Abstract
A key feature of biomineralization processes is that they take place within confined volumes, in which the local environment can have significant effects on mineral formation. Herein, we investigate the influence of confinement on the formation mechanism and structure of calcium phosphate (CaP). This is of particular relevance to the formation of dentine and bone, structures of which are based on highly mineralized collagen fibrils. CaP was precipitated within 25–300 nm diameter, cylindrical pores of track etched and anodised alumina membranes under physiological conditions, in which this system enables systematic study of the effects of the pore size in the absence of a structural match between the matrix and the growing crystals. Our results show that the main products were polycrystalline hydroxapatite (HAP) rods, together with some single crystal octacalcium phosphate (OCP) rods. Notably, we demonstrate that these were generated though an intermediate amorphous calcium phosphate (ACP) phase, and that ACP is significantly stabilised in confinement. This effect may have significance to the mineralization of bone, which can occur through a transient ACP phase. We also show that orientation of the HAP comparable, or even superior to that seen in bone can be achieved through confinement effects alone. Although this simple experimental system cannot be considered, a direct mimic of the in vivo formation of ultrathin HAP platelets within collagen fibrils, our results show that the effects of physical confinement should not be neglected when considering the mechanisms of formation of structures, such as bones and teeth.
Keywords: amorphous calcium phosphate, biomimetic synthesis, biomineralization, collagen, hydroxapatite
Introduction
Driven by the need to fabricate synthetic bone substitutes and to develop tissue-engineering strategies, which promote bone healing in vivo, there is a considerable interest in understanding the structure of bone and its formation mechanisms. Bone is a composite material that principally comprises collagen and nonstoichiometric carbonated hydroxyapatite (HAP). It exhibits a remarkable hierarchical structure that spans the self-assembly of collagen fibrils[1] through the formation of co-oriented arrays of plate-like HAP crystals within the fibrils, to the ultimate generation of the macroscopic bone structure.[2] Originating within the gap regions in the collagen fibrils, the HAP crystallites are the smallest known biogenic crystals, taking the form of platelets 30– 50 nm wide, 60–100 nm long and a mere 2–6 nm thick.[2a, 3] These distinct crystallites are also preferentially oriented within the collagen matrix, such that their c axes lie parallel to the long axis of the collagen fibril.[2b, 3]
The ability to achieve a similar degree of control over mineral formation in synthetic systems is clearly highly attractive and can be facilitated by identification of the key strategies, by which this is achieved in vivo. Therefore, significant efforts have been made to identify how mineralization occurs within collagen fibrils, in which orientation of the HAP crystals has generally been considered to be mediated by a structural relationship between the insoluble organic matrix and the developing crystals.[4] However, a factor, which has received much less attention, is the influence of the confinement provided by the channels, and only a few reports have suggested that this may place a role in controlling the orientation of HAP in mineralized collagen.[5]
Although effects of nanoscale confinement on crystallization are well recognized, the majority of studies have focused on the freezing of substances, such as water and small organics in nanoscale pores, revealing a depression in the freezing point, and a stabilisation of amorphous forms.[6] However, studies of crystallization from solution have shown that confinement can have dramatic effects on features including morphology,[7] polymorph,[8] orientation[9] and single-crystal/polycrystalline character.[10] Further, stabilization of amorphous calcium carbonate[11] and amorphous calcium sulfate[8c] between surfaces separated by up to amicron have demonstrated that effects can be observed at surprising large-length scales. Looking specifically at the effects of confinement on CaP precipitation, sequential transformation of ACP to HAP through octacalcium phosphate (OCP) has been observed within the confines of crosslinked gelatine nanoparticles.[12] Further, preferential alignment of HAP crystals has been observed within uniaxially deformed gelatine films,[13] and in bundles of nanofibres,[14] in which the [001] axis of HAP showed preferential alignment with the long axes of the fibres.
