Abstract
Cocaine, through its activation of dopamine (DA) signaling, usurps pathways that process natural rewards. However, the extent to which there is overlap between the networks that process natural and drug rewards and whether DA signaling associated with cocaine abuse influences these networks have not been investigated in humans. We measured brain activation responses to food and cocaine cues with fMRI, and D2/D3 receptors in the striatum with [11C]raclopride and Positron emission tomography in 20 active cocaine abusers. Compared to neutral cues, food and cocaine cues increasingly engaged cerebellum, orbitofrontal, inferior frontal, and premotor cortices and insula and disengaged cuneus and default mode network (DMN). These fMRI signals were proportional to striatal D2/D3 receptors. Surprisingly cocaine and food cues also deactivated ventral striatum and hypothalamus. Compared to food cues, cocaine cues produced lower activation in insula and postcentral gyrus, and less deactivation in hypothalamus and DMN regions. Activation in cortical regions and cerebellum increased in proportion to the valence of the cues, and activation to food cues in somatosensory and orbitofrontal cortices also increased in proportion to body mass. Longer exposure to cocaine was associated with lower activation to both cues in occipital cortex and cerebellum, which could reflect the decreases in D2/D3 receptors associated with chronicity. These findings show that cocaine cues activate similar, though not identical, pathways to those activated by food cues and that striatal D2/D3 receptors modulate these responses, suggesting that chronic cocaine exposure might influence brain sensitivity not just to drugs but also to food cues. Hum Brain Mapp, 36:120–136, 2015. © 2014 Wiley Periodicals, Inc.
Keywords: reward, addiction, obesity, fMRI, PET
INTRODUCTION
The mesolimbic dopamine (DA) pathway reinforces behaviors necessary for survival in part by activating brain circuits involved with reward and conditioning. Drugs of abuse such as cocaine stimulate these DA pathways [Bernier et al., 2011; Mameli et al., 2009] triggering neuroadaptations with repeated use [Thomas et al., 2008]. Specifically, preclinical studies show that chronic cocaine decreases tonic DA cell firing and enhances phasic DA cell firing in response to drug cues [Grace, 2000; Wanat et al., 2009] and reduces DA signaling during cocaine intoxication [Park et al., 2013], and imaging studies in humans reported reductions in striatal D2/D3 receptor availability [Volkow et al., 1990] and reduced DA signaling during intoxication in cocaine abusers [Volkow et al., 1997b, 2012a]. Positron emission tomography (PET) and functional magnetic resonance imaging (fMRI) studies have also shown that drug addiction impairs the limbic system and regions involved in salience attribution, conditioning, motivation, executive function, and interoception, which mediate responses to natural rewards [Volkow et al., 2011b]. However, little is known about the role of striatal D2/D3 receptors in modulating responses to drug or natural cues, and there is also limited knowledge on the overlap between the brain networks that process them in the human brain [Tomasi and Volkow, 2013].
Foods and drugs increase DA release in the nucleus accumbens (NAc) [Norgren et al., 2006; Volkow et al., 2011a; Wise, 2009], which is associated with their rewarding effects [Koob, 1992]. With repeated exposures to food or drugs these DA responses shift to the cues that predict them [Schultz et al., 1997]. Indeed when neutral stimuli are paired with a rewarding drug they will, with repeated associations, acquire the ability to increase DA in NAc and dorsal striatum (becoming conditioned cues) and these neurochemical responses are associated with drug‐seeking behavior in laboratory animals [Di Ciano and Everitt, 2004; Phillips et al., 2003; Weiss et al., 2000] and with craving in humans [Volkow et al., 2006; Wong et al., 2006]. In humans, drug conditioned cues trigger craving (desire to take the drug), playing a critical role in the cycle of relapse in addiction (O'Brien et al., 1998]. The mechanisms underlying conditioning responses to natural and drug cues involve striatal regions (dorsal and ventral) modulated by DA [reviewed Tomasi and Volkow, 2013].
Prior studies using 18Fluorodeoxyglucose‐PET documented that cocaine cues (pictures of cocaine and related objects) activate visual cortex, ventral striatum and orbitofrontal cortex (OFC) [Grant et al., 1996]. However, using a similar paradigm we showed lower glucose metabolism in OFC and ventral striatum when cocaine subjects watched a cocaine‐cue video than when they watched a neutral‐cue video [Volkow et al., 2011bb] though, paradoxically, we had previously shown that when stimulant drugs induced craving in cocaine abusers this was associated with increased activation of the OFC [Volkow et al., 1999a]. These opposite findings might reflect differences in cue reactivity paradigms (objects vs. videos). Lesions studies [Naqvi et al., 2007] and studies on cue‐induced craving [Bonson et al., 2002; Garavan et al., 2000; Kilts et al., 2001, 2004; Potenza et al., 2012; Wang et al., 1999] have also implicated the insula in drug addiction. Conversely, fMRI studies on food stimulation that contrasted brain responses to sucrose taste and tasteless water, associated hunger with fMRI activation in insula as well as cortical and subcortical brain regions [Haase et al., 2009].
Thus, drug and food cues likely activate similar but not identical pathways. However, to our knowledge no study reported a direct comparison of the effects of drug and food cues on brain activation in humans. Here, we compared the responses to cocaine and food cues in cocaine abusers in whom we hypothesized that drug (cocaine) and natural (food) cues would activate brain networks with significant, but not complete, spatial overlap. Since eating behaviors are modulated by both the homeostatic (responding to energetic and nutritional needs) and the reward pathways [Volkow et al., 2013], food cues are likely to engage circuits other than those activated by cocaine cues. Conversely, drugs might cause greater disruption of DA pathways than those triggered by excessive food consumption as they directly activate these pathways through their pharmacological actions [Tomasi and Volkow, 2013].
The aim of the present study was to assess the modulation effect of D2/D3 receptors on brain activation, independently for food and for drug cues and in the same participants. Thus we tested 20 chronic active cocaine abusers with PET and [11C]raclopride to measure DA D2/D3 receptor availability in the striatum, and with fMRi and a novel cue video paradigm to assess overlapping and differential patterns of brain activation to cocaine cues, food cues, and neutral cues. Videos are optimal for engaging human emotions because they capture motion, making life scenes more vivid and appealing. Cue video paradigms were previously proposed for neuroimaging on addiction [Crockford et al., 2005; Volkow et al., 2006] also because the saliency of a given cue may take several seconds to increase brain activity in a given region. Previous fMRI studies have shown that exposure to a cocaine‐cue video induced craving and consequent fMRI responses in cocaine subjects [Garavan et al., 2000], and that relapse to cocaine abuse is associated with increased activation in sensory association, motor and posterior cingulate cortices [Kosten et al., 2006]. Others and we have shown that compared to neutral cues, exposure to a cocaine‐cue video decreased glucose metabolism in limbic brain regions in cocaine addicts [Volkow et al., 2010bb] and increased DA release in the dorsal striatum [Volkow et al., 2006; Wong et al., 2006].
The fMRI measures were repeated in identical conditions on a different day to assess their test–retest reproducibility. We hypothesized that compared to neutral cues, cocaine and food cues would produce stronger activation in brain regions involved in reward, motivation and conditioning and that striatal DA D2/D3 receptors would modulate these responses. We further hypothesized that compared to cocaine cues, food cues would produce stronger fMRI signals in the insula and in somatosensory regions involved with palatability [Wang et al., 2002].
MATERIALS AND METHODS
Subjects
The study participants were 20 active cocaine‐abusing males (46.4 ± 3.3 years old; 12.8 ± 1.4 years of education; body mass index (BMI) of 26 ± 4 kg/m2; mean ± SD). Participants were recruited from advertisements on public bulletin boards, in local newspapers and by word‐of‐mouth. All subjects provided written informed consent as approved by the local Institutional Review Board (Stony Brook University's Committee on Research Involving Human Subjects), and were screened for absence of medical, psychiatric or neurological diseases. A clinical psychologist conducted a semistructured diagnostic interview which included the Structured Clinical Interview for DSM‐IV Axis I Disorders [research version First et al., 1996; Ventura et al., 1998] and the Addiction Severity Index [McLellan et al., 1992].
Standard laboratory tests (e.g., electrocardiogram, blood lab, and urine drug screen) were performed during the screening visit to ensure the inclusion/exclusion criteria of the study. Male subjects were included if they were (1) able to understand and give informed consent; had (2) DSM IV diagnosis for active cocaine dependence; (3) at least a 2 year history of cocaine abuse using at least 3 g of cocaine/week; (4) predominant use of cocaine by smoked or iv route, and (5) not seeking cocaine treatment. Subjects were excluded if they had (6) present or past history of neurological disease of central origin or psychiatric disease including abuse or dependence to alcohol or to drugs other than cocaine and nicotine, (7) high levels of anxiety, panic attacks, psychosis, apart from those associated with cocaine abuse; (8) current medical illness that may affect brain function; (9) current or past history of cardiovascular disease including heart disease and high blood pressure or endocrinological disease; (10) head trauma with loss of consciousness >30 min; (11) history of vascular headaches; (12) metal implants or other contraindications for MRI.
Thirteen of the subjects were cigarette smokers (17 ± 7 years of smoking; 8 ± 7 cigarettes per day). All subjects had a positive urine toxicology screen for cocaine on both study days, indicating that they have used cocaine during the prior 72 h.
Cocaine‐Cue and Food‐Cue Video Paradigms
Two novel cue video paradigms were used in the present fMRI study. The 6 min long cocaine‐cue video stimulation task (Fig. 1A,B) was composed by six cocaine, six neutral, and six control (black screen with a fixation center cross) epochs, each lasting 20 s and occurring in pseudo random order. The cocaine epochs featured nonrepeating video segments portraying scenes that simulated purchase, preparation, and smoking of cocaine that were previously published [Volkow et al., 2006, 2010bb]. The neutral epochs featured routine administrative/technical work as control items.
Figure 1.
