Abstract
In bladder cancer, increased Caveolin-1 (Cav-1) expression and decreased Src expression and kinase activity correlate with tumor aggressiveness. Here, we investigate the clinical and functional significance if any, of this reciprocal expression in bladder cancer metastasis. We evaluated the ability of tumor Cav-1 and Src RNA and protein expression to predict outcome following cystectomy in 257 patients enrolled in two independent clinical studies. In both, high Cav-1 and low Src levels were associated with metastasis development. We overexpressed or depleted Cav-1 and Src protein levels in UMUC-3 and, RT4 human bladder cancer cells and evaluated the effect of this on actin stress fibers, migration using transwells and lung metastasis following tail vein inoculation. Cav-1 depletion or expression of active Src in metastatic UMUC-3 cells decreases actin stress fibers, cell migration and metastasis, while, Cav-1 overexpression or Src depletion increased the migration of non-metastatic RT4 cells. Biochemical studies indicated Cav-1 mediates these effects via its phosphorylated form (pY14), whereas Src effects are mediated through phosphorylation of p190RhoGAP and these pathways converge to reduce activity of RhoA, RhoC and Rho effector ROCK1. Treatment with a ROCK inhibitor reduced UMUC-3 lung metastasis in vivo, phenocopying the effect of Cav-1 depletion or expression of active Src. Src suppresses while Cav-1 promotes metastasis of bladder cancer through a pharmacologically tractable common downstream signaling pathway. Clinical evaluation of personalized therapy to suppress metastasis development based on Cav-1 and Src profiles appears warranted.
Keywords: Caveolin-1, Src, Rho A, Rho C, metastasis, bladder neoplasms
INTRODUCTION
Caveolin-1 is the major coat protein of caveolae, membrane invaginations that play important roles in transport of cellular lipids, cell adhesion and signal transduction (1, 2). Additionally, non-caveolar Cav-1 may carry out additional signaling or transport functions (3). Cav-1 regulates the activity of many signaling molecules involved in cancer initiation and progression including G-proteins, H-Ras, Protein Kinase C, integrins, nitric oxide synthase and epidermal growth factor receptor (4). Cav-1 has been identified as a tumor suppressor in some cancers, while in others its expression is elevated and it promotes cell survival, adhesion and migration (5, 6). Cav-1 expression is elevated in some breast and prostate cancers (5, 6), colon cancer, thyroid carcinoma, ovarian cancer, myeloma, pancreatic ductal adenocarcinoma and lung cancer (7–11). In bladder cancer, Cav-1 expression is undetectable in benign urothelium, higher in urothelial carcinomas, and maximal in tumor cells in the invading front (12). Cav-1 expression is also correlated with tumor grade (13). However, these studies are all correlative, hence, the functional role for Cav-1 in human tumor progression is unknown.
The c-Src tyrosine kinase promotes cell growth and migration, and its activity or expression is elevated in a number of human tumors (14). In striking contrast to other cancer types, the tyrosine kinase activity and expression of pp60c-Src is elevated in low grade bladder lesions while high grade cancers display decreased expression and kinase activity (15). These results suggest a different role of Src kinase activity in bladder cancer progression that remains unexplored. Here, we study the functional consequence of Src mediated signaling in urothelial cancers to address this knowledge gap. Interestingly, Cav-1 was first described as the major substrate for Src in v-Src transformed cell lines (16, 17) and Cav-1 phosphorylated on Tyr 14 (pY14 Cav-1) can either inhibit Src through the recruitment of C-terminal Src kinase (CSK) (18) or promote Src activation through an unknown mechanism (19). Thus, while Src and Cav-1 are interconnected they may have contextually different effects.
Here, we study the roles and interactions of Cav-1 and Src in models of human bladder cancer and identify a novel and pharmacologically tractable signaling pathway and associated biomarkers. These data lay the foundation for novel personalized clinical trials for patients with locally advanced bladder cancer at high risk of metastasis development.
MATERIALS AND METHODS
Cell Culture and Statistics
All bladder cancer cell lines used except UMUC-3 Luc and Lul-2 were purchased from ATCC (Manassas, USA), cultured and profiled as described (20). UMUC3-Luc cells were generated by transfecting pCMV-Lux and pBabe (21) into UMUC3 cells using Fugene 6.0 (Roche, Indianapolis, IN) and stably selected with puromycin (2µg/ml). Iterative tail vein injections of UMUC-3 Luc generated the highly lung metastatic UMUC-3 derivative called Lul-2 (Overdevest and Theodorescu, in preparation) as reported (22). UMUC-3 Src cells and UMUC-3 Src-DN cells were generated by stably transfecting Src-527 and Src-DN construct (23) in pcDNA 3.1 vector. Cav-1 short hairpin RNA (shRNA) stable UMUC-3 cell line was generated by transfecting Cav-1 shRNA in PLKO1 vector (Sigma). Comparisons made using one way ANOVA and Newman-Keuls test unless otherwise indicated.
Small interfering RNAs
siRNA duplexes were synthesized by Dharmacon (Lafayette, CO) as follows:
Cav-1 siRNA duplex: 5’-AGACGAGCUGAGCGAGAAGCA-3’;
Cav-1 siRNA duplex 2: 5’-CAUCUACAAGCCCAACAACTT-3’
P190RhoGAP siRNA duplex: 5’-GAUCACAUUGUGGAGCAG-3’
Src siRNA duplex: 5’- ACUCGCCUUCUUAGAGUUU- 3’
Luciferase (GL2) siRNA: 5'-GTACGCGGAATACTTCGA-3'.
Cav-1 siRNA duplex is described by Sunaga et. al (24). Luciferase (GL2) siRNA served as negative control. Bladder cancer cells were transfected with Cav-1 as well as GL-2 siRNA duplexes (100 nmol/L) using OligofectAMINE (Invitrogen, Carlsbad, CA).
Additional Materials and Methods
Detailed description of materials and methods can be found in Supplementary Information section.
