Abstract
Antibody profiles have the potential to revolutionize personalized medicine by providing important information related to autoimmunity against self-proteins and exposure to infectious agents. One immunoassay technology, luciferase immunoprecipitation systems (LIPS), harnesses light-emitting recombinant proteins to generate robust, high-quality antibody data often spanning a large dynamic range of detection. Here, we describe the general format of LIPS and discuss studies using the technology to measure autoantibodies in several human autoimmune diseases including type 1 diabetes, Sjögren's syndrome, systemic lupus erythematosus, and immunodeficiencies secondary to anticytokine autoantibodies. We also describe the usefulness of evaluating antibodies against single or multiple antigens from infectious agents for diagnosis, pathogen discovery, and for obtaining individual exposure profiles. These diverse findings support the notion that the LIPS is a useful technology for generating antibody profiles for personalized diagnosis and monitoring of human health.
Abbreviations: ARDS, acute respiratory distress syndrome; dNTM, disseminated nontuberculous mycobacterial; EBV, Epstein-Barr virus; ELISA, Enzyme-linked immunoassay; HCV, hepatitis C virus; HIV, human immunodeficiency virus; HTLV, human T-lymphotropic virus; IL, interleukin; KSHV, Kaposi sarcoma-associated herpes virus; LIPS, luciferase immunoprecipitation systems; MERS, Middle East Respiratory Virus; NPHV, nonprimate hepatitis C-like virus; RIP, radioimmunoprecipitation assay; Ruc, Renilla luciferase; SLE, systemic lupus erythematosus; SS, Sjögren's syndrome; T1D, type I diabetes
Introduction
Antibodies are key components of the immune system that are able to bind with great specificity to an extremely large variety of target molecules. In addition to their important function in the adaptive immune response, antibody testing represents a major tool for the diagnosis of many infectious agents including current and past exposures.1 The detection of antibodies against self-proteins, autoantibodies, is also important for the diagnosis of a variety of autoimmune diseases. In some autoimmune diseases, autoantibodies are present before the onset of clinical symptoms, and for certain targets, autoantibodies can play a direct role in causing pathogenesis. Although the full spectrum of diagnostically useful antibodies is not currently known, generating comprehensive antibody profiles will likely represent an important next step in understanding human health. In this review, we describe the antibody profiling technology of luciferase immunoprecipitation systems (LIPS) and discuss its wide range of applications and the types of information that can be obtained from using the technology. An in-depth discussion of other immunoassay technologies used to measure antibodies can be found in a recent review.1
LIPS Technology
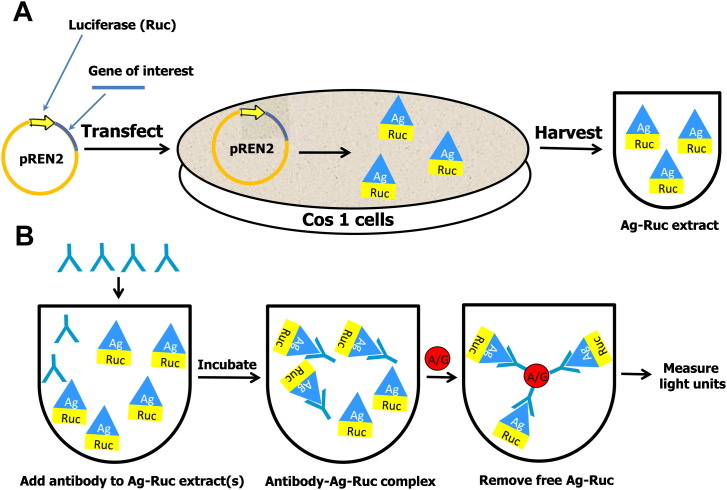
The clinical information provided by quantifying antibodies has long been recognized, but the most common immunoassay technologies such as Western blotting and enzyme-linked immunosorbent assay (ELISA), used to measure the amount of antibodies against particular targets, have a number of drawbacks including a limited ability to efficiently detect conformational epitopes, a limited dynamic range of detection, and high backgrounds frequently associated with using crude protein preparations or bacterial recombinant proteins.2 In contrast, fluid-phase radioimmunoprecipitation assays using radiolabeled antigens can overcome many of these limitations and are the method of choice for measuring autoantibodies in autoimmune diseases.3 Despite the usefulness of the radioimmunoprecipitation assay, the requirement for radioisotopes limits its widespread application. An alternative to radioimmunoprecipitation assays for measuring antibodies is the fluid-phase LIPS technology, which is based on luciferase-tagged antigens produced in mammalian cells.1, 2 With the LIPS technology, the gene encoding the 30-kDa luciferase, isolated from the soft coral Renilla reniformis, is typically used as the reporter because this light-producing enzyme has a highly linear output spanning over 7 orders of magnitude. The construction of Renilla luciferase (Ruc) chimeric genes involves standard molecular techniques with mammalian expression vectors (eg, pREN2) in which the antigen of interest is fused in-frame with Ruc.4, 5 A variety of recombinant protein targets can be used in LIPS including full-length proteins, protein variants and fragments, and short peptides. Nonprotein targets such as phospholipids, DNA, and RNA cannot be used in LIPS.
