Abstract

We report the discovery of a fluorogenic dye, N1,N3-di(2-aminidonaphthalen-6-yl) propane-1,3-diamine, MitoBlue, which selectively stains functional mitochondria while displaying low toxicity, bright blue emission, and high resistance to photobleaching. Additionally, we show that a biotin-labeled MitoBlue derivative can be used as a handle for the delivery of streptavidin-tagged species to the mitochondria.
Fluorescence imaging techniques, allowing the visualization of molecular events with extraordinary sensitivity and selectivity, have become a fundamental tool in the study of living cells.1−3 The success of these techniques derives in great part from the development of cell-permeable probes that associate with particular cellular structures.4,5 Among them, mitochondria are key organelles that integrate many essential functions in eukaryotic cells.6,7 As expected for such a metabolic focal point, mitochondria have been linked with a number of diseases and have become a major therapeutic target.8,9 Consequently, developing new fluorescent probes that can shed light on mitochondrial biology is a major goal in both basic and applied research. Currently used mitochondrial stains are only available in a few emission wavelengths in the green and red channels and suffer from important shortcomings, such as relatively high toxicity and poor (photo)chemical stability.10 We report a new mitochondrial dye, MitoBlue, which, in addition to being readily accessible, overcomes many of these drawbacks, selectively staining functional mitochondria with both low toxicity and high photostability. Furthermore, MitoBlue displays bright blue emission at 490 nm upon excitation at 329 nm, thus providing a complementary emission channel to commercial Mitotracker dyes.11,12 Moreover, to our knowledge only two blue-emissinve mitochondrial dyes have been described: one relying on the use of well-established triphenylphophonium targeting groups,13,14 and the other, a coumarine derivative, which displays relatively poor mitochondrial specificity.14
MitoBlue was initially designed as a fluorogenic DNA binding agent. We observed that DNA-binding aza-bisbenzamidines display enhanced fluorescence emission upon insertion into the minor groove of A/T-rich DNA sequences,15−19 but despite its good solvatochromic properties, the aminobenzamidine fluorophore displays relatively short excitation and emission wavelengths that are not ideal for cellular imaging applications. Therefore, we decided to explore aza-propamidine analogues with extended aromatic units, such as the bisnaphthalene derivative 6-({3-[(6-carba mimidoylnaphthalen-2-yl)amino]propyl}amino)naphthalene-2-carboximidamide (1), as potential fluorogenic DNA probes with improved spectroscopic properties.20
The target bisamidine 1 was readily synthesized following the procedure outlined in Figure 1. Methyl 6-bromo-2-naphthoate (2) was hydrolyzed, and the resulting acid transformed into the corresponding 6-bromo-2-naphthamide (3). Dehydration of 3,21 followed by Hartwig–Buchwald amination22 and conversion of the nitriles to amidines,19,23 yielded the desired product (1), which was purified by reverse-phase chromatography and isolated as a TFA salt with an overall yield for the whole synthetic sequence of ∼14%. The control monoamidines 5 and 6 were synthesized following similar procedures from the intermediate 4 (Figure 1).
Figure 1.
Top: Synthesis of amino-naphthimidamides. Bottom: Normalized excitation (dashed line) and emission spectra (thin solid line) of 6·TFA in 20 mM TrisHCl, pH 7.5, and 100 mM NaCl, and lambda scan (thick solid line) of chicken embryo fibroblasts (CEF) incubated with 5 μM bisamidine 1 for 20 min.
Compound 6 was used to characterize the photophysical properties of the 6-amino-2-naphthimidamide fluorophore. As expected for a push–pull conjugated system,24 this fluorophore is environment-sensitive, displaying a relatively weak emission in aqueous media (Φ[H2O] = 0.49), but increasing its quantum yield in more hydrophobic solvents (Φ[iPrOH] = 0.78; Φ[EtOH] = 0.67) and particularly in nonprotic media (Φ[dioxane] = 0.94; Φ[THF] = 0.92). Application of the Lippert–Mataga model allowed us to calculate the difference between the dipole moments in the ground and the excited states as a measure of the sensitivity to the polarity.25−28 The resulting value of Δμ = 7.8 D is of the same order as those measured for most environment-sensitive fluorophores, such as NBD (Δμ = 3.6)29 or 6-DMN (Δμ = 5.5),30 and accounts for the significant sensitivity to the solvent polarity. Furthermore, as expected for this type of cationic bisamidines,15,31 compound 1 behaved in vitro as a DNA minor groove binder, exhibiting good affinity for extended A/T-rich sites (Supporting Information).