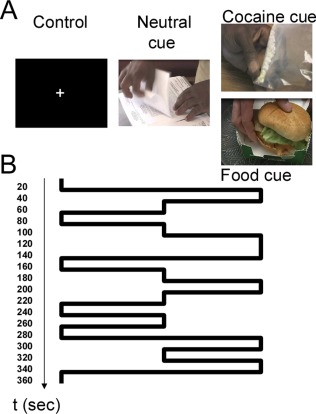
(A) The cue video stimulation tasks features control (black screen with and a fixation center cross), neutral and either cocaine or food video epochs (20 s long) portraying scenes that simulated purchase, preparation, and smoking of cocaine (cocaine cue video), or serving and consumption of the restaurant foods (food cue video). (B) Time course of the 6‐min long stimulation paradigm showing that each video epoch lasted 20 s and occurred in pseudo random order. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Similarly, the 6 min long food‐cue video stimulation task was composed by six “food,” six “neutral” (routine administrative/technical work) and six “control” (black screen with a fixation cross) epochs, each lasting 20 sec and occurring in pseudo random order. The food epochs featured nonrepeating video segments that were recently published [Wang et al., 2014], which portray scenes of serving and consumption of prepared ready to eat foods (i.e., meatballs, pasta, omelets, burger, and pancakes).
The subjects were instructed to watch the screen continuously and press a response button with their right thumb whenever they liked features of the scenes. The cue video fragments were recorded indoors and saved in Audio Video Interleave format by professional video personnel at Brookhaven National Laboratory. These cue videos were presented to the subjects on MRI‐compatible goggles (Resonance Technology, Northridge, CA) connected to a personal computer. The display software was written in Visual Basic and C languages in the Visual Studio package (Microsoft Corp., Redmond, WA) and was synchronized precisely with the MRI acquisition using a trigger pulse.
Food and Cocaine Valences
The more the subjects pressed the response button during the food, cocaine, and/or neutral epochs the more they liked the features displayed in the respective scenes. The number of button presses was used to compute relative valences in a scale from 0 to 10. Specifically, the number of button presses during food (f), neutral (n) and control baseline (b) epochs in the food‐cue video were used to compute the food = f/(f + n + b) and the neutral = n/(n + f + b) valences corresponding to the food‐cue video. Similarly, the number of button presses during the cocaine (c) epochs were used to compute the cocaine = c/(c + n + b) as well as the neutral = n/(n + c + b) valences during the cocaine‐cue video. Note that food and cocaine valences are normalized measures that have negative correlation with the corresponding neutral valence, and that b (number of button presses during the fixation baseline epochs) models the noise level and reduces the negative correlation between these valences from the perfect negative correlation.
MRI Data Acquisition
The subjects checked‐in the day before the study in an effort to avoid use of drugs the night before the study. They were brought to the Guest Housing Facility at Brookhaven National Laboratory at 5:00 p.m., where they had dinner and stayed overnight. The next morning, between 8:00 a.m. and 8:30 a.m., the subjects had a light breakfast consisting of water and a bagel, roll, or cereal depending on their preferences. Brain activation to cocaine cues, food cues and neutral cues was assessed between 9:00 a.m. and 10:00 a.m. twice on two different study days, 2 weeks apart. The presentation order of the food‐ and cocaine‐cue videos was randomized across subjects. A 4‐Tesla whole‐body Varian (Palo Alto, CA)/Siemens (Erlangen, Germany) MRI scanner with a T2*‐weighted single‐shot gradient‐echo planar imaging (EPI) pulse sequence (TE/TR = 20/1600 ms, 4‐mm slice thickness, 1‐mm gap, 35 coronal slices, 64 × 64 matrix size, 3.125 × 3.125 mm2 in‐plane resolution, 90°‐flip angle, 226 time points, 200.00 kHz bandwidth) with ramp‐sampling and whole brain coverage was used to collect functional images with blood‐oxygenation‐level‐dependent (BOLD) contrast. Padding was used to minimize motion. Subject's motion was monitored immediately after each fMRI run using a k‐space motion detection algorithm [Caparelli et al., 2003] written in Interactive Data Language (ITT Visual Information Solutions, Boulder, CO). Earplugs (−28 dB sound pressure level attenuation; Aearo Ear TaperFit 2; Aearo, Indianapolis, IN), headphones (−30 dB sound pressure level attenuation; Commander XG MRI Audio System, Resonance Technology, Northridge, CA) and a “quiet” acquisition approach were used to minimize the interference effect of scanner noise during fMRI [Tomasi et al., 2005]. Anatomical images were collected using a T1‐weighted three‐dimensional modified driven equilibrium Fourier transform pulse sequence (TE/TR = 7/15 ms, 0.94 × 0.94 × 1.00 mm3 spatial resolution, axial orientation, 256 readout and 192 × 96 phase‐encoding steps, 16 min scan time) and a modified T2‐weighted hyperecho sequence (TE/TR = 0.042/10 s, echo train length = 16, 256×256 matrix size, 30 coronal slices, 0.86 × 0.86 mm2 in‐plane resolution, 5 mm thickness, no gap, 2 min scan time) to rule out gross morphological abnormalities of the brain.
Data Processing
An iterative phase correction method that minimizes signal‐loss artifacts in EPI was used for image reconstruction [Caparelli and Tomasi, 2008]. The first four imaging time points were discarded to avoid non‐equilibrium effects in the fMRI signal. The statistical parametric mapping package SPM8 (Wellcome Trust Centre for Neuroimaging, London, UK) was used for subsequent analyses. Image realignment was performed with a fourth degree B‐spline function without weighting and without warping; head motion was less than 2‐mm translations and 2°‐rotations for all scans. Spatial normalization to the stereotactic space of the Montreal Neurological Institute (MNI) was performed using a 12‐parameter affine transformation with medium regularization, 16‐nonlinear iterations and voxel size of 3 × 3 × 3 mm3 and the standard SPM8 EPI template. Spatial smoothing was carried out using an 8‐mm full‐width‐half‐maximum (FWHM) Gaussian kernel. The fMRI responses during the video stimulation paradigms were estimated using a general linear model [Friston et al., 1995] and a design matrix with two regressors, modeling the onsets of the 20 s long cocaine/food epochs and the 20 s long neutral epochs (Fig. 1B), convolved with the canonical hemodynamic response function and a high‐pass (cutoff frequency: 1/800 Hz) filter. Thus, two contrast maps reflecting the % BOLD‐fMRI signal change from baseline (black screen with a fixation cross) caused by the cocaine/food cues and neutral cues were obtained from each fMRI run for each subject.
Test–Retest Reliability
The reliability of the brain activation responses to the cues was evaluated for each imaging voxel using two‐way mixed single measures intraclass correlation (ICC) [Shrout and Fleiss, 1979].
Specifically, ICC(3,1) was mapped in the brain in terms of between‐subjects (BMS) and residuals (EMS) mean square values computed for each voxel using the IPN test–retest reliability matlab toolbox (http://www.mathworks.com/matlabcentral/fileexchange/22122-ipn-tools-for-test-retest-reliability-analysis) and the fMRI contrast maps corresponding to cocaine/food cues from all subjects and sessions (k = 2). Note that ICC(3, 1) coefficients range from 0 (no reliability) to 1 (perfect reliability).
PET Scanning
Thirty minutes after MRI scanning (approximately 60 min after the end of the fMRI session) the subjects underwent a PET scan to map the availability of DA D2/D3 receptors in the brain. We used an HR+ tomograph (resolution 4.5 × 4.5 × 4.5 mm3 full width half‐maximum, 63 slices) with [11C]raclopride, a radiotracer that binds to DA D2/D3 receptors, and methods previously described [Volkow et al., 1993a]. Briefly, emission scans were started immediately after injection of 4–8 mCi (specific activity 0.5–1.5 Ci/µM). Twenty dynamic emission scans were obtained from time of injection up to 54 min. Arterial sampling was used to quantify total carbon‐11 and unchanged [11C]raclopride in plasma. The distribution volume (DV), which corresponds to the equilibrium measurement of the ratio of the radiotracer's tissue concentration to that of its plasma concentration, was estimated for each voxel using a graphical analysis technique for reversible systems that does not require blood sampling [Logan et al., 1990]. These images were then spatially normalized to the MNI stereotactic space using SPM8 and resliced using 2‐mm isotropic voxels. A custom MNI template, which was previously developed using DV images from 34 healthy subjects that were acquired with [11C]raclopride and the same PET scanning methodology [Wang et al., 2011], was used for this purpose. DV ratios, which correspond to the nondisplaceable binding potential (BPND) in each voxel, were obtained by normalizing the intensity of the DV images to that in the cerebellum (left and right regions‐of‐interest). The automated anatomical labeling atlas [Tzourio‐Mazoyer et al., 2002] was used to locate the MNI coordinates of the centers of mass for putamen and caudate; the center coordinates of the boundary between caudate and putamen was selected for the ventral striatum. Thus, isotropic (cubic) masks with a volume 1 ml (125 imaging voxels) were centered at the putamen [xyz = (±26, 8, 2) mm], caudate [xyz = (±12, 12, 8) mm] and ventral striatum [xyz = (±20, 10, −12) mm] to compute the average availability of D2/D3 receptors for each individual in these striatal regions (Fig. 2A).
Figure 2.
