Abstract
A phase I trial of PF-03084014, an oral reversible γ-secretase inhibitor (GSI), in solid tumor malignancies shows drug tolerability in patients. Evidence of Notch pathway inhibition was demonstrated in peripheral blood. A surprisingly high rate of response was seen in desmoid tumors, a rare but often locally aggressive sarcoma.
In this issue of Clinical Cancer Research, Messersmith and colleagues report the outcomes of a phase I dose finding study of PF-03084014, an oral reversible γ-secretase inhibitor (GSI) with better tolerability than previously tested GSIs. A surprisingly high rate of response to monotherapy was observed for desmoid tumor, a rare sarcoma with a propensity for local recurrence that can be recalcitrant to multiple treatment modalities (1). Originally developed to inhibit β-amyloid accumulation in Alzheimer's patients, GSIs have been repurposed as anti-cancer agents, largely due to their potential to inhibit Notch pathway signaling.
The Notch pathway in humans consists of four receptors – Notch1 through Notch4 – and two families of ligands: the Jagged and Delta-like (DLL) ligands. While some tumors contain activating mutations in the Notch pathway, others depend upon activation with Notch ligand. Stromal cells, particularly tumor-associated blood vessels, are a frequent and rich source of Notch ligand for tumor cells, and Notch signaling is a critical regulator of angiogenesis (reviewed in ref. 2).
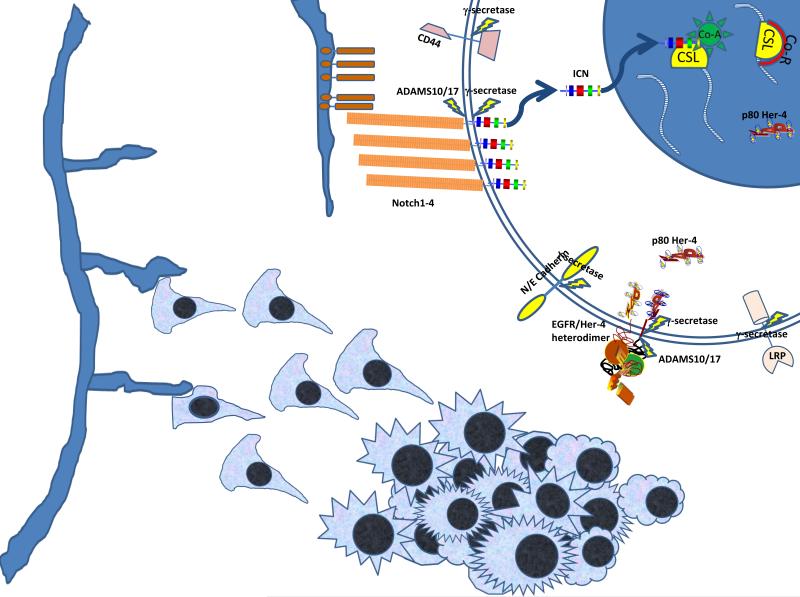
Upon exposure to ligand, Notch receptors undergo a choreographed two-step proteolytic cleavage, first by ADAMS10 or ADAMS17, which shed the extracellular domain of the protein, followed by γ-secretase, a component of the presenilin complex that cleaves within the transmembrane domain (reviewed in ref. 3). This cleavage liberates the Notch intracellular domain (NICD) from the plasma membrane to traffic to the nucleus, where NICD acts as a transcription factor with the CSL transcription complex. In the absence of NICD, co-repressor elements bind to CSL, inhibiting transcription of CSL target genes. NICD displaces the corepressor elements and recruits a coactivator complex, including the Mastermind-like protein (MAML), which activates transcription of CSL target genes (see Fig. 1; ref. 3). Many Notch target genes, such as the HES, HERP and HEY families, are themselves transcriptional regulators, often acting to suppress transcription of target genes. Notch target genes can have diverse actions, and may regulate themselves and each other (4). Notch signaling is seen in a wide range of cancers, usually as an oncogene but sometimes as a tumor suppressor (reviewed in ref. 5). Which role Notch plays can depend on its function in the development of the corresponding normal tissue. For cancers where Notch signaling is thought to drive malignant behavior, the hope is that GSI, by blocking Notch activation, will have an anti-tumor effect.
Figure 1.