Herein, we address this issue and use a simple system to explore the effects of confinement on calcium phosphate (CaP) precipitation. We stress that the system used—precipitation within the cylindrical pores of polycarbonate track etched or alumina membranes—is not considered as a direct mimic of the collagen system. Indeed, the pore sizes employed (25–300 nm) are considerably larger than the 2–6 nm deep gap regions or intermolecular spaces between triple helices in collagen fibrils. However, in facilitating a systematic investigation of the effect of pore size on CaP precipitation in the absence of any possible structural match between the matrix and the growing CaP crystals, we gain unique insight into the roles of confinement in this system. In particular, we show that orientation comparable or even superior to that seen in bone can be achieved through confinement effects alone, as can a significant stabilization of amorphous calcium phosphate (ACP).
Results and Discussion
CaP was precipitated within the pores of track etched membranes by using a double-diffusion method, in which a membrane was located between a pair of U-tube arms containing buffered 9 mm CaCl2·2H2O and 4.2 mm K2HPO4·3H2O solutions at pH 7.4 at 37 °C (Figure 1). The apparatus was sealed and was then placed in an incubator at 37 °C. After reaction, the intra-membrane particles were isolated by dissolution of the membranes in dichloromethane. The effects of confinement were demonstrated by comparison with control experiments, in which CaP was precipitated under identical reaction conditions, but in bulk solution rather than in the membrane pores. In all cases, particle morphologies were determined by using transmission electron microscopy (TEM), and polymorphs were typically confirmed by using electron diffraction, XRD and Raman microscopy. All statistical data were obtained from analysis of populations of at least ten rods.
Figure 1.
Schematic diagrams of a) the precipitation of CaP within the membrane pores by using a double-diffusion configuration and b) the formation of HAP crystals within the membrane pores. The ACP particles (yellow) begin to transform to HAP (red), which under competitive growth become preferentially oriented with their [001] axis parallel to the long axis of the pore.
Precipitation of CaP in bulk solution gave 10 nm spherical amorphous CaP particles after one hour, which had partially transformed to 50 nm octacalcium phosphate (OCP) platelets after 2 h (Figure S1 in the Supporting Information). Plate-like HAP crystals of approximate dimensions 150× 200 nm (Figure S1 in the Supporting Information) were isolated after 3 h, in which HAP was identified by using Raman microscopy and the presence of characteristic peaks at 961 cm−1 (ν̃1), 610 cm−1 and 590 cm−1 (ν̃4), at 443 cm−1 and 429 cm−1 (ν̃2) and a very weak peak at 1043 cm−1 (ν̃3 ; Figure S in the Supporting Information).[15] XRD analysis was also used to confirm this assignment, in which the {121} peak at 31.78° confirmed the HAP polymorph (Figure S2 in the Supporting Information).[15] In contrast, the CaP particles isolated from the membrane pores were entirely distinct in size, morphology and structure, demonstrating the effect of confinement on the precipitation process (Figure 2). A high yield of rods with average lengths of around 1 µm in the 200 nm pores and about 2 µm in the 50 nm pores were obtained, indicating that the CaP only partially infiltrates into the 10 µm long pore. In should also be noted that these membranes are sold for use in filtration, such that the pore diameter quoted is the pore size at the membrane surface, rather than the internal diameter. The rods precipitated in the “200 nm” pores therefore have widths of 200–250 nm, giving overall aspect ratios of five, whereas the rods formed in the “50 nm” pores have widths of 50–100 nm, corresponding to aspect ratios of about 40 (Figure 2).
Figure 2.
a) SEM and TEM (inset) images of rods formed in 200 nm pores after six days. b) Rods formed in 50 nm pores after one day, which are composed of small particles (inset). c) HAP rod isolated after one day from 50 nm pores.