RESULTS
Cav-1 and Src are reciprocal biomarkers of human bladder cancer behavior
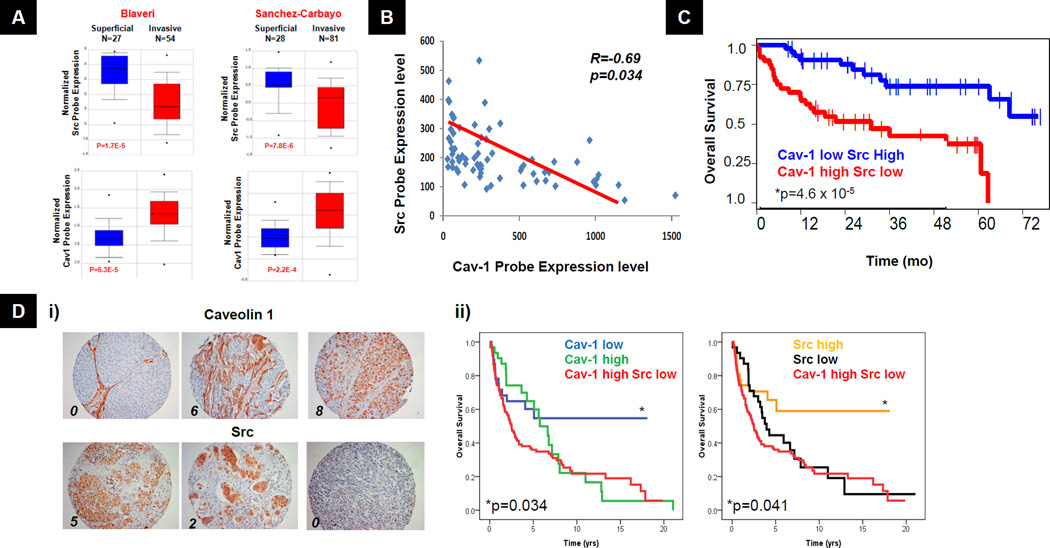
Immunohistochemical studies using human bladder cancer specimens showed that Cav-1 expression correlates with tumor aggressiveness (12, 13). Conversely, Src tyrosine kinase expression and activity decreases with tumor progression (15). However, no studies have examined both molecules in the same patient samples. To examine this relationship at the RNA level, we identified two bladder cancer studies in Oncomine (www.oncomine.org) (25, 26) that measured expression of both Cav-1 and Src. These studies found that Src expression is higher in superficial and lower in invasive bladder cancers, while Cav-1 expression is lower in superficial and higher in invasive bladder cancers (Fig 1A). Examination of a microarray from an additional dataset human bladder cancer specimens (20) showed an inverse correlation (R=-0.69, p=0.034) between Cav-1 and Src expression in the same tumors (Fig 1B). We then evaluated the association of the overall survival following radical cystectomy with Cav-1 and Src mRNA expression in a study of 109 bladder cancer patients (26). Patients with high Cav-1 and low Src mRNA tumor expression had worse survival than those with low Cav-1 and high Src (Fig 1C). Since tumor recurrence after cystectomy is usually metastatic and in most cases incurable (27), overall survival is an accurate surrogate for metastasis development. The prognostic value of Cav-1 and Src protein expression in tumors was then evaluated using immunohistochemistry in a second independent cohort of 151 bladder cancer patients that had undergone radical cystectomy. These results showed that both high Cav-1 and low Src staining correlate with poor survival while reciprocal expression in both proteins (high Cav-1 and low Src) had no further impact on prognosis (Fig 1D). Together, data from these two independent studies confirm the reciprocal role for these two proteins in metastasis and strongly support their utility as prognostic biomarkers in patients following cystectomy.
Figure 1. Expression of Cav-1 and Src in bladder cancer tissues and cell lines.
A) The relationship of Src and Cav-1 expression to tumor stage in human bladder cancer (25, 26). (from www.oncomine.org). B) Scatter plot and linear regression analysis of 65 bladder cancer specimens on HG-U133A Gene Chip array (Affymetrix)(20). Y-axis shows normalized Src probe expression (Src HG-U133A expression divided by β-actin HG-U133A expression level) against normalized Cav-1 probe expression on X-axis. C) Kaplan Meier curves showing overall survival of 109 bladder cancer patients following cystectomy as a function of Cav-1 and Src RNA expression determined by Sanchez-Carbayo et. al (26). D) (i) Representative immunohistochemical (IHC) evaluation and scoring of Cav-1 and Src as described in methods. Numbers indicate compound score (see methods) for each example. (ii) Kaplan Meier curves showing overall survival of 151 bladder cancer patients as a function of Cav-1 and Src expression determined by immunohistochemistry. Src High Score >5; Cav-1 High Score >8. * Log-rank for comparison to other two groups.
Next, we analyzed a panel of human bladder carcinoma cell lines derived from different stage tumors and having different invasive and metastatic behavior in animal models. We again observed an inverse relationship between Cav-1 and Src, where high Cav-1 and low Src were found in the more metastatic lines. For example, RNA (Fig S1A) and protein expression (Fig S1B) showed undetectable expression of Cav-1 and high expression of Src in the RT4 noninvasive urothelial carcinoma cell line, while Cav-1 was high and Src was low to undetectable in lines derived from muscle invasive (KU-7) or metastatic (UMUC-3 series) cancers. RT4 cells also displayed high levels of active Src (pY418-Src) and undetectable levels of Tyr phosphorylated Cav-1 (pY14-Cav-1), whereas aggressive cell lines had high pY14 Cav-1 and low to undetectable pY418 Src (Fig S1B). We also calculated the ratio of pY14 Cav-1 to total Cav-1 protein and pY418 Src to total Src protein from these immunoblots. Highly metastatic Lul-2 cells showed a 2-fold higher ratio compared to parental UMUC-3 or UMUC3 Luc cells (p=0.0076) (Fig S1C-D). Together, these data support a reciprocal relationship between Cav-1 and Src expression and activity.
Cav-1 and Src mediate migration and actin stress fibers in bladder cancer cells
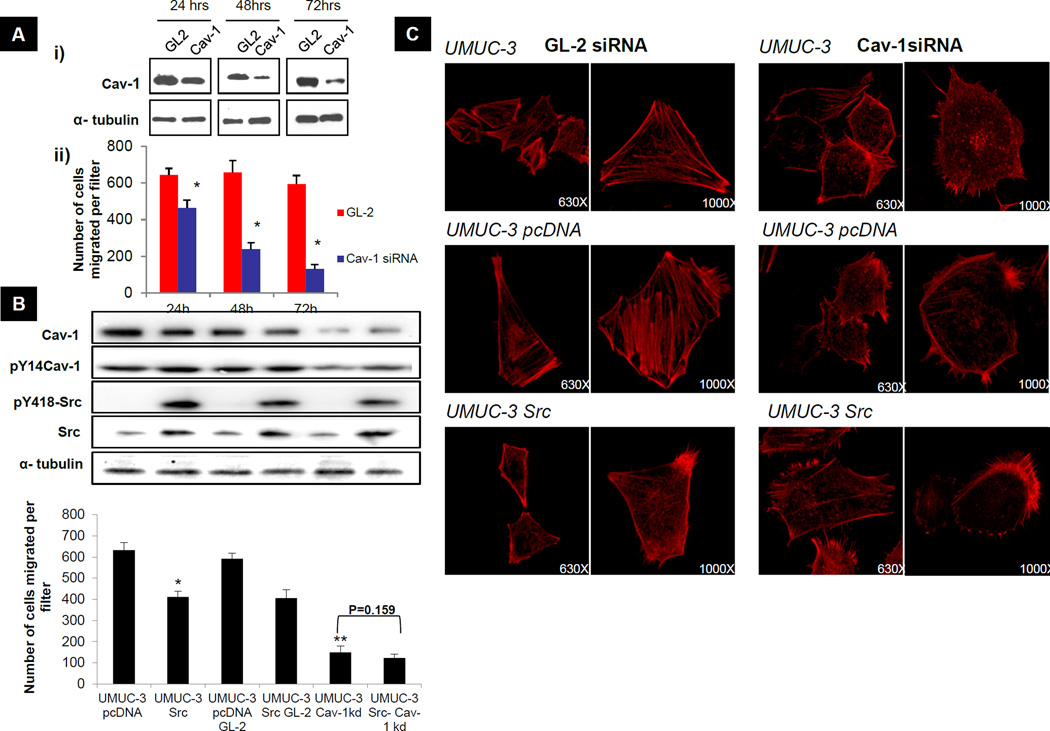
pY14 Cav-1 localizes to membrane protrusions at the leading edge of migrating cells (28) and has been implicated in cell migration. Hence, we sought to determine if Cav-1 expression promotes migration of human bladder cancer cells. UMUC-3 and KU-7 cells were transfected with Cav-1 siRNA and transwell migration was examined 24, 48 and 72 hours later. Cav-1 depletion dramatically reduced chemotaxis in the Boyden chamber assay for both cell lines in proportion to the degree of Cav-1 knockdown at different times (Fig 2A & S2A). Cav-1 depletion has been associated with apoptosis (10). However, identical cells in plating controls maintained in parallel showed no change in cell number over the same time period after Cav-1 depletion (CyQUANT fluorescence UMUC-3 GL-2 97± 4.32; UMUC-3 Cav-1 siRNA 92 ± 3.85). Thus, effects on cell migration were not due to alteration in cell numbers.