γ-secretase: Multiple targets including Notch. Since blood vessels (endothelial cells and pericytes) are a rich source of Notch ligands, Notch activity likely varies across a tumor based on vascularity and distance from a vessel. Upon binding Notch ligand, Notch receptors undergo proteolytic activation. After cleavage, the intracellular domain translocates to the nucleus, turning on transcription of CSL targets by displacing co-repressors and recruiting co-activators. There are several other known γ-secretase targets, including CD44, E-Cadherin and N-Cadherin, ERBB4 and the Low Density Lipoprotein Receptor-Related Protein (LRP). For each of these, the ectodomain of the protein is first removed, often by an ADAMS family protease, which facilitates γ-secretase activity. The effects of γ-secretase can result both from a loss of function of the full length protein and from unique activities of the intracellular fragment. In sporadic desmoid tumors, mutated β-catenin encoded by CTNNB1 accumulates in the nucleus and de-represses Wnt responsive genes. This appears to be the major oncogenic driver of desmoid. How and if Notch and β-catenin signaling interact is desmoid tumor biology is not known.
Without dismissing the role of Notch in tumors, it is important to recognize that γ-secretase has other protein targets in cancer cells, including CD44, N- and E-Cadherin, ERBB4 and LRP (reviewed in ref. 6). For some of these proteins, such as CD44 binding to hyaluronic acid, the impact of γ-secretase could be to allow cells to become mobile by losing attachment to matrix components. For others like ERBB4, γ-secretase converts the protein from a membrane-bound receptor tyrosine kinase to an intracellular protein with nuclear localization and the ability to bind and regulate multiple transcription factors. Cleavage of ERBB4 is associated with increased resistance to chemotherapy, anoikis and other cellular stresses. With Cadherins, γ-secretase activity may impact the epithelial to mesenchymal transition (EMT) or the mesenchymal to epithelial transition (MET), depending upon which cadherin is targeted. Thus while the clinician rightly applauds the tumor response seen from a GSI, it remains important to determine which γ-secretase target protein mediates the effects. The answer may vary for different tumor types.
Multiple agents targeting the Notch pathway are in clinical development, including small molecule inhibitors of γ-secretase (e.g. MK-0752; RO4929097; PF-03084014), and monoclonal antibodies targeting either the Notch receptors (OMP-59R5) or their ligands (demcizumab, a humanized IgG2 monoclonal antibody against Delta-like ligand 4 (DLL4) (7). Identification of Notch 1 activating mutations in T-acute lymphocytic leukemia (T-ALL) and activity in preclinical models suggested clinical trials of GSIs. However, an early phase trial of MK-0752 in patients with T-ALL was disappointing, even in those carrying Notch-1 mutations. However, objective responses have been observed in a variety of solid tumors, such as anaplastic astrocytoma, papillary thyroid cancer, and colorectal cancer with neuroendocrine features with GSIs as single agents and combined with chemotherapy. Considerations such as autoinduction of CYP3A4, toxicities, and desire to achieve higher Cmax to facilitate CNS penetration have resulted in clinical evaluation of various dosing schedules. Common side effects have included hypophosphatemia, diarrhea (presumed secondary to goblet cell hyperplasia), nausea, vomiting, fatigue, rash, and headache. Hypertension was observed with single agent demcizumab across all dose levels in the phase 1 trial.
This present first-in-human phase 1 trial of PF-03084014 in patients with advanced solid tumors used continuous daily dosing that was tolerable and safely administered, unlike the experience with MK-0752. Excessive toxicities, GI distress and fatigue, observed in the continuous daily dosing schedule of MK-0752 resulted in further development of the agent on more intermittent schedules (8). Impressive clinical activity was observed with PF-03084014; objective responses being observed in 5 of 9 desmoid patients and 1 of 3 patients with uterine leiomyosarcoma. Ten sarcoma patients were treated on the phase 1 study of RO4929097 with no objective responses; no patients with desmoid tumors were enrolled (9). Based on the promising activity observed in desmoid, a rare disease, NCI is conducting a phase II trial of PF-03084014 in adults with desmoid tumors [NCT01981551] with clinical response as the primary endpoint. Exome sequencing samples and evaluation of Notch target gene expression in paired tumor biopsies is planned.
Efforts to develop pharmacodynamics (PD) markers of target engagement and downstream effects have focused on evaluating surrogate tissues such as hair follicles and peripheral blood mononuclear cells. Though developed gene signature panels have shown correlation with plasma exposure previously and with HES in this present trial, target modulation specifically in tumor has not been adequately investigated (8). Given the multiple known proteolytic targets of γ-secretase, the relevant therapeutic target is not certain. In the current trial of PF-03084014, NOTCH-related gene expression patterns were assessed in paired tumor biopsies but no consistent changes were demonstrated, possibly due to small sample size (n=5 pairs). Therefore, in spite of scores of patients treated on trials investigating GSI and other inhibitors of the Notch pathway, there is a profound lack of actual tumor PD data to guide dose and schedule selection for future studies.