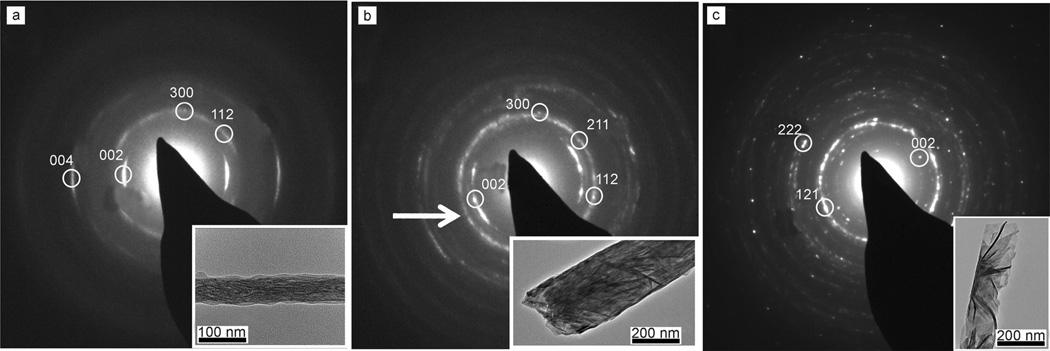
TEM examination of these rods revealed that almost all were polycrystalline HAP after one day reaction time, in which these were constructed of small particles of about 100 nm in length for rods precipitated in the 200 nm pores and 30 nm in length for rods precipitated in the 50 nm pores (Figure 2 a and b insets, respectively). The remainder of the rods were single crystals of OCP, which were comparable in size to the polycrystalline HAP rods (Figure S3a in the Supporting Information). No preferential orientation of these rods with respect to the membrane-pore axis was observed. Earlier reaction times (3–6 h) gave particles that were either partially or fully amorphous, as was determined by electrondiffraction analysis (Figure S3b in the Supporting Information). In contrast, selected-area electron diffraction (SAED) analysis of the polycrystalline HAP rods revealed an interesting and surprising feature; although the surface chemistry of the membrane itself cannot direct the orientation of the HAP crystal at nucleation, many rods exhibited a marked degree of orientation. This is shown by the presence of arcs in the SAED patterns, corresponding to a preferential alignment of the [001] axis of HAP with the long axis of the rod (Figures 2 c and 3 a and b). This alignment could be observed for rods formed in both the 50 and 200 nm pores, but was more significant for the 50 nm pores, in which 95% of the HAP rods were oriented as compared with 75% in the 200 nm pores (Figure 3 a and b). A diffraction pattern from a non-oriented rod of HAP is shown in Figure 3 c. Analysis of multiple samples showed that the angular spread of the arcs was ±15–20° for the 50 nm and ±15–25° for the 200 nm oriented rods, which is comparable to that found in bone.[2b] However, the oriented 200 nm rods often showed evidence of a sub-population of HAP crystallites oriented in different directions, as was indicated by additional weak reflections in the SAED pattern (shown with arrows in Figure 3 b). This further shows that this orientation effect was dependent on the size of the pores.
Figure 3.
SAED images with corresponding TEM images of templated HAP rods, precipitated in a) 50 nm and b) 200 nm pores, in which the arrow indicates a sub-population of HAP crystallites oriented in different directions. c) Non-oriented rod formed in a 200 nm pore.
These simple experiments clearly show that confinement on the length scales used herein (50–200 nm) can significantly affect the precipitation of CaP, influencing the size, morphology, orientation and rate of crystallization. Although bundles of nanoplatelets of HAP were generated in bulk solution, morphologically distinct polycrystalline rods of HAP with aspect ratios of up to 20–40 times were generated in the membrane pores, in which the ability of the rods to remain completely intact during isolation from the membrane suggests significant intergrowth. Such morphological control over the CaP precipitates is consistent with templating studies, which have widely demonstrated that an appropriate reaction volume can mould the morphology of inorganic solids.[16]
A further significant effect of confinement was on the observed rate of crystallization. Although HAP was produced in bulk solutions after 3 h, only amorphous calcium phosphate (ACP) was isolated from the membrane pores after the same time (Figure S3b in the Supporting Information). A similar effect has been observed for CaCO3 precipitation in confinement, in which amorphous calcium carbonate (ACC) was stabilised with respect to the crystalline polymorphs of CaCO3.[10c, 11] This was shown to be due to kinetic rather than thermodynamic factors, and limiting the contact of ACC particles with the solution, as occurs in confinement, was suggested to be the origin of its stabilization. ACP exhibits a variable composition, which is determined by the precipitation conditions, such that it can comprise 10–20 wt% water and exhibit a Ca/P ratio of between 1.18 and 2.50.