Figure 2. Effect of Cav-1 depletion and Src expression on transwell migration and the actin cytoskeleton.
A) UMUC-3 cells were transfected with GL-2 and Cav-1 siRNA duplex. (i) Lysates were collected 24, 48 and 72 hours after transfection and analyzed for Cav-1 protein expression. Data shown are representative blots of three separate experiments. (ii) Transwell migration was performed as previously described (43) on cells collected 24, 48 and 72 hours post siRNA transfection. Columns, mean; bar, ± SD. *p <0.05 significantly different from control GL-2 transfected cells at each time point. B). Transwell migration. *p<0.05 significantly different from control UMUC-3 pcDNA transfected cells. **p<0.05 significantly different from UMUC-3 GL-2 transfected cells 72 hours after transfection. Inset: Western analysis for Cav-1, pY14Cav-1, pY418 Src, total Src and tubulin expression was carried out on lysates collected from cells used for transwell migration at 72 hours after transfection. C) Actin cytoskeletal staining with phalloidin-AlexaFluor 594 (red) 72 hours after transfection with Cav-1 siRNA or GL-2 duplex. Magnification indicated.
Src promotes tumor progression in many systems (29). However, its reduced expression and activity as a function of urothelial tumor stage question such a role in this tumor type. Invasive and metastatic UMUC-3 bladder cancer cells stably transfected with active Src (c-Src527) displayed a >30% reduction in transwell migration compared to vector control transfected UMUC-3 cells (Fig 2B). While Cav-1 silencing in UMUC-3 cells reduced their migratory capacity, UMUC-3 Src cells were not significantly affected (p=0.159). KU-7 cells behaved similarly (Fig S2B). To investigate if Cav-1 and Src affect migration by altering the actin cytoskeleton, we examined cells stained for F-actin by confocal microscopy. Depletion of Cav-1 led to significant loss of stress fibers compared to control (irrelevant siRNA or empty vector, respectively) cells and overexpression of Src led to a noticeable reduction in stress fibers (Fig 2C). No further reduction in stress fibers was noted with depletion of Cav-1 in Src overexpressing cells (p=0.786) (Fig S2C). Taken together, these data show Cav-1 and Src are causally involved in bladder tumor cell migration and have similar effects on the actin cytoskeleton.
Cav-1 and Src modulate Rho GTPases through distinct yet convergent molecular pathways
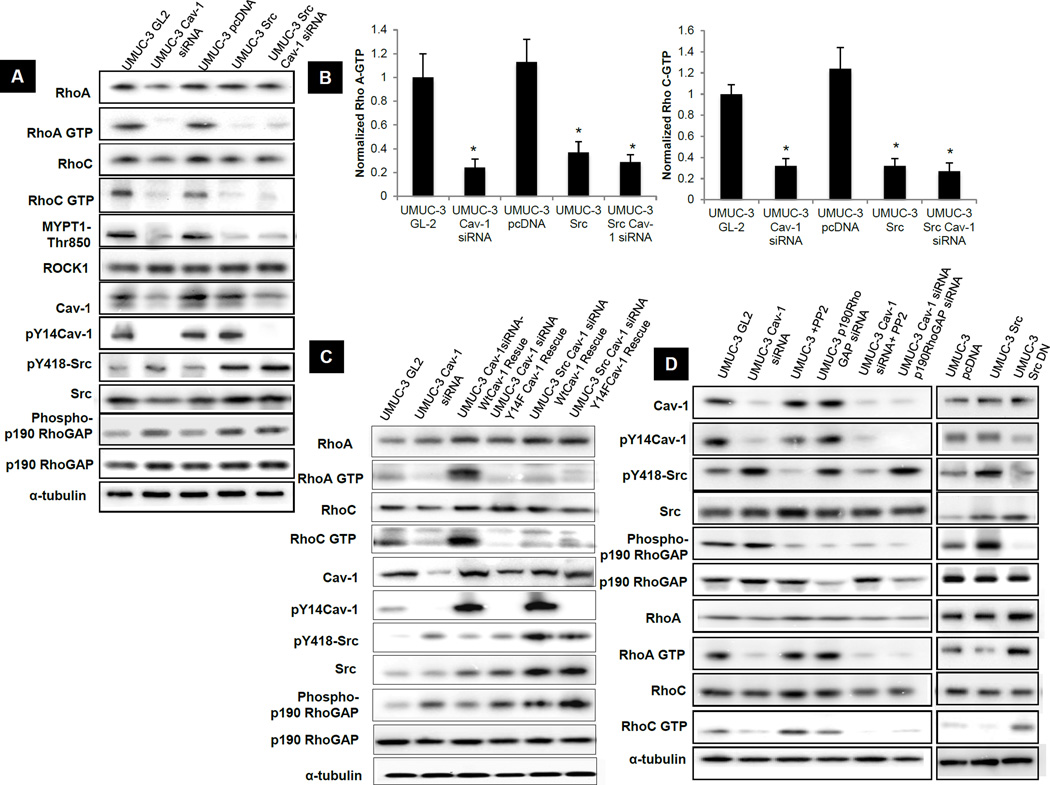
The effects on actin stress fibers and cell migration suggest that the Rho GTPases may be involved. Indeed, high expression of RhoA and RhoC, and their effector ROCK1 are associated with poor prognosis and poor patient survival in bladder cancers (30). Interestingly, when we evaluated RhoA, RhoC and ROCK1 mRNA (Fig S3A) and protein (Fig S3B) expression in the five human bladder carcinoma cell lines used earlier (Fig S1B), all three genes increased as a function of invasiveness. We therefore sought to determine if Cav-1 or Src controlled the levels or activity of these two GTPases. Cav-1 depletion or Src overexpression in UMUC-3 cells had no significant effect on expression of RhoA (Fig S3 E&F), RhoC or ROCK1 but led to a profound decrease in their activity (Fig 3A and 3B). Since activated Src can inhibit Rho via phosphorylation and activation of p190RhoGAP (19, 31), we evaluated this protein as well. Src overexpression in UMUC-3 cells increased phospho-p190RhoGAP whereas Cav-1 depletion showed only a modest increase (Fig 3A).
Figure 3. Effect of Src up-regulation and Cav-1 down regulation on Rho-ROCK pathway in UMUC-3 cells.
A) Rho A, RhoC and ROCK1 activation assays and western blots for lysates collected from cells 72 hours post transfection. B) RhoA-GTP and RhoC -GTP from Figure 3A were quantified relative to total RhoA and RhoC respectively by densitometry. *, p< 0.01 compared to their respective controls. C&D) Rho A and C activation assays and western blots for lysates collected from cells 72 hours post transfection.
