Abstract
Background
The tumor microenvironment (TME) plays an essential role in supporting and promoting tumor growth and progression. An inflammatory stroma is a widespread hallmark of the prostate TME, and prostate tumors are known to co-evolve with their reactive stroma. Cancer-associated fibroblasts (CAFs) within the reactive stroma play a salient role in secreting cytokines that contribute to this inflammatory TME. Although a number of inflammatory mediators have been identified, a clear understanding of key factors initiating the formation of reactive stroma is lacking.
Methods
We explored whether tumor secreted extracellular Hsp90 alpha (eHsp90α) may initiate a reactive stroma. Prostate stromal fibroblasts (PrSFs) were exposed to exogenous Hsp90α protein, or to conditioned medium (CM) from eHsp90α-expressing prostate cancer cells, and evaluated for signaling, motility, and expression of prototypic reactive markers. In tandem, ELISA assays were utilized to characterize Hsp90α-mediated secreted factors.
Results
We report that exposure of PrSFs to eHsp90 upregulates the transcription and protein secretion of IL-6 and IL-8, key inflammatory cytokines known to play a causative role in prostate cancer progression. Cytokine secretion was regulated in part via a MEK/ERK and NF-κB dependent pathway. Secreted eHsp90α also promoted the rapid and durable activation of the oncogenic inflammatory mediator signal transducer and activator of transcription (STAT3). Finally, eHsp90 induced the expression of MMP-3, a well-known mediator of fibrosis and the myofibroblast phenotype.
Conclusions
Our results provide compelling support for eHsp90α as a transducer of signaling events culminating in an inflammatory and reactive stroma, thereby conferring properties associated with prostate cancer progression.
Keywords: CAF, STAT3, MMP, ERK, prostate cancer
INTRODUCTION
The host microenvironment is profoundly altered during tumor growth, acquiring a reactive and inflammatory phenotype inextricably linked to its tumor supportive activity. This is observed in prostate cancer (PCa) wherein tumors co-evolve with their reactive or inflammatory tumor stroma (1). Lesions indicative of reactive stroma appear early in PCa etiology, originating during prostatic intraepithelial neoplasia (PIN) (2), a pre-neoplastic condition affecting prostatic ducts. The reactive stroma initiated during PIN represents a critical step in PCa adenocarcinoma progression, a premise supported by its co-existence with advanced prostate cancer tissue (2), and as a predictor of reduced recurrence free survival (3,4). Although the key tumor supportive mechanisms of reactive stroma are not well defined, reactive cancer associated fibroblasts (CAFs) play a central role in this process (5–7). The conversion of stromal fibroblasts to a reactive myofibroblast or CAF-like phenotype is triggered either by direct contact with cancer cells or upon exposure to tumor secreted factors (8,9). As CAFs are prominent modifiers of tumor progression, it is essential to define factors driving this phenotype.
The similarity of reactive stroma in both wound repair and cancer (10,11) is in large part due to the secretion of angiogenic and inflammatory cytokines from reactive stromal fibroblasts (6–8). Interleukins, potent mediators of inflammatory processes, are commonly found in the tissue microenvironment of both wound repair and cancer. In particular, IL-6 and IL-8 are multifunctional chemokines associated with prostatic reactive stroma (9,12). The expression of IL-6 and IL-8 is regulated in part by NF-kappB (NF-κB) (13,14), a transcriptional master regulator of inflammatory processes, many of which play widespread and causative roles in cancer progression (15,16). Importantly, NF-κB has also been identified as a prominent factor involved in conferring the reactive phenotype of CAFs (8). Similar to NF-κB, STAT3 is a transcriptional mediator of immune responses, inflammation and tumorigenesis (17). The NF-κB and STAT3 pathways collaborate in cancer progression and engage in extensive crosstalk, due in part to the production of IL-6, which serves as a major cytokine for STAT3 activation (18).
Emerging reports indicate that many tumor types secrete extracellular Hsp90α (eHsp90α), a normally intracellular molecular chaperone (19). We have shown that PCa cells with increased aggressiveness secrete more eHsp90α relative to their less aggressive counterparts (20), a trend mirrored in breast cancer cells (21). The presence of secreted eHsp90α in patients with tumor burden has been widely reported in diverse cancer types (21), including PCa (22), implicating a potential clinical role for eHsp90α secretion. Functionally, eHsp90α plays a role in sustaining cancer cell motility, invasion, and metastatic spread (21,23–26). We have previously demonstrated that eHsp90α signals in an autocrine pathway to initiate epithelial to mesenchymal transition (EMT) events in prostate epithelial cells (20). For this study, we explored whether eHsp90α may utilize paracrine signaling mechanisms to modulate the tumor stroma. Several EMT-inducing growth factors, such as TGFβ, exhibit autocrine and paracrine signaling modalities, and are potent modifiers of the tumor stroma. Although no prior reports demonstrate the ability of eHsp90α to promote a tumor reactive stroma, eHsp90α has been shown to stimulate the motility and subsequent wound healing of dermal fibroblasts (27,28) and initiate signaling in endothelial cells (29). We have also shown that eHsp90α induces signaling in vascular endothelial cell types (30,31). Collectively, these findings indicate that eHsp90 has the potential to modify the properties of stromal cell types.
We now demonstrate that eHsp90α initiates signaling events in prostate stromal cells (PrSCs), conferring properties of increased cell motility and elevated expression of molecular markers indicative of a reactive phenotype. Importantly, eHsp90α increased the expression and secretion of the inflammatory mediators IL-6 and IL-8 in a pathway partially dependent upon NF-κB. Evidence of NF-κB activation was further validated by detection of eHsp90α-dependent phosphorylation of the NF-κB effector RelA/p65. An eHsp90α-initiated inflammatory stromal phenotype was reinforced by the presence of STAT3 phosphorylation. Our findings support the notion that eHsp90α transduces a cascade of signaling events to promote the conversion of normal PrSCs to reactive and inflammatory stromal cells with the characteristics of CAFs. Moreover, our findings indicate that tumor eHsp90α utilizes paracrine signaling mechanisms to alter the TME, thereby enabling a niche favorable for subsequent cancer progression.
MATERIALS AND METHODS
Reagents and antibodies
Hsp90α human recombinant protein was purchased from Enzo Life Sciences (ADI-SPP-776). The F-5 Hsp90α and control peptide was provided by Wei Li (University of Southern California, Los Angeles, CA), and has been described elsewhere (32). The MEK/ERK inhibitor UO216 was purchased from Promega (V112A). MMP inhibitors GM6001 and the 2/9 inhibitor were obtained from EMD Millipore Chemicals (CC1010 and 444274). DMAG-N-oxide modified geldanamycin, (herein referred to as non-permeable GA, NPGA) was synthesized by Chris Lindsey and Craig Beeson (Pharmaceutical Sciences, Medical University of South Carolina, Charleston, SC). The NF-κB inhibitor Bay11-7082 (B5556), and antibodies to Tenascin-C (HPA004823) and GAPDH (G9295) were from Sigma-Aldrich. Cell Signaling antibodies included P-AKT (4058), AKT (4685), P-ERK (4370), ERK (4695), P-p65 (3033), p65 (4764), P-STAT3 (9138), and STAT3 (4904). Antibodies to Vimentin (ab8978) and SMA (ab5694) were purchased from Abcam. FAP antibody (H00002191-M01) was purchased from Abnova. The cytokine array (ARY005 R&D) was from R&D Systems and the MMP array (ab134004) was from Abcam.
Cell culture
Human primary prostate stromal cells (PrSC; Clonetics) were grown in stromal cell growth medium (SCGM; Clonetics) and were used between passages 5 to 10. Immortalized normal human prostatic fibroblasts (NPFs) were obtained from Dr. Simon Hayward (33), (Vanderbilt-Ingram Cancer Center); 6015N and 5905N PrSFs were provided by Dr. David Rowley (Baylor College of Medicine); immortalized PSC27 PrSFs were provided by Dr. Peter Nelson (34), (Fred Hutchinson Cancer Research Center). ARCaPE-LacZ and ARCapE-eHsp90 cells were generated as previously described (20). All cells were maintained at 37°C with 5% CO2.
Western blots
Nuclear preparations were prepared as previously described (35). For whole cell lysates, cells were washed with phosphate-buffered saline, and lysates prepared in lysis buffer as previously described (20,36). All blots are representative of a minimum of two independent experiments.
Immunofluorescent microscopy
Cells were treated as indicated, fixed with 4% paraformaldehyde and permeabilized with 0.1% Triton X-100 in PBS. Immunofluorescent microscopy was performed as described (36).
qRT-PCR analysis
PrSFs were pretreated (6 hr) with the indicated inhibitors, followed by treatment with Hsp90α protein, F-5 peptide, or conditioned medium (CM). Total RNA was extracted, converted to cDNA and amplified as previously described (20). Primers were purchased from IDT and sequences are as follows: MMP3 F-AGGTGTGGAGTTCCTGATGTTGGT R-TACAGCCTGGAGAATGTGAGTGGA; IL-6 F-AAGCCAGAGCTGTGCAGATGAGTA R-GCTGCGCAGAATGAGATGAGTTGT; IL-8 F-CCTTGTTCCACTGTGCCTTGGTTT R-GTGCTTCCACATGTCCTCACAACA; GAPDHF-TCGACAGTCAGCCGCATCTTCTTT R-ACCAAATCCGTTGACTCCGACCTT
Immunoassays
Equal numbers of PrSFs or PrSCs were starved overnight in low serum (0.1% FBS), and treated as indicated, also under in low serum conditions. Prior to treatment with Hsp90α protein, F-5 peptide, or conditioned medium, cells were rinsed with PBS and incubated with freshly prepared treatments of similar medium for 16 hr. Conditioned medium was collected following brief centrifugation, and IL-6, IL-8, and MMP-3 concentrations were measured by ELISA (R&D Systems) according to the manufacturer’s instructions.