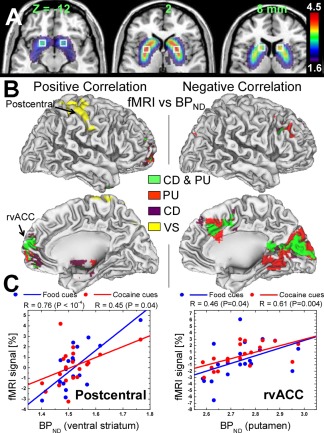
(A) Binding potential superimposed on axial MRI views of the human brain showing the availability of DA D2/D3 receptors in the striatum. PET with [11C]raclopride was used to compute DVs relative to values in the cerebellum, which correspond to the nondisplaceable binding potential in each voxel (BPND). White squares highlight the regions‐of‐interest in ventral striatum (Z = −12 mm), putamen (Z = +2 mm) and caudate (Z = +8 mm). (B) Brain regions showing significant positive (left panel) and negative (right panel) correlations between brain activation and the availability of DA D2/D3 receptors in ventral striatum (VS), putamen (PU) and caudate (CD) and their overlapping correlation patterns (CD & PU). (C) Scatter plots showing the association between the availability of DA D2/D3 receptors in the striatum and fMRI responses in postcentral gyrus and rostal ventral PFC across subjects. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Statistical Analyses
A one‐way within‐subjects analysis of variance model in SPM8 with age, BMI and years of cocaine use covariates (ANCOVA) was used to test the significance of common and differential brain activation signals to neutral, food and cocaine cues. Voxelwise SPM8 regression analyses were additionally used to test the linear association of brain activation signals with the availability of D2/D3 receptor (BPND) in caudate, putamen and ventral striatum, as well as with years of cocaine use, cue valence and BMI across subjects. Statistical significance was set as P FWE < 0.05, corrected for multiple comparisons with the random field theory and a family‐wise error (FWE) correction at the cluster level. A cluster‐forming threshold P < 0.005 and a minimum cluster size of 200 voxels were used for this purpose. The conservative Bonferroni method for multiple comparisons was additionally used to control for the number of independent SPM regression analyses. A stringent cluster‐level corrected threshold Pc < 0.05 that simultaneously accounted for Bonferroni corrections and whole‐brain FWE‐corrections was used for this purpose.
Functional Region‐of‐Interest‐Analyses
Brain activation and deactivation clusters were further evaluated with region‐of‐interest (ROI) analyses to identify outliers that might influence strongly correlation analyses, and to report average values in a volume comparable to the image smoothness (e.g., resolution elements or “resels” [Worsley et al., 1992]) rather than single‐voxel peak values. The volume of the resels was estimated using the random field calculation in SPM8 as a near cubic volume with Cartesian FWHM = 12.7, 12.3, 13.1 mm. Thus, 9‐mm isotropic masks containing 27 voxels (0.73 ml) were defined at the centers of relevant activation/deactivation/correlation clusters to extract the average % BOLD signal from individual contrast maps. These masks were created and centered at the precise coordinates listed in Tables 1–IV.
Table 1.
Statistical significance for brain activation clusters that were commonly activated by cocaine (C) and food (F) cues compared to neutral (N) cues
| MNI coordinates [mm] | Cluster | t‐score | ||||||
|---|---|---|---|---|---|---|---|---|
| Region | BA | x | y | x | [Voxels] | C > N | F > N | C > N & F > N |
| Cerebellum | −42 | −70 | −29 | 2242 | 3.5 | 4.4 | 5.3 | |
| Cerebellum | 36 | −64 | −26 | 4.2 | 3.8 | 5.3 | ||
| Cerebellum | 39 | −76 | −29 | 3.1 | 3.1 | 4.1 | ||
| Inferior Frontal | 44 | 45 | 8 | 28 | 452 | 4.8 | 1.9 | 4.4 |
| Insula | 47 | 42 | 20 | −8 | 2.5 | 2.6 | 3.4 | |
| Inferior Frontal | 45 | 51 | 20 | 10 | 3.1 | 2.0 | 3.4 | |
| Supramarginal | 42 | −57 | −19 | 22 | NS | 5.5 | 3.8 | |
| OFC | 47 | −45 | 20 | −5 | 185 | 2.2 | 3.7 | 4.0 |
| Inferior Frontal | 44 | −54 | 14 | 19 | 106 | 1.6 | 3.2 | 3.1 |
| Precentral | 6 | −42 | −4 | 52 | 2.6 | 1.6 | 2.7 | |
| Ventral striatum | −21 | 11 | −14 | −2.1 | −3.2 | −4.3 | ||
| Hypothalamus | −3 | −1 | −5 | NS | −3.5 | −3.7 | ||
| rvACC | 32 | 9 | 44 | 4 | 163 | −3.8 | −1.8 | −3.7 |
| rvACC | 11 | −3 | 41 | −5 | −2.5 | −1.8 | −2.8 | |
| Calcarine | 18 | 9 | −67 | 19 | 517 | NS | −4.3 | −3.6 |
| Calcarine | 17 | −9 | −61 | 13 | NS | −4.2 | −3.5 | |
| Calcarine | 18 | 21 | −64 | 13 | NS | −4.3 | −3.4 | |
Average statistical values computed within functional ROIs (9‐mm isotropic) with 27 voxels.
Statistical significance for C > N & F > N conjunction analyses: cluster level PFWE < 0.05.
RESULTS
Behavior
The valences were lower for neutral cues than for food or cocaine cues (P < 10−6, t > 7.4, df = 19, paired t‐test; Fig. 3A) but did not differ for food and cocaine cues. There was a negative correlation across subjects between the valence of the neutral cues and that of the cocaine/food cues, such that the more the subjects liked the cocaine/food cues the less they liked the neutral cues (R < −0.8, P < 0.0001, df = 18, Pearson correlation; Fig. 3B).
Figure 3.
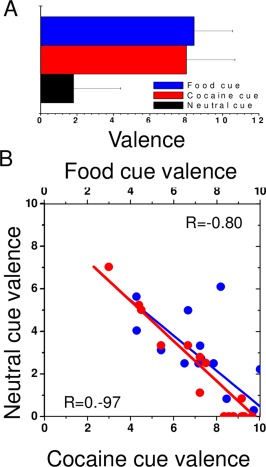
Behavioral responses during cue video stimulation. (A) The subjects were instructed to press a response button whenever they liked features of the scene. The number of button presses was used to determine how much the subjects liked the cocaine, food, and neutral scenes in the videos in a scale from 0 to 10. (B) Scatter plots showing the strong negative correlation between cocaine liking scores and neutral liking scores (red) and the weak negative correlation between food liking scores and neutral liking scores (blue) across subjects. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Striatal DA D2/D3 Receptors
The average availability of DA D2/D3 receptors in the striatal ROIs was higher for putamen than for caudate, and for caudate than for ventral striatum (P < 10−9, left and right hemispheres values averaged). The availability of D2/D3 receptors in the striatum did not show significant correlation with age, BMI, chronicity, or with the valence of the cues.
Brain Activation
Compared to the fixation baseline, neutral cues produced bilateral activation in middle occipital, fusiform and superior frontal gyri (BAs 19 and 6), cerebellum (posterior lobe), inferior parietal cortex (BA 40), inferior frontal operculum (BA 44) and the hippocampus, and bilateral deactivation in posterior default mode network (DMN) regions (cuneus, precuneus, and angular gyrus) (P FWE < 0.0005; Fig. 4).
Figure 4.
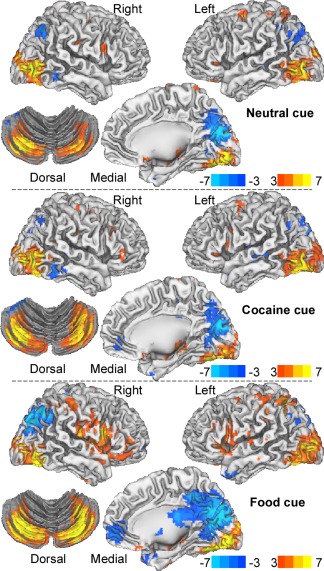
Statistical significance of brain activation (red‐yellow)/deactivation (blue‐cyan) responses to the cue videos relative to the fixation baseline epochs, rendered on lateral and ventral views of the cerebrum and a dorsal view of the cerebellum. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Compared to the fixation baseline, cocaine cues produced bilateral activation in calcarine and inferior parietal cortices (BAs 18 and 40), fusiform (BA 19), precentral (BA 6) and middle frontal gyri (BA 44), and the hippocampus, and bilateral deactivation in posterior DMN regions (cuneus, precuneus, posterior cingulum, and angular gyrus) (P FWE < 0.0005; Fig. 4).
Compared to the fixation baseline, food cues produced bilateral activation in calcarine cortex (BA 18), fusiform gyrus (BA 19), temporal pole (BA 38), inferior parietal cortex (BA 40), inferior frontal operculum (BA 45), OFC (BA 11) and the hippocampus, and bilateral deactivation in rostral/ventral anterior cingulate cortex (rvACC, BAs 10, 11 and 32), cuneus (BAs 18 and 19), precuneus (BA 7), and the angular gyrus (BA 39) (P FWE < 0.0005; Fig. 4).
Test–Retest Reliability
The ICC analysis of the test–retest fMRI data demonstrated moderate to high reliability for the BOLD‐fMRI responses to the cues. Specifically, the fMRI signals in rvACC, occipital cortex, ventral striatum, cerebellum, inferior frontal operculum, postcentral, precentral and inferior frontal gyri, cuneus, precuneus, and the angular gyrus had ICC(3,1) > 0.5 (Fig. 5).
Figure 5.
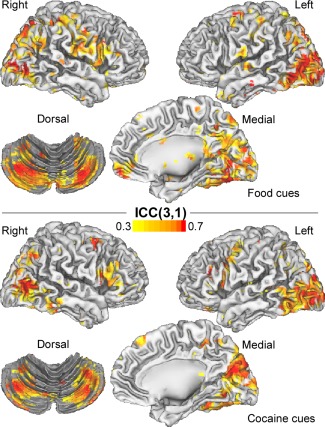
Intraclass correlation (ICC) maps, rendered on lateral and ventral views of the cerebrum and a dorsal view of the cerebellum, depicting the reliability of the fMRI signals. The ICC(3,1) voxel values were computed from BOLD‐fMRI responses to food and cocaine cues recorded on two study days under identical conditions using the same MRI acquisition parameters. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Common Activation Patterns for Food and Cocaine Cues
Cocaine and food cues produced higher activation than neutral cues in cerebellum, inferior frontal and precentral gyri, OFC and the insula, and lower activation than neutral cues in ventral striatum, rvACC, and the calcarine cortex (P FWE < 0.0005; ANCOVA; Fig. 6 and Table 1).
Figure 6.