[17] Similar to ACC, ACP also crystallizes in the presence of water, possibly by dissociation into clusters rather than complete ionic solvation, and the rate of crystallization is recognized to depend on variables including the pH, ionic strength, temperature and the presence of additives.[17–18] Therefore, confinement presents an alternative mechanism of stabilization of ACP, in which this could arise from a reduced ability of ACP to dissolve/dissociate into clusters and to undergo subsequent structural reorganisation into a crystalline phase in a constrained reaction volume. We further suggest that this may also have significance to the mineralization of bone, in which a number of studies have now demonstrated that bone tissue formation can occur through transient ACP phase.[19]
A really striking observation is the marked degree of orientation of the intra-membrane crystals. This can only arise from the confinement offered by the pores, because the functional groups on the membrane surface are randomly oriented and thus could not define the in-plane orientation of the HAP crystals. However, the geometry of the membrane pores examined would not be expected to define the crystal orientation at the point of nucleation, because the curvature of the pores is such that a 2–5 nm crystal nucleus in a 100 nm pore would effectively see a flat surface. We therefore attribute the preferred orientation to competitive growth of the individual crystallites (Figure 1 b), an effect which can give rise to textured polycrystalline materials[20] and the formation of oriented β-glycine crystals within nanoporous polymer monoliths.[21] Indeed, such competitive growth has been observed to give rise to orientation effects and elongation of OCP and HAP crystals in a range of gel and membrane systems.[22]
In growing within the membrane pores, if the HAP crystals impinge on the membrane walls, their growth will be retarded, whereas those [001] axis of which lies closer to the long axis of the pore will be able to grow unrestricted. This can only occur because there is a strong anisotropy in the HAP lattice, such that it typically forms as elongated plates or needles, in which the [001] axis is coincident with its direction of most rapid growth.[23] Some re-orientation of the HAP crystals may also occur during growth, such that alignment with the axis of the pore would facilitate continued growth. Although directional ion flow can give rise to the orientation and elongation of OCP crystals,[22a, 24] this effect is not expected to contribute to the effects seen herein, as the HAP crystals form through intermediate ACP rods.
Strong support for this mechanism is provided by the relationship between the pore size and the degree of crystallographic orientation, in which competitive growth is expected to lead to superior orientation in smaller pores. Building on the data obtained from the 50 and 200 nm pores, which clearly show that the rods formed within the 50 nm crystals exhibit superior orientation to their 200 nm counterparts, CaP was also precipitated in larger 300 nm pores in anodised aluminium oxide (AAO) membranes and smaller 25 nm pores in track etched membrane pores. Although the 300 nm pores gave particles that exhibited almost no evidence of orientation (Figure 4 a), the rods precipitated in the 25 nm pores were highly oriented, with angular spreads of ±5–12° (Figure 4 b).
Figure 4.
SAED images with corresponding TEM images (inset) of a) a randomly oriented HAP rod precipitated in a 300 nm pore of an AAO membrane; b) highly oriented rod grown in a 25 nm TE membrane pore, in which the axis of the rod is coincident with the [001] axis.