Cell Migration Analysis
Cell migration analyses were performed as previously described (20,36).
Statistical analysis
Statistical significance was determined using one-way ANOVA followed by one-tailed student’s T-tests. All cell motility and quantitative real time-PCR experiments were performed in triplicate. Data shown are presented as mean % S.D.; differences in treatment groups are defined as statistically significant at p< 0.05 value, as calculated from Student’s t test.
RESULTS
eHsp90α promotes ERK-dependent prostate stromal fibroblast cell motility
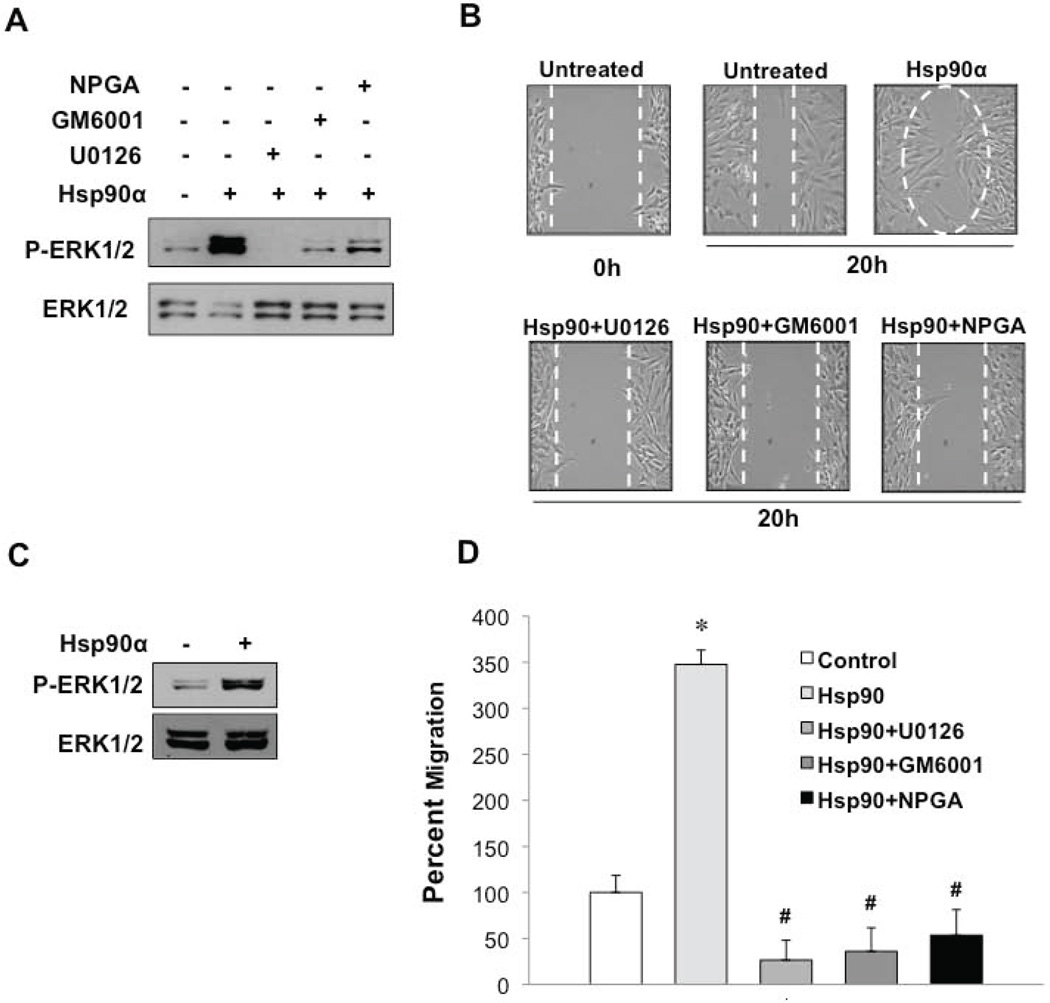
To investigate a possible paracrine function for eHsp90α within the context of PCa, we utilized human primary PrSCs and PrSFs as representative models of stromal cell types found in the TME. We have shown that eHsp90α stimulates ERK activation in prostate epithelial cells, an event required for cell motility (20). Addition of Hsp90α protein to PrSCs elicited the robust and rapid activation of ERK, which was blocked by the ERK inhibitor UO126 (Fig. 1A). Treatment of cells with either the pan-MMP inhibitor GM6001 or a non-permeable geldanamycin (GA) derivative (NPGA) that specifically blocks eHsp90 function (20,25,36), similarly attenuated eHsp90-mediated ERK activity. We then investigated whether eHsp90 affected PrSC cell motility. As shown, eHsp90 enhanced PrSC motility by over 50% (Fig. 1B, Supp Fig. 1A). PrSC motility was comparably blocked by NPGA, UO126, and GM6001. Given that both NPGA and GM6001 blocked ERK phosphorylation, our results indicate that ERK activity is required for the pro-motility function of eHsp90α. eHsp90α-mediated ERK activation was also observed in normal prostatic fibroblasts (NPFs) (Fig. 1C), consistent with its ability to stimulate cell motility (Fig. 1D, Supp Fig. 1B). Moreover, this pro-motility function of eHsp90α was similarly inhibited by UO126, GM6001 and NPGA, thereby supporting a shared mechanism of action. We also observed eHsp90-mediated ERK activation in the immortalized stromal fibroblast cell line, PSC27 (34) (Supp Fig. 1C).
Figure 1. eHsp90 promotes ERK-dependent prostate stromal fibroblast cell motility.
A) Prostate stromal cells (PrSCs, Clonetics) were serum starved (0.1% FBS) overnight, treated with the indicated inhibitors for 6 hr, and exposed to Hsp90α protein (3 ug/ml) for 10 min. Resultant cell lysates were analyzed for phosphorylated and total ERK1/2 by SDS-PAGE and Western blot. B) Scratch wound assays of serum starved PrSCs. PrSCs were pre-treated for 4 hours in the absence or presence of NPGA (1 µM), U0261 (10 µM), or GM6001 (1 µM), followed by stimulation with Hsp90α protein. Cell migration into the wound area was determined at 20 hours. Cell motility was quantified using ImageJ and significance (*) determined by ANOVA and Student’s t-test (p < 0.05). C) Immortalized NPFs were treated as in A and lysates analyzed for activated and total ERK1/2. D) NPFs were treated as in B and eHsp90α–mediated cell motility similarly determined. (#) denotes p < 0.05 with respect to inhibitory effects of treatments relative to Hsp90 treated cells.
eHsp90α initiates molecular changes associated with a reactive stromal phenotype
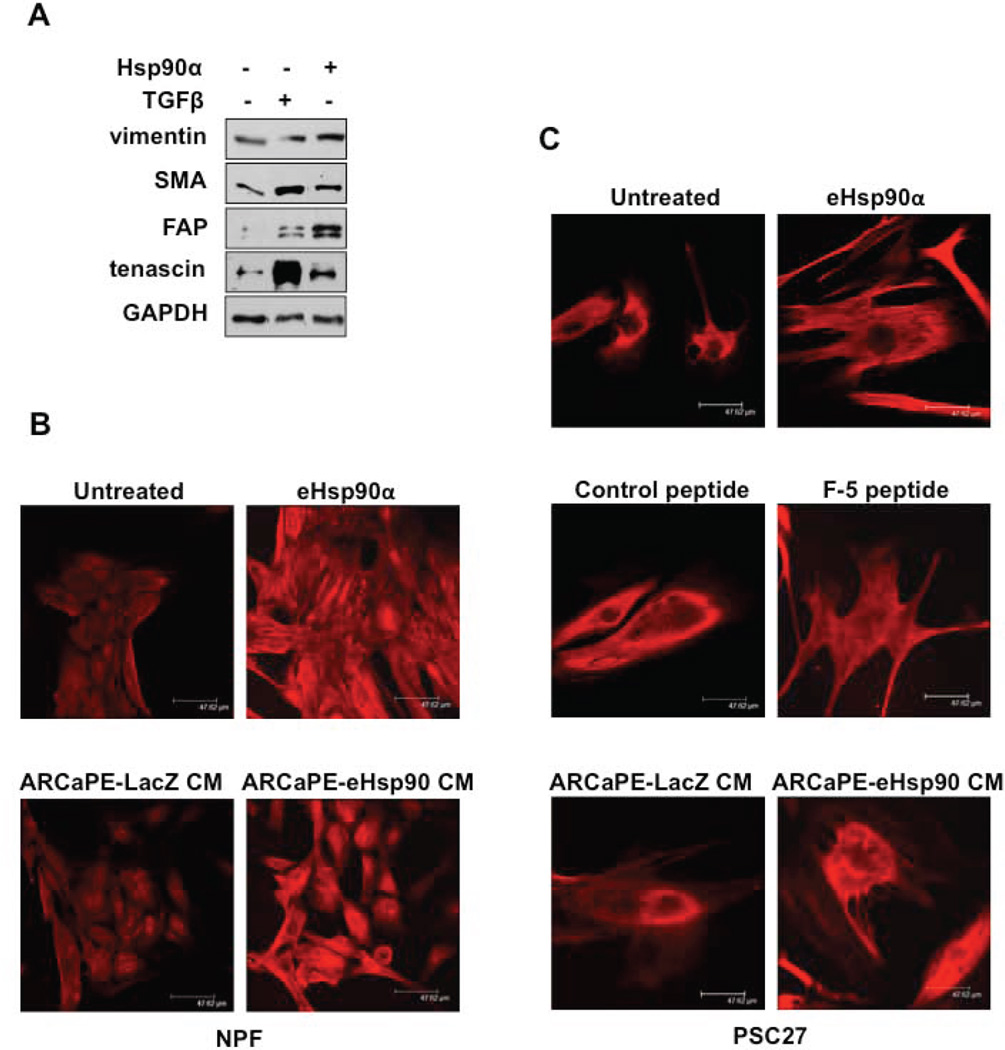
We next asked whether exposure of PrSFs to eHsp90α conferred expression of molecular markers associated with a reactive phenotype. Given that TGF-β can convert fibroblasts into reactive myofibroblasts or CAFs (37,38), we used TGF-β as a positive control. As expected, TGF-β induced smooth muscle actin (SMA) and tenascin C, whereas eHsp90α modestly upregulated vimentin, SMA, fibroblast activation factor (FAP) and tenascin C (Fig. 2A). Therefore, eHsp90α stimulated the expression of markers associated with a CAF-like phenotype, although the profile exhibited some differences from TGF-β -treated cells. We next utilized fluorescent microscopy to evaluate the cellular distribution of SMA. As shown, addition of eHsp90α to NPFs induced SMA (Fig. 2B), which also appeared to be polymerized and recruited to stress fibers, consistent with changes associated with reactive stroma (39). To evaluate the effects of eHsp90α within a more physiological context, we exposed stromal cells to the conditioned medium (CM) of cells expressing eHsp90α, using ARCaPE tumor cells with low basal secretion (20) that were transduced with lentivirus expressing either LacZ (control) virus or eHsp90α virus as described (20). The CM from a clonal population of ARCAPE-eHsp90α that exhibited a 6-fold increase in eHsp90α secretion over LacZ cells (Supp Fig. 2) similarly upregulated SMA expression. A similar analysis was performed for PSC27 cells. In keeping with these trends, eHsp90α protein and CM from ARCaPE-eHsp90α induced polymerization of SMA (Fig. 2C). In addition, we evaluated the activity of an eHsp90α peptide (F-5 peptide) representing an N-terminally truncated Hsp90α protein deficient in the ATPase domain (32) critical for Hsp90 chaperone activity (19). The F-5 peptide elicited similar effects, indicating that eHsp90α-dependent chaperone function was not required for these effects. A control peptide 27-mer) derived from the middle domain of Hsp90α (32) had no effect. Notably, Hsp90α protein, F-5 peptide, and Hsp90α derived from ARCaPE CM dramatically altered PSC cell morphology and promoted the formation of multiple protrusions.
