Abstract
In mammalian transcriptomes approximately 25% of 5’ ends determined by Capped Analysis of Gene Expression (CAGE) map to locations within spliced exons. The current study sought to determine if the cytoplasmic capping complex participates in generating these downstream CAGE tags. 5’-RACE was used to amplify the uncapped ends of target transcripts that accumulate when cytoplasmic capping is blocked. Sequencing of these RACE products mapped the positions of uncapped ends either exactly to or just downstream of archived CAGE tags. These findings support a role for cytoplasmic capping in generating the downstream capped ends identified by CAGE.
Keywords: mRNA capping, cytoplasm, CAGE, uncapped 5’ ends, cytoplasmic capping, mammalian cells
Introduction
The 5’ ends of all mRNAs and lncRNAs have a methylguanosine (m7G) ‘cap’ that is added co-transcriptionally as the first step of post-transcriptional processing. This served as the basis for development of Capped Analysis of Gene Expression (CAGE) as a method of identifying transcription start sites. However, in the first broad scale application of CAGE to mammalian transcriptomes a large number of capped ends were identified that did not match to known sites of transcription initiation [1]. At the time, we were studying the decay of nonsense-containing β-globin mRNA in erythroid cells [2]. In these cells, nonsense-containing human β-globin mRNA is cleaved by SMG6 to generate metastable intermediates [3] that were described as having a 5’ cap [4]. The concept of capped decay products raised several questions, including whether these indeed had an m7G cap and how this might be added when all of the proteins that catalyze capping were thought to be present only in the nucleus. Further complicating matters was the fact that capping enzyme transfers covalently-bound GMP onto RNA with a 5’-diphosphate end, and there was no known mechanism for generating this substrate from 5’-monophosphate RNA. In pursuing this question we identified the cytoplasmic complex that includes capping enzyme and a kinase that generates a capping substrate from 5’-monophosphate RNA [5]. We subsequently showed that the cytoplasmic capping complex assembles on adapter protein Nck1, a protein with 3 SH3 domains and a single SH2 domain that functions primarily in transducing receptor tyrosine kinase signaling [6]. Importantly, Nck1 is only found in the cytoplasm, and the binding of capping enzyme and the 5’-kinase to adjacent SH3 domains juxtaposes these two critical enzymes in a manner that facilitates cytoplasmic capping.
In [7], cytoplasmic capping targets were identified by the susceptibility of uncapped 5’ ends to in vitro degradation by Xrn1 when recovered from cells expressing a dominant negative form of capping enzyme, termed K294A, that was modified to restrict its distribution to the cytoplasm. Three classes of targets were identified by position-dependent changes in probe intensity on human exon arrays, two of which accumulate uncapped forms when cytoplasmic capping is blocked. The accumulation of uncapped forms of these mRNAs was confirmed by 4 independent methods; increased susceptibility to in vitro degradation by Xrn1, selective recovery of uncapped RNAs following ligation of an RNA adapter and hybridization to a biotin-tagged antisense DNA, selective exclusion from a cap affinity column containing a heterodimer of eIF4E bound to eIF4G, and the appearance of products by 5’-RACE only when cytoplasmic capping is blocked. The latter proved to be particularly important in that it identified full-length transcripts and 5’-truncated forms of the same RNAs.
We wondered if the uncapped ends of shorter transcripts that appeared by 5’-RACE of RNA from capping inhibited cells might correspond to downstream capping sites identified by CAGE. Using positional data of CAGE tags from ENCODE [8] we designed primers to several of the transcripts for which shortened forms appeared by 5’-RACE [7]. We show that uncapped ends that accumulate when cytoplasmic capping is blocked map either at or near CAGE tags, thus providing the first direct evidence of a functional role for cytoplasmic capping in generating this form of transcriptome diversity.
Materials and Methods
Downstream CAGE tag correlations
Poly-A +/−, hg19-aligned cytoplasmic CAGE tags were downloaded from the UCSC FTP data server [9] for the K562 cell line (Table 1). Reads were combined across the 4 samples (one poly-A-, three poly-A+ samples), and genomic coordinates for 5’-ends of reads were mapped to transcript coordinates for Gencode v19 transcripts [10], downloaded from the UCSC Table Browser [11]. Transcripts in the top quartile of total CAGE expression (which had no significant difference in total CAGE tags across categories, compared using Student's t-test) were classified as recapping targets or controls based on [7], and as containing a downstream CAGE tag when at least one location downstream of the annotated translation start site had a minimum CAGE coverage of 10 reads. Fisher's exact test was performed on the distribution of the number of transcripts across these categories using the R Statistical Computing Package, version 3.1.1 [12].
