measure is that it is objective; however, it does not capture conditions for which one might not be taking prescription medication. Future research should replicate the present results using different operationalizations of both affect and disease. Finally, this was a generally healthy sample, which was likely to have restricted the range of the chronic disease measure. This may have contributed to the more limited results with this measure compared with those for age. Likewise, SRH was higher than in population-based studies of older adults (e.g., Idler & Kasl, 1991; McCullough & Laurenceau, 2004). Therefore, the present models are more concerned with declines in health than improvements. Although declines in SRH are more common in older age than improvements (Miller & Wolinsky, 2007), predictors of the latter are of interest and, indeed, the present study did find that such improvements are likely to correlate with a combination of improving physical health and positive state affect.
Future Directions and Conclusion
This study focused on the predictors of SRH in affect, age, and chronic disease in a dynamic model that incorporated both stability and change. One important quality of SRH is that it, in turn, predicts important health endpoints. Recent studies have suggested that the change component of SRH may be as important, if not more so, than the stable component in predicting morbidity and mortality (Benyamini et al., 2003; Bopp et al., 2012; Diehr et al., 2001; Miller & Wolinsky, 2007; Singh-Manoux et al., 2007). An important future direction for SRH and mortality research is in distinguishing between the amplification model and the zero-sum model. Which piece of SRH predicts morbidity and mortality? The decline in SRH associated with age that is amplified by affectivity, or the change in SRH that is driven by state affect but most present in the young-old and those with better health?
Finally, these findings speak to the clinical utility of SRH. For example, primary care clinicians may not weight SRH highly in their assessments because it can be perceived as a “soft” measure of somatic anxiety or depression. However, the suggestion to measure SRH repeatedly on older adults (Diehr et al., 2001) should be considered seriously in the clinical setting as an additional piece of prognostic information. In old-old adults, the influence of changes in state affect on change in SRH is minimal, and a decline – particularly to “fair” or “poor” health – could presage a sentinel health event. In young-old adults, however, a decline in SRH might also be associated with changes in state affect requiring clinical attention, such as new-onset depression. Knowledge of how SRH relates to age and affect therefore increases the clinical utility of this deceptively simple measure of health.
Acknowledgments
This research was supported by the Dana Foundation and the National Institute of Aging (AG026307-R01, AG033629-K02, AG028383-P30). The author thanks Audrey Darville, Tory Eisenlohr-Moul, and Ian Boggero for coding and generating the chronic disease score and for comments on a previous version of this work and Peggy Keller for statistical consultation.
References
- Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. Thousand Oaks, CA: Sage; 1991. [Google Scholar]
- Andreasson AN, Szulkin R, Unden AL, von Essen J, Nilsson LG, Lekander M. Inflammation and positive affect are associated with subjective health in women of the general population. Journal of Health Psychology. doi: 10.1177/1359105311435428. (in press) Published ahead of print. [DOI] [PubMed] [Google Scholar]
- Benyamini Y, Blumstein T, Lusky A, Modan B. Gender differences in the self-rated health-mortality association: Is it poor self-rated health that predicts mortality or excellent self-rated health that predicts survival? The Gerontologist. 2003;43:396–405. doi: 10.1093/geront/43.3.396. [DOI] [PubMed] [Google Scholar]
- Bopp M, Braun J, Gutzwiller F, Faeh D for the Swiss National Cohort Study Group. Health risk or resource? Graduate and independent association between self-rated health and mortality persists over 30 years. PLoS ONE. 2012;7:e30795. doi: 10.1371/journal.pone.0030795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DO, con Korff M, Saunders K, Baluch WM, Simon GE. A chronic disease score with empirically derived weights. Medical Care. 1995;33:783–795. doi: 10.1097/00005650-199508000-00004. [DOI] [PubMed] [Google Scholar]
- Cohen S, Doyle WJ, Skoner DP, Fireman P, Gwaltney JM, Jr, Newson JT. State and trait negative affect as predictors of objective and subjective symptoms of respiratory viral infections. Journal of Personality and Social Psychology. 1995;68:159–169. doi: 10.1037//0022-3514.68.1.159. [DOI] [PubMed] [Google Scholar]
- Cole DA, Nolen-Hoeksema S, Girgus J, Paul G. Stress exposure and stress generation in child and adolescent depression: A latent trait-state-error approach to longitudinal analyses. Journal of Abnormal Psychology. 2006;115:40–51. doi: 10.1037/0021-843X.115.1.40. [DOI] [PubMed] [Google Scholar]