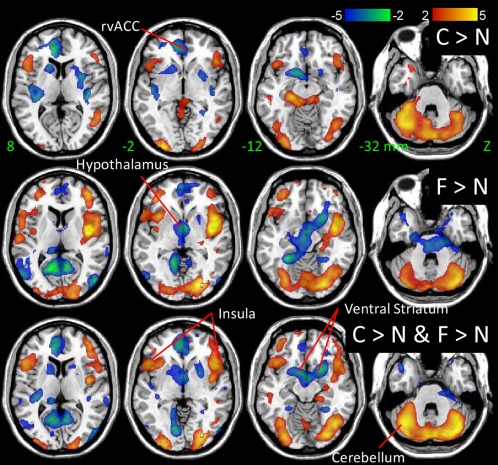
Statistical significance of brain coactivation responses to the cocaine and food cues relative to that of the neutral cues rendered on axial views of the human brain. SPM8 model: ANCOVA. Color bars are t‐scores. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Specific Activation Patterns for Food and Cocaine Cues
Cocaine cues produced higher activation than neutral cues in inferior frontal and occipital, parahippocampal and postcentral gyri and the cerebellum, and lower activation than neutral cues in visual areas, auditory cortex, OFC, rvACC, posterior insula, paracentral lobule and precentral gyrus, caudate, putamen and ventral striatum (location of NAc) (P FWE < 0.05, ANCOVA; Supporting Information Table S1, Figs. 6 and 7). Similarly, food cues produced higher activation than neutral cues in postcentral gyrus, temporal pole inferior and superior frontal cortex, insula and the cerebellum, and lower activation than neutral cues in primary visual cortex, precuneus, cuneus, middle occipital gyrus, ventral striatum, hypothalamus, and the midbrain [location of ventral tegmental area and substantia nigra (SN); P FWE < 0.01; Supporting Information Table S1 and Fig. 7].
Figure 7.
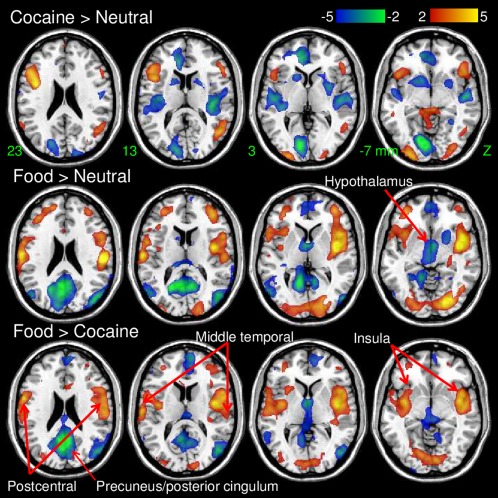
Statistical significance of differential activation responses to the cues rendered on axial views of the human brain. SPM8 model: ANCOVA. Color bars are t‐scores. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Compared to food cues, cocaine cues produced lower activation in insula and postcentral gyrus, lower deactivation in hypothalamus, precuneus and posterior cingulum and higher activation in middle temporal gyrus and the inferior parietal cortex (Table 2; P FWE < 0.005; Fig. 7). In contrast compared to cocaine cues, food cues produced greater deactivation in hypothalamus/midbrain and in posterior cingulum and they deactivated the posterior cingulum whereas cocaine cues activated it.
Table 2.
Statistical significance for brain activation clusters that were differentially activated by cocaine, food and neutral cues
| Region | BA | MNI coordinates [mm] | Cluster | T‐score | ||||
|---|---|---|---|---|---|---|---|---|
| x | y | z | [Voxels] | C > N | F > N | F > C | ||
| Middle Temporal | 21 | −51 | −58 | 19 | 480 | 3.7 | n.s. | −2.6 |
| Middle Temporal | 22 | −57 | −49 | 13 | 3.4 | n.s. | −2.1 | |
| Inferior Parietal | 40 | −54 | −52 | 37 | 2.4 | n.s. | −3.6 | |
| Hypothalamus/VTA | −3 | −10 | −8 | n.s. | −3.6 | −2.0 | ||
| Insula | 13 | −45 | 2 | −5 | 1101 | n.s. | 4.7 | 3.1 |
| Insula | 13 | −39 | −4 | 10 | n.s. | 4.1 | 2.7 | |
| Insula | 13 | −45 | 14 | 1 | n.s. | 3.8 | 2.9 | |
| Postcentral | 43 | 63 | −13 | 19 | 707 | n.s. | 4.2 | 2.0 |
| Postcentral | 43 | 60 | −1 | 16 | n.s. | 3.3 | 2.4 | |
| Posterior cingulum | 26/29 | 0 | −55 | 28 | 994 | n.s. | −2.6 | −4.3 |
| Precuneus | 23 | −9 | −49 | 43 | n.s. | n.s. | −3.4 | |
| Cingulum | 23 | 6 | −52 | 37 | 2.0 | −2.6 | −3.6 | |
Average statistical values computed within functional ROIs (9‐mm isotropic) with 27 voxels. Statistical significance for F > C: cluster level P FWE < 0.05.
Striatal D2/D3 Receptor Availability and Brain Activation
We assessed the linear association between brain activation and D2/D3 receptors independently for dorsal caudate and putamen and ventral striatum because different regions of the striatum have demonstrated different cortical projections, and have different modulatory effects on brain regions involved with control of behavior [Grahn et al., 2008], salience attribution and reward processing [Volkow et al., 2011b]. There were significant correlations between the availability of DA D2/D3 receptors in the striatum and the average coactivation responses elicited by food and cocaine cues (P FWE < 0.05; Table 3; Fig. 2B,C). Specifically, increased BPND in caudate was associated with stronger activation in hippocampus and parahippocampus, rvACC, and OFC, and weaker activation in cuneus, superior frontal gyrus and caudal dorsal ACC (cdACC). Increased BPND in putamen was associated with stronger activation in OFC, midbrain, cerebellum and superior frontal and parahippocampal gyri and with weaker activation in cdACC and middle frontal gyrus, cuneus and superior occipital and lingual gyri. The linear associations with BPND in caudate and putamen survived additional Bonferroni corrections for the number of BP regressions (Pc < 0.05, cluster level corrected in whole‐brain with the FWE correction and for the three BP regressions with the Bonferroni method). Increased BPND in ventral striatum was associated with stronger activation in inferior and superior parietal cortices, paracentral lobule, postcentral gyrus, and precentral gyrus and weaker activation in the cerebellum. However, the linear associations with BPND in ventral striatum did not survive additional Bonferroni corrections for the number of BP regressions. These correlations were not significantly different for cocaine and food cues (Fig. 2C). The correlation patterns for caudate and putamen had significant overlap in occipital cortex, cdACC and rvACC (Fig. 2B). The correlation patterns for ventral striatum did not show significant overlap with those for caudate and putamen.
Table 3.
Statistical significance for the correlation between average fMRI responses to food (F) and cocaine (C) cues and the availability of DA D2 receptors (D2R) in caudate, putamen and ventral striatum
| Region | BA | MNI coordinates [mm] | Cluster‐level | D2R‐corr [t] | ||||
|---|---|---|---|---|---|---|---|---|
| x | y | z | P FWE | Voxels | F & C | (F/C) | ||
| D2R in caudate | ||||||||
| Parahippocampal | 28 | −18 | 5 | −23 | 0.016 | 599 | 4.2 | 3.0/2.8 |
| Hippocampus | 34 | 18 | −7 | −11 | 3.9 | 2.8/2.7 | ||
| OFC | 10 | −6 | 47 | −8 | 0.016 | 534 | 3.9 | 4.0/NS |
| rvACC | 32 | 9 | 53 | 25 | 3.9 | 2.7/2.7 | ||
| Cuneus | 18 | −9 | −88 | 19 | 0.002 | 775 | −6.0 | −4.6/−3.9 |
| Superior frontal | 9 | 12 | 47 | 43 | 0.012 | 515 | −4.0 | −2.8/−2.9 |
| cdACC | 32 | 6 | 26 | 37 | −3.7 | −3.1/−2.2 | ||
| D2R in putamen | ||||||||
| OFC | 10 | −12 | 47 | 1 | 0.004 | 637 | 4.0 | 2.5/3.3 |
| Superior Frontal | 10 | 18 | 50 | 13 | 3.7 | 1.9/3.4 | ||
| Parahippocampal | 28 | −18 | 5 | −23 | 0.003 | 745 | 3.7 | 2.0/3.3 |
| Midbrain | −3 | −25 | −11 | 3.5 | 2.1/3.1 | |||
| Cerebellum | −21 | −46 | −44 | 3.5 | 2.5/2.5 | |||
| Lingual | 37 | 24 | −52 | −5 | <0.001 | 1576 | −5.5 | −4.0/−3.7 |
| Superior Occipital | 19 | −3 | −85 | 43 | −5.4 | −4.0/−3.6 | ||
| Cuneus | 18 | −9 | −85 | 16 | −5.2 | −4.2/−3.1 | ||
| cdACC | 32 | 6 | 26 | 37 | 0.005 | 612 | −4.6 | −3.0/−3.7 |
| Middle frontal | 8 | 27 | 14 | 49 | −3.6 | −2.4/−2.8 | ||
| D2R in ventral striatum | ||||||||
| Precentral | 4 | −33 | −31 | 64 | 0.029 | 450 | 5.0 | 4.7/−3.1 |
| Inferior parietal | 2 | −30 | −46 | 55 | 4.4 | 4.5/NS | ||
| Postcentral | 3 | −51 | −22 | 52 | 3.2 | 2.8/NS | ||
| Superior parietal | 7 | 21 | −67 | 55 | 0.024 | 536 | 4.9 | 3.1/NS |
| Paracentral lobule | 4 | 6 | −37 | 79 | 3.5 | 4.2/NS | ||
| Cerebellum | 42 | −76 | −50 | 0.051 | 441 | −6.5 | −4.2/NS | |
P FWE : P‐value corrected for multiple comparisons at the cluster level.
Associations with Chronicity, Behavioral Responses, and BMI
Linear regression analyses revealed associations between the average coactivation elicited by food and cocaine cues, the number of years of cocaine use and valences of food and cocaine cues (P FWE < 0.05; Table 4; Fig. 8). Specifically, longer cocaine exposure was associated with lower activation in a cluster region that contained the right calcarine cortex and the right and left cerebellum to both food and cocaine cues (Table 4, Fig. 8). Increased valence for food and cocaine cues was associated with increased activation in inferior and superior parietal and middle and inferior temporal cortices, cerebellum and the postcentral gyrus, and with lower activation in cuneus for both cocaine and food cues. In addition higher BMI was associated with increased activation to food cues in OFC (BA 11) and the postcentral gyrus (P FWE < 0.05; Table 4; Fig. 8). These linear associations with years of cocaine use, cue valence and BMI survived additional Bonferroni corrections for the number of regressions (Pc < 0.05).