To initiate LIPS, plasmids encoding these light-emitting antigen fusions are first transfected into Cos1 mammalian cells (Fig 1 ). Because the antigen is directly tagged with luciferase, crude extracts are used without the need for time-consuming protein purification. Importantly, many of the crude extracts containing the Ruc-tagged antigens can be stored as frozen aliquots and then thawed for use at a later time. For antibody testing, a defined amount of the Ruc-tagged recombinant protein based on light units is first incubated with each serum sample typically for 1 hour. In these assays, 1.0 μL of serum is used, potentially allowing up to 1000 determinations to be made from 1 mL of serum or plasma. During this first incubation step, antibodies in serum, if present, bind to the target antigen fused to Ruc (Fig 1). The reaction mixture is then transferred for an additional hour to a filter plate containing antibody capturing reagents such as protein A/G beads or other secondary immunoglobulin-immobilized beads. Although these beads can bind both free immunoglobulins and antibodies bound to the Ruc-tagged antigen, free unbound luciferase-tagged antigen is removed from the microtiter filter plate by multiple washing steps. Next, the relative amount of antibody bound to the Ruc-tagged antigen can be determined by measuring the light produced when adding coelenterazine, the substrate for Ruc (Fig 1). It should be noted that a variety of LIPS formats can be performed to collect highly quantitative antibody data including single tube assays,5 96-well plates,4 rapid tests,6, 7 arrays,8 and even a microfluidic device.9 The time required to perform LIPS testing is less than 2.5 hours and typically faster than ELISA and Western blotting. Although there are currently no commercial products available for specific LIPS tests, the LIPS vectors used to generate light-emitting proteins are available on request (from Dr. Peter D. Burbelo).
Fig 1.
Schematic of the general steps involved in luciferase immunoprecipitation systems. (A) The DNA sequence of the antigen of interest is genetically fused to the C-terminus of Renilla luciferase (Ruc). These recombinant plasmids are then used to transfect Cos1 cells and cell lysate is harvested 48 hours later without purification. (B) Aliquots of a single extract for a Ruc-antigen or a mixture of multiple extracts for different Ruc-antigens are then incubated with serum samples. The antibody complexes are then captured by protein A/G beads and the unbound luciferase-tagged antigen is washed away. The amount of specific antibodies present is then determined by the amount of bound antigen present by adding luciferase substrate.
Detection and Analysis of Autoantibodies by LIPSs in Autoimmune Conditions
Autoimmune diseases are quite common conditions and are associated with significant morbidity and mortality costs. For many autoimmune diseases, genetic information offers limited diagnostic or predictive clinical value because these complex diseases are not caused by single genetic alterations, but rather involve multiple weakly associated gene polymorphisms interacting with various environmental factors.10 On the contrary, autoantibody detection in autoimmune conditions represents an important tool for personalized care providing information for diagnosis, monitoring, and even disease prediction. Here, we describe the application of LIPS for measuring autoantibodies in a wide range of autoimmune studies yielding improved diagnostic performance or new information (Table I ).
Table I.
LIPS for autoantibody detection
| Disease/infection | Examples of important findings made by LIPS |
|---|---|
| Type 1 diabetes | |
| Immunodysregulation, polyendocrinopathy, enteropathy X-linked syndrome |
|
| Stiff person syndrome | |
| SS |
|
| SLE |
|
| Patients with thymoma | |
| dNTM | |
| ARDS and sepsis |
|
Abbreviations: ARDS, acute respiratory distress syndrome; dNTM, disseminated nontuberculous mycobacterial; LIPS, luciferase immunoprecipitation system; SLE, systemic lupus erythematosus; SS, Sjögren's syndrome.