- Costa PT, Jr, McCrae RR. Neuroticism, somatic complaints, and disease: Is the bark worse than the bite? Journal of Personality. 1987;55:297–316. doi: 10.1111/j.1467-6494.1987.tb00438.x. [DOI] [PubMed] [Google Scholar]
- Costa PT, Jr, McCrae RR. Revised NEO Personality Inventory and NEO Five-Factor Inventory. Odessa, FL: Psychological Assessment Resources; 1992. [Google Scholar]
- Cranford JA, Shrout PE, Iida M, Rafaeli E, Yip T, Bolger N. A procedure for evaluating sensitivity to within-person change: Can mood measures in diary studies detect change reliably? Personality and Social Psychology Bulletin. 2006;32:917–929. doi: 10.1177/0146167206287721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeg DJH, van Zonneveld RJ, van der Maas PJ, Habbema JDF. Medical and social predictors of longevity in the elderly: Total predictive value and interdependence. Social Science and Medicine. 1989;29:1271–1280. doi: 10.1016/0277-9536(89)90067-1. [DOI] [PubMed] [Google Scholar]
- DeSalvo KB, Bloser N, Reynolds K, He J, Muntner P. Mortality prediction with a single general self-rated health question: A meta-analysis. Journal of General Internal Medicine. 2005;20:267–275. doi: 10.1111/j.1525-1497.2005.00291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl MK, Wahl HW. Awareness of age-related change: Examination of a (mostly) unexplored concept. Journal of Gerontology: Social Sciences. 2010;65B:340–350. doi: 10.1093/geronb/gbp110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehr P, Williamson J, Patrick DL, Bild DE, Burke GL. Patterns of self-rated health in older adults before and after sentinel health events. Journal of the American Geriatric Society. 2001;49:36–44. doi: 10.1046/j.1532-5415.2001.49007.x. [DOI] [PubMed] [Google Scholar]
- Enders CK, Tofighi D. Centering predictor variables in cross-sectional multilevel models: A new look at an old issue. Psychological Methods. 2007;12:121–138. doi: 10.1037/1082-989X.12.2.121. [DOI] [PubMed] [Google Scholar]
- Gander J, Lee DC, Sui X, Hébert JR, Hooker SP, Blair SN. Self-rated health status and cardiorespiratory fitness as predictors of mortality in men. British Journal of Sports Medicine. 2011;45:1095–1100. doi: 10.1136/bjsm.2010.079855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giltay EJ, Vollaard AM, Kromhout D. Self-rated health and physician-rated health as independent predictors of mortality in elderly men. Age and Ageing. 2012;41:165–171. doi: 10.1093/ageing/afr161. [DOI] [PubMed] [Google Scholar]
- Gijsbers van Wijk CMT, Huisman H, Kolk AM. Gender differences in physical symptoms and illness behavior: A health diary study. Social Science and Medicine. 1999;49:1061–1074. doi: 10.1016/s0277-9536(99)00196-3. [DOI] [PubMed] [Google Scholar]
- Hamaker EL. Why researchers should think “within person”: A paradigmatic rationale. In: Mehl MR, Conner TS, editors. Handbook of Research Methods for Studying Daily Life. New York: Guilford; 2012. pp. 43–61. [Google Scholar]
- Han B, Jylha M. Improvement in depressive symptoms and changes in self-rated health among community-dwelling disabled older adults. Aging and Mental Health. 2006;10:599–605. doi: 10.1080/13607860600641077. [DOI] [PubMed] [Google Scholar]
- Hankin BL, Fraley RC, Abela JRZ. Daily depression and cognitions about stress: Evidence for a traitlike depressogenic cognitive style and the prediction of depressive symptoms in a prospective daily diary study. Journal of Personality and Social Psychology. 2004;88:673–685. doi: 10.1037/0022-3514.88.4.673. [DOI] [PubMed] [Google Scholar]
- Haring R, Feng YS, Moock J, Kohlmann T. Self-perceived quality of life predicts mortality risk better than a multi-biomarker panel, but the combination of both does best. BMC Medical Research Methodology. 2011;11:103. doi: 10.1186/1471-2288-11-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertzog C, Van Alstine J, Usala PD, Hultsch DF, Dixon R. Measurement properties of the Center for Epidemiological Studies Depression Scale (CES-D) in older populations. Psychological Assessment: A Journal of Consulting and Clinical Psychology. 1990;2:64–72. [Google Scholar]
- Idler EL, Benyamini Y. Self-rated health and mortality: A review of twenty-seven community studies. Journal of Health and Social Behavior. 1997;38:21–37. [PubMed] [Google Scholar]
- Idler EL, Kasl S. Health perceptions and survival: Do global evaluations of health status really predict mortality? Journal of Gerontology: Social Sciences. 1991;46:S55–S65. doi: 10.1093/geronj/46.2.s55. [DOI] [PubMed] [Google Scholar]
- Kaplan G, Barell V, Lusky A. Subjective state of health and survival in elderly adults. Journal of Gerontology: Social Sciences. 1988;43:S114–S120. doi: 10.1093/geronj/43.4.s114. [DOI] [PubMed] [Google Scholar]