Table 1.
CAGE Libraries Used
| wgEncodeRikenCageK562CytosolPapAln.bam | Fig. 1A |
| wgEncodeRikenCageK562CytosolPapAlnRep1.bam | Fig. 1A |
| wgEncodeRikenCageK562CytosolPapAlnRep2.bam | Fig. 1A |
| wgEncodeRikenCageK562CytosolPamAln.bam | Fig. 1A |
| wgEncodeRikenCageAlignmentsGm12878CytosolLongnonpolya | Figs. 1B-D, 3, 4 |
| wgEncodeRikenCageAlignmentsGm12878NucleolusTotal | Figs. 1B-D, 3, 4 |
| wgEncodeRikenCageAlignmentsGm12878NucleusLongnonpolya | Figs. 1B-D, 3, 4 |
| wgEncodeRikenCageAlignmentsH1hescCellLongnonpolya | Figs. 1B-D, 3, 4 |
| wgEncodeRikenCageAlignmentsHepg2CytosolLongnonpolya | Figs. 1B-D, 3, 4 |
| wgEncodeRikenCageAlignmentsHepg2NucleolusTotal | Figs. 1B-D, 3, 4 |
| wgEncodeRikenCageAlignmentsHepg2NucleusLongnonpolya | Figs. 1B-D, 3, 4 |
| wgEncodeRikenCageAlignmentsHuvecCytosolLongnonpolya | Figs. 1B-D, 3, 4 |
| wgEncodeRikenCageAlignmentsK562ChromatinTotal | Figs. 1B-D, 3, 4 |
| wgEncodeRikenCageAlignmentsK562CytosolLongnonpolya | Figs. 1B-D, 3, 4 |
| wgEncodeRikenCageAlignmentsK562CytosolLongpolya | Figs. 1B-D, 3, 4 |
| wgEncodeRikenCageAlignmentsK562NucleolusTotal | Figs. 1B-D, 3, 4 |
| wgEncodeRikenCageAlignmentsK562NucleoplasmTotal | Figs. 1B-D, 3, 4 |
| wgEncodeRikenCageAlignmentsK562NucleusLongnonpolya | Figs. 1B-D, 3, 4 |
| wgEncodeRikenCageAlignmentsK562NucleusLongpolya | Figs. 1B-D, 3, 4 |
| wgEncodeRikenCageAlignmentsK562PolysomeLongnonpolya | Figs. 1B-D, 3, 4 |
| wgEncodeRikenCageAlignmentsNhekCytosolLongnonpolya | Figs. 1B-D, 3, 4 |
| wgEncodeRikenCageAlignmentsNhekNucleusLongnonpolya | Figs. 1B-D, 3, 4 |
| wgEncodeRikenCageAlignmentsProstateCellLongnonpolya | Figs. 1B-D, 3, 4 |
Cell culture and preparation of cytoplasmic RNA
Tetracycline-inducible U2OS cells stably transfected with pcDNA4/TO/myc-K294-ΔNLS+NES-Flag (K294A) were cultured in McCoy's medium (Gibco) supplemented with 10% fetal bovine serum [5, 7]. 3 × 106 log-phase cells were split into 150 mm tissue culture dishes followed 24 hr later by addition of 1 μg/ml doxycycline to induce K294A. The medium was removed 24 hr later, the cells were rinsed twice with ice-cold phosphate buffered saline and suspended with a cell scraper. These were recovered by centrifugation for 5 min at 1000 xg, the pellet was suspended in 5 volumes of lysis buffer (20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 5 mM MgCl2, 1 mM DTT, 0.2% NP-40, 80 U/ml RNaseOUT (Invitrogen)) and incubated on ice for 10 min with gentle agitation. Nuclei were removed by centrifuging at 16,000 xg for 10 minutes at 4°C and cytoplasmic RNA was recovered from the supernatant fraction with Trizol (Life Technologies) according to the manufacturer's instructions. The recovered RNA was resuspended in water and treated with DNase I (5U/50 μl) (Life Technologies) according to the manufacturer's instructions, and the reaction was stopped by the addition of EDTA and denaturation at 65°C for 10 minutes.