In type 1 diabetes (T1D), an autoimmune disease involving the destruction of insulin-producing pancreatic beta cells, several different autoantibodies have been identified including insulin, glutamic decarboxylase-65, insulinoma-associated protein-2, insulinoma-associated protein-2 beta and zinc transporter-8. Although the radioimmunoprecipitation assay (RIP), a fluid-phase immunoassay, is the gold standard for detecting T1D-associated autoantibodies,3 LIPS represents a promising nonradioactive alternative. Comparative studies have shown that both LIPS and RIP have similar sensitivity and specificity for detecting autoantibodies against several major T1D autoantigens.11, 12 For example, the detection of anti-IA2 autoantibodies in patients with T1D by the LIPS demonstrated 85% sensitivity and 100% specificity, and autoantibody values obtained correlated well with radioimmunoprecipitation assay.12 In these studies, the dynamic range of detection for the LIPS assays was greater than the radioimmunoprecipitation assay and spanned 103–105 light units. Several other investigators have successfully used the LIPS as a nonradioactive alternative for measuring autoantibodies in T1D.13, 14, 15, 16, 17 Autoantibodies against the relatively newly identified autoantigen, pancreatic and duodenal homeobox-1, were shown by the LIPS to be more prevalent in patients with T1D harboring autoantibodies against multiple autoantigens.13 Lampasona et al detected high levels of robust autoantibodies by the LIPS against harmonin and villin in most patients with the autoimmune condition immunodysregulation, polyendocrinopathy, enteropathy X-linked syndrome, but found no evidence of these autoantibodies in T1D.14 It is also important to point out that other immunoassays including ELISA and protein array are often inadequate for detecting autoantibodies in T1D and other autoimmune conditions.3 The lack of diagnostic utility of these solid-phase assays is exemplified by a recent study showing that a protein array technology was unable to detect any significant autoantibody responses against the 2 major known autoantigens, GAD65 and IA2, which were detectable by the LIPS in the serum samples of the same patient with T1D.16
Acquiring autoantibody profiles against multiple autoantigen targets in T1D is highly desirable because studies have shown that the number of different islet autoantibodies present in a given individual has the greatest predictive value for determining which children will go on to develop diabetes.18 In addition, patients with T1D can also show evidence of other comorbid autoimmune conditions that can be detected by the presence of autoantibodies associated with these conditions. Because of the modular nature of the assay and the ability of using crude extracts without purification, the LIPS is ideal for testing multiple autoantigens. Additionally, the assay development time is generally much lesser than other solid-phase immunoassays that use native or recombinant proteins. This is because the development of the different light-emitting protein detectors for the LIPS simply involves cloning and transfection in which the extracts can generally be used in a standard format without optimization. In one investigation autoantibodies against 9 targets were measured in patients with T1D.19 Not only did the patients with T1D show a high frequency of T1D-associated autoantibodies, but approximately 50% of the patients with T1D had autoantibody responses against at least one other extrapancreatic target including the thyroid peroxidase associated with Hashimoto's thyroiditis, transglutaminase 2 associated with celiac disease, and the gastric adenosine triphosphatase associated with autoimmune gastritis. Although the clinical data for this cohort were not available for analysis, this study demonstrates the possibility of using the LIPS to identify patient subgroups with different clinical symptoms or for further examining potential genetic associations.
In addition to the high frequency of GAD65 autoantibodies found in patients with T1D, autoantibodies against GAD65 can also be detected in several neurologic diseases including stiff person syndrome and ataxia.20 In stiff person syndrome, LIPS detected highly robust levels of GAD65 autoantibodies and achieved diagnostic accuracy of 100% sensitivity and 100% specificity.21 In another study, LIPS was also used to screen a cohort of psychiatric patients for autoantibodies.22 High levels of GAD65 autoantibodies were detected in a female patient with major depressive disorder who showed signs of psychomotor slowing, a clinical condition characterized by slow movement of her extremities. Increased GAD65 autoantibodies were also highly detectable in cerebrospinal fluid of the patient, and the autoantibody levels correlated over time with clinical severity suggesting that central nervous system autoimmunity might be responsible for psychomotor impairment. These findings support the idea of uncovering unrecognized autoimmune pathogenesis in human disease by autoantibody profiling.
Sjögren's syndrome (SS) is an autoimmune disease characterized by autoimmune attack on the salivary and lacrimal glands leading to decreased saliva and tear production, respectively.23 One objective criterion for the diagnosis of SS involves measuring autoantibodies against SSB (La) and SSA (Ro52 and Ro60). LIPS showed 75% sensitivity for the detection of La autoantibodies compared with 45% sensitivity for an established ELISA in a small cohort of patients with SS and healthy controls.24 For SSA, the LIPS separately detected autoantibodies against Ro52 and Ro60 and showed similar diagnostic performance to that of the ELISA, which measured them together. Measuring autoantibodies against other targets also revealed that some patients also had significant autoantibodies associated with other autoimmune conditions. For example, 16% of patients with SS had autoantibodies against thyroid peroxidase, an autoantigen associated with Hashimoto's thyroiditis. Similarly, 14% and 12% of the patients with SS had autoantibodies against gastric adenosine triphosphatase and aquaporin 4, respectively, representing potential autoimmune attack on the stomach and nervous system.24 Compared with the standard 2.5 hour–testing format, a rapid 15-minute LIPS test showed promise for detecting anti-Ro52 serum autoantibodies for the diagnosis of SS.6 The highly robust LIPS assay can be used for noninvasive testing by using saliva instead of a serum as the clinical sample. Using saliva, Ro60 autoantibodies showed 75% sensitivity and 96% specificity for the diagnosis of SS and correlated with serum levels of Ro60 autoantibody.25 The ability to examine antibodies in saliva has additional applications for noninvasively studying humoral responses against infectious agents.