Following the photophysical and in vitro characterization of the fluorophore, we next studied the behavior of 1 in cellular settings. Therefore, cells from a primary culture of chicken embryo fibroblasts (CEF) were incubated with 5 μM of bisamidine 1 for 20 min. The cells were then washed and directly observed under the microscope without fixation. Surprisingly, despite its significant in vitro DNA binding affinity, the intracellular emission was not concentrated in the cell nuclei, but showed instead a cytosolic filamentous pattern, consistent with mitochondrial localization of the dye (Figure 2, top row, left panel). Indeed, counter-stained cells with the mitochondrial marker Mitotracker Red (Figure 2, top row, middle) confirm that both dyes display superimposable distributions (Figure 2, top right panel), thus demonstrating that 1, henceforward MitoBlue, represents both a new mitochondrial-targeting structural motif and a selective mitochondrial dye. Quantitative analysis of the colocalization of both dyes consistently resulted in Pearson’s Correlation Coefficients in the order of 0.9.32,33 To confirm that MitoBlue can be used as a general marker for mitochondria, we carried out the same double-staining experiment with Vero, BHK, DF1, and HeLa cell lines, and in all cases, the staining is coincident with that of Mitotracker Red (Supporting Information).34 In contrast with MitoBlue, the control monoamidine 5 shows less selectivity in its mitochondrial staining and significant background emission in the cytoplasm (Figure 2, bottom row); the more hydrophilic monoamidine 6 does not show any intracellular staining, demonstrating that the dicationic nature of MitoBlue is relevant for its selectivity. Cytotoxicity was evaluated with trypan blue,35 and no significant differences are observed between the cell culture stained with MitoBlue and control cells (see Supporting Information). Incidentally, unlike many other mitochondrial stains, MitoBlue is well retained after fixation with 4% paraformaldehyde (PFA).36
Figure 2.
Double staining of living CEF cells with Mitotracker Red. Top row images correspond to MitoBlue (1), and bottom row to mononaphtylamidine 5. The Mitotracker images and the corresponding overlays are in the center and right columns, respectively.
The cellular distribution of MitoBlue is consistent with quantitative structure–activity relationship models that point to mitochondrial targeting for compounds with pKa > 10, charge (Z) > 0, largest conjugate fragment (LCF) < 17, conjugated bond number (CBN) < 40, and 2 > Log Pcation > 0.37−39 MitoBlue, with pKa ≈ 11.3, Z = 2, LCF = 15, CBN = 30, and Log Pcation ≈ −2.9,40 meets all these criteria except the Log P value, which is included in those models as a measure of efficient membrane translocation. However, despite their high polarity, amidinium derivatives exhibit excellent transport properties. Additionally, the distribution of MitoBlue is comparable to that of other delocalized lipophilic cations (DLCs),41,42 such as triphenylphosphonium derivatives,43 rhodamine 123,44 or even certain bisbenzamidines.45
As expected for an electrostatic trapping mechanism, no staining is observed with MitoBlue if the mitochondria are previously depolarized with CCCP (carbonyl cyanide 3-chlorophenyl hydrazone).46,47 In that case, only dead cells seem to efficiently uptake the dye, which is then mainly located in nuclei. Furthermore, the addition of CCCP after the cells have been labeled with MitoBlue leads to dye leakage and complete loss of fluorescence after 30 min (Supporting Information).48 Interestingly, a lambda scan performed with a confocal microscopy on MitoBlue-labeled CEF cells is consistent with the MitoBlue dye partially inserted in a hydrophobic environment, as evidenced by the slight displacement of the maximum emission to shorter wavelengths (see Figure 1, bottom). Taken together, these data suggest that MitoBlue is electrostatically driven to the mitochondria by the large electrochemical potential generated by the electron transport chain,49 and most probably accumulates in the mitochondrial membrane, where it displays enhanced emission due to the surrounding hydrophobic environment.