- Kreft IGG, de Leeuw J, Aiken LS. The effect of different forms of centering in hierarchical linear models. Multivariate Behavioral Research. 1995;30:1–21. doi: 10.1207/s15327906mbr3001_1. [DOI] [PubMed] [Google Scholar]
- Lyyra TM, Heikkinen E, Lyyra AL, Jylha M. Self-rated health and mortality: Could clinical and performance-based measures of health and functioning explain the association? Archives of Gerontology and Geriatrics. 2006;42:277–288. doi: 10.1016/j.archger.2005.08.001. [DOI] [PubMed] [Google Scholar]
- McCullough ME, Laurenceau JP. Gender and the natural history of self-rated health: A 59-year longitudinal study. Health Psychology. 2004;23:651–655. doi: 10.1037/0278-6133.23.6.651. [DOI] [PubMed] [Google Scholar]
- Miller TR, Wolinsky FD. Self-rated health trajectories and mortality among older adults. Journal of Gerontology: Social Sciences. 2007;62B:S22–S27. doi: 10.1093/geronb/62.1.s22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera S, Studenski S, Chandler JM, Guralnik JM. Magnitude and patterns of decline in health and function in 1 year affect subsequent 5-year survival. Journal of Gerontology: Medical Science. 2005;60A:894–900. doi: 10.1093/gerona/60.7.894. [DOI] [PubMed] [Google Scholar]
- Pettit JW, Kline JP, Gencoz T, Gencoz F, Joiner TE., Jr Are happy people healthier? The specific role of positive affect in predicting self-reported health symptoms. Journal of Research in Personality. 2001;35:521–536. [Google Scholar]
- Rodin J, McAvay G. Determinants of change in perceived health in a longitudinal study of older adults. Journal of Gerontology: Psychological Sciences. 1992;47:P373–P384. doi: 10.1093/geronj/47.6.p373. [DOI] [PubMed] [Google Scholar]
- Ryu E, West SG, Sousa KH. Distinguishing between-person and within-person relationships in longitudinal health research: Arthritis and quality of life. Annals of Behavioral Medicine. 2012;43:330–342. doi: 10.1007/s12160-011-9341-6. [DOI] [PubMed] [Google Scholar]
- Sargent-Cox KA, Anstey KJ, Luszcz MA. Patterns of longitudinal change in older adults’ self-rated health: The effect of the point of reference. Health Psychology. 2010;29:143–152. doi: 10.1037/a0017652. [DOI] [PubMed] [Google Scholar]
- Schöllgen I, Huxhold O, Schmiedek F. Emotions and physical health in the second half of life: Interindividual differences in age-related trajectories and dynamic associations according to socioeconomic status. Psychology and Aging. 2011;27:338–352. doi: 10.1037/a0026115. [DOI] [PubMed] [Google Scholar]
- Singer JD. Fitting individual growth models using SAS PROC MIXED. In: Moskowitz DS, Hershberger SL, editors. Modeling intraindividual variability with repeated measures data: Methods and applications. Mahwah, NJ: Lawrence Erlbaum Associates; 2002. pp. 135–170. [Google Scholar]
- Singer JD, Willett JB. Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence. New York: Oxford University Press; 2003. [Google Scholar]
- Singh-Manoux A, Gueguen A, Martikainen P, Ferrie J, Marmot M, Shipley M. Self-rated health and mortality: Short-and long-term associations in the Whitehall II study. Psychosomatic Medicine. 2007;69:138–143. doi: 10.1097/PSY.0b013e318030483a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snijders T, Bosker R. Multilevel modeling. Thousand Oaks, CA: Sage; 1999. [Google Scholar]
- Stewart AL, Ware JE. Measuring functioning and well-being: The Medical Outcomes Study approach. Durham, NC: Duke University Press; 1992. [Google Scholar]
- Strawbridge WJ, Wallhagen MI. Self-rated health and mortality over three decades: Results from a time-dependent covariate analysis. Research on Aging. 1999;21:402–416. [Google Scholar]
- Suls J, Howren MB. Understanding the physical-symptom experience: The distinctive contributions of anxiety and depression. Current Directions in Psychological Science. 2012;21:129–134. [Google Scholar]
- Watson D. Intraindividual and interindividual analyses of positive and negative affect: Their relation to health complaints, perceived stress, and daily activities. Journal of Personality and Social Psychology. 1988;54:1020–1030. doi: 10.1037//0022-3514.54.6.1020. [DOI] [PubMed] [Google Scholar]
- Watson D, Pennebaker JW. Health complaints, stress, and distress: Exploring the central role of negative affectivity. Psychological Review. 1989;96:234–254. doi: 10.1037/0033-295x.96.2.234. [DOI] [PubMed] [Google Scholar]
- Williams JMG, Mathews A, MacLeod C. The emotional Stroop task and psychopathology. Psychological Bulletin. 1996;120:3–24. doi: 10.1037/0033-2909.120.1.3. [DOI] [PubMed] [Google Scholar]
- Winter L, Lawton MP, Langston CA, Ruckdeschel K, Sando R. Symptoms, affect, and self-rated health: Evidence for a subjective trajectory of health. Journal of Aging and Health. 2007;19:453–469. doi: 10.1177/0898264307300167. [DOI] [PubMed] [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO. Development and validation of a geriatric depression screening scale: A preliminary report. Journal of Psychiatric Research. 1983;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]