Systemic lupus erythematosus (SLE) represents a relatively common autoimmune disease characterized by inflammation and chronic immune attack on various cells and tissues.23 The detection of autoantibodies in SLE is critical for diagnosis, disease monitoring, and can predict disease onset.26, 27 Ching et al28 used the LIPS to detect autoantibodies against a panel of autoantigens revealing that most patients with SLE had either a cluster I or cluster II antibody phenotype. Patients with a cluster I phenotype showed enriched autoantibodies against Sm, U1-RNP-A1, and U1-70K RNP RNA-binding proteins, whereas patients with a cluster II phenotype had enriched autoantibodies against Ro52, Ro60, and La. The exact method for analyzing the LIPS autoantibody data from the patients with SLE simply involved comparing the responses against Sm, U1-RNP-A1, and U1-70K RNP vs Ro52, Ro60, and La. In contrast, a previous study based on ELISA immunoassay results identified the 2 similar autoantibody clusters in SLE using the relatively complex K-means clustering algorithm.29 Overall, these findings highlight the ease of antibody analysis when robust and highly quantitative values are generated by the LIPS technology. Another unique feature of the LIPS is the possibility of using antigen mixtures for achieving high diagnostic performance from a single test.28, 30, 31 The mixture format is based on the fact that the protein A/G beads used in the assay bind many different immunoglobulins present in serum. When incubated with multiple light-emitting antigens, these bound immunoglobulins can interact with multiple detectors yielding an overall signal similar to performing these tests separately (Fig 1). In the case of the diagnosis of lupus, a single LIPS mixture test incorporating 6 antigens (Sm, U1-RNP-A1, U1-70K RNP, Ro52, Ro60, and La) combined with 1 μL of serum matched the sensitivity and specificity of performing these 6 individual LIPS tests separately.28 Because this approach does not provide information about the specific target proteins that are immunoreactive in seropositive samples, if needed, additional follow-up testing of individual antigens would need to be performed. Nevertheless, this LIPS mixture approach saves time and resources and has also been used to simplify the diagnosis of several infectious agents.30, 31
Besides autoantibodies as biomarkers, pathogenic autoantibodies can interfere with normal processes and directly cause human disease. Pathogenic autoantibodies can be generated against cytokines, which are secreted molecules that play critical roles in regulating the immune response to infection. The LIPS detected high levels of anticytokine autoantibodies in several diseases including in certain patients with acute respiratory distress syndrome and sepsis,32 SLE,28 and in opportunistic infections secondary to anticytokine autoantibodies.33, 34 In patients with thymoma exhibiting opportunistic infections such as mucocandidiasis and disseminated varicella-zoster virus, the LIPS identified multiple anticytokine autoantibodies as the likely culprits involved in pathogenesis.34 Although the LIPS detected known increased levels of anti–interferon alfa autoantibodies, high levels of autoantibodies against interleukin (IL)-12 p35, IL-12–p40, and IL-17 correlated better with the presence of opportunistic infections in these patients. The levels of autoantibodies detected by the LIPS against these cytokines also matched functional assays for signal transducer and activator of transcription neutralization experiments. Lastly, this study also highlighted how the LIPS can be used for autoantigen discovery. In this study, systematic LIPS screening of 39 different candidate cytokines resulted in the discovery of 2 patients with previously undescribed autoantibodies against the B cell-activating factor cytokine.34
Because of the ability to screen many different antigens in parallel, the LIPS has been used to analyze a cohort of patients with disseminated nontuberculous mycobacterial (dNTM) infections to understand the full spectrum of anticytokine autoantibodies.33 Patients with dNTM infections are severely immunosuppressed and harbor infections with numerous rapid and slow growing mycobacteria. From analyzing autoantibodies against 41 cytokine and other immune targets, approximately 90% of the patients with dNTM infections demonstrated interferon gamma (IFN-γ) autoantibodies, which routinely were 1000 times greater than the levels found in the controls. The levels of IFN-γ autoantibodies detected by the LIPS in the serum samples of patients with dNTM infections also tracked their ability to neutralize downstream signaling activity as detected by in vitro assays. One patient with dNTM infection did not have IFN-γ autoantibodies, but showed high levels of anti-granulocyte-macrophage colony-stimulating factor autoantibodies.33 Remarkably, no significant autoantibody seropositivity was detected against the other cytokines. These results suggest that the LIPS technology is useful for identifying pathogenic autoantibodies against cytokines and potentially other extracellular targets.
Because the full spectrum of human diseases showing autoantibodies is not known, there are likely to be other diseases that may have an unrecognized autoimmune component. Two such diseases fitting this category are acute respiratory distress syndrome and sepsis, conditions characterized by intense immune activation leading to organ failure. The LIPS not only detected evidence of robust autoantibodies against several autoantigens including cytokines and known autoantigens, but also demonstrated that there was rapid induction of some autoantibodies in only a few days and suggests that ongoing inflammation may mediate the break in tolerance to self-proteins.32 Collectively, the results presented highlight the possibility of using the LIPS to detect new biomarkers for many other diseases including conditions that are not classically thought to involve autoimmune responses.