Tyr phosphorylated Cav-1 (pY14Cav-1) is present in focal adhesions of migrating cells and may directly interact with RhoA and RhoC (32, 33). Cav-1 depletion in UMUC-3 cells led to a corresponding and dramatic decrease of pY14 Cav-1 (Fig 3A and S3C). However, Src overexpression in UMUC-3 cells did not affect Cav-1 phosphorylation (Fig 3A and S3C). Other studies have reported that Cav-1 is a substrate of Src kinases in Rous Sarcoma virus-transformed chick embryo fibroblasts (16). However, in our studies Src overexpression did not appear to contribute to Cav-1 phosphorylation in UMUC3 cells. To determine the importance of pY14 Cav-1 for RhoA and RhoC activity, we knocked down Cav-1 and rescued its expression using wild type or non-phosphorylatable Y14F Cav-1. These experiments were carried out in both vector control and Src overexpressing UMUC-3 cells. Whereas wild type Cav-1 restored RhoA and RhoC activity in knockdown cells, Cav-1 Y14F completely failed to rescue (Fig 3C).
It is worth noting that the profound increase in RhoGTPase activation after Cav-1 rescue corresponds to the increase in pY14 Cav-1, while the change in pY418 Src is minimal (Fig 3C). Thus, pY14 Cav-1 is critical for Cav-1-dependent Rho activation in these cells.
Cav-1−/− mouse fibroblasts show constitutive activation of Src kinase (19), which suggests that in cells lacking Cav-1, active Src could increase p190RhoGAP activity to inhibit Rho. We therefore tested whether this occurs in bladder cancer by different approaches. UMUC-3 cells in which Cav-1 was depleted were treated with the Src inhibitor PP2 or co-transfected with p190RhoGAP siRNA. Cav-1-silenced cells showed increased Src activity, a modest but reproducible increase in phospho-p190RhoGAP, and the expected decrease in Rho GTP loading compared to GL-2 siRNA transfected cells. However, neither PP2 treatment, forced expression of dominant negative mutant of Src (Src DN) nor siRNA-mediated p190RhoGAP depletion in Cav-1 knocked down cells restored Rho activity. This result shows that, although Cav-1 still affects Src activity, the pathway linking Cav-1 to Rho is largely independent of Src and p190RhoGAP (Fig 3D).
Src overexpression did not appear to increase phosphorylation of Cav-1, however, we sought to determine the contribution of endogenous Src activity to Cav-1 phosphorylation in bladder cancer. A dose response with PP2 revealed a much steeper reduction in pY418 Src than pY14Cav-1 (Fig S3D). Since Src, Fyn and Abl have been shown to phosphorylate Cav-1(34), and PP2 inhibits Src and Fyn, these results suggests that other kinases, possibly including Abl, may contribute to pY14Cav-1 levels in bladder cancer cells.
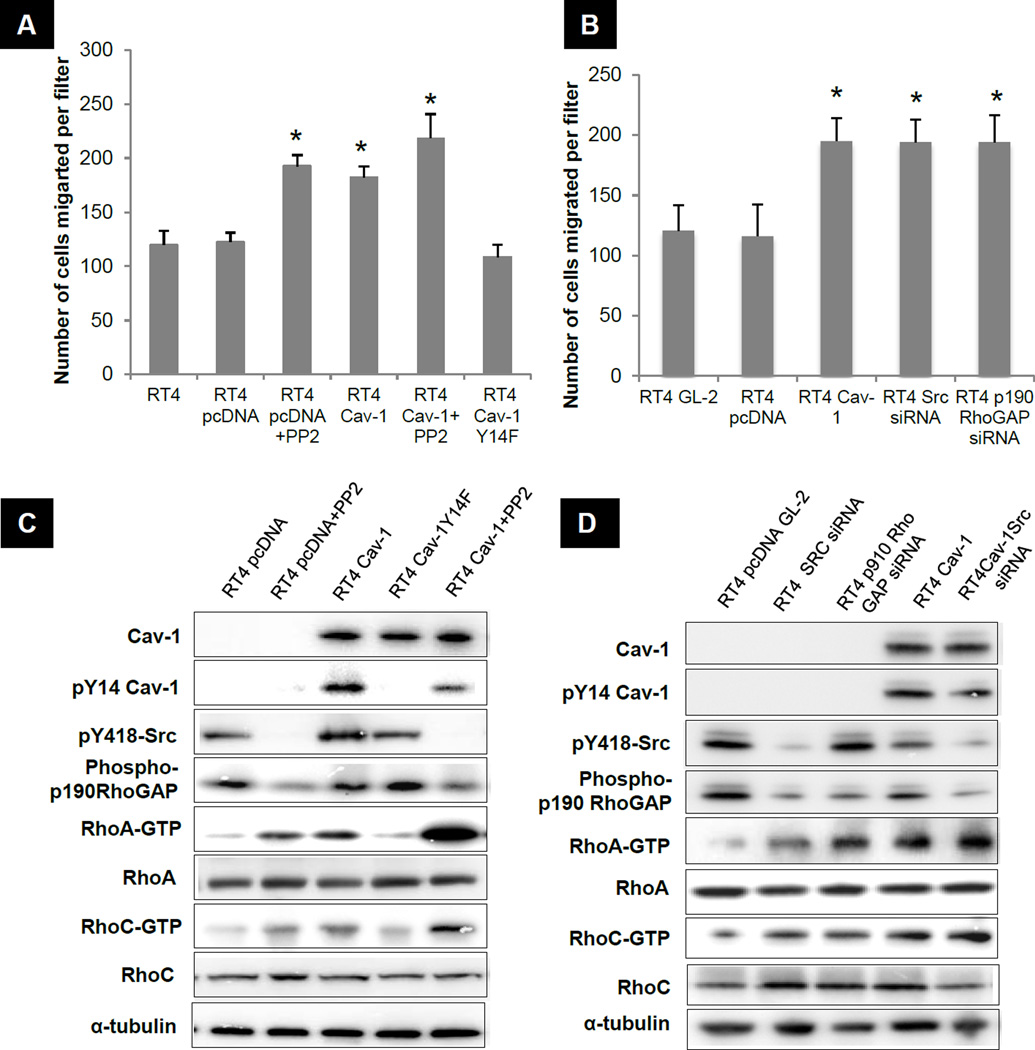
To complement the results carried out above in the metastatic cell line UMUC-3, we investigated whether non invasive RT4 human urothelial cells that express abundant Src but lack Cav-1 (Fig S1A&B) show altered migration in response to Src inhibition or Cav-1 over-expression. Stable expression of Cav-1 increased migration by 42% whereas inhibiting Src with PP2 increased migration 47% (Fig 4A). Depletion of Src with siRNA also increased migration 51% compared to GL-2 transfected RT4 cells (Fig 4B). Conversely, introduction of non-phosrphorylatable Y1-4F Cav-1 failed to increase migration (Fig 4A) or activate Rho GTPases (Fig 4C). These results confirm the importance of Tyr 14 phosphorylation of Cav-1 in this pathway.
Figure 4. Effect of Src inhibition and Cav-1 introduction on migration of RT4.
A) Transwell migration. *p <0.05 significantly different from RT4 and RT4 pcDNA transfected cells at each time point. B) Transwell migration *p <0.05 significantly different from RT4 GL-2 and RT4 pcDNA transfected cells. C & D) Western blotting and Rho activation assays as described. Data shown are representative blots of three separate experiments.
