Abstract
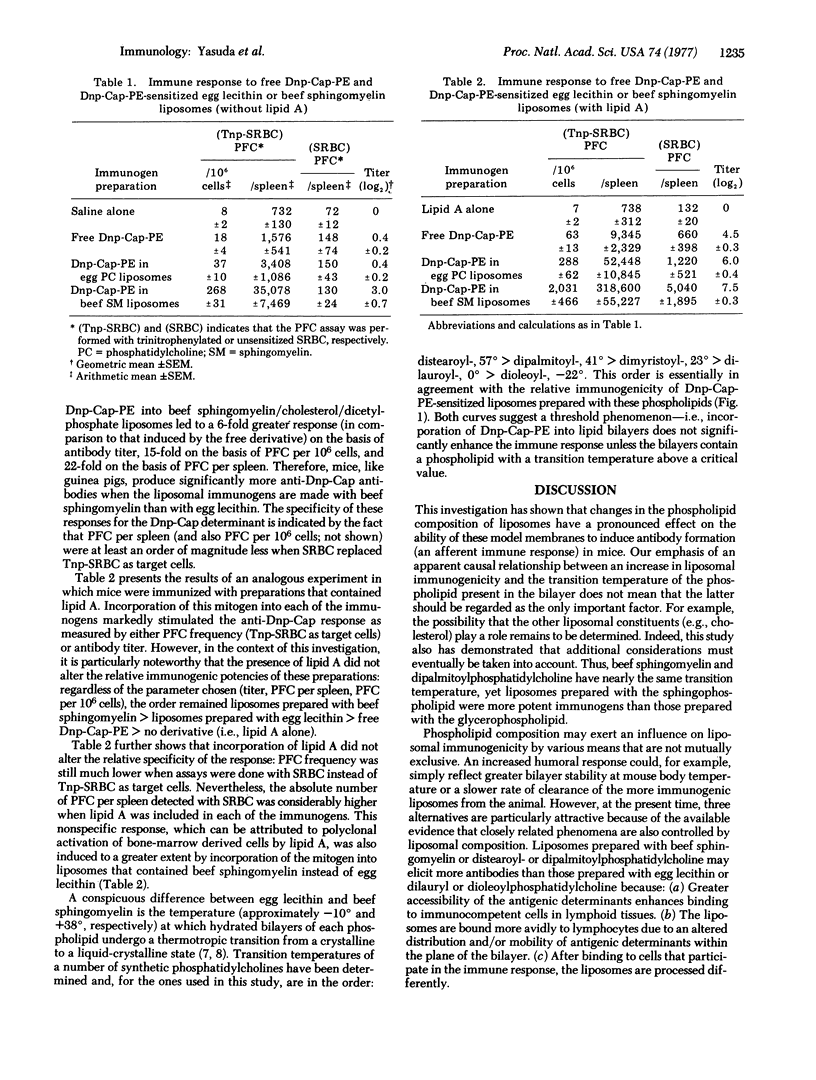
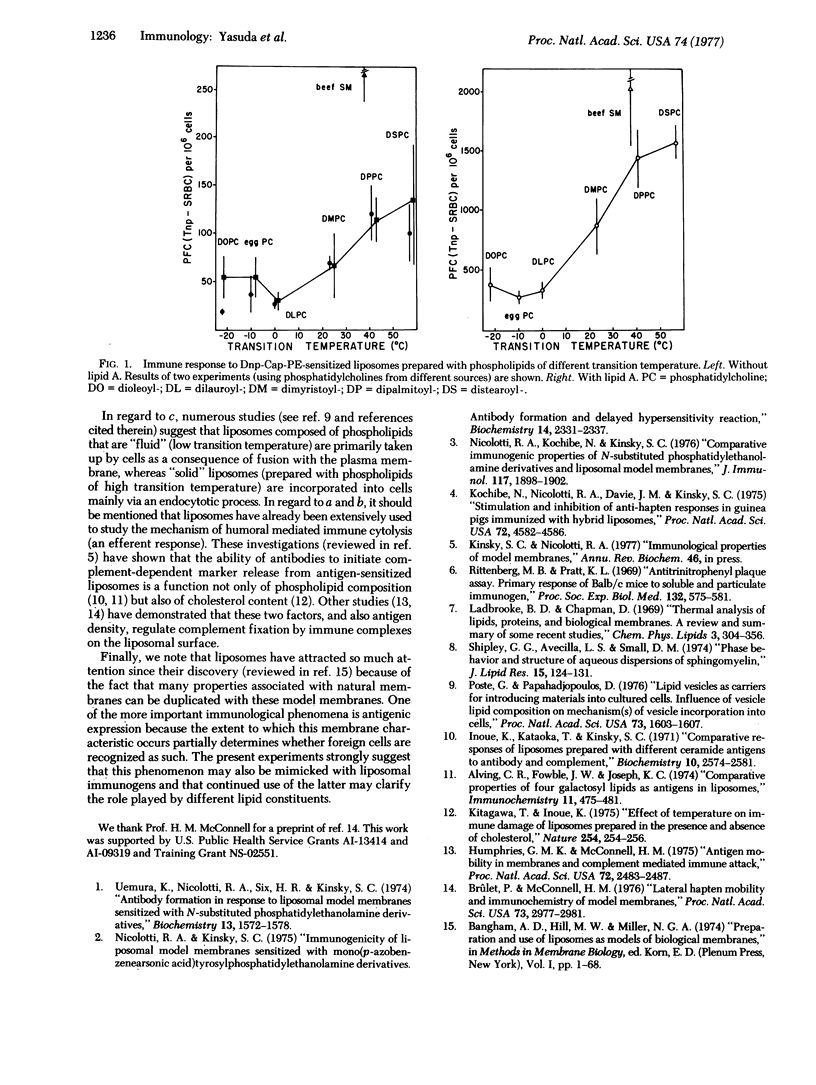
This investigation demonstrated that antigenic expression in liposomal model membranes may be markedly influenced by phospholipid composition. Incorporation of dinitrophenylaminocaproylphosphatidylethanolamine (Dnp-Cap-PE) into egg lecithin/cholesterol/dicetylphosphate bilayers did not significantly enhance the response of AKR mice to this synthetic amphipathic antigen. In contrast, the immunogenicity of Dnp-Cap-PE was increased (as measured by either plaque-forming cell frequency of hemagglutination titer) after its insertion into liposomes prepared with beef sphingomyelin instead of egg lecithin. We also show that the response to the Dnp-Cap determinant can be stimulated by the presence of lipid A in the same bilayers without altering the relative immunogenic potency of the sphingomyelin and lecithin liposomes; similarly, incorporation of this mitogen into sphingomyelin liposomes produced a greater polyclonal (nonspecific) response. The response to Dnp-Cap-PE-sensitized liposomes (with or without lipid A) prepared with a series of synthetic phosphatidylcholines (distearoyl-, dipalmitoyl-, dimyristoyl-, dilauroyl-, dioleoyl-) suggests a direct correlation between liposomal immunogenicity and transition temperature of the phospholipid.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alving C. R., Fowble J. W., Joseph K. C. Comparative properties of four galactosyl lipids as antigens in liposomes. Immunochemistry. 1974 Aug;11(8):475–481. doi: 10.1016/0019-2791(74)90118-9. [DOI] [PubMed] [Google Scholar]
- Brûlet P., McConnell H. M. Lateral hapten mobility and immunochemistry of model membranes. Proc Natl Acad Sci U S A. 1976 Sep;73(9):2977–2981. doi: 10.1073/pnas.73.9.2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphires G. M., McConnell H. M. Antigen mobility in membranes and complement-medical immune attack. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2483–2487. doi: 10.1073/pnas.72.7.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K., Kataoka T., Kinsky S. C. Comparative responses of liposomes prepared with different ceramide antigens to antibody and complement. Biochemistry. 1971 Jun 22;10(13):2574–2581. doi: 10.1021/bi00789a025. [DOI] [PubMed] [Google Scholar]