Figure 2. eHsp90α initiates molecular changes associated with a reactive stromal phenotype.
A) Serum starved (0.1%) NPFs were treated with TGFβ (5 ng/mL) or Hsp90α (3 µg/mL) for 16 hr. Whole cell lysates were collected and analyzed for vimentin, SMA, FAP, and tenascin C. B) NPF cells were serum starved overnight as in A and subsequently incubated (3 days) with either Hsp90α protein, or with conditioned media (CM) from transduced ARCaPE-LacZ or ARCaPE-eHsp90 cells. C) PSC27 cells were treated as in B. Comparative effects of Hsp90α F-5 peptide are shown. The control peptide is derived from the middle domain of Hsp90α.
eHsp90α initiates a pro-inflammatory phenotype in stromal prostate fibroblasts
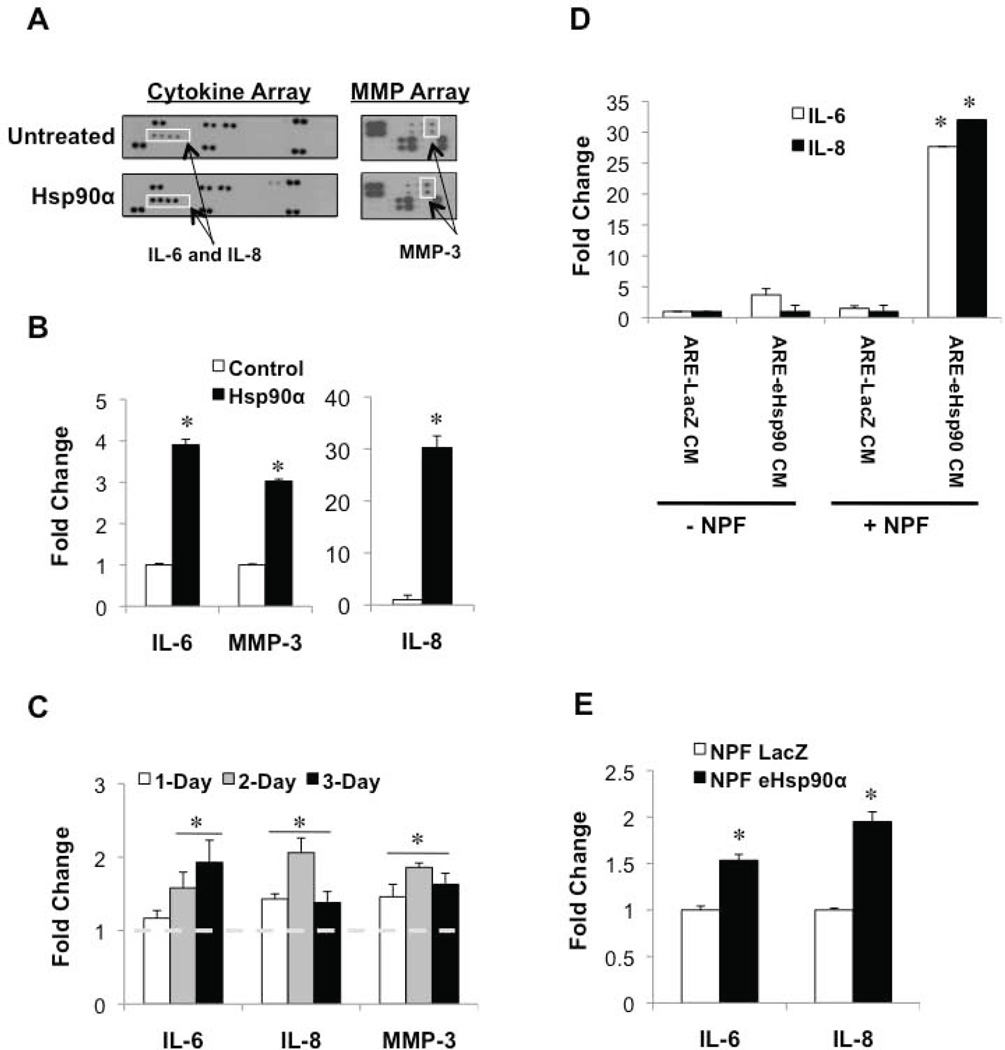
We next investigated whether eHsp90 confers an inflammatory phenotype resembling the PCa TME. Towards this end, NPFs were treated with Hsp90α protein, and resultant CM was analyzed via a cytokine and MMP array. As shown, Hsp90α induced the secretion of inflammatory cytokines IL-6 and IL-8, as well as the proteolytic enzyme MMP-3 (Fig. 3A). These findings were subsequently validated by ELISA analysis (Fig. 3B). Similar trends of eHsp90α action were observed in PSC27 cells (Fig. 3C). The eHsp90α-mediated effects were generally sustained over a 3-day treatment period, indicating the ability of eHsp90α to initiate a durable response.
Figure 3. eHsp90α initiates a pro-inflammatory phenotype in stromal prostate fibroblasts.
A) Serum starved (0.1%) NPFs were treated with Hsp90α protein overnight. CM was collected and analyzed via a cytokine and MMP-based array. B) ELISA confirmation of the Hsp90α-mediated secretion of IL-6, IL-8, and MMP3 in NPFs. C) Similar analysis of Hsp90α-mediated secretion of IL-6, IL-8, and MMP3 in PSC27 following the indicated treatment times. D) Analysis of IL-6 and IL-8 secretion in ARCaPE-LacZ and ARCaPE-eHsp90 in CM, and effects of addition of this CM (1:1) to NPFs (16 hr). Secreted protein values were normalized and are presented as fold change over untreated control. Hsp90α protein significantly induced IL-6, IL-8, and MMP3 secretion (*) as determined by ANOVA and Student’s t-test (p < 0.05). E) RNA was isolated from cells in D and the indicated targets analyzed via qPCR in triplicate.