We next compared the photostability of MitoBlue against standard mitochondrial dyes. Vero cells were incubated with 5 μM MitoBlue, 2 μM rhodamine 123, or 0.5 μM Mitotracker Red; each sample was then continuously irradiated with the fluorescence microscope through the 100× objective, and images were collected after specific times. The green rhodamine 123 displays very poor photostability, being almost completely photolyzed after 30 s of irradiation (Figure 3, top row). Mitotraker Red shows more resistance to fading, but photobleaching is complete before 60 s under the light source (Figure 3, center row). In contrast with these, MitoBlue shows almost no degradation in its emission after 2 min under the same conditions and is still clearly visible even after 8 min of steady irradiation (Figure 3, bottom row).
Figure 3.
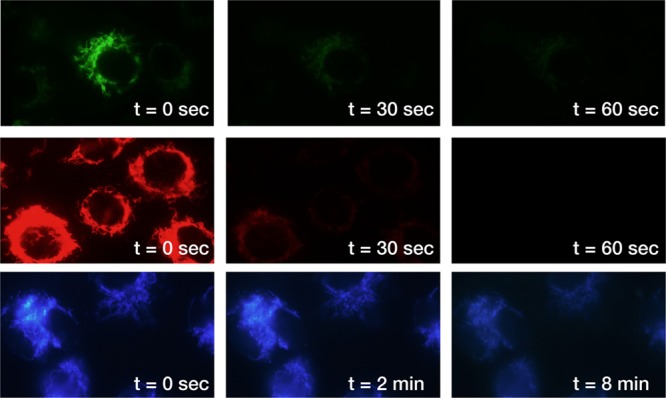
Vero cells incubated with rhodamine 123 (top row), Mitotracker Red (middle row), and MitoBlue (bottom row). Images were taken after the indicated irradiation times. All micrographs were taken using the same settings for comparison.
Another advantage of MitoBlue derives from its straightforward and versatile synthesis, which can be easily tailored for introducing new functionalities into its structure. In this context, we decided to synthesize the biotinylated MitoBlue derivative (7, bt-MitoBlue, Figure 4) as a potential affinity tag for capturing streptavidin conjugates in the mitochondria.50
Figure 4.
Biotinylated MitoBlue (bt-MitoBlue) is synthesized from intermediate 4 in five operational steps (see Supporting Information). Mitochondrial capture with bt-MitoBlue. (A) CEF cells treated with MitoBlue and bt-MitoBlue using stvAF594 as secondary label. Left image, blue channel; center, red channel; right, superposition of both images. (B) CEF cells treated with bt-MitoBlue. Left image, stvAF405 as secondary label (blue channel); center, Qdot565 as secondary label (green channel); right, no secondary marker (blue channel).
In order to assess the viability of this strategy, we incubated CEF cells for 2 h with 20 μM bt-MitoBlue, allowed the cells to stand for 5 h in DMEM medium for its effective intracellular redistribution, and then treated them with 10 μM MitoBlue for 20 min as a control for mitochondrial localization.51 After washing with PBS buffer, fixation with 4% PFA, and permeabilization with 0.5% Triton X100 for 3 min, cells were blocked with 2% BSA and incubated with the streptavidin-Alexa Fluor 594 conjugate (stvAF594) for 1.5 h.52 As expected, observation of the blue channel, corresponding to the MitoBlue emission, shows the typical mitochondrial network pattern (Figure 4A, left); more importantly, the distribution of the stvAF594 conjugate is also consistent with mitochondrial targeting (red channel, Figure 4A, center) and fully coincident with that of MitoBlue (merge, Figure 4A, right). This result can be reproduced with other streptavidin conjugates, such as with Alexa Fluor 405 (stvAF405) or the quantum dot conjugate Qdot565, both of which also localize in the mitochondria in the presence of bt-MitoBlue (Figure 4B, left and center, respectively). Importantly, control experiments demonstrated that stvAF405 does not concentrate in the mitochondria in the absence of bt-MitoBlue, but instead displays poor labeling and weak emission, barely visible at the same exposure settings in the microscope (Supporting Information). Also, as expected, no emission is observed with bt-MitoBlue if the secondary labeling with the fluorescent streptavidin conjugates is omitted (Figure 4B, right). Taken together these results support the use of bt-MitoBlue as an effective and versatile mitochondrial affinity tag.