- Kitagawa T., Inoue K. Effect of temperature on immune damage of liposomes prepared in the presence and absence of cholesterol. Nature. 1975 Mar 20;254(5497):254–256. doi: 10.1038/254254a0. [DOI] [PubMed] [Google Scholar]
- Kochibe N., Nicolotti R. A., Davie J. M., Kinsky S. C. Stimulation and inhibition of anti-hapten responses in guinea pigs immunized with hybrid liposomes. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4582–4586. doi: 10.1073/pnas.72.11.4582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladbrooke B. D., Chapman D. Thermal analysis of lipids, proteins and biological membranes. A review and summary of some recent studies. Chem Phys Lipids. 1969 Dec;3(4):304–356. doi: 10.1016/0009-3084(69)90040-1. [DOI] [PubMed] [Google Scholar]
- Nicolotti R. A., Kinsky S. C. Immunogenicity of liposomal model membranes sensitized with mono(p-azobenzenearsonic acid)tyrosylphosphatidylethanolamine derivatives. Antibody formation and delayed hypersensitivity reaction. Biochemistry. 1975 Jun 3;14(11):2331–2337. doi: 10.1021/bi00682a009. [DOI] [PubMed] [Google Scholar]
- Nicolotti R. A., Kochibe N., Kinsky S. C. Comparative immunogenic properties of N-substituted phosphatidylethanolamine derivatives and liposomal model membranes. J Immunol. 1976 Nov;117(5 PT2):1898–1902. [PubMed] [Google Scholar]
- Poste G., Papahadjopoulos D. Lipid vesicles as carriers for introducing materials into cultured cells: influence of vesicle lipid composition on mechanism(s) of vesicle incorporation into cells. Proc Natl Acad Sci U S A. 1976 May;73(5):1603–1607. doi: 10.1073/pnas.73.5.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittenberg M. B., Pratt K. L. Antitrinitrophenyl (TNP) plaque assay. Primary response of Balb/c mice to soluble and particulate immunogen. Proc Soc Exp Biol Med. 1969 Nov;132(2):575–581. doi: 10.3181/00379727-132-34264. [DOI] [PubMed] [Google Scholar]
- Shipley G. G., Avecilla L. S., Small D. M. Phase behavior and structure of aqueous dispersions of sphingomyelin. J Lipid Res. 1974 Mar;15(2):124–131. [PubMed] [Google Scholar]
- Uemura K., Nicolotti R. A., Six H. R., Kinsky S. C. Antibody formation in response to liposomal model membranes sensitized with N-substituted phosphatidylethanolamine derivatives. Biochemistry. 1974 Apr 9;13(8):1572–1578. doi: 10.1021/bi00705a003. [DOI] [PubMed] [Google Scholar]