We have demonstrated that more aggressive prostate cancer cells exhibit increased secretion of Hsp90α (20), raising the question of whether these cell types may have an increased propensity to impact the behavior of adjacent stromal cells. To assess the ability of tumor-derived eHsp90 to elicit a pro-inflammatory response, stromal cells were exposed to CM derived from either ARCaPE-LacZ or ARCaPE-eHsp90α. As shown, IL-6 and IL-8 secretion was absent or nominal in both ARCaPE-LacZ and ARCaPE-eHsp90α (Fig. 3D, note -NPF data sets), indicating that eHsp90 does not appreciably induce these cytokines in an autocrine manner in this cell line. Remarkably, a dramatic increase in both IL-6 and IL-8 secretion was observed following co-incubation of NPFs with CM from eHsp90α-expressing ARCaPE-eHsp90 (Fig. 3D, compare left and right columns for +NPF data sets). These data demonstrate that eHsp90α initiates paracrine signaling mechanisms to elicit a robust pro-inflammatory phenotype in PrSFs. We next evaluated whether the increased cytokine secretion was controlled at the transcriptional level. As shown, ARCaPE-eHsp90 CM modestly induced IL-6 and IL-8 message levels in NPFs (Fig. 3E). Thus, although transcriptional mechanisms participate in modulating increased cytokine secretion, the magnitude of the increase implicates additional collaborating mechanisms.
eHsp90α promotes the stromal secretion of inflammatory mediators in a chaperone-independent manner
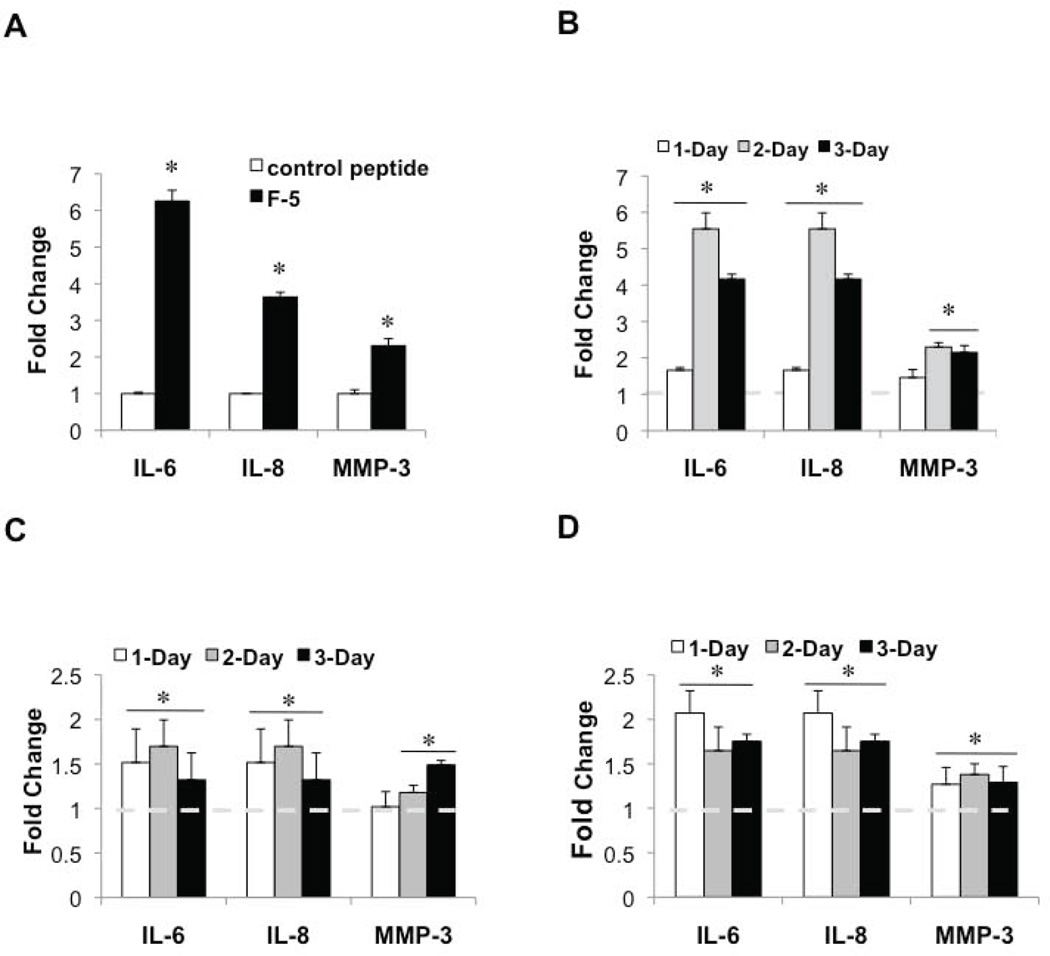
Hsp90α recombinant protein has been shown to promote the motility of dermal fibroblasts (27). Moreover, the truncated Hsp90α peptide (F-5) retains comparable pro-motility activity (28,40), indicating that eHsp90α activity may be driven by chaperone-independent signaling events. Given that the F-5 peptide elicited molecular and morphologic changes consistent with a reactive phenotype (Fig. 2), we next investigated whether it was sufficient to elicit a pro-inflammatory response. As shown, while a control Hsp90α peptide (32) had no effect, exposure of NPFs to F-5 peptide induced secretion of IL-6, IL-8 and MMP-3 (Fig. 4A). This analysis was expanded to additional prostate stromal cell lines, including PSC27 cells (Fig. 4B) and two primary prostate fibroblast lines (Figs. 4C, D). In all instances, F-5 promoted IL-6 and IL-8 secretion, and also induced modest MMP-3 secretion. These results confirm that eHsp90α signaling activity is responsible for the secretion of these inflammatory mediators.
Figure 4. eHsp90α promotes the stromal secretion of inflammatory mediators in a chaperone-independent manner.
A) Serum starved NPFs were treated with either control or Hsp90α F-5 peptide (at a concentration equimolar to 3 µg/mL Hsp90α protein) overnight, and CM analyzed as in Fig. 3. B–D) Immortalized PSC27 (B) or human primary PrSF cells 5905N (C) and 6105N (D) were treated with F-5 peptide as in A, for the indicated times, and CM similarly analyzed. Statistics were performed as in Fig. 3.
Hsp90α regulates stromal IL-6 and IL-8 secretion via an ERK1/2 and MMP-2/9 dependent pathway
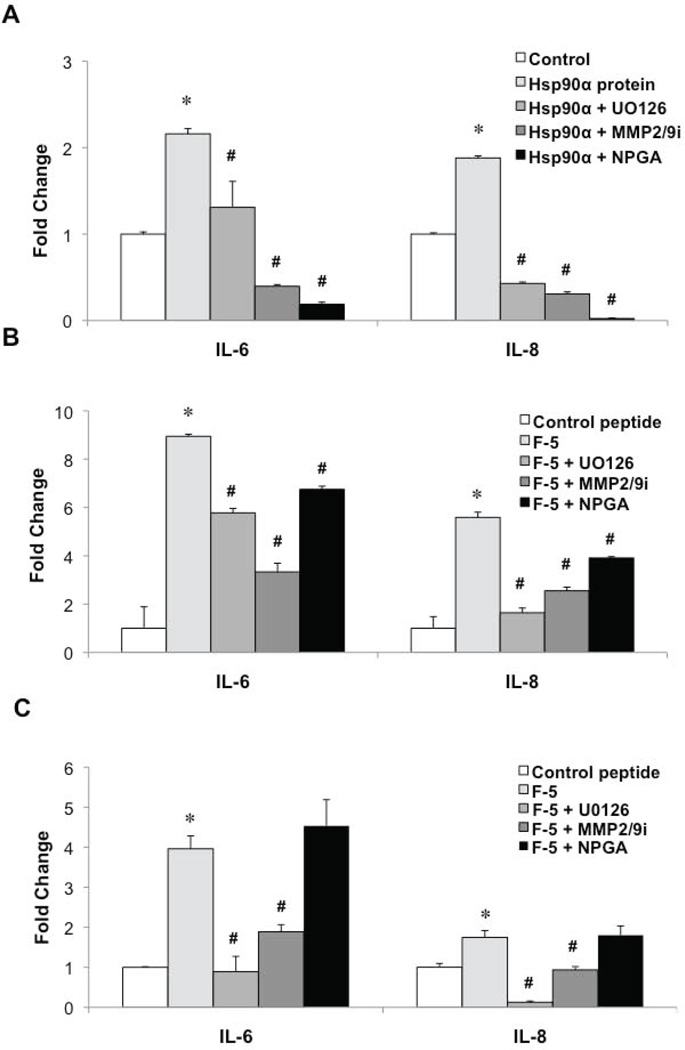
We next focused on potential mechanisms involved in eHsp90α-initiated cytokine secretion. Inhibition of either ERK or MMP-2/9 attenuated the ability of Hsp90α protein to induce cytokine secretion, with blockade of eHsp90α eliciting the most robust suppression (Fig. 5A). Inhibition of either ERK or MMP-2/9 similarly diminished the Hsp90α F-5-mediated increase in cytokine secretion (Fig. 5B). As expected, NPGA was much less effective at blocking the activity of the F5 peptide relative to its ability to suppress the effects of eHsp90α protein, due to the absence of the ATP binding site in the F5 peptide that is targeted by NPGA. We confirmed that MMP-2/9 was required for eHsp90α-mediated ERK activation (Supplemental Fig. 3A). Interestingly, although eHsp90α-ERK had no effect upon MMP-2/9 transcription (not shown), eHsp90α-ERK appears to regulate MMP-3 at the transcriptional level, in an MMP-2/9 dependent pathway (Supplemental Fig. 3B). Finally, we demonstrate that blockade of ERK or MMP-2/9 reduced the transcriptional message levels of IL-6 and IL-8 in response to the F-5 peptide (Fig. 5C). Mirroring trends for the secretion data, NPGA did not appreciably dampen IL-6 and IL-8 mRNA levels induced by F-5.
Figure 5. Hsp90α regulates stromal IL-6 and IL-8 secretion via an ERK1/2 and MMP2/9 dependent pathway.
A, B) Serum starved NPFs were pre-treated (6 hr) with either U0126 (10 µM), MMP-2/9 inhibitor IV (1 µM) or NPGA (1 µM) prior to incubation (16 hr) with either Hsp90α protein (A) or F-5 peptide (B). ELISA analysis was used to evaluate secreted levels of IL-6 and IL-8. C) RNA was harvested from the treated cells and transcript levels corresponding to IL-6 and IL-8 were determined by qRT-PCR. Values were normalized to untreated control and significance (*, #) was determined by ANOVA and Student’s t-test (p < 0.05). (#) denotes significance of inhibitory effects of treatments relative to Hsp90 protein or F-5 peptide treated cells.