Having shown that confinement, at the mesoscale, can significantly influence the orientation of hydroxyapatite, the question arises as to whether this mechanism has any relevance to the orientation of the HAP crystallites in biological hierarchical nanocomposites, such as bone and dentine. Strikingly, although mineralized collagen fibrils are organized over much smaller length scales, they exhibit a number of features in common with system studied herein. Both bone[2, 5] and mineralised collagen fibrils generated through in vitro mineralisation of reconstituted collagen fibrils[5, 25] exhibit preferential orientation of the c axes of the mineral crystallites with the long axis of the collagen fibrils such that the typical angular spread in HAP orientation is ±15° compared with ±5–25° in our system. The origin of this orientation effect has been much discussed and is generally attributed to a structural match between the HAP nanocrystals and the amino acid groups on the collagen,[14b, 25b] although the possibility that the confines of the gaps in the collagen may direct orientation has also been suggested.[26] It has also been considered that the angular spread in the diffraction spots for collagen-confined HAP may arise from the helical twist of the collagen microfibrils, such that they can still be highly oriented with respect to the collagen molecules.[2b, 27] This clearly provides a different mechanism for generating angular spread than the synthetic system described herein. Finally, it is noted that there are some reports of the oriented growth of CaP crystals on collagen fibrils, although it is unclear whether this effect is chemical or topographical in origin.[28]
Although structural characterization of collagen indicates that groups of charged amino acids are located within the hole zones that could act as effective nucleation sites,[1, 29] it is very unlikely that these could provide an epitaxial match which would direct the alignment of the HAP crystals with the collagen fibril axis. Therefore, it is possible that the confinement provided by the intermolecular spaces between the triple helices in collagen fibrils may contribute to the observed orientation effects. In addition to a competitive growth mechanism of the type suggested to operate in the track etched membrane pores, the collagen triple helix also has very different curvature properties in the axial plane compared with the cross-sectional plane. This extreme structural anisotropy may also contribute to the co-alignment of the c axes of the HAP crystals with the axis of the collagen molecule.
Conclusion
Our results clearly demonstrate that spatial constraints can both stabilize amorphous calcium phosphate and impose a degree of structural organization on apatitic crystals formed in vitro. Notably, this occurs even at length scales of 100 nm, which is two orders of magnitude greater than the thickness of the gap regions in collagen. Further, pores of 25 nm can direct orientation comparable, or even superior to that seen in bone. Although the simplicity of the experimental system employed herein is such that it cannot be considered a direct mimic of HAP precipitation in collagen, the results presented do give some insight into the control mechanisms, which may operate in vivo. Indeed, although the orientation of the HAP crystallites in bone is frequently attributed to a structural match between hydroxyapatite and the collagen matrix, our results suggest that this is not a pre-requisite to orientation. Further, confinement effects may also contribute to the stabilization of ACP seen during bone formation in vivo. A striking feature of mineralization of collagen fibrils, which we have not been able to investigate herein, is the ability of CaP to infiltrate so effectively into the 2–6 nm gaps in collagen fibrils. It is hard to imagine that this does not derive from the specific chemical structures, and indeed interplay between the collagen matrix and non-collagenous proteins, as is fully consistent with the literature.[5,25b, 30] Therefore, our simple experiments emphasise that the role of the physical confinement imposed by the collagen fibril structure should not be neglected in considering the mechanism of formation of highly organized mineralized collagen fibrils, which provide the building blocks of dentine and bone. Future work will further investigate the potential role of soluble additives on calcium phosphate precipitation in constrained volumes.
Experimental Section
General
Calcium phosphate (CaP) particles were precipitated within the confined environments of track etched (TE) membrane and anodised aluminium oxide (AAO) membrane pores, and the influence of the pore diameter and the reaction conditions on the resulting particles was investigated.
Precipitation of CaP in track etched (TE) and anodised aluminium oxide (AAO) membrane pores
CaP precipitation was carried out in a trissaline buffer prepared by dissolving NaCl (8.77 g), Tris-HCl (6.61 g) and Tris-base (0.96 g) in ultra-pure H2O (1 L) to give a final solution of pH 7.59 at 25°C[5]. This buffer stock solution was then used to prepare separate solutions of CaCl2·2H2O (9mm) and K2HPO4 3H2O (4.2 mm), in which after the pH of both solutions was adjusted using NaOH to 7.4 at 37°C. Two types of polycarbonate track etched membranes were used. Isopore GTTP membranes with 50 and 200 nm pore sizes (Millipore) and ipPORE membranes with 25 nm pores. AAO membranes (commercially available membranes, “anodic 25” from Whatman) with pore sizes of 300 nm were used to study larger pore sizes. The membranes were initially treated in a plasma cleaner for 1 min under an oxygen plasma to increase their hydrophilic character. The membranes were then transferred into vials containing the buffered CaCl2·2H2O solution (4.5 mm), and were then degassed under vacuum and left overnight to ensure filling of the membrane pores with the solution.