The role of the Notch pathway in desmoid tumor is not understood. Most sporadic desmoids appear to be driven primarily by specific activating mutations in exon 3 of CTNNB1, the gene encoding β-catenin. These prevent β-catenin phosphorylation by the APC complex and allow nuclear accumulation (10). Nuclear β-catenin releases multiple Wnt-responsive genes from constitutive inhibition modulating downstream pathways. Notch and Wnt pathways are activated together and potentially co-regulate many processes from tissue development to stem cell maintenance. Yet, how they interact one with another and whether both are required in desmoid tumors is unclear. This leads to several intriguing questions: Is the effect of GSI on desmoid by interrupting Wnt/β-catenin signaling? If so, how does GSI interrupt β-catenin signaling—by withdrawing Notch activity or by protecting another protein target of γ-secretase? Or, is the effect of GSI on desmoids independent of the β-catenin pathway or perhaps even the Notch pathway? GSI might also interrupt critical tumor-stroma interactions. More mechanistic studies of GSI in desmoid tumors is needed, and based on this present trial more leiomyosarcoma patients should be treated. An additional consideration would be treatment of angiosarcoma, another sarcoma with strong activation of Notch (11).
Acknowledgments
Grant Support
D.P.M. Hughes is supported by the NCI of the NIH under award numbers R01CA149501 and R01CA141208.
Footnotes
Disclosure of Potential Conflicts of Interest
S. Kummar is the principal investigator of a phase II trial of PF-03084014 in desmoid tumors. No potential conflicts of interest were disclosed by the other authors.
References
- 1.Messersmith WA, Shapiro GI, Cleary JM, Jimeno A, Dasari A, Huang B, et al. A phase I, dose-finding study in patients with advanced solid malignancies of the oral γ-Secretase Inhibitor PF-03084014. Clin Cancer Res. 2014;21:xx–xx. doi: 10.1158/1078-0432.CCR-14-0607. [DOI] [PubMed] [Google Scholar]
- 2.Benedito R, Hellström M. Notch as a hub for signaling in angiogenesis. Exp Cell Res. 2013;319:1281–1288. doi: 10.1016/j.yexcr.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 3.Capaccione KM, Pine SR. The Notch signaling pathway as a mediator of tumor survival. Carcinogenesis. 2013;34:1420–1430. doi: 10.1093/carcin/bgt127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang P, Yang Y, Nolo R, Zweidler-McKay PA, Hughes DP. Regulation of NOTCH signaling by reciprocal inhibition of HES1 and Deltex 1 and its role in osteosarcoma invasiveness. Oncogene. 2010;29:2916–26. doi: 10.1038/onc.2010.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.South AP, Cho RJ, Aster JC. The double-edged sword of Notch signaling in cancer. Semin Cell Dev Biol. 2012;23:458–464. doi: 10.1016/j.semcdb.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Strooper B, Iwatsubo T, Wolfe MS. Presenilins and γ-secretase: structure, function, and role in Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2:a006304. doi: 10.1101/cshperspect.a006304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takebe N, Nguyen D, Yang SX. Targeting Notch signaling pathway in cancer: Clinical development advances and challenges. Pharmacol Ther. 2014;141:140–9. doi: 10.1016/j.pharmthera.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krop I, Demuth T, Guthrie T, Wen PY, Mason WP, Chinnaiyan P, et al. Phase I pharmacologic and pharmacodynamic study of the gamma secretase (Notch) inhibitor MK-0752 in adult patients with advanced solid tumors. J Clin Oncol. 2012;30:2307–13. doi: 10.1200/JCO.2011.39.1540. [DOI] [PubMed] [Google Scholar]
- 9.Tolcher AW, Messersmith WA, Mikulski SM, Papadopoulos KP, Kwak EL, Gibbon DG, et al. Phase I study of RO4929097, a gamma secretase inhibitor of Notch signaling, in patients with refractory metastatic or locally advanced solid tumors. J Clin Oncol. 2012;30:2348–53. doi: 10.1200/JCO.2011.36.8282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lazar AJ, Tuvin D, Hajibashi S, Habeeb S, Bolshakov S, Mayordomo-Aranda E, et al. Specific mutations in the beta-catenin gene (CTNNB1) correlate with local recurrence in sporadic desmoid tumors. Am J Pathol. 2008;173:1518–27. doi: 10.2353/ajpath.2008.080475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kluk MJ, Ashworth T, Wang H, Knoechel B, Mason EF, Morgan EA, et al. Gauging NOTCH1 activation in cancer using immunohistochemistry. PLoS One. 2013;8:e67306. doi: 10.1371/journal.pone.0067306. [DOI] [PMC free article] [PubMed] [Google Scholar]