Table 4.
Statistical significance for the correlations between average fMRI responses to food (F) and cocaine (C) cues and years of cocaine, liking scores and body mass index (BMI)
| Region | BA | MNI coordinates [mm] | Cluster‐level | Correlation [T] | ||||
|---|---|---|---|---|---|---|---|---|
| x | y | z | P FWE | k | F & C | F/C | ||
| Years of cocaine | ||||||||
| Calcarine | 17 | 3 | −103 | 1 | <0.001 | 1366 | −5.9 | −6.3/−2.6 |
| Cerebellum | −30 | −73 | −23 | −4.7 | −3.9/−1.7 | |||
| Cerebellum | 36 | −82 | −26 | −4.7 | −4.9/NS | |||
| Valence | ||||||||
| Inferior parietal | 2 | −30 | −46 | 55 | 0.008 | 1296 | 4.5 | 2.6/3.6 |
| Postcentral | 3 | 39 | −28 | 49 | 3.7 | 2.9/NS | ||
| Superior parietal | 40 | 39 | −55 | 61 | 3.6 | 2.7/2.7 | ||
| Cerebellum | 33 | −49 | −47 | 0.003 | 1693 | 3.7 | NS/NS | |
| Inferior temporal | 20 | 51 | −43 | −17 | 3.7 | 2.5/1.9 | ||
| Middle temporal | 22 | 63 | −46 | 7 | 3.6 | 3.3/2.4 | ||
| Cuneus | 18 | 12 | −76 | 28 | 0.002 | 1779 | −4.3 | −2.7/−3.6 |
| Lingual | 18 | 12 | −61 | 4 | −3.8 | NS/−2.9 | ||
| BMI | ||||||||
| Postcentral | 43 | 63 | −22 | 43 | 0.004 | 508 | 4.4 | 4.3/NS |
| Postcentral | 4 | 33 | −31 | 73 | NS | 3.8/NS | ||
| Postcentral | 1 | 18 | −43 | 73 | 2.6 | 3.7/NS | ||
| OFC | 11 | −24 | 44 | −11 | 0 | 784 | NS | 4.0/NS |
T : t‐score.
P FWE: P‐value corrected for multiple comparisons at the cluster level.
k : number of voxels in the cluster.
Figure 8.
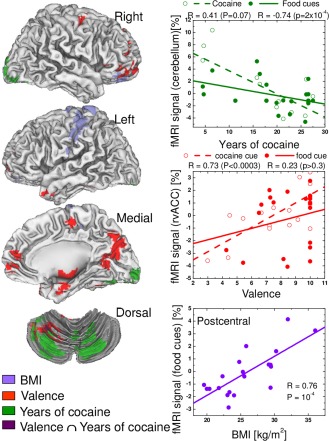
Correlation patterns between the average activation to cocaine and food cues and BMI, cue valence and years of cocaine use and their overlap (Valence ∩ Years of cocaine use), superimposed on lateral and ventral views of the cerebrum and a dorsal view of the cerebellum (left), as well as the corresponding linear regression plots showing the significant correlation of these variables (right). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
DISCUSSION
The current study demonstrates for the first time common and distinct functional circuits involved in drug (cocaine cues) and natural (food cues) reward for men that actively abuse cocaine, and shows significant correlation between striatal D2/D3 receptors and brain activation to cocaine and food cues.
D2/D3 Receptors and Brain Activation
The availability of DA D2/D3 receptors in striatum was associated with brain activation to cocaine and food cues. Interestingly, while the correlation patterns were similar for cocaine and food cues, the linear associations between striatal D2/D3 receptor availability and BOLD responses had significant overlap for caudate and putamen (dorsal striatum) but ventral striatum showed a distinct pattern. These findings are consistent with the modulatory role of DA and of D2/D3 receptors in reactivity to food and drug cues [Salamone and Correa, 2012] and with the distinct role that dorsal and ventral striatal region have in modulating cue responses [Di Ciano et al., 2008].
The pattern of correlations between striatal D2/D3 receptors and BOLD activation included cortical areas (parietal cortex) and cerebellum, which are brain regions that have relatively low levels of D2/D3 receptors [Mukherjee et al., 2002]. This widespread pattern of correlations is likely to reflect the modulatory role that D2/D3 receptors containing neurons in the striatum have in cortical activity through their thalamocortical projections [Haber and Calzavara, 2009]. Thus, the strength of the correlation between D2/D3 receptors and BOLD activation in a given region would reflect the modulatory role of striatal D2 and D3 receptors expressing projections into the relevant cortical and subcortical networks activated by the cues.
The role of D2/D3 receptors in reactivity to food and drug cues is consistent with prior clinical findings. Specifically, using PET and [11C]raclopride we and others have shown that exposure to drug cues increases DA following exposure to cocaine [Volkow et al., 2006; Wong et al., 2006], amphetamine [Boileau et al., 2007], and heroin [Zijlstra et al., 2008] cues. Pharmacological studies with haloperidol and amisulpiride have also shown that D2/D3 receptor blockade reduces attentional bias to heroin cues in heroin addicts [Franken et al., 2004], and normalizes hypoactivation to smoking cues in ACC and prefrontal cortex (PFC) in smokers [Luijten et al., 2012] and to alcohol cues in ACC and OFC in alcoholics [Hermann et al., 2006]. Thus, our findings along with those of others [Saunders and Robinson, 2013] indicate that DA, in part through D2 receptors but presumably also D3 receptors, has a key role in the processing of drug and food cues. Different from our prior studies [Wang et al., 2001], striatal BPND was not associated with BMI in the present study, which might reflect differences between samples. Specifically, whereas the present study includes only a small fraction of obese individuals (3/20 subjects with BMI > 30 kg/m2; BMI range: 20–35 kg/m2) and all of them were cocaine abusers, our previous study included 10 severely nondrug abusing obese individuals with a BMI greater than 40 kg/m2 (range: 42–60 kg/m2) and 10 healthy nondrug abusing controls (range: 21–28 kg/m2).
The Common Network
Identification of overlapping brain circuits which are activated by food and drug cues could help identify treatment strategies that may benefit both drug addicts and obese individuals. Natural rewards release DA in the ventral striatum, which is believed to underlie their rewarding effects. However, with repeated exposure to the reward the DA increases are transferred from the reward to the cues that predict them [Schultz et al., 1997], thus triggering the motivational drive required to ensure behaviors necessary for reward consumption [Pasquereau and Turner, 2013]. Repeated exposure to drugs of abuse also results in conditioning. In this way, conditioned responses for food and drugs shift the incentive motivation to the conditioned cue stimuli that predict the reward [Berridge and Robinson, 2003].
Interestingly, we show that dopaminergic regions where deactivated by exposure to the reward cues, including ventral striatum (to both food and drug cues), hypothalamus and midbrain (to food cues) when compared to neutral cues (Table 2 and Fig. 4), which is consistent with the inhibitory properties of DA in non‐human primates [West and Grace, 2002] and in humans [Volkow et al., 2010bb], and with the increases in DA in the striatum following drug cues in cocaine abusers [Volkow et al., 2006] and food cues in controls [Volkow et al., 2002]. All addictive drugs increase DA in the ventral striatum (NAc) [Koob, 1992], and their rewarding effects are associated with these increases in DA release [Drevets et al., 2001; Volkow et al., 1999b; Wise, 2009]. Foods can also increase DA in ventral striatum [de Araujo et al., 2008; Norgren et al., 2006] and are potently rewarding [Lenoir et al., 2007]. The cerebellum and the insula, conversely, showed stronger activation to cocaine and food cues than to neutral cues (Table 2 and Fig. 4). These findings are consistent with the activation of the cerebellum and the insula during taste perception in hunger conditions [Haase et al., 2009] and with cerebellar [Grant et al., 1996] and insular activation in cocaine abusers exposed to cocaine cues [Wang et al., 1999]. Moreover, when exposed to cocaine cues, cocaine abusers instructed to inhibit their craving deactivate the insula [Volkow et al., 2010a], and damage to the insula can disrupt addiction to cigarette smoking [Naqvi et al., 2007]. Indeed, the insula is increasingly recognized as being a critical neural substrate for addiction in part by mediating interoceptive awareness of drug craving [Naqvi and Bechara, 2010]. Our results differ from those obtained in rats trained to associate odor cues with the availability of a reinforcer (intravenous cocaine/oral sucrose), which show different brain activity in NAc for cocaine than for sucrose [Liu et al., 2013]. This discrepancy might reflect differences between species (addicted humans vs. rats exposed to cocaine), the use of odors versus visual cues and confounds from the effects of anesthesia used for the rodent studies.
Cerebellar activation was stronger for cocaine and food cues than for neutral cues, which is consistent with prior studies documenting a role of the cerebellum in reward‐based learning [Thoma et al., 2008], cocaine‐induced memory [Carbo‐Gas et al., 2013] and in the regulation of visceral functions and feeding control [Haines et al., 1984]. Cerebellar activation to food and cocaine cues decreased with years of cocaine use (Table 4). This finding is consistent with the cocaine subjects' weaker brain responses compared to controls [Bolla et al., 2004; Goldstein et al., 2009; Hester and Garavan, 2004; Li et al., 2008; Moeller et al., 2010; Volkow et al., 2010bb], and with our prior findings showing that the increases in cerebellar metabolism observed after a challenge with an intravenous stimulant drug (methylphenidate) were correlated with striatal D2/D3 receptor availability [Volkow et al., 1997a], which tend to be decreased in cocaine abusers [Martinez et al., 2004; Volkow et al., 1990, 1993b].