Nuclear extracts and western blotting
The nuclear extract was made by resuspending the pelleted nuclei from above in the same lysis buffer and sonicating four times using a micro tip at 40% amplitude on continuous setting for four seconds. Each sample was incubated on ice for one minute between sonications. Samples were then centrifuged at 16,000 xg for 10 minutes at 4°C and the supernatant was used for further analysis. Protein levels of nuclear and reserved cytoplasmic extracts were determined by Bradford assay (Biorad). Western blotting was performed by loading either 10 or 20 μg of protein into precast 4-15% gradient or 10% Mini-PROTEAN TGX gels (Biorad) and transferring to nitrocellulose (Amersham) or Immobilon-FL PVDF (EMD Millipore) membranes. The resulting membranes were blocked in 5% non-fat milk in Tris-buffered saline (TBS). Blots were probed (overnight at 4°C) with primary antibodies targeting Histone H3 (rabbit, Cell Signalling), -tubulin (mouse, Sigma), or c-Myc (mouse, Santa Cruz), that were diluted 1:1000, 1:15,000, 1:1000 respectively with 1% milk in TBS +0.05% Tween-20 (TBST). Secondary antibodies, goat anti-Mouse IRDye 800CW (Rockland) or goat anti-Rabbit Alexa-flour 680 (Invitrogen), were used at 1:10,000 dilutions in 1% milk in TBST and visualized with a Licor Odyssey imager.
Identification of CAGE tags for analysis
Sequences of the most common isoforms of ITGB1, SARS, and ZNF207 from [7] were aligned against human genome hg18 using BLAT [13] and the result was parsed for the genomic locations of all exons of the transcripts. Raw sequence data files from 18 ENCODE CAGE libraries (aligned to hg18) [8] that were available at the time were obtained from the UCSC Genome Browser in tag-aligned format (Table 1). A custom script was used to extract all tags within 100 nucleotides of the target transcripts, associate the position of each CAGE tag with the 5’ end and 3’ end of the read for genes in the forward and reverse strand, respectively, using the exon positions to convert the genomic coordinates into transcript coordinates, remove tags from transcript positions with encoded GGs as well as tags supported by fewer than 10 reads, and finally tally the tag counts across all experimental conditions. Primers selected for use in 5’ RACE were located ~150 bases 3’ to sizeable peaks or clusters of CAGE tags.
5’ RACE and PCR
Cytoplasmic RNA (5 μg) was depleted of ribosomal RNA using a RiboZero kit as directed by the manufacturer (Epicentre). One fifth of the resulting RNA (5 μl) was mixed with 1.5 μl of 100 μM RNA RACE Adaptor oligonucleotide (Table 2), 3.5 μl DMSO, and 15 μl of water. Reactions were incubated at 65°C for five minutes followed by rapid chilling on ice. 10 μl of ligation mix two (3.5 μl 10x RNA Ligase 1 buffer, 3.5 μl of 10 mM ATP, and 3 μl of RNA Ligase 1) was added to each sample, followed by gentle mixing and incubation for 16 hours at 16°C. Reactions were stopped with the addition of 5 μl of 25 mM EDTA and inactivated by incubation at 65°C for 15 minutes. One fourth of the ligation reaction was used for reverse transcription with Superscript III (Life Technologies) according to the manufacturer's instructions. cDNA synthesis was primed using gene-specific primers listed in Table 2, followed by nested PCR performed using 5% of the cDNA, the adapter-specifc RACE primer and the indicated transcript-specific primers. Primary nested PCR products were purified using DNA Clean and Concentrator-5 columns (Zymo) and secondary PCRs were performed using CAGE-directed primers (Table 2). The reactions were separated on 2% agarose gels and each of the amplified products was recovered using a gel extraction kit (Syd Labs). Purified DNA was sequenced at the Plant-Microbe Genomics Facility at The Ohio State University. The resulting sequences were mapped to their respective genes using BLAST [14] from NCBI.
Table 2.