We next tested the role of p190RhoGAP in suppression of migration by Src in RT4 cells. Knocking down p190RhoGAP increased migration by 48% (Fig 4B). Additionally, p190RhoGAP knockdown in RT4 cells increased RhoA and C activation to approximately the same extent as Cav-1 expression or Src inhibition (Fig 4C & 4D). Importantly, simultaneous expression of Cav-1 and Src blockade (PP2 or siRNA) provided additive effects on RhoA and RhoC activity but not p190RhoGAP phosphorylation (Fig 4C & 4D). These data confirm that both Cav-1 and Src activate Rho predominantly through independent pathways to stimulate cell migration, but only Src acts through p190RhoGAP.
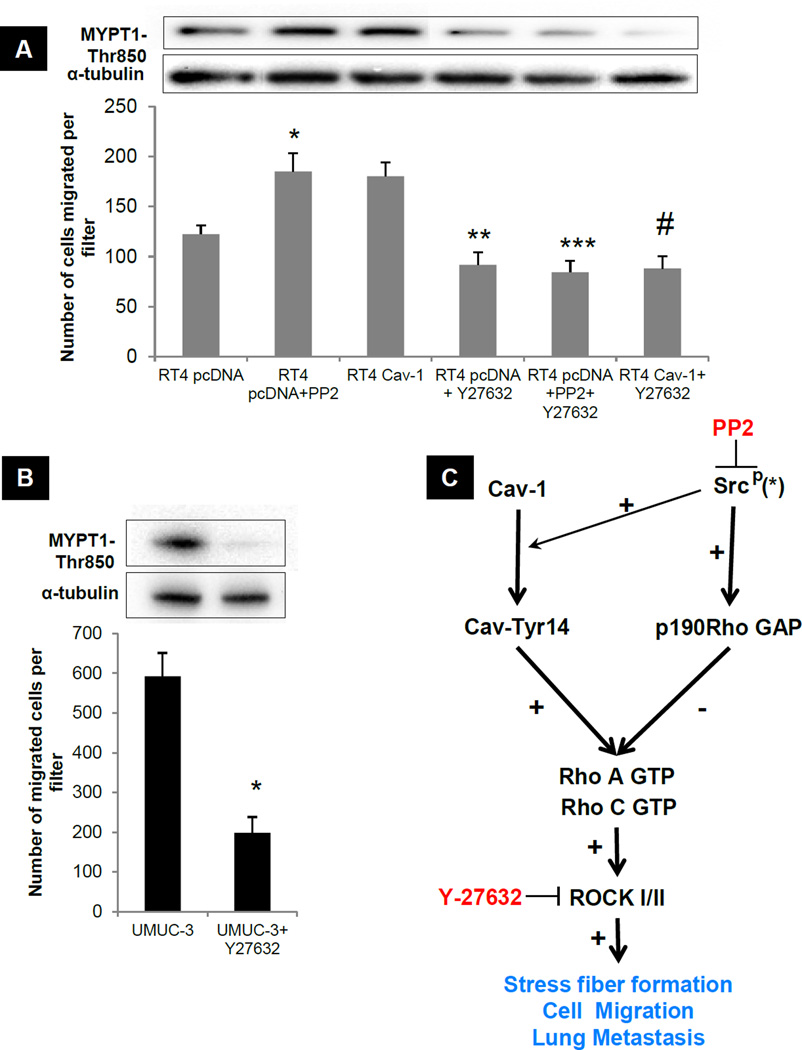
Given the association of ROCK1 expression with human bladder cancer progression (30), we tested its role in bladder cancer cell migration downstream of Rho. Treating RT4 cells with Y27632, a selective ROCK inhibitor (35), reduced the migration of untreated cells, Cav-1 over-expressors or Src-inhibited cells to the same level (Fig 5A). Similarly, treatment of UMUC-3 cells with Y27632 strongly suppressed migration (Fig 5B). In parallel experiments, Y27632 caused no change in cell number over the same time period, excluding cytotoxicity or changes in cell number (CyQUANT fluorescence UMUC-3 vehicle treated 95± 6.1; UMUC-3 Y27632 treated 89.2 ± 8.35). These data suggest ROCK1 is a common downstream element that lies downstream of both Cav-1 and Src/p190RhoGAP (Fig 5C).
Figure 5. Effect of Src and Cav-1 on migration is mediated via Rho-ROCK pathway.
A) Transwell migration. *p <0.05 significantly different from RT4 pcDNA transfected cells. **p<0.05 significantly different from RT4 pcDNA transfected cells ***p<0.05 significantly different from RT4 pcDNA+PP2 transfected cells #p<0.05 significantly different from RT4-Cav-1 transfected cells. Inset: Corresponding ROCK1 activity. B) Transwell migration. *p <0.05 significantly different from UMUC-3 vehicle treated cells. Inset: Corresponding ROCK1 activity. C) Proposed Cav-1 and Src mediated regulation of Rho/ROCK in bladder cancer. Cav-1 at Tyr 14 results in GTPase activation of RhoA and RhoC leading to ROCK1 activation subsequently resulting in increased stress fiber formation, migration and metastasis. Activated Src leads to 190RhoGAP phosphorylation resulting in the inhibition of Rho/ROCK pathway leading to loss of stress fibers, reduced migration and metastasis. * PP2 inhibits the activity of other members of the Src family kinases (SFK) and hence these may also be involved.
Cav-1 and Src are reciprocal regulators of lung colonization
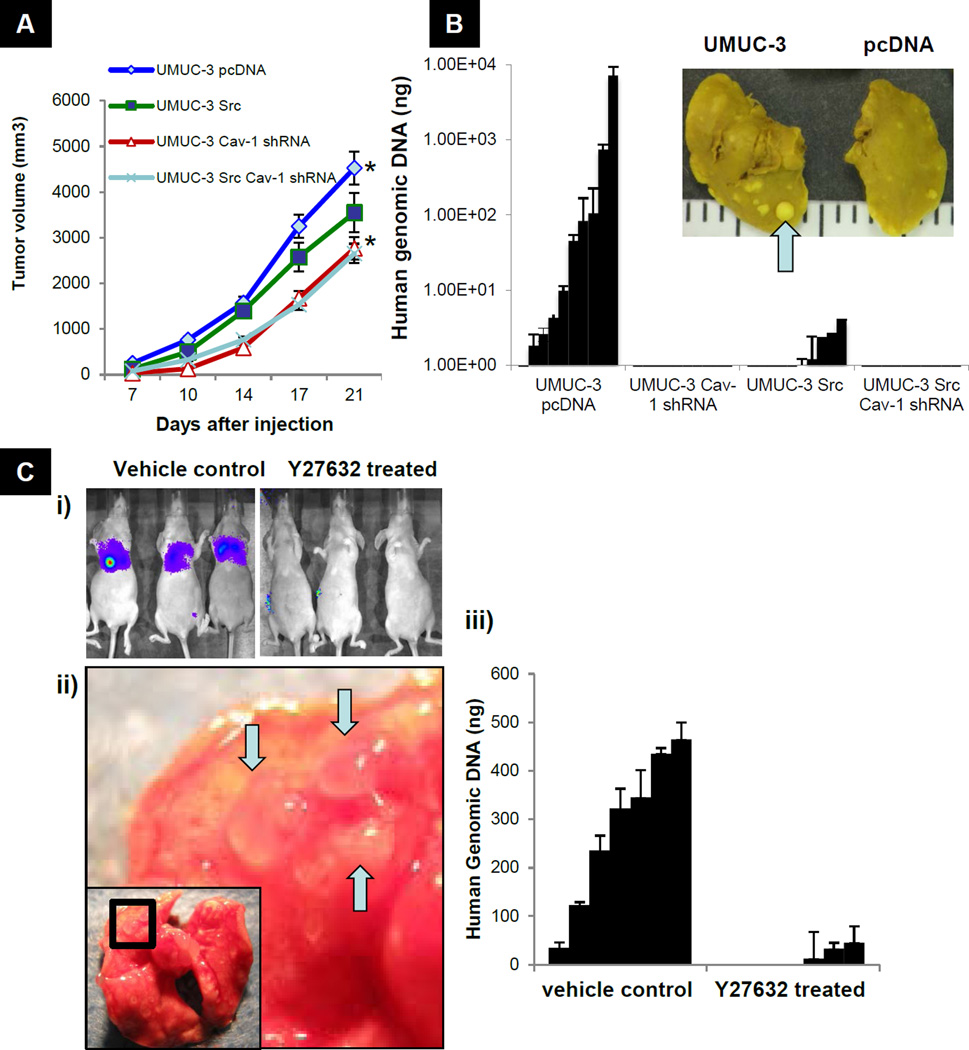
We next investigated whether Cav-1 and Src affect in vivo tumor growth and metastasis. UMUC-3 cells in which Cav-1 was stably depleted or Src was stably overexpressed were developed and characterized in vitro. Western analysis confirmed the expected expression pattern of Cav-1, pY14 Cav-1, Src and pY418 Src (Fig S4A). Cav-1 shRNA cells (Cav-1shRNA) showed a modest but significant reduction in both monolayer growth (p= 0.014) and soft agar colony formation (p=0.0081) (Fig S4B & S4C). Cav-1 depletion also resulted in a nearly 6-fold decrease in matrigel invasion compared to vector control cells (Fig S4D). When injected subcutaneously into mice, UMUC-3 Cav-1 shRNA cells produced tumors that were slightly (38%) smaller compared to UMUC-3 pcDNA cells at 21 days after injection (p=0.0134) (Fig 6A). Importantly, both pY14 Cav-1 and Rho GTP loading were maintained in vivo as determined in tissue excised from these tumors (Fig S4E). Expression of active Src in UMUC-3 cells marginally decreased in monolayer growth (14%, p=0.014), anchorage independent growth (33%, p=0.009) and subcutaneous growth (22%, p=0.021) compared to vector controls (Fig S4B, S4C, and 6A). In contrast to these mild phenotypes, a more substantial reduction in matrigel invasion was seen with overexpression of active Src (p=0.0081) (Fig S4D).
Figure 6. Effect of Src upregulation and Cav-1 downregulation on growth and lung colonization of UMUC-3 cells.
A) In vivo subcutaneous growth of cells in Figure 5a. 106 cells were injected subcutaneously in 10 mice per group. Mean tumor volume ± SEM (44). *p <0.036 compared to controls at 21 days. B) In vivo lung metastasis by 12p quantitative PCR in mice injected UMUC-3 cells. Inset: Appearance of lungs with metastatic nodules (arrow) found at necropsy. C) In vivo lung metastasis of mice injected 8 weeks previously with Lul-2 cells with or without Y27632 treatment. i) Representative Xenogen images (D-luciferase) of vehicle treated and Y27632 treated animals ii) Representative gross lung pictures at 8 weeks. Arrows indicate lung metastases. iii) 12p quantitative PCR from vehicle treated and Y27632 treated mice at 8 weeks.