In summary, MitoBlue is a nontoxic, blue-emitting dye that stains functional mitochondria with high selectivity, retaining its localization and emissive properties after cell fixation with PFA. MitoBlue displays high resistance to photobleaching, making it particularly appropriate for experiments requiring long or multiple exposures. This set of advantages makes MitoBlue quite unique among most mitochondrial dyes. In addition to that, MitoBlue represents a new mitochondrial targeting system that can be readily derivatized for mitochondrial delivery of chemical cargoes and, in the form of a biotin conjugate, can be used as an organelle affinity tag, selectively driving streptavidin conjugates into the mitochondria in fixated cell cultures.
Acknowledgments
We are thankful for the support given by the Spanish grants SAF2013-41943-R, CTQ2012-31341, and BFU2013-43513-R, the Xunta de Galicia, GRC2013-041, the ERDF and the European Research Council (Advanced Grant Na 340055). M.I.S. thanks the Spanish MCINN for his Ph.D. fellowship. We also thank the insights and advice kindly provided by Prof. M. Murphy (MRC Mitochondrial Biology Unit).
Supporting Information Available
Synthesis and characterization, fluorescence spectroscopy, DNA binding studies, and in vitro cellular experiments, including costaining experiments, toxicity assays, and controls. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Contributions
M.I.S. and J.M.-C. carried out the experiments; J.M.-C. also designed and analyzed the biological experiments and advised on cell biology; J.M.C., J.L.M., and M.E.V. supervised the work and prepared the manuscript.
The authors declare the following competing financial interest(s): A patent application describing the synthesis and applications of MitoBlue as mitochondrial stain has been filed.
Supplementary Material
References
- Giepmans B. N. G.; Adams S. R.; Ellisman M. H.; Tsien R. Y. (2006) The fluorescent toolbox for assessing protein location and function. Science 312, 217–224. [DOI] [PubMed] [Google Scholar]
- Mason W. T., Dempster J., Hoyland J., McCann T. J., Somasundaram B., and O’Brien W. (1999) Fluorescent and Luminescent Probes for Biological Activity (Mason W.T., Ed.) pp 175, Academic Press, London. [Google Scholar]
- Stephens D. J.; Allan V. J. (2003) Light microscopy techniques for live cell imaging. Science 300, 82–86. [DOI] [PubMed] [Google Scholar]
- Satori C. P.; Henderson M. M.; Krautkramer E. A.; Kostal V.; Distefano M. M.; Arriaga E. A. (2013) Bioanalysis of eukaryotic organelles. Chem. Rev. 113, 2733–2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazelwood K. L., Olenych S. G., Murphy C. S., and Davidson M. W. (2008) Microscopic Image Analysis, (Rittscher J., Machiraju R., Wong S. T. C., Eds.) pp 19, Artech House, Inc., Norwood MA. [Google Scholar]
- Wallace D. C. (1999) Mitochondrial diseases in man and mouse. Science 283, 1482–1488. [DOI] [PubMed] [Google Scholar]
- Youle R. J.; Karbowski M. (2005) Mitochondrial fission in apoptosis. Nat. Rev. Mol. Cell Biol. 6, 657–663. [DOI] [PubMed] [Google Scholar]
- Armstrong J. S. (2009) Mitochondrial medicine: Pharmacological targeting of mitochondria in disease. Br. J. Pharmacol. 151, 1154–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulda S.; Galluzzi L.; Kroemer G. (2010) Targeting mitochondria for cancer therapy. Nat. Rev. Drug Discovery 9, 447–464. [DOI] [PubMed] [Google Scholar]
- Current dyes usually incorporate a reactive chloromethyl moiety that results in higher toxicity and reduced stability.Minamikawa T.; Sriratana A.; Williams D. A.; Bowser D. N.; Hill J. S.; Nagley P. (1999) Chloromethyl-X-rosamine (MitoTracker Red) photosensitises mitochondria and induces apoptosis in intact human cells. J. Cell Sci. 112, 2419–2430. [DOI] [PubMed] [Google Scholar]