Diagnosis and Monitoring Antibody Responses to Infectious Agents
Antibody testing represents a major tool for the diagnosis of many infectious agents and provides insight into current and past exposure and even response to vaccines. Liquid-phase immunoassays such as radioimmunoprecipitation assays have not been generally used to measure antibodies against infectious agents because of the requirement for radioactive labeling. With the recent development of the LIPS, this liquid-phase immunoassay has been used to interrogate antibodies against a wide range of different infectious agents including fungal, filarial, bacterial, and viral agents.1, 2 Subsequently, we describe these studies and discuss how the LIPS has provided new information for the detection of antibodies against infectious agents (Table II ).
Table II.
LIPS for antibody detection of infectious agents
| Disease/infection | Examples of important findings made by LIPS |
|---|---|
| Strongyloides stercoralis | |
| Onchocerca volvulus |
|
| Loa loa | |
| EBV | |
| HIV |
|
| HTLV-1 | |
| HCV |
|
| Wuchereria bancrofti | |
| KSHV | |
| NPHV |
|
| MERS |
|
Abbreviations: EBV, Epstein-Barr virus; ELISA, enzyme-linked immunosorbent assay; HCV, hepatitis C virus; HIV, human immunodeficiency virus; HTLV, human T-lymphotropic virus; LIPS, luciferase immunoprecipitation system; MERS, Middle East respiratory syndrome; NPHV, nonprimate HCV-like virus.
One key advantage of LIPS is the highly robust antibody levels that are often seen against multiple proteins from a given infectious agent, which is helpful in distinguishing infected patients from uninfected controls. For several infectious agents, side-by-side comparison has shown improved diagnostic potential of LIPS over classic ELISA tests. For example, LIPSs showed greater sensitivity compared with ELISA and several other immunoassays for the detection of Strongyloides stercoralis,35, 36 Onchocerciasis,31 and Loa loa infection.7 For other agents, the diagnostic performance of LIPS has been comparable with ELISA tests but has shown additional useful features.30, 37, 38 For example, an LIPS test for Borrelia burgdorferi, the bacterial cause of Lyme disease, also matched the diagnostic performance of an established Lyme ELISA test37 and has been used to exclude a major role of Lyme disease in the pathogenesis of autism.39 An LIPS test for a common herpesvirus infection, varicella-zoster virus, was able to distinguish vaccinated from unvaccinated subjects and matched the gold standard fluorescent antibody to a membrane antigen test.40 For the Epstein-Barr virus (EBV), herpesvirus, an LIPS test detecting antibodies to EBV gp350 correlated with antibody neutralization assays, which could be useful for measuring responses to EBV vaccines.41
The ability of using crude lysates from transfected cells without protein purification also makes the LIPS a practical approach to measure antibodies against different proteins from a given infectious agent including the whole proteome of human immunodeficiency virus (HIV)8, 42 and partial proteomes of human T-lymphotropic virus (HTLV)-1, hepatitis C virus (HCV),8, 43, 44 and EBV.8 This approach is also useful for screening and identifying new antigenic proteins from infectious agents. For example, a screen of 16 antigens from Wuchereria bancrofti identified Wb123 as a highly informative antigen for the diagnosis of this filarial infection.45 Similarly, screening of 20 proteins from Kaposi-associated herpesvirus (KSHV) identified robust antibodies against v-cyclin in 75% of patients with Kaposi's sarcoma.30 Interestingly, antibodies against v-cyclin were not detected in several solid-phase formats including Western blot46 and protein array47 consistent with the better detection of conformational antibodies by the liquid-phase immunoassays compared with solid-phase assays.3
An LIPS mixture assay for 4 KSHV antigenic targets efficiently detected infection and matched the sensitivity and specificity of performing 2 separate ELISA tests.30
Owing to the ability of LIPS to detect antibodies against multiple antigens from different viruses, unique antibody profiles were identified in patient subsets including patients infected with HTLV-1,48 EBV,49 KSHV,50 and HIV.51 For example, patients with HTLV-I–associated myelopathy, a debilitating neurologic disease caused by HTLV-1 viral infection, showed high levels of anti-envelope HTLV-1 antibodies compared with asymptomatic infected individuals or patients with HTLV-1–associated lymphoma.48, 52 In patients with chronic active EBV, anti-EBV antibody profiling showed greater antibody levels against several lytic EBV antigens compared with healthy controls, which is consistent with increased EBV replication in these patients.49 In 2 patients with KSHV-associated diseases, the relative antibody levels against lytic vs latent viral antigens were markedly greater in multicentric Castlemen's disease compared with patients with Kaposi's sarcoma.50 The different antibody patterns seen in the patient subgroups likely reflect altered protein expression and/or immune recognition of these infection agents.
The LIPS has also provided simple biomarkers for studying clinical subsets of patients with HIV. Mendoza et al51 analyzed a cohort of elite HIV controllers, patients who showed exquisite control over HIV infection, and found a novel low antibody response signature against reverse transcriptase, protease, and integrase consistent with the possibility that these patients had low levels of replicating HIV virus. In a subsequent HIV study, humoral responses were examined by the LIPS against 9 HIV proteins in patients with low HIV viral load including elite controllers, antiretroviral-treated patients, and in the Berlin patient, the first patient cured.53 A key finding was that the Berlin patient had undetectable antibodies against p24 and 5 other HIV proteins, but still had weak HIV antibody responses against tat, gp41, and reverse transcriptase. In another study of patients coinfected with HCV-HIV, a novel HCV antibody biomarker profile was identified by the LIPS, which correlated with response to treatment with interferon alfa and ribavirin.43 Overall, these results with HIV and HCV suggest that these quantitative antibody profiles generated by the LIPS could be used to monitor therapy.