We next tested the effect of Cav-1 and Src expression on experimental lung metastasis by injecting bladder cancer cells into the tail veins of 6-week-old nude mice. At 8 weeks post-injection, 60% of the mice receiving the empty vector-transfected UMUC-3 cells displayed visible lung metastases. Remarkably, only 10% of the mice receiving UMUC3-Src cells had metastases were none were detectable when Cav-1 depleted cells were inoculated (Table S1). A more sensitive and quantitative analysis using PCR with a 12p human specific probe (36)showed a drastic reduction in human genomic DNA in Src-overexpressors compared to vector controls (SEM 47.8± 27 vs. 4320.2 ±1256 respectively, p= 0.0081) (Fig 6B) no human genomic DNA was detected in the lungs of mice inoculated with Cav-1 shRNA UMUC-3 cells. To study the involvement of Src and Cav-1 model in bladder cancer metastasis in detail, we used an alternative approach. Stable Cav-1 knock-down and Src overexpressing MB49 mouse bladder cancer model cell lines were injected subcutaniously in C57B6 mice (37). Control MB49 cells injected mice displayed metastasis in lung and visceral organs 21 days after injection, while Cav-1 depleted cells failed to display any metastasis. Also, stable Src overexpressed MB49 cells showed a marked abrogation in their metastatic ability compared to control cells (Fig S5). These results once again established the significance of the proposed Cav-1-Src bladder cancer metastasis model.
Finally, we evaluated effects of ROCK inhibition on the metastatic colonization of the lung by bladder cancer cells by treating mice injected with highly metastatic Lul-2 human bladder cancer cells with Y27632 for a 28 day period. We observed approximately 80–90% reduction in lung colonization in the Y27632 treated group compared to the vehicle treated group as assessed by bioluminescent imaging, visual lung examination and PCR using 12p probe (Fig 6C). These results establish the therapeutic rationale of Rho-ROCK pathway blockade in bladder cancer metastasis. Since this agent is approved for human use for the treatment of cerebral vasospasm, it provides a rapid path to safe evaluation of this agent in treatment of metastatic bladder cancer in high-risk patients.
DISCUSSION
Cav-1 is a highly multifunctional protein that can make both positive and negative contributions to cancer progression and metastasis. In bladder cancer, Cav-1 expression is elevated at the onset of oncogenesis (12) and rises even further as a function of stage and grade (12, 13, 38). This finding is particularly relevant because metastatic urothelial carcinoma is associated with poor prognosis and there are presently few molecular markers to identify localized tumors with high metastatic potential (39). Thus Cav-1 may be a clinically useful marker. Our results take this idea a step further by showing that Cav-1 makes a critical functional contribution to bladder cancer migration and metastasis. Thus Cav-1 is both a biomarker and a driver of tumor progression.
Src was first identified as a proto-oncogene and is well known to promote cell growth and motility in many systems (40). A previous study showed that Src kinase activity is elevated in early stage bladder cancer; however, it was lower in poorly differentiated urothelial carcinoma (15). These results raised some questions about the role of Src in urothelial cancer. Our data confirmed that Src is decreased in advanced bladder cancer and, in addition, showed that inhibiting Src expression or kinase activity increased migration and metastatic capability of bladder cancer cells. Thus Src is a negative regulator of bladder cancer metastasis.
When Cav-1 and Src expression were analyzed in two independent cohorts of human bladder cancer specimens and a panel of cell lines, a striking inverse correlation was noted. Several links have been reported between Cav-1 and Src. Cav-1 is a substrate for non-receptor tyrosine kinases, including Src (16, 17). Although we failed to observe any increase in Cav-1 phosphorylation followed by Src over-expression in metastatic UMUC-3 cells, Src depletion in non-metastatic RT4 cells or inhibition of Src by PP2 in UMUC-3 cells moderately decreased pY14 Cav-1 levels, suggesting that Src is probably one of several kinases that mediate Cav-1 phosphorylation. Thus, other kinases such as Abl may be responsible for maintaining pY14 Cav-1 in bladder cancer cells (34). Further, Cav-1 phosphorylated on Tyr 14 (pY14 Cav-1) can inhibit Src through the recruitment of C-terminal Src kinase (CSK) (18). Some decrease in Src activity after Cav-1 expression was visible in UMUC-3 cells but not obvious in RT4 cells, confirming that this mechanism exists but is probably not a major determinant in bladder cancer. Thus we considered alternative mechanisms by which Src and Cav-1 might interact.