CaP particles was precipitated within the membrane pores by using a double-diffusion method (Figure 1a), in which a membrane was sealed between a pair of U-tube arms and buffered CaCl2·2H2O (9mm) and K2HPO4·3H2O (4.2 mm) solutions were added to the two U-tube arms, which were finally sealed with Parafilm to prevent evaporation of the solution. In this way final concentrations of 4.5 and 2.1 mm were obtained of respectively CaCl2·2H2O and K2HPO4·3H2O. Both of these experimental set-ups were then incubated in an oven at 37 °C to mimic body temperature, for times between three hours and six days.
At the end of the reaction, the membranes were removed from the reaction solution, washed with ethanol, and their surfaces were wiped with the edge of glass cover slip to remove the crystals located on the surface of the membrane. The membranes were then rinsed in ethanol and dried with air. To isolate the CaP particles from the membrane pores, the TE membranes were dissolved in dichloromethane, sonicated for two minutes and then centrifuged at 13.2 rpm to separate the inorganic precipitate from the solution. The dichloromethane was subsequently changed, and the sonication, centrifugation and dichloromethane exchange protocol was then repeated a further four times to ensure complete removal of the dissolved polymer from the CaP particles. Finally, the remaining dichloromethane was removed from the centrifuged sample and was exchanged for methanol. The sample was sonicated for two minutes and centrifuged for four minutes, before exchanging for ethanol. This process was repeated two more times with ethanol. The AAO membranes were dissolved by placing them in NaOH (0.5m) for one day. Afterwards, the NaOH solution was removed, and the CaP particles were washed with ethanol. The sample was then sonicated for two minutes and centrifuged for four minutes, before exchanging the ethanol. This step was then repeated three more times.
Control experiments
Control experiments were also carried out under identical reaction conditions, but in bulk solution as opposed to with a membrane present. C-coated Ni TEM grids were placed in the solution, after which they were washed in ethanol and left to dry. The grids were removed at a range of times, and TEM analysis was used to compare the particles precipitated with those formed in bulk solution. For Raman and XRD analyses, the precipitated particles were isolated by filtration, washed with ethanol and left to dry before analysis.
Characterization of the CaP particles
The CaP particles were characterized by using scanning electron microscopy (SEM), TEM, Raman, and XRD techniques to determine their structures, polymorph and morphologies. Samples for SEM were prepared by placing one or two droplets of an ethanol suspension of the isolated intra-membrane CaP particles onto a glass slide, allowing it to dry, mounting the slide on an SEM stub by using adhesive carbon pads, and sputter coating with 10 nm Pt/Pd. SEM was then performed using a LEO 1530 Gemini field-emission gun SEM (FEG-SEM) operating at 2.00 kV. TEM was carried out by placing an ethanol suspension of the isolated CaP particles on a carbon-covered Ni TEM grid, and then studying the dried sample in a Tecnai TF20 transmission electron microscope, operating at 200 kV. Identification of the polymorphs present and orientation of the samples was achieved by using electron diffraction in the TEM by using SAED. Arcs in the diffraction patterns were measured with imageJ after optimization of the brightness and contrast. For each type of pore, at least ten rods were measured to obtain statistically relevant data. Raman microscopy was performed by using a Renishaw in Via-Raman microscope, operating with a 785 nm diode laser as excitation source. XRD was performed by using a Bruker D8 Advanced diffractometer equipped with an X-ray source emitting CuKα1 radiation. Samples were placed on a piece of corundum wafer, and XRD data were collected in an angular range from 5 to 60° in intervals of 0.02°, with a scan rate of 1°min−1.
Acknowledgements
We thank the Engineering and Physical Sciences Research Council (EPSRC) for funding of a Leadership Fellowship (F.C.M. and B.C., EP/ H005374/1) and the NIH for financial support by grant NIH R56 DE016703 (E.B.). This work was also funded under an EPSRC Programme Grant (FCM, EP/I001514/1) which funds the Materials Interface with Biology (MIB) consortium.
Footnotes
Supporting information for this article is available on the WWW under http://dx.doi.org/10.1002/chem.201302835.