- Neto B. A. D.; Carvalho P. H. P. R.; Santos D. C. B. D.; Gatto C. C.; Ramos L. M.; Vasconcelos N. M.; Corrêa J. R.; deCosta M. B.; de Oliveira H. C. B.; Silva R. G. (2012) Synthesis, properties and highly selective mitochondria staining with novel, stable and superior benzothiadiazole fluorescent probes. RSC Adv. 2, 1524–1532. [Google Scholar]
- Plymale D. R.; Haskins J. R.; de la Iglesia F. A. (1999) Monitoring simultaneous subcellular events in vitro by means of coherent multiprobe fluorescence. Nat. Med. 5, 351–355. [DOI] [PubMed] [Google Scholar]
- Leung C. W. T.; Hong Y.; Chen S.; Zhao E.; Lam J. W. Y.; Tang B. Z. (2013) A photostable AIE luminogen for specific mitochondrial imaging and tracking. J. Am. Chem. Soc. 135, 62–65. [DOI] [PubMed] [Google Scholar]
- Guo D.; Chen T.; Ye D.; Xu J.; Jiang H.; Chen K.; Wang H.; Liu H. (2011) Cell-permeable iminocoumarine-based fluorescent dyes for mitochondria. Org. Lett. 13, 2884–2887. [DOI] [PubMed] [Google Scholar]
- Sánchez M. I.; Vazquez O.; Martinez-Costas J.; Vazquez M. E.; Mascareñas J. L. (2012) Straightforward access to bisbenzamidine DNA binders and their use as versatile adaptors for DNA-promoted processes. Chem. Sci. 3, 2383–2387. [Google Scholar]
- Sánchez M. I.; Martinez-Costas J.; Gonzalez F.; Bermudez M. A.; Vazquez M. E.; Mascareñas J. L. (2012) In vivo light-driven DNA binding and cellular uptake of nucleic acid stains. ACS Chem. Biol. 7, 1276–1280. [DOI] [PubMed] [Google Scholar]
- Bordello J.; Sánchez M. I.; Vazquez M. E.; Mascareñas J. L.; Al-Soufi W.; Novo M. (2012) Single-molecule approach to DNA minor-groove association dynamics. Angew. Chem., Int. Ed. 51, 7541–7544. [DOI] [PubMed] [Google Scholar]
- Vazquez O.; Sánchez M. I.; Mascareñas J. L.; Vazquez M. E. (2010) dsDNA-triggered energy transfer and lanthanide sensitization processes. Luminescent probing of specific A/T sequences. Chem. Commun. 46, 5518–5520. [DOI] [PubMed] [Google Scholar]
- Vazquez O.; Sánchez M. I.; Martinez-Costas J.; Vazquez M. E.; Mascareñas J. L. (2010) Bis-4-aminobenzamidines: versatile, fluorogenic A/T-selective dsDNA binders. Org. Lett. 12, 216–219. [DOI] [PubMed] [Google Scholar]
- Lu Z.; Lord S. J.; Wang H.; Moerner W. E.; Twieg R. J. (2006) Long-wavelength analogue of PRODAN: synthesis and properties of Anthradan, a fluorophore with a 2,6-donor-acceptor anthracene structure. J. Org. Chem. 71, 9651–9657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campagna F.; Carotti A.; Casini G. (1977) A convenient synthesis of nitriles from primary amides under mild conditions. Tetrahedron Lett. 18, 1813–1815. [Google Scholar]
- Zhang H.; Cai Q.; Ma D. (2005) Amino acid promoted CuI-catalyzed C–N bond formation between aryl halides and amines or N-containing heterocycles. J. Org. Chem. 70, 5164–5173. [DOI] [PubMed] [Google Scholar]
- Ismail M. A.; Brun R.; Wenzler T.; Tanious F. A.; Wilson W. D.; Boykin D. W. (2004) Dicationic biphenyl benzimidazole derivatives as antiprotozoal agents. Bioorg. Med. Chem. 12, 5405–5413. [DOI] [PubMed] [Google Scholar]
- Painelli A.; Terenziani F. (2000) Optical spectra of push-pull chromophores in solution: A simple model. J. Phys. Chem. A 104, 11041–11048. [Google Scholar]
- Mataga N.; Kaifu Y.; Koizumi M. (1955) The solvent effect on fluorescence spectrum, change of solute-solvent interaction during the lifetime of excited solute molecule. Bull. Chem. Soc. Jpn. 28, 690–691. [Google Scholar]
- Mataga N.; Kaifu Y.; Koizumi M. (1956) Solvent effects upon fluorescence spectra and the dipolemoments of excited molecules. Bull. Chem. Soc. Jpn. 29, 465–475. [Google Scholar]