Compared to neutral cues, cocaine/food cues also elicited increased activation in lateral OFC, inferior frontal and premotor cortices and stronger deactivation in rvACC, precuneus and visual areas (Table 1). Previous studies have shown that compared to neutral cues, food cues elicit significant activation responses in the insula, somatosensory cortex, parietal and visual cortices [Cornier et al., 2013], and children at risk for obesity show stronger activation to food cues in the somatosensory cortex [Stice et al., 2011]. Furthermore, the anterior insula, and inferior frontal and OFC are interconnected to the striatum by corticostriatal projections modulated by DA [Haber, 2003] and play important roles in inhibitory control, decision making, emotional regulation, motivation, and salience attribution [Goldstein and Volkow, 2002; Phan et al., 2002; Volkow et al., 1996]. Moreover, OFC gray matter volume demonstrated negative correlations with BMI in cocaine addicts and controls as well as with years of cocaine use in cocaine addicts [Smith et al., in press], which could also reflect the effects of cocaine in regions underlying natural rewards responses such as OFC.
Differential Networks
Cocaine cues produced stronger fMRI activation in cerebellum, occipital and prefrontal cortices and greater deactivation in rvACC and ventral striatum than neutral cues. These findings are consistent with the craving‐related metabolic increases in PFC, medial temporal lobe and cerebellum [Grant et al., 1996] and with the metabolic decreases in ventral striatum [Volkow et al., 2010bb] and the cerebral blood flow decreases in basal ganglia [Childress et al., 1999] in cocaine addicts during cocaine‐cue stimulation paradigms.
Food cues produced stronger fMRI activation than neutral cues in the insula, gustatory, and visual association cortices, and greater deactivation in rvACC, hypothalamus, midbrain and primary visual cortex, precuneus, and angular gyrus. Whereas cocaine cues did not activate BA 43 (gustatory cortex; Table 2) significantly across subjects, the fMRI responses to food cues in BA 43 were significant (Table 2) and positively correlated with the availability of DA D2/D3 receptors in ventral striatum (Fig. 2C), which would suggest dopaminergic modulation of this brain region. Supporting this were the significant correlations between fMRI activation responses in the gustatory cortex and food cue valence (Table 4), as DA modulates the value of food rewards [Volkow et al., 2012b].
Deactivation in posterior DMN regions was higher for food than for cocaine cues. Activation of the DMN has been associated with the generation of spontaneous thoughts during mind‐wandering [Mason et al., 2007] and its deactivation occurs during the performance of attention‐demanding cognitive tasks [Fox et al., 2005]. Importantly, the degree of DMN deactivation during attention demanding cognitive tasks varies across tasks [Tomasi et al., 2006], likely reflecting the degree of suppression of spontaneous thoughts. Thus, weaker DMN deactivation for cocaine cues than for food cues could reflect higher degree of generation of spontaneous thoughts during cocaine cues than during food cues. This could reflect in part differences in DA release between food cues and cocaine cues because DA increases are associated with DMN deactivation [Thanos et al., 2013; Tomasi et al., 2009]. The negative correlation observed between D2/D3 receptors in dorsal striatum and fMRI responses in cuneus, such that the higher the receptor levels the greater the deactivation of the cuneus, is consistent with the inhibitory role of DA in the DMN [Thanos et al., 2013; Tomasi et al., 2009].
The BOLD‐fMRI signals in this study were not significantly different across study days, suggesting lower variability within‐ than between‐subjects. Furthermore, the test–retest reliability of the activation and deactivation patterns elicited by the cues was similar to that of standard working memory fMRI tasks that use blocked designs [Bennett and Miller, 2013]. Specifically, the reliability of the fMRI signals ranged from 0.4 (moderate reliability) to 0.8 (high reliability), also suggesting lower variability of brain activation to food and cocaine cues for within‐subject than for between‐subjects measures.
In interpreting our results, we considered the possibility that cocaine abusers might be particularly sensitive to reward‐cues (natural and drug reward), which in turns might contribute to their vulnerability for addiction [Saunders and Robinson, 2013]. Moreover, in our results the valence of the cocaine cues correlated with the valence of the food cues, consistent with a common sensitivity to general cue reactivity [Saunders and Robinson, 2013]. Thus we cannot exclude the possibility that the differences we observe in the cocaine abusers might have preceded their drug use and might have made them more vulnerable to cocaine abuse. In this respect, it would have been desirable to include a control group to assess the specificity of the effects to food and cocaine cues in addicted versus nonaddicted individuals and to determine if their sensitivity to food cues also differed between the groups. We postulate that differences in behavioral responses and brain activation elicited by food cues versus cocaine cues would be significantly larger for controls than for cocaine abusers. Furthermore, we used [11C]raclopride, which maps D2/D3 receptor availability, and it would have been desirable to use radiotracers that would help us to distinguish between the contribution of D2 receptors and that of D3 receptors. Also, [11C]raclopride is sensitive to competition to endogenous DA [Volkow et al., 1994], so we cannot determine if the association with brain activation reflects differences in D2/D3 receptors levels or competition of DA with the radiotracer for binding to D2/D3 receptors. However, as we and others have consistently shown that cocaine abusers show decreased DA release [Volkow et al., 2011b] it is very likely that differences in brain activation reflect different levels of D2/D3 receptors in striatum. In addition, the fMRI session preceded PET scanning by 60 min and could have increased endogenous DA release, systematically reducing the BPND measures. However, increases in DA release triggered by cues are fast and short‐lasting (2–3 min) [Erhardt et al., 2002] and thus it is expected that DA release would have returned to baseline by the time of the PET scan procedure. Nonetheless, because we cannot corroborate its absence, DA release during fMRI is a confounding factor in our study.
Our results show that food and cocaine cues engaged a common network modulated by DA D2/D3 receptors that includes cerebellum, insula, inferior frontal, OFC, ACC, somatosensory and occipital cortices, ventral striatum, and DMN. Food cues produced stronger activation responses than cocaine cues in the posterior insula and the postcentral gyrus, higher deactivation in DMN and hypothalamic regions and lower activation in temporal and parietal cortices. Brain activation responses to food and cocaine cues in prefrontal and temporal cortical regions involved with reward processes increased with the valence of the cues and was correlated with D2/D3 receptors; consistent with a common neuronal substrate for the value of natural and drug cues that is modulated via D2/D3 receptor mediated signaling in addiction.
Supporting information
Supplementary Information
ACKNOWLEDGMENT
The authors report no biomedical financial interests or potential conflicts of interest.
REFERENCES
- Bennett C, Miller M (2013): fMRI reliability: Influences of task and experimental design. Cogn Affect Behav Neurosci 13:690–702. Doi: 10.3758/s13415-013-0195-1. [DOI] [PubMed] [Google Scholar]
- Bernier B, Whitaker L, Morikawa H (2011): Previous ethanol experience enhances synaptic plasticity of NMDA receptors in the ventral tegmental area. J Neurosci 31:5205–5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge K, Robinson T (2003): Parsing reward. Trends Neurosci 26:507–513. [DOI] [PubMed] [Google Scholar]
- Boileau I, Dagher A, Leyton M, Welfeld K, Booij L, Diksic M, Benkelfat C (2007): Conditioned dopamine release in humans: A positron emission tomography [11C]raclopride study with amphetamine. J Neurosci 27:3998–4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolla K, Ernst M, Kiehl K, Mouratidis M, Eldreth D, Contoreggi C, Matochik J, Kurian V, Cadet J, Kimes A, Funderburk F, London E (2004): Prefrontal cortical dysfunction in abstinent cocaine abusers. J Neuropsychiatry Clin Neurosci 16:456–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonson K, Grant S, Contoreggi C, Links J, Metcalfe J, Weyl H, Kurian V, Ernst M, London E (2002): Neural systems and cue‐induced cocaine craving. Neuropsychopharmacology 26:376–386. [DOI] [PubMed] [Google Scholar]
- Caparelli E, Tomasi D (2008): K‐space spatial low‐pass filters can increase signal loss artifacts in echo‐planar imaging. Biomed Signal Process Control 3:107–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caparelli EC, Tomasi D, Arnold S, Chang L, Ernst T (2003): K‐Space based summary motion detection for functional magnetic resonance imaging. NeuroImage 20:1411–1418. [DOI] [PubMed] [Google Scholar]
- Carbo‐Gas M, Vazquez‐Sanroman D, Aguirre‐Manzo L, Coria‐Avila G, Manzo J, Sanchis‐Segura C, Miquel M (2013): Involving the cerebellum in cocaine‐induced memory: Pattern of cFos expression in mice trained to acquire conditioned preference for cocaine. Addict Biol 19:61–76. [DOI] [PubMed] [Google Scholar]
- Childress A, Mozley P, McElgin W, Fitzgerald J, Reivich M, O'Brien C (1999): Limbic activation during cue‐induced cocaine craving. Am J Psychiatry 156:11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornier M, McFadden K, Thomas E, Bechtell J, Eichman L, Bessesen D, Tregellas J (2013): Differences in the neuronal response to food in obesity‐resistant as compared to obesity‐prone individuals. Physiol Behav 110–111:122–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crockford D, Goodyear B, Edwards J, Quickfall J, el‐Guebaly N (2005): Cue‐induced brain activity in pathological gamblers. Biol Psychiatry 58:787–795. [DOI] [PubMed] [Google Scholar]