In spite of the identification of novel infectious agents by nucleic acid amplification and DNA sequencing approaches, it is important to point out that the full spectrum of infectious agents and their impact on health remains largely incomplete.54 Because the detection of specific antibody responses can provide in vivo evidence of infection, the robust antibody responses obtained by the LIPS can be used as another tool for pathogen discovery. For example, numerous astroviruses have been identified by metagenomic sequencing of human stool,55, 56 but the in vivo relevance of these agents is unknown. LIPS testing of one of the new astroviruses, HMOAstV-C, revealed significant antibodies to the capsid of this virus in humans, but not in several animal species including pigs and rabbits. Further analysis of samples from children revealed that approximately 20% of 1-year-old children and approximately 65% of adults showed antibodies against HMOAstV-C.57 These findings support the incorporation of high-quality serologic LIPS data for accelerating the discovery of new pathogens obtained from nucleic acid discovery efforts.
LIPS can also be helpful for identifying animal reservoirs of human-related viruses. In one study, the LIPS was used to identify the natural reservoir of HCV-like viruses in nonprimates.58 The area of research was initially started by Kapoor et al,59 who discovered the first nonprimate homolog of HCV called canine hepativirus in 2 dogs. No serology was performed at that time, and the development of a subsequent LIPS test against the capsid and helicase regions of canine hepacivirus failed to detect antibodies in more than 120 canine serum samples suggesting that infection by this virus may be a rare event in dogs. However, additional testing of a variety of other animals revealed that approximately 40% of horses showed robust antibody responses against the helicase. DNA analysis of the seropositive horses identified 8 new genetically distinct nonhuman primate hepatitis-like viruses in these equine samples and established horses as a major reservoir of HCV-like viruses.58 More recently, LIPS was used to investigate the animal reservoir of Middle East respiratory syndrome (MERS) virus, a potential lethal viral infection in humans occurring in Saudi Arabia.60 Although no serologic evidence for MERS infection was observed in sheep and goats, camels were identified as a source of MERS. These results highlight how the LIPS can be used to discover novel pathogens in animals, which can cause zoonotic infections in humans.
Increasing evidence suggests that the complicated interaction of our bodies with microbial agents and even exposure against many infectious agents that do not cause overt disease may influence human health. One important opportunity for personalized health profiles will be to define individual exposure profiles to multiple infectious agents. As a proof of concept, the LIPS was used to measure antibodies against 13 common infectious agents in 3 different chronic diseases, patients with HIV, IFN-γ autoantibodies, and SS.61 Rather than focusing on antibody responses to any one individual infectious agents, the cumulative antibody data were modeled by principal component analysis. For both patients with HIV and patients with high levels of autoantibodies against IFN-γ, a distinct antibody profile was observed compared with healthy control subjects. Moreover, there was a noticeable difference between these profiles highlighting the fact that each disease perturbs different specific immune pathways.61 In contrast, the SS cohort did not reveal an informative profile suggesting that these infectious agents might be less relevant for this disease.61 On the basis of these promising findings, it is likely that the incorporation of additional infectious agent targets into the panel might make this approach even more informative. Finally, the ability to profile so many different infectious agents in a single format presents a powerful tool for diagnosis and personalized medicine and might be configured into a novel immune readout of overall immune health.
Conclusions
In this review, we have discussed how the LIPS, a liquid-phase immunoassay, provides important information for diagnosis, monitoring, and insight into disease pathogenesis. These many diverse studies provide a platform for more extensive interrogation of antibody profiles in human disease. Four major attributes make LIPS a highly desirable technology for studying antibodies: (1) an assay that does not require radioactivity, (2) the possibility of stably storing the fusion antigen extracts for facile testing on demand, (3) the high signal-to-noise ratio, and (4) the wide dynamic range of antibody detection. Because the LIPS requires small amounts of serum, hundreds of targets could theoretically be profiled with the serum obtained from several milliliters of processed blood. Besides testing one protein at a time, LIPS arrays offer an alternative format for simultaneously multiple antibodies in which different light-emitting protein targets are tested in individual wells of a 96-well plate with control and test serum samples. As a proof of concept, LIPS arrays detected highly robust immunoreactivity against multiple proteins derived from HCV, HIV, and EBV proteomes in infected subjects compared with uninfected controls.8 On the basis of these results, LIPS arrays could be used as a discovery tool for simultaneously measuring antibody levels against candidate target proteins in autoimmune and infectious diseases.