Oligonucleotides used
| Oligonucletide name | Sequence | Use(s) |
|---|---|---|
| RNA_RACE_Adaptor | GUUCAGAGUUCUACAGUCCGACGAUC | ligation reaction |
| RACE_Forward | GTTCAGAGTTCTACAGTCCGACGATC | 1° and 2° nested PCR |
| ZNF207_nTail (1725- | GTCCAATAGTTCCTGGTATGTGGGAAG | cDNA synthesis and 1° |
| SARS_3′ (1838-1815)Rev | ATCAATGATGGGTCCCTATGCCCA | nDNA synthesis and 1° |
| ITGB1_sTail (3678-3653)Rev | GGGCAACTCAAATGGTGAGAAGTAAA | nDNA synthesis and 1° |
| ZNF207-A (326-301)Rev | ACCTGCATGCAATGAATAGCTAAGC | 2° nested PCR |
| ZNF207-B (649-627)Rev | TGGCATTAATGGAGGTATGCCT | 2° nested PCR |
| ZNF207-C (1192-1169)Rev | GTACTATTTAAGGGTTTGAAATC | 2° nested PCR |
| SARS (160-134)Rev | CTTGAAGCGCTTCTCCTGCGTCTCTC | 2° nested PCR |
| ITGB1-A (469-446)Rev | AATTTTAATGTAAATGTCTGTGG | 2° nested PCR |
| ITGB1-B (1705-1681)Rev | TTTCATTTGTATTATCCCTCTTCC | 2° nested PCR |
| ITGB1-C (2296-2273)Rev | CCAATAAGAACAATTCCAGCAAC | 2° nested PCR |
Results and Discussion
Target selection for 5’-RACE
In [7], cytoplasmic capping targets were identified by the susceptibility of uncapped ends to in vitro degradation by Xrn1, particularly when cap homeostasis was disrupted by expression of an inactive form of cytoplasmic capping enzyme (K294A). For the most part, 5’-RACE products were only observed with RNA that was recovered from K294A-expressing cells, and some were missing portions from their 5’ ends. While it was conceivable that the 5’-truncated transcripts were degradation products, the fact that they were discrete, reproducible, and accentuated by inhibition of cytoplasmic capping suggested they might instead correspond to downstream capped ends identified by CAGE [1, 8].
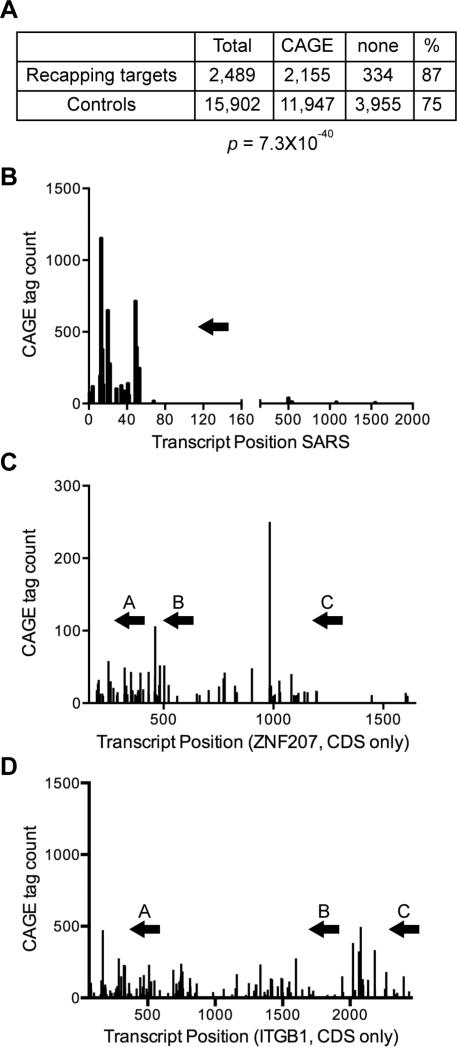
We first determined if there was any difference in downstream CAGE tags in recapping targets versus control transcripts identified in [7]. In Figure 1A the datasets of cytoplasmic capping targets and control transcripts were compared with the CAGE databases indicated in Table 1 using Fisher's Exact Test. The results showed there is a significantly greater representation of downstream CAGE tags in cytoplasmic capping targets (87%) than control transcripts (75%, p = 7.3×10−40).
Figure 1. mRNA targets of cytoplasmic capping have a greater representation of downstream CAGE tags than non-target mRNAs.