- de Araujo I, Oliveira‐Maia A, Sotnikova T, Gainetdinov R, Caron M, Nicolelis M, Simon S (2008): Food reward in the absence of taste receptor signaling. Neuron 57:930–941. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Everitt B (2004): Direct interactions between the basolateral amygdala and nucleus accumbens core underlie cocaine‐seeking behavior by rats. J Neurosci 24:7167–7173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Ciano P, Robbins T, Everitt B (2008): Differential effects of nucleus accumbens core, shell, or dorsal striatal inactivations on the persistence, reacquisition, or reinstatement of responding for a drug‐paired conditioned reinforcer. Neuropsychopharmacology 33:1413–1425. [DOI] [PubMed] [Google Scholar]
- Drevets W, Gautier C, Price J, Kupfer D, Kinahan P, Grace A, Price J, Mathis C (2001): Amphetamine‐induced dopamine release in human ventral striatum correlates with euphoria. Biol Psychiatry 49:81–96. [DOI] [PubMed] [Google Scholar]
- Erhardt S, Schwieler L, Engberg G (2002): Excitatory and inhibitory responses of dopamine neurons in the ventral tegmental area to nicotine. Synapse 43:227–237. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J (1996): Structured Clinical Interview for DSM‐IV Axis I disorders ‐Patient Edition (SCID‐I/P, Version 2.0). New York: Biometrics Research Department, New York State Psychiatric Institute. [Google Scholar]
- Fox M, Snyder A, Vincent J, Corbetta M, Van Essen D, Raichle M (2005): The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA 102:9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franken I, Hendriks V, Stam C, Van den Brink W (2004): A role for dopamine in the processing of drug cues in heroin dependent patients. Eur Neuropsychopharmacol 14:503–508. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Ashburner J, Frith CD, Poline JB, Heather JD, Frackowiak RSJ (1995): Spatial registration and normalization of images. Hum Brain Mapp 2:165–189. [Google Scholar]
- Garavan H, Pankiewicz J, Bloom A, Cho JK, Sperry L, Ross TJ, Salmeron BJ, Risinger R, Kelley D, Stein EA (2000): Cue‐induced cocaine craving: Neuroanatomical specificity for drug users and drug stimuli. Am J Psychiatry 157:1789–1798. [DOI] [PubMed] [Google Scholar]
- Goldstein R, Volkow N (2002): Drug addiction and its underlying neurobiological basis: Neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry 159:1642–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein R, Alia‐Klein N, Tomasi D, Carrillo J, Maloney T, Woicik P, Wang R, Telang F, Volkow N (2009): Anterior cingulate cortex hypoactivations to an emotionally salient task in cocaine addiction. Proc Natl Acad Sci USA 106:9453–9458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace A (2000): The tonic/phasic model of dopamine system regulation and its implications for understanding alcohol and psychostimulant craving. Addiction 95 Supp 2:S119–S128. [DOI] [PubMed] [Google Scholar]
- Grahn J, Parkinson J, Owen A (2008): The cognitive functions of the caudate nucleus. Prog Neurobiol 86:141–155. [DOI] [PubMed] [Google Scholar]
- Grant S, London E, Newlin D, Villemagne V, Liu X, Contoreggi C, Phillips R, Kimes A, Margolin A (1996): Activation of memory circuits during cue‐elicited cocaine craving. Proc Natl Acad Sci USA 93:12040–12045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase L, Cerf‐Ducastel B, Murphy C (2009): Cortical activation in response to pure taste stimuli during the physiological states of hunger and satiety. Neuroimage 44:1008–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber S (2003): The primate basal ganglia: Parallel and integrative networks. J Chem Neuroanat 26:317–330. [DOI] [PubMed] [Google Scholar]
- Haber S, Calzavara R (2009): The cortico‐basal ganglia integrative network: The role of the thalamus. Brain Res Bull 78:69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haines D, Dietrichs E, Sowa T (1984): Hypothalamo‐cerebellar and cerebello‐hypothalamic pathways: A review and hypothesis concerning cerebellar circuits which may influence autonomic centers affective behavior. Brain Behav Evol 24:198–220. [DOI] [PubMed] [Google Scholar]
- Hermann D, Smolka M, Wrase J, Klein S, Nikitopoulos J, Georgi A, Braus D, Flor H, Mann K, Heinz A (2006): Blockade of cue‐induced brain activation of abstinent alcoholics by a single administration of amisulpride as measured with fMRI. Alcohol Clin Exp Res 30:1349–1354. [DOI] [PubMed] [Google Scholar]
- Hester R, Garavan H (2004): Executive dysfunction in cocaine addiction: Evidence for discordant frontal, cingulate, and cerebellar activity. J Neurosci 24:11017–11022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilts C, Schweitzer J, Quinn C, Gross R, Faber T, Muhammad F, Ely T, Hoffman J, Drexler K (2001): Neural activity related to drug craving in cocaine addiction. Arch Gen Psychiatry 58:334–341. [DOI] [PubMed] [Google Scholar]
- Kilts C, Gross R, Ely T, Drexler K (2004): The neural correlates of cue‐induced craving in cocaine‐dependent women. Am J Psychiatry 161:233–241. [DOI] [PubMed] [Google Scholar]
- Koob G (1992): Neural mechanisms of drug reinforcement. Ann N Y Acad Sci 654:171–191. [DOI] [PubMed] [Google Scholar]
- Kosten T, Scanley B, Tucker K, Oliveto A, Prince C, Sinha R, Potenza M, Skudlarski P, Wexler B (2006): Cue‐induced brain activity changes and relapse in cocaine‐dependent patients. Neuropsychopharmacology 31:644–650. [DOI] [PubMed] [Google Scholar]
- Lenoir M, Serre F, Cantin L, Ahmed S (2007): Intense sweetness surpasses cocaine reward. PLos One 2:e698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Huang C, Yan P, Bhagwagar Z, Milivojevic V, Sinha R (2008): Neural correlates of impulse control during stop signal inhibition in cocaine‐dependent men. Neuropsychopharmacology 33:1798–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Chefer S, Lu H, Guillem K, Rea W, Kurup P, Yang Y, Peoples L, Stein E (2013): Dorsolateral caudate nucleus differentiates cocaine from natural reward‐associated contextual cues. Proc Natl Acad Sci USA 110:4093–4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan J FJ, Volkow ND, Wolf AP, Dewey SL, Schlyer DJ, MacGregor RR, Hitzemann R, Bendriem B, Gatley SJ, Christman DR (1990): Graphical analysis of reversible radioligand binding from time‐activity measurements applied to [N‐11C‐methyl]‐(‐)‐cocaine PET studies in human subjects. J Cereb Blood Flow Metab 10:740–747. [DOI] [PubMed] [Google Scholar]
- Luijten M, Veltman D, Hester R, Smits M, Pepplinkhuizen L, Franken I (2012): Brain activation associated with attentional bias in smokers is modulated by a dopamine antagonist. Neuropsychopharmacology 37:2772–2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mameli M, Halbout B, Creton C, Engblom D, Parkitna J, Spanagel R, Lüscher C (2009): Cocaine‐evoked synaptic plasticity: Persistence in the VTA triggers adaptations in the NAc. Nat Neurosci 12:1036–1041. [DOI] [PubMed] [Google Scholar]
- Martinez D, Broft A, Foltin R, Slifstein M, Hwang D, Huang Y, Perez A, Frankle W, Cooper T, Kleber H, Fischman MW, Laruelle M (2004): Cocaine dependence and d2 receptor availability in the functional subdivisions of the striatum: Relationship with cocaine‐seeking behavior. Neuropsychopharmacology 29:1190–1202. [DOI] [PubMed] [Google Scholar]
- Mason M, Norton M, Van Horn J, Wegner D, Grafton S, Macrae C (2007): Wandering minds: The default network and stimulus‐independent thought. Science 315:393–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan A, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, Argeriou M (1992): The fifth edition of the addiction severity index. J Subst Abuse Treat 9:199–213. [DOI] [PubMed] [Google Scholar]
- Moeller F, Steinberg J, Schmitz J, Ma L, Liu S, Kjome K, Rathnayaka N, Kramer L, Narayana P (2010): Working memory fMRI activation in cocaine dependent subjects: Association with treatment response. Psych Res Neuroimaging 181:174–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee J, Christian B, Dunigan K, Shi B, Narayanan T, Satter M, Mantil J (2002): Brain imaging of 18F‐fallypride in normal volunteers: Blood analysis, distribution, test‐retest studies, and preliminary assessment of sensitivity to aging effects on dopamine D‐2/D‐3 receptors. Synapse 46:170–188. [DOI] [PubMed] [Google Scholar]
- Naqvi N, Bechara A (2010): The insula and drug addiction: An interoceptive view of pleasure, urges, and decision‐making. Brain Struct Funct 214:435–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi N, Rudrauf D, Damasio H, Bechara A (2007): Damage to the insula disrupts addiction to cigarette smoking. Science 315:531–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norgren R, Hajnal A, Mungarndee S (2006): Gustatory reward and the nucleus accumbens. Physiol Behav 89:531–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien C, Childress A, Ehrman R, Robbins S (1998): Conditioning factors in drug abuse: Can they explain compulsion? J Psychopharmacol 12:15–22. [DOI] [PubMed] [Google Scholar]
- Park K, Volkow N, Pan Y, Du C (2013): Chronic cocaine dampens dopamine signaling during cocaine intoxication and unbalances D1 over D2 receptor signaling. J Neurosci 33:15827–15836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquereau B, Turner R (2013): Limited encoding of effort by dopamine neurons in a cost‐benefit trade‐off task. 33:8288–82300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan K, Wager T, Taylor S, Liberzon I (2002): Functional neuroanatomy of emotion: A meta‐analysis of emotion activation studies in PET and fMRI. Neuroimage 16:331–348. [DOI] [PubMed] [Google Scholar]
- Phillips P, Stuber G, Heien M, Wightman R, Carelli R (2003): Subsecond dopamine release promotes cocaine seeking. Nature 422:614–618. [DOI] [PubMed] [Google Scholar]