It is expected that in the coming years, antibody profiles generated by LIPSs will continue to provide important information related to pathogenesis and diagnosis. One strategy for predictive medicine involves monitoring antibodies longitudinally over time to many different targets. Along these lines, it is conceivable that a library of known autoantigen targets used in LIPS could prove sufficient for the broad diagnosis of many common autoimmune disorders. Because it is currently known that autoantibodies are present years before clinical onset of several autoimmune conditions including T1D,62 SLE,26 and SS,63 and it is possible that LIPS and other assays, along with clinical information including association with family members and the presence of risk genes, could be efficiently used to identify individuals at high risk for developing these and other conditions including cancer and other age-related neurologic diseases. In addition, the approach of using LIPS serology to profile antibodies against multiple infectious agents could be used to identify novel agents that might contribute to inflammation and impact the likelihood of developing certain diseases. Most importantly, these antibody profiles might be used for early detection thereby identifying patients before the clinical onset, which may allow a change in lifestyle or treatment options that might delay or further prevent disease onset or progression.
Acknowledgments
Conflicts of Interest: All authors have read the journal's policy on disclosure of potential conflicts of interest. One of the authors (P.D.B.) has 3 patent applications submitted regarding the use of luciferase immunoprecipitation systems for detecting antibodies against KSHV/HHV-8, Wucheria bancrofti, and Lyme disease. The 2 other authors have no potential conflicts of interest to declare.
All authors have also read the journal's authorship agreement and have reviewed and approved the manuscript. No editorial support was used in the preparation of the manuscript.
This work was supported by the intramural research program of the National Institute of Dental and Craniofacial Research, National Institutes of Health.
References
- 1.Burbelo P.D., Ching K.H., Bush E.R., Han B.L., Iadarola M.J. Antibody-profiling technologies for studying humoral responses to infectious agents. Expert Rev Vaccines. 2010;9:567–578. doi: 10.1586/erv.10.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burbelo P.D., Ching K.H., Bren K.E., Iadarola M.J. Searching for biomarkers: humoral response profiling with luciferase immunoprecipitation systems. Expert Rev Proteomics. 2011;8:309–316. doi: 10.1586/epr.11.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu E., Eisenbarth G.S. Accepting clocks that tell time poorly: fluid-phase versus standard ELISA autoantibody assays. Clin Immunol. 2007;125:120–126. doi: 10.1016/j.clim.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burbelo P.D., Ching K.H., Klimavicz C.M., Iadarola M.J. Antibody profiling by luciferase immunoprecipitation systems (LIPS) J Vis Exp. 2009;32:e1549. doi: 10.3791/1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burbelo P.D., Goldman R., Mattson T.L. A simplified immunoprecipitation method for quantitatively measuring antibody responses in clinical sera samples by using mammalian-produced Renilla luciferase-antigen fusion proteins. BMC Biotechnol. 2005;5:22. doi: 10.1186/1472-6750-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burbelo P.D., Ching K.H., Issa A.T. Rapid serological detection of autoantibodies associated with Sjögren's syndrome. J Transl Med. 2009;7:83. doi: 10.1186/1479-5876-7-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burbelo P.D., Ramanathan R., Klion A.D., Iadarola M.J., Nutman T.B. Rapid, novel, specific, high-throughput assay for diagnosis of Loa loa infection. J Clin Microbiol. 2008;46:2298–2304. doi: 10.1128/JCM.00490-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burbelo P.D., Bren K.E., Ching K.H. LIPS arrays for simultaneous detection of antibodies against partial and whole proteomes of HCV, HIV and EBV. Mol Biosyst. 2011;7:1453–1462. doi: 10.1039/c0mb00342e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zubair A., Burbelo P.D., Vincent L.G. Microfluidic LIPS for serum antibody detection: demonstration of a rapid test for HSV-2 infection. Biomed Microdevices. 2011;13:1053–1062. doi: 10.1007/s10544-011-9575-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Visscher P.M., Brown M.A., McCarthy M.I., Yang J. Five years of GWAS discovery. Am J Hum Genet. 2012;90:7–24. doi: 10.1016/j.ajhg.2011.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burbelo P.D., Hirai H., Issa A.T. Comparison of radioimmunoprecipitation with luciferase immunoprecipitation for autoantibodies to GAD65 and IA-2beta. Diabetes Care. 2010;33:754–756. doi: 10.2337/dc09-1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burbelo P.D., Hirai H., Leahy H. A new luminescence assay for autoantibodies to mammalian cell-prepared insulinoma-associated protein 2. Diabetes Care. 2008;31:1824–1826. doi: 10.2337/dc08-0286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donelan W., Wang H., Li S.W. Novel detection of pancreatic and duodenal homeobox 1 autoantibodies (PAA) in human sera using luciferase immunoprecipitation systems (LIPS) assay. Int J Clin Exp Pathol. 2013;6:1202–1210. [PMC free article] [PubMed] [Google Scholar]