A. The datasets of cytoplasmic capping targets and control transcripts from [7] and from the databases of K562 cells in Table 1 were compared using Fisher's Exact Test to determine the correlation between cytoplasmic capping targets and the presence of downstream CAGE tags within spliced exons. B-D. The positions and number of CAGE tags are shown across the length of the predominant isoforms of SARS (B), ZNF207 (C) and ITGB1 mRNA (D). The location of primers used for 5’-RACE are identified by arrows above the plots for each transcript.
Relationship of CAGE tags to recapping sites
Three transcripts (SARS, ZNF207 and ITGB1) were selected for further analysis. Each of these was shown previously by 5’-RACE to accumulate shortened, uncapped forms when cytoplasmic capping is blocked [7]. The locations of downstream CAGE tags relative to mRNA 5’ ends of these mRNAs are shown in Figure 1B-D. Each has a cluster of CAGE tags toward the 5’-end, and ZNF207 and ITGB1 have clusters of CAGE tags further downstream (Figure 1B-D). The arrows in each of these figures identify the locations of primers that were used for PCR amplification of 5’-RACE products for each of these clusters.
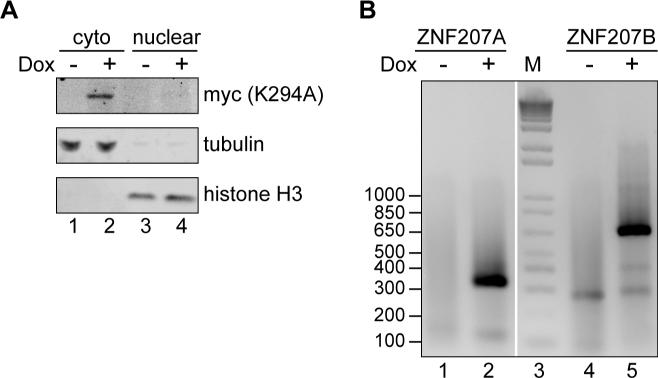
Prior to mapping uncapped ends we confirmed that uncapped forms appear as a result of inhibiting cytoplasmic capping. We previously described the development and application of a line of U2OS cells that expresses the inhibitory K294A form of cytoplasmic capping enzyme under a tetracycline-inducible promoter [5, 7]. The Western blots in Figure 2A show K294A is only expressed in doxycycline-treated cells, that its distribution is restricted to the cytoplasm, and that the cytoplasmic extract used to recover RNA for 5’-RACE was free of nuclear contamination. To confirm that uncapped transcripts appear when cytoplasmic capping is blocked, cytoplasmic RNA recovered from extracts of uninduced and K294A-expressing cells was depleted of ribosomal RNA and ligated to an RNA adapter. 5’-RACE was then performed using two of the primers for ZNF207 mRNA shown in Figure 1C. The selective appearance of products from K294A-expressing cells (Figure 2B) confirmed that uncapped 5’ ends appear within the body of ZNF207 mRNA as a consequence of inhibiting cytoplasmic capping.
Figure 2. Appearance of uncapped 5’-ends as a consequence of inhibiting cytoplasmic capping.
A. Cytoplasmic and nuclear extracts were prepared from uninduced and doxycycline-treated U2OS cells stably transfected with a tetracycline-inducible K294A transgene [5]. Restricted expression of K294A to doxycycline-treated cells was demonstrated by Western blotting with antibody to the N-terminal Myc tag on the protein, and Western blotting was performed with antibodies to -tubulin and histone H3 to demonstrate that cytoplasmic extract was free of nuclear contamination. B. RNA recovered from cytoplasmic extracts of control and doxycycline-treated cells was depleted of ribosomal RNA, followed by primer ligation onto uncapped 5’-ends. 5’-RACE was performed for ZNF207 mRNA using primers A and B in Figure 1C, followed by electrophoresis on a 2% agarose gel. Lane 3 contains a 100 bp ladder (M).