References
- 1.Orgel J, Irving TC, Miller A, Wess TJ. Proc. Natl. Acad. Sci. USA. 2006;103:9001–9005. doi: 10.1073/pnas.0502718103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.a) Fratzl P, Weinkamer R. Prog. Mater. Sci. 2007;52:1263–1334. [Google Scholar]; b) Weiner S, Traub W, Wagner HD. J. Struct. Biol. 1999;126:241–255. doi: 10.1006/jsbi.1999.4107. [DOI] [PubMed] [Google Scholar]
- 3.Landis WJ, Hodgens KJ, Arena J, Song MJ, McEwen BF. Microsc. Res. Tech. 1996;33:192–202. doi: 10.1002/(SICI)1097-0029(19960201)33:2<192::AID-JEMT9>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 4.Beniash E. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2011;3:47–69. doi: 10.1002/wnan.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olszta MJ, Cheng X, Jee SS, Kumar R, Kim Y-Y, Kaufman MJ, Douglas EP, Gower LB. Mater. Sci. Eng. R. 2007;58:77–116. [Google Scholar]
- 6.Christenson HK. J. Phys. Condens. Matter. 2001;13:R95–R133. [Google Scholar]
- 7.a) Finnemore AS, Scherer MRJ, Langford R, Mahajan S, Ludwigs S, Meldrum FC, Steiner U. Adv. Mater. 2009;21:3928–3932. [Google Scholar]; b) Wucher B, Yue W, Kulak AN, Meldrum FC. Chem. Mater. 2007;19:1111–1119. [Google Scholar]
- 8.a) Stephens CJ, Kim Y-Y, Evans SD, Meldrum FC, Christenson HK. J. Am. Chem. Soc. 2011;133:5210–5213. doi: 10.1021/ja200309m. [DOI] [PubMed] [Google Scholar]; b) Tester CC, Brock RE, Wu C-H, Krejci MR, Weigand S, Joester D. Cryst Eng Comm. 2011;13:3975–3978. [Google Scholar]; c) Wang Y-W, Christenson HK, Meldrum FC. Adv. Func. Mater. 2013 [Google Scholar]; d) Ha JM, Wolf JH, Hillmyer MA, Ward MD. J. Am. Chem. Soc. 2004;126:3382–3383. doi: 10.1021/ja049724r. [DOI] [PubMed] [Google Scholar]
- 9.Hamilton BD, Weissbuch I, Lahav M, Hillmyer MA, Ward MD. J. Am. Chem. Soc. 2009;131:2588–2596. doi: 10.1021/ja807193s. [DOI] [PubMed] [Google Scholar]
- 10.a) Loste E, Meldrum FC. Chem. Commun. 2001:901–902. [Google Scholar]; b) Loste E, Park RJ, Warren J, Meldrum FC. Adv. Funct. Mater. 2004;14:1211–1220. [Google Scholar]; c) Kim YY, Hetherington NBJ, Noel EH, Kroger R, Charnock JM, Christenson HK, Meldrum FC. Angew. Chem. 2011;123:12780–12785. doi: 10.1002/anie.201104407. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2011;50:12572–12577. doi: 10.1002/anie.201104407. [DOI] [PubMed] [Google Scholar]; d) Yadlovker D, Berger S. J. Appl. Phys. 2007;101 [Google Scholar]
- 11.Stephens CJ, Ladden SF, Meldrum FC, Christenson HK. Adv. Funct. Mater. 2010;20:2108–2115. [Google Scholar]
- 12.Ethirajan A, Ziener U, Chuvilin A, Kaiser U, Colfen H, Land-fester K. Adv. Funct. Mater. 2008;18:2221–2227. [Google Scholar]
- 13.Falini G, Gazzano M, Ripamonti A. J. Mater. Chem. 2000;10:535–538. [Google Scholar]