NF-κB signaling participates in eHsp90α-mediated cytokine secretion
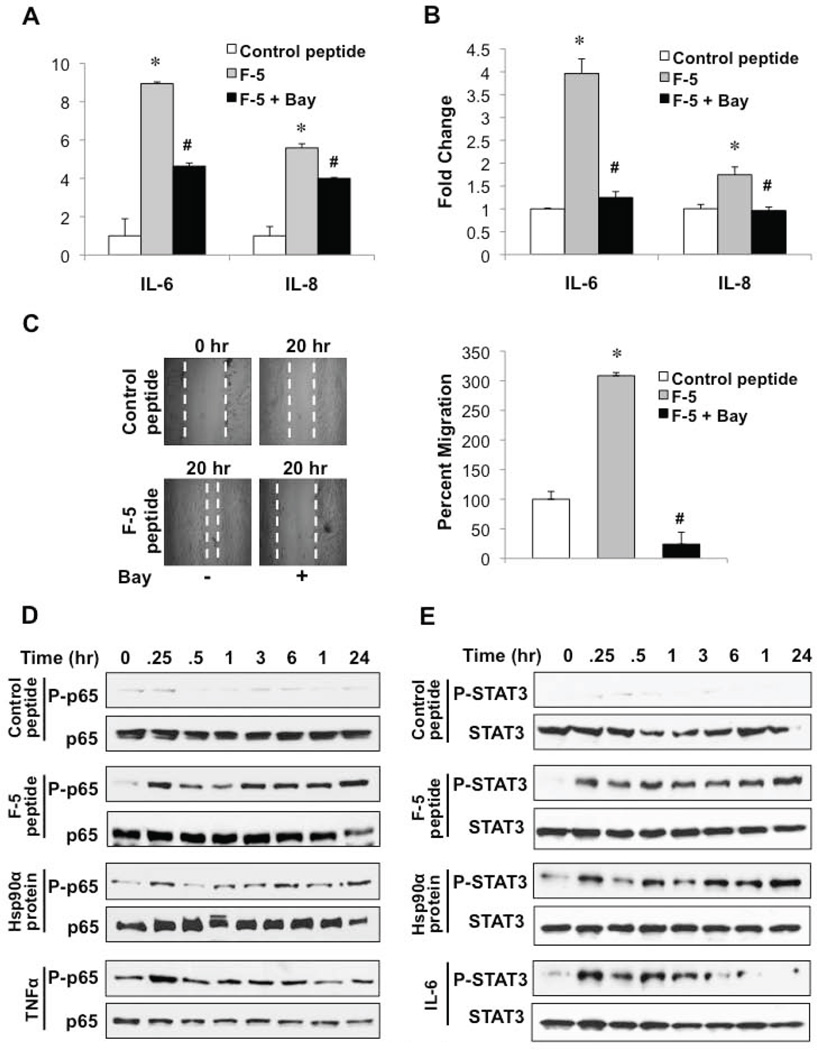
Given that both IL-6 and IL-8 are regulated in part by NF-κB-dependent signaling mechanisms (41), we investigated whether NF-κB served as a molecular effector for Hsp90α-mediated cytokine secretion. In normal unstimulated cells, NF-κB is maintained in an inactive cytoplasmic complex by its association with the IkB kinases. In the canonical activation pathway, inflammatory cytokines such as TNFα promote the phosphorylation and degradation of IkB proteins, resulting in the subsequent phosphorylation and nuclear translocation of p65/RelA. As shown, pharmacologic inhibition of NF-κB activity attenuated the eHsp90α-mediated induction of IL-6 and IL-8 mRNA and protein secretion (Figs. 6A, 6B), highlighting a role for NF-κB in eHsp90α-dependent cytokine expression. We next evaluated whether NF-κB signaling participated in eHsp90α-dependent NPF cell motility. As shown, NF-κB inhibition (Bay11-7082) dramatically impaired the pro-motility function of F-5 peptide (Fig. 6C). These findings support the notion that NF-κB plays a functional role in promoting the reactive inflammatory phenotype of prostate stromal cells.
Figure 6. NF-κB signaling participates in eHsp90α-mediated cytokine secretion.
A) Serum starved NPF cells were pre-treated with the NF-κB inhibitor Bay11-7082 (2 μM), for 6 h prior to stimulation with control or Hsp90α peptide (16 h). ELISA was used to assess levels of IL-6 and IL-8 in CM. B) NPFs were treated as in A and transcript levels determined by qRT-PCR. C) NPFs were treated as in A and subjected to a scratch wounding assay. Representative images and quantified values of migration are indicated. As in Fig. 5, (#) denotes significance of inhibitory effects of treatments relative to F-5 peptide treated cells. D, E) Serum starved NPFs were stimulated with Hsp90α protein or F5 peptide for the indicated times. TNFα (20 ng/mL) and IL-6 (20 ng/mL) were used as positive controls for P-p65 and P-STAT3, respectively. Immunoblot analysis was performed on whole cell lysates for phosphorylated and total p65 (D) and phosphorylated and total STAT3 (E).
To further confirm the connection between eHsp90 and NF-κB activation, we examined the phosphorylation status of the NF-κB subunit p65. Using TNFα as a positive control, we demonstrate the rapid phosphorylation of p65 (Fig. 6D). We then evaluated the ability of eHsp90 protein and F-5 peptide to activate p65. As shown, both treatments similarly elicited the rapid and durable phosphorylation of p65. The control peptide, which was not effective in promoting cytokine secretion, was also unable to induce p65 phosphorylation. Extensive crosstalk exists between IL-6 and STAT3 signaling (42), due in large part to the ability of IL-6 to function as a major effector for STAT3 (18,43). STAT3 activation requires phosphorylation (Tyr705), which mediates its dimerization and subsequent nuclear entry. Using IL-6 treatment as a positive control, we show the expected phosphorylation of STAT3 (Fig. 6E). Surprisingly, both eHsp90α protein and the F-5 peptide rapidly induced STAT3 phosphorylation, and moreover, this activation was sustained over a 24 hr period, indicative of a durable and chronic inflammatory response. Treatment with UO126 did not attenuate F-5-mediated STAT3 phosphorylation (not shown) indicating the involvement of additional pathways.
DISCUSSION
We report the novel finding that eHsp90α activates and initiates crosstalk between proteolytic effectors (MMP-2/9) and signaling effectors (MEK/ERK) to activate NF-κB and consequently induce expression of the inflammatory mediators IL-6 and IL-8. To our knowledge, our findings are the first to demonstrate the role of eHsp90α as an initiator of NF-κB signaling in prostate stromal fibroblasts. Our data also indicate that these inflammatory changes were inextricably linked with the conversion of stromal cells to a reactive phenotype. This was supported by the increased expression of molecular markers consistent with a reactive phenotype, coupled with profound changes in cell morphology, including the recruitment of SMA to stress fibers. Moreover, MMP-2/9, ERK and NF-κB collaborated to regulate eHsp90α-driven stromal cell motility. Thus, our collective findings indicate that an eHsp90-initiated MMP-2/9-ERK cascade is critical for the NF-κB driven stromal inflammation and fibroblast activation.