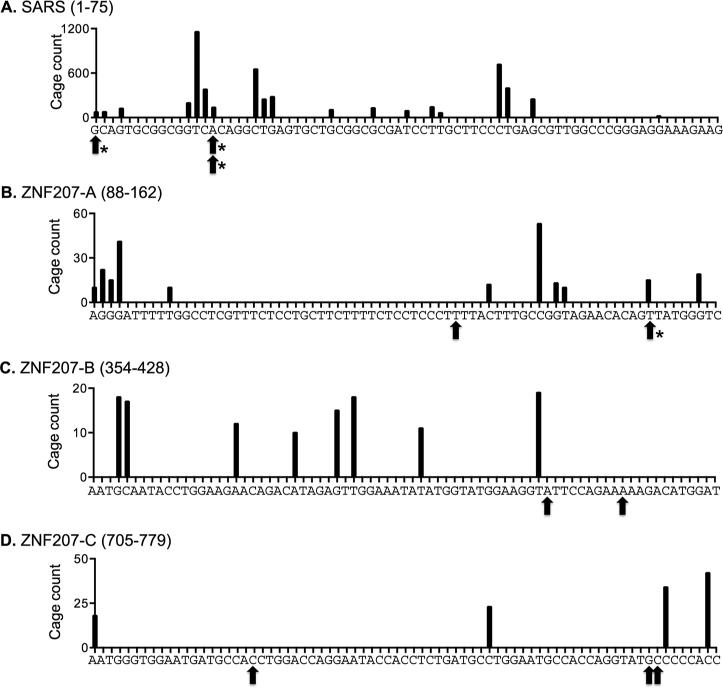
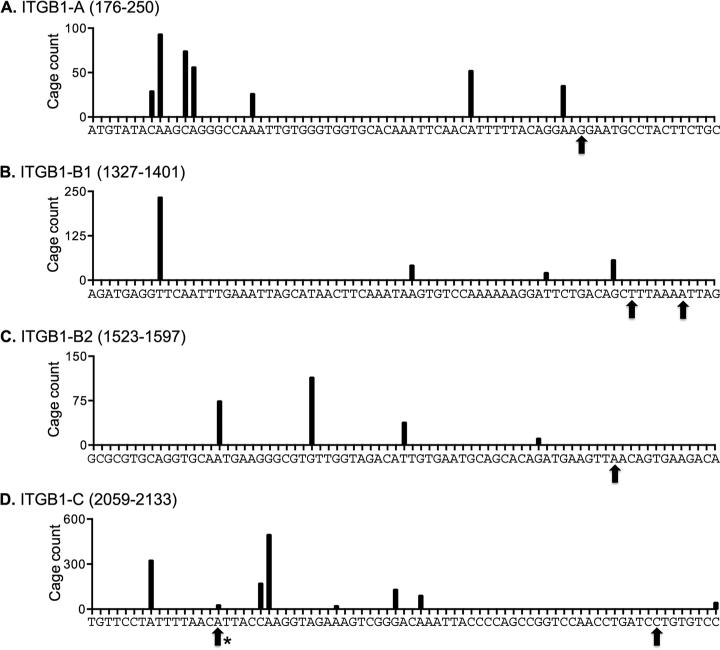
Next, each of the 3 mRNAs in Fig. 1B-D were amplified by 5’-RACE using the primers indicated on the figure, the products were gel purified, sequenced, and the positioning of the ligated primer was used to identify the location of uncapped ends. SARS was the first mRNA to be examined because it has a limited number of CAGE tags that cluster toward the 5’ end. The black bars in Figure 3A show the locations of the CAGE tags assembled from the ENCODE data listed in Table 1, and the arrows indicate the locations of uncapped 5’ ends as determined by the junction between the ligated primer and the mRNA. In agreement with the 5’-RACE data in [7] this captured the uncapped 5’ end as well as an uncapped end located in the first major cluster of CAGE tags (arrows). This analysis was then extended to ZNF207 (Figure 3B-D) and ITGB1 (Figure 4). Because each of these mRNAs have 3 major clusters of downstream CAGE tags 5’-RACE was performed with 3 different downstream primers (Figures 1C and D). Here the results were more variable. For both ZNF207 and ITGB1 5’-RACE identified uncapped ends that mapped to CAGE tags but also to other sites. U2OS cells have less Xrn1 than most commonly used cell lines, and to be consistent with our previous work [7] no steps were taken to inactivate Xrn1. Because of this, some of the ends identified here may not match precisely to CAGE tag locations due to in vivo 5’ end trimming.
Figure 3. Map of 5’ RACE products of SARS and ZNF207 mRNA.
The sequences upstream of primers used for 5’-RACE of SARS (A) and ZNF207 (B-D) are shown on the horizontal axes with the positions of CAGE tags compiled from the data sets in Table 1 identified with vertical lines. The vertical axis represents the number of CAGE tags for any given location. Arrows under each sequence denote the locations of uncapped ends as determined by the junction between the ligated adapter and the target mRNA. Asterisks denote RACE products that exactly match CAGE tags.