Src regulates Rho family of GTPases, activating Rac and Cdc42 (41), and inhibiting Rho via activation of p190RhoGAP (19, 31). Consistent with published data (31, 42), we observed inhibition of RhoA and C activity by Src in both UMUC-3 and RT4 cells. Additional results support a model in which Src controls bladder cancer metastasis through p190 RhoGAP activation and subsequent inactivation of Rho and ROCK1. Interestingly, we also found that Cav-1 expression controls activation of Rho, in this case as a positive regulator. As with Src, ROCK1 was critical for Cav-1-induced cell motility and invasion. However, these effects required pY14 Cav-1 but were largely independent of Src and p190RhoGAP. Thus, both Src and Cav-1 control bladder cancer metastasis through a common pathway involving Rho and ROCK1 but do so via distinct upstream elements which are likely of significance in most, but likely not all bladder cancers. Notwithstanding the two separate upstream pathways, our results indicate that inhibiting the Rho-ROCK pathway using Y27632 in vivo could be a viable therapeutic strategy in patients. This approach is particularly appealing and timely since there are several Rho kinase inhibitors such as fasudil that are orally available, safe and approved for human use for treatment of cerebral vasospasm. This approach would permit rapid deployment of personalized adjuvant clinical studies in patients at high risk for metastasis development after radical cystectomy. The biomarkers developed here would be used for patient selection, optimizing the chances for therapeutic success.
In summary, these results provide a mechanistic explanation for our observed association of high Cav-1 and low Src expression with metastasis development and poor survival in human bladder tumors. This finding also demonstrates the utility of Cav-1 and Src as predictive biomarkers of clinical outcome. Most importantly, it offers the opportunity for rapid translation of this molecular knowledge into personalized therapy.
ACKNOWLEDGEMENTS
This work was supported by National Institute of Health grants T32DK069264 to ST and R01CA075115 to DT. The authors wish to thank Drs N. Balasubramanian for helpful suggestions, S J Parsons for providing Src constructs and A.V. Somlyo for providing Y27632. None of the authors have any financial conflict of interest that might be construed to influence the results or interpretation of the manuscript.
Abbreviations
- Cav-1
caveolin-1
- p190RhoGAP
p190 RhoGTPase activating protein
- ROCK1
Rho Kinase
Footnotes
AUTHOR CONTRIBUTIONS
S.T, J.B.O, M.N, M.A.S. and D.T. designed the experiments and analyzed the data. S.T, J.B.O, M.N, P.D.W, C.O, M. S-C and H. F.F performed the experiments. S.T, M.A.S. and D.T wrote the manuscript.
REFERENCES
- 1.Anderson RG, Jacobson K. A role for lipid shells in targeting proteins to caveolae, rafts, and other lipid domains. Science. 2002;296:1821–1825. doi: 10.1126/science.1068886. [DOI] [PubMed] [Google Scholar]
- 2.Ikonen E, Parton RG. Caveolins and cellular cholesterol balance. Traffic. 2000;1:212–217. doi: 10.1034/j.1600-0854.2000.010303.x. [DOI] [PubMed] [Google Scholar]
- 3.Del Pozo MA, Schwartz MA. Rac, membrane heterogeneity, caveolin and regulation of growth by integrins. Trends Cell Biol. 2007;17:246–250. doi: 10.1016/j.tcb.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Liu P, Rudick M, Anderson RG. Multiple functions of caveolin-1. J Biol Chem. 2002;277:41295–41298. doi: 10.1074/jbc.R200020200. [DOI] [PubMed] [Google Scholar]
- 5.Navarro A, Anand-Apte B, Parat MO. A role for caveolae in cell migration. FASEB J. 2004;18:1801–1811. doi: 10.1096/fj.04-2516rev. [DOI] [PubMed] [Google Scholar]
- 6.Williams TM, Lisanti MP. Caveolin-1 in oncogenic transformation, cancer, and metastasis. Am J Physiol Cell Physiol. 2005;288:C494–C506. doi: 10.1152/ajpcell.00458.2004. [DOI] [PubMed] [Google Scholar]
- 7.Fine SW, Lisanti MP, Galbiati F, Li M. Elevated expression of caveolin-1 in adenocarcinoma of the colon. Am J Clin Pathol. 2001;115:719–724. doi: 10.1309/YL54-CCU7-4V0P-FDUT. [DOI] [PubMed] [Google Scholar]
- 8.Ho CC, Huang PH, Huang HY, Chen YH, Yang PC, Hsu SM. Up-regulated caveolin-1 accentuates the metastasis capability of lung adenocarcinoma by inducing filopodia formation. Am J Pathol. 2002;161:1647–1656. doi: 10.1016/S0002-9440(10)64442-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ito Y, Yoshida H, Nakano K, et al. Caveolin-1 overexpression is an early event in the progression of papillary carcinoma of the thyroid. Br J Cancer. 2002;86:912–916. doi: 10.1038/sj.bjc.6600172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Podar K, Tai YT, Cole CE, et al. Essential role of caveolae in interleukin-6- and insulin-like growth factor I-triggered Akt-1-mediated survival of multiple myeloma cells. J Biol Chem. 2003;278:5794–5801. doi: 10.1074/jbc.M208636200. [DOI] [PubMed] [Google Scholar]
- 11.Suzuoki M, Miyamoto M, Kato K, et al. Impact of caveolin-1 expression on prognosis of pancreatic ductal adenocarcinoma. Br J Cancer. 2002;87:1140–1144. doi: 10.1038/sj.bjc.6600619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fong A, Garcia E, Gwynn L, Lisanti MP, Fazzari MJ, Li M. Expression of caveolin-1 and caveolin-2 in urothelial carcinoma of the urinary bladder correlates with tumor grade and squamous differentiation. Am J Clin Pathol. 2003;120:93–100. doi: 10.1309/292N-HAYN-WAVR-EJ37. [DOI] [PubMed] [Google Scholar]
- 13.Rajjayabun PH, Garg S, Durkan GC, Charlton R, Robinson MC, Mellon JK. Caveolin-1 expression is associated with high-grade bladder cancer. Urology. 2001;58:811–814. doi: 10.1016/s0090-4295(01)01337-1. [DOI] [PubMed] [Google Scholar]
- 14.Irby RB, Yeatman TJ. Role of Src expression and activation in human cancer. Oncogene. 2000;19:5636–5642. doi: 10.1038/sj.onc.1203912. [DOI] [PubMed] [Google Scholar]
- 15.Fanning P, Bulovas K, Saini KS, Libertino JA, Joyce AD, Summerhayes IC. Elevated expression of pp60c-src in low grade human bladder carcinoma. Cancer Res. 1992;52:1457–1462. [PubMed] [Google Scholar]
- 16.Glenney JR, Jr, Zokas L. Novel tyrosine kinase substrates from Rous sarcoma virus-transformed cells are present in the membrane skeleton. J Cell Biol. 1989;108:2401–2408. doi: 10.1083/jcb.108.6.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee H, Volonte D, Galbiati F, et al. Constitutive and growth factor-regulated phosphorylation of caveolin-1 occurs at the same site (Tyr-14) in vivo: identification of a c-Src/Cav-1/Grb7 signaling cassette. Mol Endocrinol. 2000;14:1750–1775. doi: 10.1210/mend.14.11.0553. [DOI] [PubMed] [Google Scholar]