- Lippert E. (1955) Dipolmoment und Elektronenstruktur von angeregten Molekülen. Z. Naturforsch., A: Phys. Sci. 10, 541–545. [Google Scholar]
- Husain M. M.; Sindhu R.; Tandon H. C. (2012) Photophysical properties and estimation of ground and excited state dipole moments of 7-diethylamino and 7-diethylamino-4-methyl coumarin dyes from absorption and emission spectra. Eur. J. Chem. 3, 87–93. [Google Scholar]
- Mukherjee S.; Chattopadhyay A.; Samanta A.; Soujanya T. (1994) Dipole-moment change of NBD group upon excitation studied using solvatochromic and quantum-chemical approaches: implications in membrane research. J. Phys. Chem. 98, 2809–2812. [Google Scholar]
- Vazquez M. E.; Blanco-Canosa J. B.; Imperiali B. (2005) Photophysics and biological applications of the environment-sensitive fluorophore 6-N,N-dimethylamino-2,3-naphthalimide. J. Am. Chem. Soc. 127, 1300–1306. [DOI] [PubMed] [Google Scholar]
- Nunn C. M.; Neidle S. (1995) Sequence-dependent drug binding to the minor groove of DNA: crystal structure of the DNA dodecamer d(CGCAAATTTGCG)2 complexed with propamidine. J. Med. Chem. 38, 2317–2325. [DOI] [PubMed] [Google Scholar]
- Dunn K. W.; Kamocka M. M.; McDonald J. H. (2011) A practical guide to evaluating colocalization in biological microscopy. Am. J. Physiol., Cell Physiol. 300, C723––C742Pearson’s correlation coefficient ranges from 0 in uncorrelated distributions to 1 for two probes with superimposable labeling patterns. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colocalization analysis was performed with the JACoP plug-in for ImageJ.Bolte S.; Cordelières F. P. (2006) A guided tour into subcellular colocalization analysis in light microscopy. J. Microsc. 224, 213–232Pearson’s coefficients with Mitotracker Red were also consistent with mitocondrial staining of MitoBlue in other cell types (0.97 for DF1; 0.97 for HeLa; and 0.88 for Vero cells). [DOI] [PubMed] [Google Scholar]
- Twenty-four hours after incubation with MitoBlue, the blue labeling is not concentrated in the cell mitochondria, but instead shows a punctuated pattern consistent with lysosomal localization. Further studies on the temporal evolution of MitoBlue staining are underway.
- Altman S. A.; Randers L.; Rao G. (1993) Comparison of trypan blue dye exclusion and fluorometric assays for mammalian cell viability determinations. Biotechnol. Prog. 9, 671–674. [DOI] [PubMed] [Google Scholar]
- Methanol or acetone, which are known to induce cell permeabilization, cause alterations in the intracellular distribution of MitoBlue (Supporting Information).Fischer A. H.; Jacobson K. A.; Rose J.; Zeller R. (2008) Fixation and permeabilization of cells and tissues. CSH Protoc. 3, 36. [DOI] [PubMed] [Google Scholar]
- Horobin R. W.; Trapp S.; Weissig V. (2007) Mitochondriotropics: A review of their mode of action, and their applications for drug and DNA delivery to mammalian mitochondria. J. Controlled Release 121, 125–136. [DOI] [PubMed] [Google Scholar]
- Horobin R. W.; Rashid F. (1990) Interactions of molecular probes with living cells and tissues. Part 1. Some general mechanistic proposals, making use of a simplistic Chinese box model. Histochemistry 94, 205–209. [DOI] [PubMed] [Google Scholar]
- Rosania G. R. (2003) Supertargeted chemistry: identifying relationships between molecular structures and their sub-cellular distribution. Curr. Top. Med. Chem. 3, 659–685. [DOI] [PubMed] [Google Scholar]
- The Log Pcation was calculated based on the PHYSPROP database values, using the partitioning tool in MarvinSketch 5.4.0.1 software, 2010, ChemAxon (http://www.chemaxon.com).