Figure 4. Map of 5’ RACE products of ITGB1 mRNA.
The locations of CAGE tags and ligated 5’ ends of ITGB1 mRNA are shown as described in the legend to Figure 3.
Finally, there were a number of instances where downstream CAGE tags did not have matching 5’-RACE products. This was to be expected, first because the CAGE tag positions were a summation of data generated using total, cytoplasmic and nuclear RNA, whereas only cytoplasmic RNA was used here for 5’-RACE. By definition this will remove from our analysis transcripts that never make it out of the nucleus, such as those with improperly methylated caps. Second, CAGE data were generated from 7 different cell and tissue types, and the number and locations of CAGE tags varied between these. Thus, cell type differences contribute to the observed differences between CAGE tags and uncapped ends detected by 5’-RACE.
In contrast to mammals, downstream CAGE tags are absent from the Drosophila transcriptome [15]. This raised the possibility that Drosophila lacks one or more of the biochemical mechanisms needed for downstream capping, and it was this idea that led us to perform the phylogenetic analysis in [6] that identified the basis for this. That analysis identified a proline rich sequence at the C-terminus of mammalian capping enzyme whose absence from Drosophila capping enzyme precludes it's binding to Nck1, and therefore, its ability to participate in cytoplasmic capping. These findings, together with results in the current study, point to cytoplasmic capping being responsible for at least a portion of the capped ends that constitute the downstream CAGE tags of vertebrate transcriptomes.
HIghlights.
~25% of the 5’ ends identified by Capped Analysis of Gene Expression (CAGE) map to sites within spliced exons.
A subset of the transcriptome undergoes cyclical decapping and recapping in the cytoplasm, with uncapped forms accumulating when cytoplasmic capping is blocked.
Sequencing of 5’-RACE products mapped uncapped ends to the vicinity downstream CAGE tags.
These findings support a role for cytoplasmic capping in generating the downstream capped ends identified by CAGE.
Acknowledgements
The authors would like to acknowledge Ken Gerien for initial experiments that helped establish ligation conditions for the 5’ RACE reactions and the Fisk Laboratory for providing the tubulin antibody. This work was supported by grants from the National Institutes of Health [grant number R01 GM084177 to D.R.S.] and the National Science Foundation [grant numbers DMR-1105458 and 1410172 to R.B.]. D.L.K. was supported by the National Cancer Institute [grant number T32 CA0093338] and a Pelotonia postdoctoral fellowship award. The content is solely the responsibility of the authors and does not necessarily represent the official views of Pelotonia, The Ohio State University, the National Science Foundation, or the National Institutes of Health.
Abbreviations
- CAGE
capped analysis of gene expression
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fejes-Toth K, Sotirova V, Sachidanandam R, Assaf G, Hannon GJ, Kapranov P, Foissac S, Willingham AT, Duttagupta R, Dumais E, Gingeras TR. Post-transcriptional processing generates a diversity of 5’-modified long and short RNAs. Nature. 2009;457:1028–1032. doi: 10.1038/nature07759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stevens A, Wang Y, Bremer K, Zhang J, Hoepfner R, Antoniou M, Schoenberg DR, Maquat LE. Beta-globin mRNA decay in erythroid cells: UG site-preferred Proc. Natl. endonucleolytic cleavage that is augmented by a premature termination codon. Acad. Sci. USA. 2002;99:12741–12746. doi: 10.1073/pnas.192442399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mascarenhas R, Dougherty JA, Schoenberg DR. SMG6 cleavage generates metastable decay intermediates from nonsense-containing -globin mRNA. PLoS One. 2013;8:e74791. doi: 10.1371/journal.pone.0074791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lim SK, Maquat LE. Human beta-globin mRNAs that