- 18.Cao H, Courchesne WE, Mastick CC. A phosphotyrosine-dependent protein interaction screen reveals a role for phosphorylation of caveolin-1 on tyrosine 14: recruitment of C-terminal Src kinase. J Biol Chem. 2002;277:8771–8774. doi: 10.1074/jbc.C100661200. [DOI] [PubMed] [Google Scholar]
- 19.Grande-Garcia A, Echarri A, de Rooij J, et al. Caveolin-1 regulates cell polarization and directional migration through Src kinase and Rho GTPases. J Cell Biol. 2007;177:683–694. doi: 10.1083/jcb.200701006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith SC, Oxford G, Baras AS, et al. Expression of ral GTPases, their effectors, and activators in human bladder cancer. Clin Cancer Res. 2007;13:3803–3813. doi: 10.1158/1078-0432.CCR-06-2419. [DOI] [PubMed] [Google Scholar]
- 21.Morgenstern JP, Land H. A series of mammalian expression vectors and characterisation of their expression of a reporter gene in stably and transiently transfected cells. Nucleic Acids Res. 1990;18:1068. doi: 10.1093/nar/18.4.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nicholson BE, Frierson HF, Conaway MR, et al. Profiling the evolution of human metastatic bladder cancer. Cancer Res. 2004;64:7813–7821. doi: 10.1158/0008-5472.CAN-04-0826. [DOI] [PubMed] [Google Scholar]
- 23.Ishizawar RC, Tice DA, Karaoli T, Parsons SJ. The C terminus of c-Src inhibits breast tumor cell growth by a kinase-independent mechanism. J Biol Chem. 2004;279:23773–23781. doi: 10.1074/jbc.M312368200. [DOI] [PubMed] [Google Scholar]
- 24.Sunaga N, Miyajima K, Suzuki M, et al. Different roles for caveolin-1 in the development of non-small cell lung cancer versus small cell lung cancer. Cancer Res. 2004;64:4277–4285. doi: 10.1158/0008-5472.CAN-03-3941. [DOI] [PubMed] [Google Scholar]
- 25.Blaveri E, Simko JP, Korkola JE, et al. Bladder cancer outcome and subtype classification by gene expression. Clin Cancer Res. 2005;11:4044–4055. doi: 10.1158/1078-0432.CCR-04-2409. [DOI] [PubMed] [Google Scholar]
- 26.Sanchez-Carbayo M, Socci ND, Lozano J, Saint F, Cordon-Cardo C. Defining molecular profiles of poor outcome in patients with invasive bladder cancer using oligonucleotide microarrays. J Clin Oncol. 2006;24:778–789. doi: 10.1200/JCO.2005.03.2375. [DOI] [PubMed] [Google Scholar]
- 27.Stein JP, Lieskovsky G, Cote R, et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol. 2001;19:666–675. doi: 10.1200/JCO.2001.19.3.666. [DOI] [PubMed] [Google Scholar]
- 28.Parat MO, Anand-Apte B, Fox PL. Differential caveolin-1 polarization in endothelial cells during migration in two and three dimensions. Mol Biol Cell. 2003;14:3156–3168. doi: 10.1091/mbc.E02-11-0761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Summy JM, Gallick GE. Src family kinases in tumor progression and metastasis. Cancer Metastasis Rev. 2003;22:337–358. doi: 10.1023/a:1023772912750. [DOI] [PubMed] [Google Scholar]
- 30.Kamai T, Tsujii T, Arai K, et al. Significant association of Rho/ROCK pathway with invasion and metastasis of bladder cancer. Clin Cancer Res. 2003;9:2632–2641. [PubMed] [Google Scholar]
- 31.Shen CH, Chen HY, Lin MS, et al. Breast tumor kinase phosphorylates p190RhoGAP to regulate rho and ras and promote breast carcinoma growth, migration, and invasion. Cancer Res. 2008;68:7779–7787. doi: 10.1158/0008-5472.CAN-08-0997. [DOI] [PubMed] [Google Scholar]
- 32.Joshi B, Strugnell SS, Goetz JG, et al. Phosphorylated caveolin-1 regulates Rho/ROCK-dependent focal adhesion dynamics and tumor cell migration and invasion. Cancer Res. 2008;68:8210–8220. doi: 10.1158/0008-5472.CAN-08-0343. [DOI] [PubMed] [Google Scholar]
- 33.Pertz O, Hodgson L, Klemke RL, Hahn KM. Spatiotemporal dynamics of RhoA activity in migrating cells. Nature. 2006;440:1069–1072. doi: 10.1038/nature04665. [DOI] [PubMed] [Google Scholar]
- 34.Sanguinetti AR, Cao H, Corley Mastick C. Fyn is required for oxidative- and hyperosmotic-stress-induced tyrosine phosphorylation of caveolin-1. Biochem J. 2003;376:159–168. doi: 10.1042/BJ20030336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uehata M, Ishizaki T, Satoh H, et al. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389:990–994. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- 36.Nitz MD, Harding MA, Theodorescu D. Invasion and metastasis models for studying RhoGDI2 in bladder cancer. Methods Enzymol. 2008;439:219–233. doi: 10.1016/S0076-6879(07)00417-X. [DOI] [PubMed] [Google Scholar]
- 37.Loskog A, Ninalga C, Hedlund T, Alimohammadi M, Malmstrom PU, Totterman TH. Optimization of the MB49 mouse bladder cancer model for adenoviral gene therapy. Lab Anim. 2005;39:384–393. doi: 10.1258/002367705774286475. [DOI] [PubMed] [Google Scholar]
- 38.Kunze E, Von Bonin F, Werner C, Wendt M, Schlott T. Transitional cell carcinomas and nonurothelial carcinomas of the urinary bladder differ in the promoter methylation status of the caveolin-1, hDAB2IP and p53 genes, but not in the global methylation of Alu elements. Int J Mol Med. 2006;17:3–13. [PubMed] [Google Scholar]
- 39.O'Donnell MA. Advances in the management of superficial bladder cancer. Semin Oncol. 2007;34:85–97. doi: 10.1053/j.seminoncol.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 40.Ishizawar R, Parsons SJ. c-Src and cooperating partners in human cancer. Cancer Cell. 2004;6:209–214. doi: 10.1016/j.ccr.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 41.Kawakatsu T, Ogita H, Fukuhara T, et al. Vav2 as a Rac-GDP/GTP exchange factor responsible for the nectin-induced, c-Src- and Cdc42-mediated activation of Rac. J Biol Chem. 2005;280:4940–4947. doi: 10.1074/jbc.M408710200. [DOI] [PubMed] [Google Scholar]
- 42.Arthur WT, Burridge K. RhoA inactivation by p190RhoGAP regulates cell spreading and migration by promoting membrane protrusion and polarity. Mol Biol Cell. 2001;12:2711–2720. doi: 10.1091/mbc.12.9.2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oxford G, Owens CR, Titus BJ, et al. RalA and RalB: antagonistic relatives in cancer cell migration. Cancer Res. 2005;65:7111–7120. doi: 10.1158/0008-5472.CAN-04-1957. [DOI] [PubMed] [Google Scholar]
- 44.Gildea JJ, Seraj MJ, Oxford G, et al. RhoGDI2 is an invasion and metastasis suppressor gene in human cancer. Cancer Res. 2002;62:6418–6423. [PubMed] [Google Scholar]