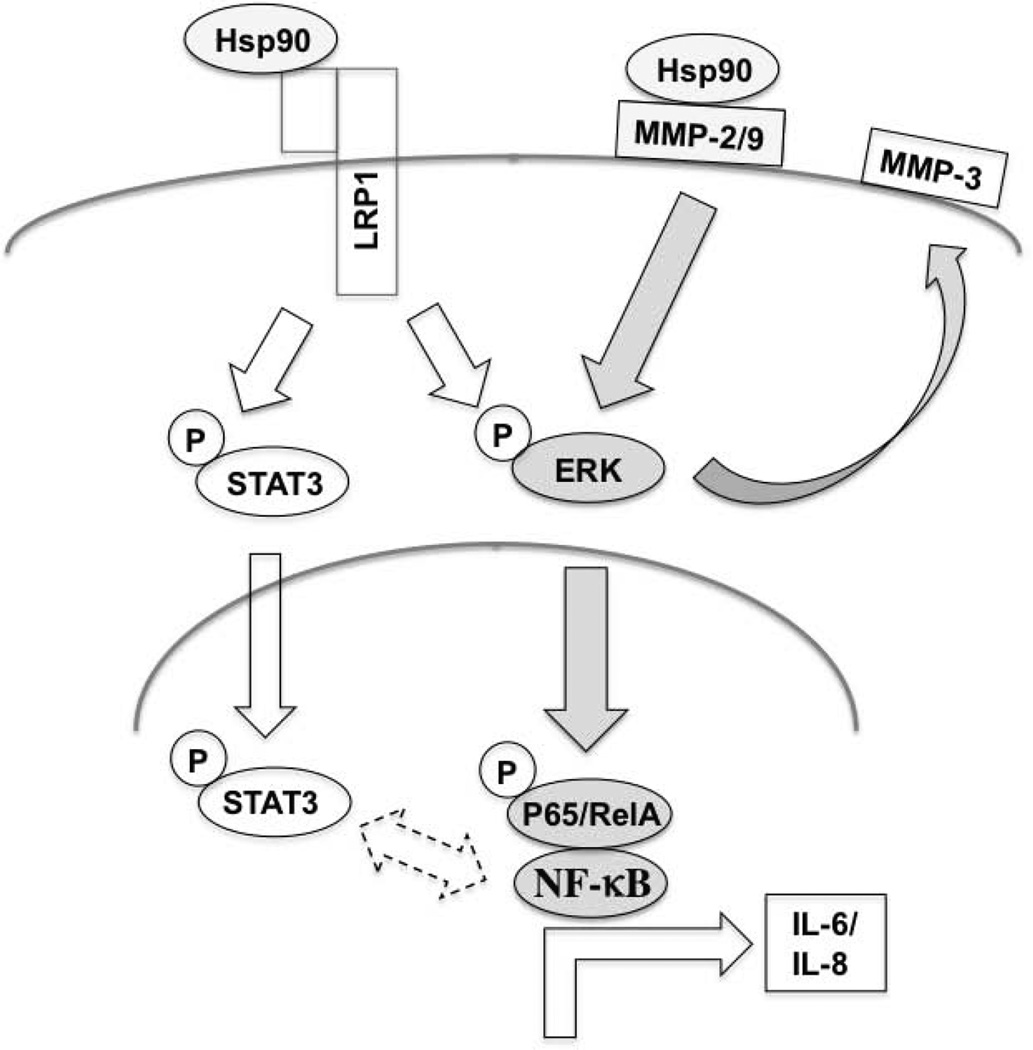
The ability of full length eHsp90α protein and ATP-ase deleted F-5 eHsp90α peptide to comparably activate ERK and NF-κB, and to elicit IL-6/IL-8 cytokine secretion, supports the notion that eHsp90α’s pro-inflammatory action is elicited via signaling modalities independent of chaperone function. A working model of our findings is presented (Fig. 7). eHsp90α plays a major role as a facilitator of stromal ERK activation. This eHsp90-ERK axis is likely reinforced by several mechanisms, including signaling via the LRP1 receptor (20). Herein, we demonstrate that MMP-2/9 is essential for eHsp90-mediated ERK activation. Our data are consistent with the current model wherein eHsp90α activates MMP-2/9 proteolytic activity via protein-protein interactions (20,23,26,44,45). Although possible that eHsp90α may similarly modulate MMP-3 proteolytic activity (46), our findings indicate that eHsp90-ERK signaling induces MMP-3 transcription. While MMP-3 inhibition had minimal impact upon IL-6/IL-8 secretion (not shown), MMP-3 is a well-known mediator of fibrosis and the myofibroblast phenotype (47). Our findings indicate that an eHsp90α-MMP-2/9-ERK cascade plays a dominant role in NF-κB-mediated induction of IL-6/IL-8. Although further studies are required to discern the precise mechanism of collaboration, MEK/ERK and p65/NF-κB have well-documented roles as collaborators of inflammatory signaling and tumorigenesis (48) and MEK/ERK has been identified as an upstream regulator of NF-κB (49). Additional mechanisms may also collaborate, such as eHsp90α-LRP1 dependent interaction with IkB kinases (50). We also demonstrate that eHsp90 induces the rapid and durable phosphorylation of STAT3. STAT3 may be activated by a diverse array of signaling mediators (51,52). As indicated, nuclear STAT3 and NF-κB may collaborate to maintain the inflammatory milieu.
Figure 7. Model for eHsp90α-mediated induction of an inflammatory stromal milieu.
eHsp90α initiates ERK signaling, herein demonstrated via MMP-2/9 activation. An eHsp90α-MMP-2/9-ERK pathway also upregulates MMP-3 transcription. The eHsp90α-MMP-2/9-ERK axis is required for NF-κB-dependent transcription of IL-6/IL-8. eHsp90 also stimulates STAT3 activation, albeit in an ERK-independent pathway. STAT-3 and NF-κB may collaborate to promote tissue inflammation.
Secretion of pro-inflammatory cytokines IL-6 and IL-8 have been shown to drive a CAF-like phenotype in PrSFs (1,9). Given the importance and prevalence of these cytokines in the reactive stroma, and the emerging role of eHsp90α in cancer progression, our findings support the notion that tumor-derived eHsp90α may be a key factor in the maintenance of an inflammatory phenotype. The ability of eHsp90α to activate stromal NF-κB and STAT3, well established master regulators of inflammatory responses, offers additional support for this premise. IL-6 is a major transducer of STAT3 signaling (18,42,43), and eHsp90α elicited a durable activation of STAT3. Whereas transient STAT3 activation is a component of normal physiological responses, sustained STAT3 hyperactivity is associated with chronic inflammation and malignant transformation (53). Thus, the ability of eHsp90α to sustain STAT3 activation may have implications for transformation and progression. The rapid activation of STAT3 by eHsp90α argues against transcriptional mechanisms driving the initial activation process.
The co-activation of NF-κB and STAT3 provides convincing evidence that eHsp90α initiates a complex interplay of potentially reinforcing signaling events. For example, although IL-6 is a downstream target of NF-κB, it also participates in a feed-forward mechanism to sustain NF-κB and STAT3 activation (54,55). The co-activation of these pathways would be expected to amplify and sustain a number of downstream events. Indeed, STAT3 activation is a known driver of MMP-3 expression, herein also shown to be an eHsp90α transcriptional target. The complexity of this signaling is expected to play a prominent role in altering tumor-stromal signaling. Our findings support this premise, as eHsp90α-dependent signaling in epithelial cells resulted in a cytokine milieu with the ability to robustly increase stromal secretion of IL-6 and IL-8. The CM from eHsp90α-expressing epithelial cells was also shown to confer morphological and molecular changes to prostate stromal fibroblasts consistent with adoption of a reactive phenotype. These data illustrate that eHsp90 dramatically modifies stromal-tumor signaling events to reinforce an inflammatory tumor microenvironment. This inflammatory function for eHsp90α has potentially broad implications for its role in prostate cancer progression. Nuclear NF-κB is found in almost half of all prostate cancers and is associated with progression, metastasis and recurrence (56–58), with similar functional roles bestowed upon the NF-κB gene products IL-6 and IL-8 (59–61). STAT3, which is activated in a majority of human PCa (62), also appears to play a prominent role. STAT3 collaborates with IL-6 in PCa progression (63,64), and is sufficient to execute many of the tumorigenic effects of IL-6 (65).
Our findings highlight a novel role for eHsp90α as a potent initiator of stromal inflammatory responses, exemplified by its robust activation of the master regulators NF-κB and STAT3. Given that eHsp90α expression was nominal in prostate stromal cells (data not shown), our data strongly support a paracrine role for tumor eHsp90α in conferring a reactive stromal phenotype. We have recently demonstrated that more aggressive PCa cells secrete elevated levels of eHsp90α, (20) indicating that PCa cells are a likely source of eHsp90α. This premise is further supported by the stromal inflammatory responses elicited by CM derived from eHsp90α-expressing PCa cells. This tumor-stromal co-culture model demonstrates that within a physiological context, modest increases in eHsp90α secretion are capable of dramatically altering the prostate stroma. Thus, the elevated eHsp90α expression observed in more aggressive PCa cells may portend their ability to more effectively initiate a profoundly reactive stroma. In summary, our findings indicate that eHsp90 is poised as a critical effector of tumor-stromal crosstalk to synergistically fuel cancer progression. Delineation of the mechanisms by which eHsp90 modifies the cancer niche to create a permissive and supportive microenvironment for tumor progression will be required to establish the potential therapeutic value of targeting eHsp90α as a means of preventing PCa progression.
Supplementary Material
Acknowledgments
We thank Simon Hayward for NPFs and David Rowley for kindly providing 6015N and 5905N PrSFs. We thank Wei Li for his generosity in providing Hsp90α F-5 and control peptide. Finally, we thank Chris Lindsey and Craig Beeson for synthesis of DMAG-N-oxide (NPGA).