harbor a nonsense codon are degraded in murine erythroid tissues to intermediates lacking regions of exon I or exons I and II that have a cap-like structure at the 5’ termini. EMBO J. 1992;11:3271–3278. doi: 10.1002/j.1460-2075.1992.tb05405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Otsuka Y, Kedersha NL, Schoenberg DR. Identification of a cytoplasmic complex that adds a cap onto 5’-monophosphate RNA. Mol. Cell. Biol. 2009;29:2155–2167. doi: 10.1128/MCB.01325-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mukherjee C, Bakthavachalu B, Schoenberg DR. The cytoplasmic capping complex assembles on adapter protein nck1 bound to the proline-rich C-terminus of Mammalian capping enzyme. PLoS Biol. 2014;12:e1001933. doi: 10.1371/journal.pbio.1001933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mukherjee C, Patil DP, Kennedy BA, Bakthavachalu B, Bundschuh R, Schoenberg DR. Identification of cytoplasmic capping targets reveals a role for cap homeostasis in translation and mRNA stability. Cell Rep. 2012;2:674–684. doi: 10.1016/j.celrep.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Djebali S, Davis CA, Merkel A, Dobin A, Lassmann T, Mortazavi A, Tanzer A, Lagarde J, Lin W, Schlesinger F, Xue C, Marinov GK, Khatun J, Williams BA, Zaleski C, Rozowsky J, Roder M, Kokocinski F, Abdelhamid RF, Alioto T, Antoshechkin I, Baer MT, Bar NS, Batut P, Bell K, Bell I, Chakrabortty S, Chen X, Chrast J, Curado J, Derrien T, Drenkow J, Dumais E, Dumais J, Duttagupta R, Falconnet E, Fastuca M, Fejes-Toth K, Ferreira P, Foissac S, Fullwood MJ, Gao H, Gonzalez D, Gordon A, Gunawardena H, Howald C, Jha S, Johnson R, Kapranov P, King B, Kingswood C, Luo OJ, Park E, Persaud K, Preall JB, Ribeca P, Risk B, Robyr D, Sammeth M, Schaffer L, See LH, Shahab A, Skancke J, Suzuki AM, Takahashi H, Tilgner H, Trout D, Walters N, Wang H, Wrobel J, Yu Y, Ruan X, Hayashizaki Y, Harrow J, Gerstein M, Hubbard T, Reymond A, Antonarakis SE, Hannon G, Giddings MC, Ruan Y, Wold B, Carninci P, Guigo R, Gingeras TR. Landscape of transcription in human cells. Nature. 2012;489:101–108. doi: 10.1038/nature11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karolchik D, Barber GP, Casper J, Clawson H, Cline MS, Diekhans M, Dreszer TR, Fujita PA, Guruvadoo L, Haeussler M, Harte RA, Heitner S, Hinrichs AS, Learned K, Lee BT, Li CH, Raney BJ, Rhead B, Rosenbloom KR, Sloan CA, Speir ML, Zweig AS, Haussler D, Kuhn RM, Kent WJ. The UCSC Genome Browser database: 2014 update. Nucleic Acids Res. 2014;42:D764–D770. doi: 10.1093/nar/gkt1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harrow J, Frankish A, Gonzalez JM, Tapanari E, Diekhans M, Kokocinski F, Aken BL, Barrell D, Zadissa A, Searle S, Barnes I, Bignell A, Boychenko V, Hunt T, Kay M, Mukherjee G, Rajan J, Despacio-Reyes G, Saunders G, Steward C, Harte R, Lin M, Howald C, Tanzer A, Derrien T, Chrast J, Walters N, Balasubramanian S, Pei B, Tress M, Rodriguez JM, Ezkurdia I, van Baren J, Brent M, Haussler D, Kellis M, Valencia A, Reymond A, Gerstein M, Guigo R, Hubbard TJ. GENCODE: the reference human genome annotation for The ENCODE Project. Genome Res. 2012;22:1760–1774. doi: 10.1101/gr.135350.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karolchik D, Hinrichs AS, Furey TS, Roskin KM, Sugnet CW, Haussler D, Kent WJ. The UCSC Table Browser data retrieval tool. Nucleic Acids Res. 2004;32:D493–D496. doi: 10.1093/nar/gkh103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Team RC. R: A Language and Environment for Statistical Computing. Vienna: 2014. 2014. [Google Scholar]
- 13.Kent WJ. BLAT--the BLAST-like alignment tool. Genome Res. 2002;12:656–664. doi: 10.1101/gr.229202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Batut P, Dobin A, Plessy C, Carninci P, Gingeras TR. High-fidelity promoter profiling reveals widespread alternative promoter usage and transposon-driven developmental gene expression. Genome Res. 2013;23:169–180. doi: 10.1101/gr.139618.112. [DOI] [PMC free article] [PubMed] [Google Scholar]