- Trapp S.; Horobin R. W. (2005) A predictive model for the selective accumulation of chemicals in tumor cells. Eur. Biophys. J. 34, 959–966. [DOI] [PubMed] [Google Scholar]
- Modica-Napolitano J. S.; Aprille J. R. (2001) Delocalized lipophilic cations selectively target the mitochondria of carcinoma cells. Adv. Drug Delivery Rev. 49, 63–70. [DOI] [PubMed] [Google Scholar]
- Ross M. F.; Kelso G. F.; Blaikie F. H.; James A. M.; Cochemé H. M.; Filipovska A.; Da Ros T.; Hurd T. R.; Smith R. A. J.; Murphy M. P. (2005) Lipophilic triphenylphosphonium cations as tools in mitochondrial bioenergetics and free radical biology. Biochemistry 70, 222–230. [DOI] [PubMed] [Google Scholar]
- Johnson L. V.; Walsh M. L.; Chen L. B. (1980) Localization of mitochondria in living cells with rhodamine 123. Proc. Natl. Acad. Sci. U.S.A. 77, 990–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansiaux A.; Tanious F.; Mishal Z.; Dassonneville L.; Kumar A.; Stephens C. E.; Hu Q.; Wilson W. D.; Boykin D. W.; Bailly C. (2002) Distribution of furamidine analogues in tumor cells: targeting of the nucleus or mitochondria depending on the amidine substitution. Cancer. Res. 62, 7219–7229. [PubMed] [Google Scholar]
- Heytler P. G.; Prichard W. W. (1962) A new class of uncoupling agents: carbonyl cyanide phenylhydrazones. Biochem. Biophys. Res. Commun. 4, 272–275. [DOI] [PubMed] [Google Scholar]
- Hirose S.; Yaginuma N.; Inada Y. (1974) Disruption of charge separation followed by that of the proton gradient in the mitochondrial membrane by CCCP. J. Biochem. 76, 213–216. [DOI] [PubMed] [Google Scholar]
- Mitochondrial leakage after treatment with CCCP is also observed with Mitotracker Red and Rhodamine 123 (Supporting Information).
- Ross M. F.; Da Ros T.; Blaikie F. H.; Prime T. A.; Porteous C. M.; Severina I. I.; Skulachev V. P.; Kjaergaard H. G.; Smith R. A. J.; Murphy M. P. (2006) Accumulation of lipophilic dications by mitochondria and cells. Biochem. J. 400, 199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilchek M.; Bayer E. A. (1988) The avidin-biotin complex in bioanalytical applications. Anal. Biochem. 171, 1–32. [DOI] [PubMed] [Google Scholar]
- bt-MitoBlue is basically nonfluorescent (see Supporting Information); this is consistent with studies reporting that conjugation to biotin induces fluorescence quenching.Jung D.; Maiti S.; Lee J.-H.; Lee J. H.; Kim J. S. (2014) Rational design of biotin–disulfide–coumarin conjugates: a cancer targeted thiol probe and bioimaging. Chem. Commun. 50, 3044–3047. [DOI] [PubMed] [Google Scholar]
- The conjugate comprises a biotin-binding streptavidin covalently attached to a fluorescent Alexa Fluor dye.Mason J. N.; Farmer H.; Tomlinson I. D.; Schwartz J. W.; Savchenko V.; DeFelice L. J.; Rosenthal S. J.; Blakely R. D. (2005) Novel fluorescence-based approaches for the study of biogenic amine transporter localization, activity, and regulation. J. Neurosci. Methods 143, 3–25. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.