Funding: NIH R01 CA135297 (JSI), ACS Postdoctoral Fellowship 124154-PF-13-024-0-CSM (MWH), and institutional funds. Support also provided by the Cell & Molecular Imaging Shared Resource, Hollings Cancer Center, P30 CA138313
Footnotes
Disclosure: The authors state no conflicts of interest
References
- 1.Barron DA, Rowley DR. The reactive stroma microenvironment and prostate cancer progression. Endocrine-related cancer. 2012;19(6):R187–R204. doi: 10.1530/ERC-12-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tuxhorn JA, Ayala GE, Smith MJ, Smith VC, Dang TD, Rowley DR. Reactive stroma in human prostate cancer: induction of myofibroblast phenotype and extracellular matrix remodeling. Clin Cancer Res. 2002;8(9):2912–2923. [PubMed] [Google Scholar]
- 3.Ayala G, Tuxhorn JA, Wheeler TM, Frolov A, Scardino PT, Ohori M, Wheeler M, Spitler J, Rowley DR. Reactive stroma as a predictor of biochemical-free recurrence in prostate cancer. Clin Cancer Res. 2003;9(13):4792–4801. [PubMed] [Google Scholar]
- 4.Yanagisawa N, Li R, Rowley D, Liu H, Kadmon D, Miles BJ, Wheeler TM, Ayala GE. Reprint of: Stromogenic prostatic carcinoma pattern (carcinomas with reactive stromal grade 3) in needle biopsies predicts biochemical recurrence-free survival in patients after radical prostatectomy. Hum Pathol. 2008;39(2):282–291. doi: 10.1016/j.humpath.2007.04.025. [DOI] [PubMed] [Google Scholar]
- 5.Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature. 2004;432(7015):332–337. doi: 10.1038/nature03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6(5):392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 7.Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer cell. 2012;21(3):309–322. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 8.Erez N, Truitt M, Olson P, Arron ST, Hanahan D. Cancer-Associated Fibroblasts Are Activated in Incipient Neoplasia to Orchestrate Tumor-Promoting Inflammation in an NF-kappaB-Dependent Manner. Cancer cell. 2010;17(2):135–147. doi: 10.1016/j.ccr.2009.12.041. [DOI] [PubMed] [Google Scholar]
- 9.Giannoni E, Bianchini F, Masieri L, Serni S, Torre E, Calorini L, Chiarugi P. Reciprocal activation of prostate cancer cells and cancer-associated fibroblasts stimulates epithelial-mesenchymal transition and cancer stemness. Cancer Res. 2010;70(17):6945–6956. doi: 10.1158/0008-5472.CAN-10-0785. [DOI] [PubMed] [Google Scholar]
- 10.Haddow A. Molecular repair, wound healing, and carcinogenesis: tumor production a possible overhealing? Advances in cancer research. 1972;16:181–234. doi: 10.1016/s0065-230x(08)60341-3. [DOI] [PubMed] [Google Scholar]
- 11.Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. The New England journal of medicine. 1986;315(26):1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 12.Schauer IG, Ressler SJ, Tuxhorn JA, Dang TD, Rowley DR. Elevated epithelial expression of interleukin-8 correlates with myofibroblast reactive stroma in benign prostatic hyperplasia. Urology. 2008;72(1):205–213. doi: 10.1016/j.urology.2007.11.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Libermann TA, Baltimore D. Activation of interleukin-6 gene expression through the NF-kappa B transcription factor. Molecular and cellular biology. 1990;10(5):2327–2334. doi: 10.1128/mcb.10.5.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nourbakhsh M, Kalble S, Dorrie A, Hauser H, Resch K, Kracht M. The NF-kappa b repressing factor is involved in basal repression and interleukin (IL)-1-induced activation of IL-8 transcription by binding to a conserved NF-kappa b-flanking sequence element. The Journal of biological chemistry. 2001;276(6):4501–4508. doi: 10.1074/jbc.M007532200. [DOI] [PubMed] [Google Scholar]
- 15.Basseres DS, Baldwin AS. Nuclear factor-kappaB and inhibitor of kappaB kinase pathways in oncogenic initiation and progression. Oncogene. 2006;25(51):6817–6830. doi: 10.1038/sj.onc.1209942. [DOI] [PubMed] [Google Scholar]
- 16.Ben-Neriah Y, Karin M. Inflammation meets cancer, with NF-kappaB as the matchmaker. Nature immunology. 2011;12(8):715–723. doi: 10.1038/ni.2060. [DOI] [PubMed] [Google Scholar]
- 17.Li N, Grivennikov SI, Karin M. The unholy trinity: inflammation, cytokines, and STAT3 shape the cancer microenvironment. Cancer cell. 2011;19(4):429–431. doi: 10.1016/j.ccr.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhong Z, Wen Z, Darnell JE., Jr Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science. 1994;264(5155):95–98. doi: 10.1126/science.8140422. [DOI] [PubMed] [Google Scholar]
- 19.Isaacs JS, Xu W, Neckers L. Heat shock protein 90 as a molecular target for cancer therapeutics. Cancer cell. 2003;3(3):213–217. doi: 10.1016/s1535-6108(03)00029-1. [DOI] [PubMed] [Google Scholar]
- 20.Hance MW, Dole K, Gopal U, Bohonowych JE, Jezierska-Drutel A, Neumann CA, Liu H, Garraway IP, Isaacs JS. Secreted Hsp90 is a novel regulator of the epithelial to mesenchymal transition (EMT) in prostate cancer. The Journal of biological chemistry. 2012;287(45):37732–37744. doi: 10.1074/jbc.M112.389015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang X, Song X, Zhuo W, Fu Y, Shi H, Liang Y, Tong M, Chang G, Luo Y. The regulatory mechanism of Hsp90alpha secretion and its function in tumor malignancy. Proc Natl Acad Sci U S A. 2009;106(50):21288–21293. doi: 10.1073/pnas.0908151106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burgess EF, Ham AJ, Tabb DL, Billheimer D, Roth BJ, Chang SS, Cookson MS, Hinton TJ, Cheek KL, Hill S, Pietenpol JA. Prostate cancer serum biomarker discovery through proteomic analysis of alpha-2 macroglobulin protein complexes. Proteomics Clin Appl. 2008;2(9):1223–1233. doi: 10.1002/prca.200780073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eustace BK, Sakurai T, Stewart JK, Yimlamai D, Unger C, Zehetmeier C, Lain B, Torella C, Henning SW, Beste G, Scroggins BT, Neckers L, Ilag LL, Jay DG. Functional proteomic screens reveal an essential extracellular role for hsp90 alpha in cancer cell invasiveness. Nature cell biology. 2004;6(6):507–514. doi: 10.1038/ncb1131. [DOI] [PubMed] [Google Scholar]
- 24.Sidera K, Gaitanou M, Stellas D, Matsas R, Patsavoudi E. A critical role for HSP90 in cancer cell invasion involves interaction with the extracellular domain of HER-2. The Journal of biological chemistry. 2008;283(4):2031–2041. doi: 10.1074/jbc.M701803200. [DOI] [PubMed] [Google Scholar]
- 25.Tsutsumi S, Scroggins B, Koga F, Lee MJ, Trepel J, Felts S, Carreras C, Neckers L. A small molecule cell-impermeant Hsp90 antagonist inhibits tumor cell motility and invasion. Oncogene. 2008;27(17):2478–2487. doi: 10.1038/sj.onc.1210897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stellas D, El Hamidieh A, Patsavoudi E. Monoclonal antibody 4C5 prevents activation of MMP2 and MMP9 by disrupting their interaction with extracellular HSP90 and inhibits formation of metastatic breast cancer cell deposits. BMC Cell Biol. 2010;11:51. doi: 10.1186/1471-2121-11-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li W, Li Y, Guan S, Fan J, Cheng CF, Bright AM, Chinn C, Chen M, Woodley DT. Extracellular heat shock protein-90alpha: linking hypoxia to skin cell motility and wound healing. Embo J. 2007;26(5):1221–1233. doi: 10.1038/sj.emboj.7601579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheng CF, Fan J, Fedesco M, Guan S, Li Y, Bandyopadhyay B, Bright AM, Yerushalmi D, Liang M, Chen M, Han YP, Woodley DT, Li W. Transforming growth factor alpha (TGFalpha)-stimulated secretion of HSP90alpha: using the receptor LRP-1/CD91 to promote human skin cell migration against a TGFbeta-rich environment during wound healing. Molecular and cellular biology. 2008;28(10):3344–3358. doi: 10.1128/MCB.01287-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liao DF, Jin ZG, Baas AS, Daum G, Gygi SP, Aebersold R, Berk BC. Purification and identification of secreted oxidative stress-induced factors from vascular smooth muscle cells. The Journal of biological chemistry. 2000;275(1):189–196. doi: 10.1074/jbc.275.1.189. [DOI] [PubMed] [Google Scholar]
- 30.Qin Z, DeFee M, Isaacs JS, Parsons C. Extracellular Hsp90 serves as a co-factor for MAPK activation and latent viral gene expression during de novo infection by KSHV. Virology. 2010;403(1):92–102. doi: 10.1016/j.virol.2010.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Defee MR, Qin Z, Dai L, Toole BP, Isaacs JS, Parsons CH. Extracellular Hsp90 serves as a co-factor for NF-kappaB activation and cellular pathogenesis induced by an oncogenic herpesvirus. American journal of cancer research. 2011;1(5):687–700. [PMC free article] [PubMed] [Google Scholar]
- 32.Sahu D, Zhao Z, Tsen F, Cheng CF, Park R, Situ AJ, Dai J, Eginli A, Shams S, Chen M, Ulmer TS, Conti P, Woodley DT, Li W. A potentially common peptide target in secreted heat shock protein-90alpha for hypoxia-inducible factor-1alpha-positive tumors. Molecular biology of the cell. 2012;23(4):602–613. doi: 10.1091/mbc.E11-06-0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olumi AF, Grossfeld GD, Hayward SW, Carroll PR, Tlsty TD, Cunha GR. Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Res. 1999;59(19):5002–5011. doi: 10.1186/bcr138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun Y, Campisi J, Higano C, Beer TM, Porter P, Coleman I, True L, Nelson PS. Treatment-induced damage to the tumor microenvironment promotes prostate cancer therapy resistance through WNT16B. Nature medicine. 2012;18(9):1359–1368. doi: 10.1038/nm.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bohonowych JE, Peng S, Gopal U, Hance MW, Wing SB, Argraves KM, Lundgren K, Isaacs JS. Comparative analysis of novel and conventional Hsp90 inhibitors on HIF activity and angiogenic potential in clear cell renal cell carcinoma: implications for clinical evaluation. BMC cancer. 2011;11:520. doi: 10.1186/1471-2407-11-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gopal U, Bohonowych JE, Lema-Tome C, Liu A, Garrett-Mayer E, Wang B, Isaacs JS. A novel extracellular Hsp90 mediated co-receptor function for LRP1 regulates EphA2 dependent glioblastoma cell invasion. PloS one. 2011;6(3):e17649. doi: 10.1371/journal.pone.0017649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bhowmick NA, Chytil A, Plieth D, Gorska AE, Dumont N, Shappell S, Washington MK, Neilson EG, Moses HL. TGF-beta signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science. 2004;303(5659):848–851. doi: 10.1126/science.1090922. [DOI] [PubMed] [Google Scholar]