- Potenza M, Hong K, Lacadie C, Fulbright R, Tuit K, Sinha R (2012): Neural correlates of stress‐induced and cue‐induced drug craving: Influences of sex and cocainedependence. Am J Psychiatry 169:406–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone J, Correa M (2012): The mysterious motivational functions of mesolimbic dopamine. Neuron 76:470–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders B, Robinson T (2013): Individual variation in resisting temptation: Implications for addiction. Neurosci Biobehav Rev 37:1955–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague P (1997): A neural substrate of prediction and reward. Science 275:1593–1599. [DOI] [PubMed] [Google Scholar]
- Shrout P, Fleiss J (1979): Intraclass correlations: Uses in assessing rater reliability. Psychol Bull 86:420–428. [DOI] [PubMed] [Google Scholar]
- Smith D, Jones P, Williams G, Bullmore E, Robbins T, Ersche K (2013): Overlapping decline in orbitofrontal gray matter volume related to cocaine use and body mass index. Addict Biol (in press). Doi: 10.1111/adb.12081. [DOI] [PubMed] [Google Scholar]
- Stice E, Yokum S, Burger K, Epstein L, Small D (2011): Youth at risk for obesity show greater activation of striatal and somatosensory regions to food. J Neurosci 31:4360–4366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanos P, Robison L, Nestler E, Kim R, Michaelides M, Lobo M, Volkow N (2013): Mapping brain metabolic connectivity in awake rats with µPET and optogenetic stimulation. J Neurosci 33:6343–6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoma P, Bellebaum C, Koch B, Schwarz M, Daum I (2008): The cerebellum is involved in reward‐based reversal learning. Cerebellum 7:433–443. [DOI] [PubMed] [Google Scholar]
- Thomas M, Kalivas P, Shaham Y (2008): Neuroplasticity in the mesolimbic dopamine system and cocaine addiction. Br J Pharmacol 154:327–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Volkow N (2013): Striatocortical pathway dysfunction in addiction and obesity: Differences and similarities. Crit Rev Biochem Mol Biol 48:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Caparelli EC, Chang L, Ernst T (2005): fMRI‐acoustic noise alters brain activation during working memory tasks. Neuroimage 27:377–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Ernst T, Caparelli E, Chang L (2006): Common deactivation patterns during working memory and visual attention tasks: An intra‐subject fMRI study at 4 Tesla. Hum Brain Mapp 27:694–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Volkow N, Wang R, Telang F, Wang G, Chang L, Ernst T, Fowler J (2009): Dopamine Transporters in Striatum Correlate with Deactivation in the Default Mode Network during Visuospatial Attention. PLoS ONE 4:e6102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, N, Mazoyer B, Joliot M (2002): Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. Neuroimage 15:273–289. [DOI] [PubMed] [Google Scholar]
- Ventura J, Liberman R, Green M, Shaner A, Mintz J (1998): Training and quality assurance with the Structured Clinical Interview for DSM‐IV (SCID‐I/P). Psychiatry Res 79:163–173. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wolf AP, Schlyer D, Shiue CY, Alpert R, Dewey SL, Logan J, Bendriem B, Christman D, Hitzemann R, Henn F (1990): Effects of chronic cocaine abuse on postsynaptic dopamine receptors. Am J Psychiatry 147:719–724. [DOI] [PubMed] [Google Scholar]
- Volkow N, Fowler J, Wang G‐J, SL Dewey, D Schlyer, R MacGregor, J Logan, D Alexoff, C Shea, R Hitzemann, B Angrist, AP Wolf (1993a): Reproducibility of repeated measures of Carbon‐11‐raclopride binding in the human brain. J Nucl Med 34:609–613. [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Hitzemann R, Logan J, Schlyer DJ, Dewey SL, Wolf AP (1993b): Decreased dopamine D2 receptor availability is associated with reduced frontal metabolism in cocaine abusers. Synapse 14:169–177. [DOI] [PubMed] [Google Scholar]
- Volkow N, Wang G, Fowler J, Logan J, Schlyer D, Hitzemann R, Lieberman J, Angrist B, Pappas N, MacGregor R (1994): Imaging endogenous dopamine competition with [11C]raclopride in the human brain. Synapse 16:255–262. [DOI] [PubMed] [Google Scholar]
- Volkow N, Ding Y, Fowler J, Wang G (1996): Cocaine addiction: Hypothesis derived from imaging studies with PET. J Addict Dis 15:55–71. [DOI] [PubMed] [Google Scholar]
- Volkow N, Wang G, Fowler J, Logan J, Angrist B, Hitzemann R, Lieberman J, Pappas N (1997a): Effects of methylphenidate on regional brain glucose metabolism in humans: Relationship to dopamine D2 receptors. Am J Psychiatry 154:50–55. [DOI] [PubMed] [Google Scholar]
- Volkow N, Wang G, Fowler J, Logan J, Gatley S, Hitzemann R, Chen A, Dewey S, Pappas N (1997b): Decreased striatal dopaminergic responsiveness in detoxified cocaine‐dependent subjects. Nature 386:830–833. [DOI] [PubMed] [Google Scholar]
- Volkow N, Wang G, Fowler J, Hitzemann R, Angrist B, Gatley S, Logan J, Ding Y, Pappas N (1999a): Association of methylphenidate‐induced craving with changes in right striato‐orbitofrontal metabolism in cocaine abusers: Implications in addiction. Am J Psychiatry 156:19–26. [DOI] [PubMed] [Google Scholar]
- Volkow N, Wang G, Fowler J, Logan J, Gatley S, Wong C, Hitzemann R, Pappas N (1999b): Reinforcing effects of psychostimulants in humans are associated with increases in brain dopamine and occupancy of D(2) receptors. J Pharmacol Exp Ther 291:409–415. [PubMed] [Google Scholar]
- Volkow N, Wang G, Fowler J, Logan J, Jayne M, Franceschi D, Wong C, Gatley S, Gifford A, Ding Y, Pappas N (2002): "Nonhedonic" food motivation in humans involves dopamine in the dorsal striatum and methylphenidate amplifies this effect. Synapse 44:175–180. [DOI] [PubMed] [Google Scholar]
- Volkow N, Wang G, Telang F, Fowler J, Logan J, Childress A, Jayne M, Ma Y, Wong C (2006): Cocaine cues and dopamine in dorsal striatum: Mechanism of craving in cocaine addiction. J Neurosci 26:6583–6588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N, Fowler J, Wang G, Telang F, Logan J, Jayne M, Ma Y, Pradhan K, Wong C, Swanson J (2010a): Cognitive control of drug craving inhibits brain reward regions in cocaine abusers. Neuroimage 49:2536–2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N, Wang G, Tomasi D, Telang F, Fowler J, Pradhan K, Jayne M, Logan J, Goldstein R, Alia‐Klein N, Wong C (2010b): Methylphenidate attenuates limbic brain inhibition after cocaine‐cues exposure in cocaine abusers. PLoS ONE 5:e11509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N, Wang G, Baler R (2011a): Reward, dopamine and the control of food intake: Implications for obesity. Trends Cogn Sci 15:37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N, Wang G, Fowler J, Tomasi D, Telang F (2011b): Addiction: Beyond dopamine reward circuitry. Proc Natl Acad Sci USA 108:15037–15042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N, Wang G, Fowler J, Tomasi D (2012a): Addiction circuitry in the human brain. Annu Rev Pharmacol Toxicol 52:321–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N, Wang G, Fowler J, Tomasi D, Baler R (2012b): Food and Drug Reward: Overlapping Circuits in Human Obesity and Addiction. Curr Top Behav Neurosci 11:1–24. DOI: 10.1007/7854_2011_169. [DOI] [PubMed] [Google Scholar]
- Volkow N, Wang G, Tomasi D, Baler R (2013): The addictive dimensionality of obesity. Biol Psychiatry 73:811–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanat M, Willuhn I, Clark J, Phillips P (2009): Phasic dopamine release in appetitive behaviors and drug addiction. Curr Drug Abuse Rev 2:195–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Smith L, Volkow N, Telang F, Logan J, Tomasi D, Wong C, Hoffman W, Jayne M, Alia‐Klein N, P Thanos, JS Fowler (2011): Decreased dopamine activity predicts relapse in methamphetamine abusers. Mol Psychiatry 17:918–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Volkow N, Fowler J, Cervany P, Hitzemann R, Pappas N, Wong C, Felder C (1999): Regional brain metabolic activation during craving elicited by recall of previous drug experiences. Life Sci 64:775–784. [DOI] [PubMed] [Google Scholar]
- Wang G, Volkow N, Logan J, Pappas N, Wong C, Zhu W, Netusil N, Fowler J (2001): Brain dopamine and obesity. Lancet 357:354–357. [DOI] [PubMed] [Google Scholar]
- Wang G, Volkow N, Felder C, Fowler J, Levy A, Pappas N, Wong C, Zhu W, Netusil N (2002): Enhanced resting activity of the oral somatosensory cortex in obese subjects. Neuroreport 13:1151–1155. [DOI] [PubMed] [Google Scholar]
- Wang G, Tomasi D, Volkow N, Wang RT, F, Caparelli E, Dunayevich E (2014): Effect of combined naltrexone and bupropion therapy on the brain's reactivity to food cues. Int J Obes 38:682–688. Doi: 10.1038/ijo.2013.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss F, Maldonado‐Vlaar C, Parsons L, Kerr T, Smith D, Ben‐Shahar O (2000): Control of cocaine‐seeking behavior by drug‐associated stimuli in rats: Effects on recovery of extinguished operant‐responding and extracellular dopamine levels in amygdala and nucleus accumbens. Proc Natl Acad Sci USA 97:4321–4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West A, Grace A (2002): Opposite influences of endogenous dopamine D1 and D2 receptor activation on activity states and electrophysiological properties of striatal neurons: Studies combining in vivo intracellular recordings and reverse microdialysis. J Neurosci 22:294–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise R (2009): Roles for nigrostriatal–not just mesocorticolimbic–dopamine in reward and addiction. Trends Neurosci 32:517–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DF Wong, H Kuwabara, DJ Schretlen, KR Bonson, Y Zhou, A Nandi, JR Brasić, AS Kimes, MA Maris, A Kumar, C Contoreggi, J Links, M Ernst, O Rousset, S Zukin, AA Grace, JS Lee, C Rohde, DR Jasinski, A Gjedde, ED London (2006): Increased occupancy of dopamine receptors in human striatum during cue‐elicited cocaine craving. Neuropsychopharmacology 31:2716–2727. [DOI] [PubMed] [Google Scholar]
- Worsley K, Evans A, Marrett S, Neelin P (1992): A three‐dimensional statistical analysis for CBF activation studies in human brain. J Cereb Blood Flow Metab 12:900–918. [DOI] [PubMed] [Google Scholar]
- Zijlstra F, Booij J, van den Brink W, Franken I (2008): Striatal dopamine D2 receptor binding and dopamine release during cue‐elicited craving in recently abstinent opiate‐dependent males. Eur Neuropsychopharmacol 18:262–270. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information