- 14.a) He T, Abbineni G, Cao BR, Mao CB. Small. 2010;6:2230–2235. doi: 10.1002/smll.201001108. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Hartgerink JD, Beniash E, Stupp SI. Science. 2001;294:1684–1688. doi: 10.1126/science.1063187. [DOI] [PubMed] [Google Scholar]; c) Cao B, Mao C. Langmuir. 2007;23:10701–10705. doi: 10.1021/la7014435. [DOI] [PubMed] [Google Scholar]; d) Newcomb CJ, Bitton R, Velichko YS, Snead ML, Stupp SI. Small. 2012;8:2195–2202. doi: 10.1002/smll.201102150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koutsopoulos S. J. Biomed. Mater. Res. 2002;62:600–612. doi: 10.1002/jbm.10280. [DOI] [PubMed] [Google Scholar]
- 16.Yue WB, Kulak AN, Meldrum FC. J. Mater. Chem. 2006;16:408–416. [Google Scholar]
- 17.Wang LJ, Nancollas GH. Chem. Rev. 2008;108:4628–4669. doi: 10.1021/cr0782574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boskey AL, Posner AS. J. Phys. Chem. 1973;77:2313–2317. [Google Scholar]
- 19.a) Crane NJ, Popescu V, Morris MD, Steenhuis P, Ignelzi MA. Bone. 2006;39:434–442. doi: 10.1016/j.bone.2006.02.059. [DOI] [PubMed] [Google Scholar]; b) Mahamid J, Aichmayer B, Shimoni E, Ziblat R, Li CH, Siegel S, Paris O, Fratzl P, Weiner S, Addadi L. Proc. Natl. Acad. Sci. USA. 2010;107:6316–6321. doi: 10.1073/pnas.0914218107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thompson CV. Ann. Acad. Med. Gedanensis Ann. Rev. Mater. Sci. 2000;30:159–190. [Google Scholar]
- 21.Hamilton BD, Ha JM, Hillmyer MA, Ward MD. Acc. Chem. Res. 2012;45:414–423. doi: 10.1021/ar200147v. [DOI] [PubMed] [Google Scholar]
- 22.a) Iijima M, Moriwaki Y. J. Cryst. Growth. 1991;112:571–579. [Google Scholar]; b) Iijima M, Moriwaki Y. J. Cryst. Growth. 1998;194:125–132. [Google Scholar]
- 23.Simmer JP, Fincham AG. Crit. Rev. Oral Biol. Med. 1995;6:84–108. doi: 10.1177/10454411950060020701. [DOI] [PubMed] [Google Scholar]
- 24.Iijima M, Moriwaki Y, Kuboki Y. Connect. Tissue Res. 1997;36:51–61. doi: 10.3109/03008209709160213. [DOI] [PubMed] [Google Scholar]
- 25.a) Beniash E, Metzler RA, Lam RSK, Gilbert P. J. Struct. Biol. 2009;166:133–143. doi: 10.1016/j.jsb.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Nudelman F, Pieterse K, George A, Bomans PHH, Friedrich H, Brylka LJ, Hilbers PAJ, de With G, Sommerdijk N. Nat. Mater. 2010;9:1004–1009. doi: 10.1038/nmat2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jee SS, Kasinath RK, DiMasi E, Kim YY, Gower L. Cryst eng comm. 2011;13:2077–2083. [Google Scholar]
- 27.Ziv V, Sabanay I, Arad T, Traub W, Weiner S. Microsc. Res. Tech. 1996;33:203–213. doi: 10.1002/(SICI)1097-0029(19960201)33:2<203::AID-JEMT10>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 28.a) Iijima M, Iijima K, Moriwaki Y, Kuboki Y. J. Cryst. Growth. 1994;140:91–99. [Google Scholar]; b) Holbrough JL, Campbell JM, Meldrum FC, Christenson HK. Cryst. Growth Des. 2012;12:750–755. [Google Scholar]; c) Campbell JM, Meldrum FC, Christenson HK. Cryst. Growth Des. 2013;13:1915–1925. [Google Scholar]
- 29.Silver FH, Landis WJ. Connect. Tissue Res. 2011;52:242–254. doi: 10.3109/03008207.2010.551567. [DOI] [PubMed] [Google Scholar]
- 30.Deshpande AS, Beniash E. Cryst. Growth Des. 2008;8:3084–3090. doi: 10.1021/cg800252f. [DOI] [PMC free article] [PubMed] [Google Scholar]