- 38.Kojima Y, Acar A, Eaton EN, Mellody KT, Scheel C, Ben-Porath I, Onder TT, Wang ZC, Richardson AL, Weinberg RA, Orimo A. Autocrine TGF-beta and stromal cell-derived factor-1 (SDF-1) signaling drives the evolution of tumor-promoting mammary stromal myofibroblasts. Proc Natl Acad Sci U S A. 2010;107(46):20009–20014. doi: 10.1073/pnas.1013805107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hinz B, Mastrangelo D, Iselin CE, Chaponnier C, Gabbiani G. Mechanical tension controls granulation tissue contractile activity and myofibroblast differentiation. The American journal of pathology. 2001;159(3):1009–1020. doi: 10.1016/S0002-9440(10)61776-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheng CF, Sahu D, Tsen F, Zhao Z, Fan J, Kim R, Wang X, O'Brien K, Li Y, Kuang Y, Chen M, Woodley DT, Li W. A fragment of secreted Hsp90alpha carries properties that enable it to accelerate effectively both acute and diabetic wound healing in mice. The Journal of clinical investigation. 2011;121(11):4348–4361. doi: 10.1172/JCI46475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441(7092):431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 42.Grivennikov SI, Karin M. Dangerous liaisons: STAT3 and NF-kappaB collaboration and crosstalk in cancer. Cytokine & growth factor reviews. 2010;21(1):11–19. doi: 10.1016/j.cytogfr.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liang J, Nagahashi M, Kim EY, Harikumar KB, Yamada A, Huang WC, Hait NC, Allegood JC, Price MM, Avni D, Takabe K, Kordula T, Milstien S, Spiegel S. Sphingosine-1-Phosphate Links Persistent STAT3 Activation, Chronic Intestinal Inflammation, and Development of Colitis-Associated Cancer. Cancer cell. 2013;23(1):107–120. doi: 10.1016/j.ccr.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lagarrigue F, Dupuis-Coronas S, Ramel D, Delsol G, Tronchere H, Payrastre B, Gaits-Iacovoni F. Matrix metalloproteinase-9 is upregulated in nucleophosmin-anaplastic lymphoma kinase-positive anaplastic lymphomas and activated at the cell surface by the chaperone heat shock protein 90 to promote cell invasion. Cancer Res. 2010;70(17):6978–6987. doi: 10.1158/0008-5472.CAN-10-0861. [DOI] [PubMed] [Google Scholar]
- 45.Song X, Wang X, Zhuo W, Shi H, Feng D, Sun Y, Liang Y, Fu Y, Zhou D, Luo Y. The regulatory mechanism of extracellular Hsp90{alpha} on matrix metalloproteinase-2 processing and tumor angiogenesis. J Biol Chem. 2010;285(51):40039–40049. doi: 10.1074/jbc.M110.181941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Correia AL, Mori H, Chen EI, Schmitt FC, Bissell MJ. The hemopexin domain of MMP3 is responsible for mammary epithelial invasion and morphogenesis through extracellular interaction with HSP90beta. Genes & development. 2013;27(7):805–817. doi: 10.1101/gad.211383.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yamashita CM, Dolgonos L, Zemans RL, Young SK, Robertson J, Briones N, Suzuki T, Campbell MN, Gauldie J, Radisky DC, Riches DW, Yu G, Kaminski N, McCulloch CA, Downey GP. Matrix metalloproteinase 3 is a mediator of pulmonary fibrosis. The American journal of pathology. 2011;179(4):1733–1745. doi: 10.1016/j.ajpath.2011.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rokavec M, Wu W, Luo JL. IL6-mediated suppression of miR-200c directs constitutive activation of inflammatory signaling circuit driving transformation and tumorigenesis. Molecular cell. 2012;45(6):777–789. doi: 10.1016/j.molcel.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Joo JH, Jetten AM. NF-kappaB-dependent transcriptional activation in lung carcinoma cells by farnesol involves p65/RelA(Ser276) phosphorylation via the MEK-MSK1 signaling pathway. The Journal of biological chemistry. 2008;283(24):16391–16399. doi: 10.1074/jbc.M800945200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen JS, Hsu YM, Chen CC, Chen LL, Lee CC, Huang TS. Secreted heat shock protein 90alpha induces colorectal cancer cell invasion through CD91/LRP-1 and NF-kappaB-mediated integrin alphaV expression. The Journal of biological chemistry. 2010;285(33):25458–25466. doi: 10.1074/jbc.M110.139345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wheeler SE, Suzuki S, Thomas SM, Sen M, Leeman-Neill RJ, Chiosea SI, Kuan CT, Bigner DD, Gooding WE, Lai SY, Grandis JR. Epidermal growth factor receptor variant III mediates head and neck cancer cell invasion via STAT3 activation. Oncogene. 2010;29(37):5135–5145. doi: 10.1038/onc.2009.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Raptis L, Arulanandam R, Geletu M, Turkson J. The R(h)oads to Stat3: Stat3 activation by the Rho GTPases. Experimental cell research. 2011;317(13):1787–1795. doi: 10.1016/j.yexcr.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yu H, Jove R. The STATs of cancer--new molecular targets come of age. Nat Rev Cancer. 2004;4(2):97–105. doi: 10.1038/nrc1275. [DOI] [PubMed] [Google Scholar]
- 54.Korkaya H, Kim GI, Davis A, Malik F, Henry NL, Ithimakin S, Quraishi AA, Tawakkol N, D'Angelo R, Paulson AK, Chung S, Luther T, Paholak HJ, Liu S, Hassan KA, Zen Q, Clouthier SG, Wicha MS. Activation of an IL6 inflammatory loop mediates trastuzumab resistance in HER2+ breast cancer by expanding the cancer stem cell population. Molecular cell. 2012;47(4):570–584. doi: 10.1016/j.molcel.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang P, Guo L, Duan ZJ, Tepper CG, Xue L, Chen X, Kung HJ, Gao AC, Zou JX, Chen HW. Histone methyltransferase NSD2/MMSET mediates constitutive NF-kappaB signaling for cancer cell proliferation, survival, and tumor growth via a feed-forward loop. Molecular and cellular biology. 2012;32(15):3121–3131. doi: 10.1128/MCB.00204-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huang S, Pettaway CA, Uehara H, Bucana CD, Fidler IJ. Blockade of NF-kappaB activity in human prostate cancer cells is associated with suppression of angiogenesis, invasion, and metastasis. Oncogene. 2001;20(31):4188–4197. doi: 10.1038/sj.onc.1204535. [DOI] [PubMed] [Google Scholar]
- 57.Ross JS, Kallakury BV, Sheehan CE, Fisher HA, Kaufman RP, Jr, Kaur P, Gray K, Stringer B. Expression of nuclear factor-kappa B and I kappa B alpha proteins in prostatic adenocarcinomas: correlation of nuclear factor-kappa B immunoreactivity with disease recurrence. Clin Cancer Res. 2004;10(7):2466–2472. doi: 10.1158/1078-0432.ccr-0543-3. [DOI] [PubMed] [Google Scholar]
- 58.Lessard L, Karakiewicz PI, Bellon-Gagnon P, Alam-Fahmy M, Ismail HA, Mes-Masson AM, Saad F. Nuclear localization of nuclear factor-kappaB p65 in primary prostate tumors is highly predictive of pelvic lymph node metastases. Clin Cancer Res. 2006;12(19):5741–5745. doi: 10.1158/1078-0432.CCR-06-0330. [DOI] [PubMed] [Google Scholar]
- 59.Inoue K, Slaton JW, Eve BY, Kim SJ, Perrotte P, Balbay MD, Yano S, Bar-Eli M, Radinsky R, Pettaway CA, Dinney CP. Interleukin 8 expression regulates tumorigenicity and metastases in androgen-independent prostate cancer. Clin Cancer Res. 2000;6(5):2104–2119. [PubMed] [Google Scholar]
- 60.Uehara H, Troncoso P, Johnston D, Bucana CD, Dinney C, Dong Z, Fidler IJ, Pettaway CA. Expression of interleukin-8 gene in radical prostatectomy specimens is associated with advanced pathologic stage. The Prostate. 2005;64(1):40–49. doi: 10.1002/pros.20223. [DOI] [PubMed] [Google Scholar]
- 61.Araki S, Omori Y, Lyn D, Singh RK, Meinbach DM, Sandman Y, Lokeshwar VB, Lokeshwar BL. Interleukin-8 is a molecular determinant of androgen independence and progression in prostate cancer. Cancer Res. 2007;67(14):6854–6862. doi: 10.1158/0008-5472.CAN-07-1162. [DOI] [PubMed] [Google Scholar]
- 62.Mora LB, Buettner R, Seigne J, Diaz J, Ahmad N, Garcia R, Bowman T, Falcone R, Fairclough R, Cantor A, Muro-Cacho C, Livingston S, Karras J, Pow-Sang J, Jove R. Constitutive activation of Stat3 in human prostate tumors and cell lines: direct inhibition of Stat3 signaling induces apoptosis of prostate cancer cells. Cancer Res. 2002;62(22):6659–6666. [PubMed] [Google Scholar]
- 63.Lou W, Ni Z, Dyer K, Tweardy DJ, Gao AC. Interleukin-6 induces prostate cancer cell growth accompanied by activation of stat3 signaling pathway. The Prostate. 2000;42(3):239–242. doi: 10.1002/(sici)1097-0045(20000215)42:3<239::aid-pros10>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 64.Barton BE, Murphy TF, Adem P, Watson RA, Irwin RJ, Huang HF. IL-6 signaling by STAT3 participates in the change from hyperplasia to neoplasia in NRP-152 and NRP-154 rat prostatic epithelial cells. BMC cancer. 2001;1:19. doi: 10.1186/1471-2407-1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rojas A, Liu G, Coleman I, Nelson PS, Zhang M, Dash R, Fisher PB, Plymate SR, Wu JD. IL-6 promotes prostate tumorigenesis and progression through autocrine cross-activation of IGF-IR. Oncogene. 2011;30(20):2345–2355. doi: 10.1038/onc.2010.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.