- 14.Lampasona V., Passerini L., Barzaghi F. Autoantibodies to harmonin and villin are diagnostic markers in children with IPEX syndrome. PLoS One. 2013;8:e78664. doi: 10.1371/journal.pone.0078664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marcus P., Yan X., Bartley B., Hagopian W. LIPS islet autoantibody assays in high-throughput format for DASP 2010. Diabetes Metab Res Rev. 2011;27:891–894. doi: 10.1002/dmrr.1268. [DOI] [PubMed] [Google Scholar]
- 16.Miersch S., Bian X., Wallstrom G. Serological autoantibody profiling of type 1 diabetes by protein arrays. J Proteomics. 2013;94:486–496. doi: 10.1016/j.jprot.2013.10.018. [DOI] [PubMed] [Google Scholar]
- 17.Ustinova J., Zusinaite E., Utt M. Development of a luciferase-based system for the detection of ZnT8 autoantibodies. J Immunol Methods. 2014;405:67–73. doi: 10.1016/j.jim.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 18.Notkins A.L. New predictors of disease. Molecules called predictive autoantibodies appear in the blood years before people show symptoms of various disorders. Tests that detected these molecules could warn of the need to take preventive action. Sci Am. 2007;296:72–79. [PubMed] [Google Scholar]
- 19.Burbelo P.D., Lebovitz E.E., Bren K.E. Extrapancreatic autoantibody profiles in type I diabetes. PLoS One. 2012;7:e45216. doi: 10.1371/journal.pone.0045216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dayalu P., Teener J.W. Stiff person syndrome and other anti-GAD-associated neurologic disorders. Semin Neurol. 2012;32:544–549. doi: 10.1055/s-0033-1334477. [DOI] [PubMed] [Google Scholar]
- 21.Burbelo P.D., Groot S., Dalakas M.C., Iadarola M.J. High definition profiling of autoantibodies to glutamic acid decarboxylases GAD65/GAD67 in stiff-person syndrome. Biochem Biophys Res Commun. 2008;366:1–7. doi: 10.1016/j.bbrc.2007.11.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ching K.H., Burbelo P.D., Carlson P.J., Drevets W.C., Iadarola M.J. High levels of anti-GAD65 and anti-Ro52 autoantibodies in a patient with major depressive disorder showing psychomotor disturbance. J Neuroimmunol. 2010;222:87–89. doi: 10.1016/j.jneuroim.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wahren-Herlenius M., Dorner T. Immunopathogenic mechanisms of systemic autoimmune disease. Lancet. 2013;382:819–831. doi: 10.1016/S0140-6736(13)60954-X. [DOI] [PubMed] [Google Scholar]
- 24.Burbelo P.D., Leahy H.P., Issa A.T. Sensitive and robust luminescent profiling of anti-La and other autoantibodies in Sjogren's syndrome. Autoimmunity. 2009;42:515–524. doi: 10.1080/08916930902911738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ching K.H., Burbelo P.D., Gonzalez-Begne M. Salivary anti-Ro60 and anti-Ro52 antibody profiles to diagnose Sjogren's syndrome. J Dent Res. 2011;90:445–449. doi: 10.1177/0022034510390811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arbuckle M.R., McClain M.T., Rubertone M.V. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med. 2003;349:1526–1533. doi: 10.1056/NEJMoa021933. [DOI] [PubMed] [Google Scholar]
- 27.Eriksson C., Kokkonen H., Johansson M. Autoantibodies predate the onset of systemic lupus erythematosus in northern Sweden. Arthritis Res Ther. 2011;13:R30. doi: 10.1186/ar3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ching K.H., Burbelo P.D., Tipton C. Two major autoantibody clusters in systemic lupus erythematosus. PLoS One. 2012;7:e32001. doi: 10.1371/journal.pone.0032001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.To C.H., Petri M. Is antibody clustering predictive of clinical subsets and damage in systemic lupus erythematosus? Arthritis Rheum. 2005;52:4003–4010. doi: 10.1002/art.21414. [DOI] [PubMed] [Google Scholar]
- 30.Burbelo P.D., Leahy H.P., Groot S. Four-antigen mixture containing v-cyclin for serological screening of human herpesvirus 8 infection. Clin Vaccine Immunol. 2009;16:621–627. doi: 10.1128/CVI.00474-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burbelo P.D., Leahy H.P., Iadarola M.J., Nutman T.B. A four-antigen mixture for rapid assessment of Onchocerca volvulus infection. PLoS Negl Trop Dis. 2009;3:e438. doi: 10.1371/journal.pntd.0000438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burbelo P.D., Seam N., Groot S. Rapid induction of autoantibodies during ARDS and septic shock. J Transl Med. 2010;8:97. doi: 10.1186/1479-5876-8-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Browne S.K., Burbelo P.D., Chetchotisakd P. Adult-onset immunodeficiency in Thailand and Taiwan. N Engl J Med. 2012;367:725–734. doi: 10.1056/NEJMoa1111160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burbelo P.D., Browne S.K., Sampaio E.P. Anti-cytokine autoantibodies are associated with opportunistic infection in patients with thymic neoplasia. Blood. 2010;116:4848–4858. doi: 10.1182/blood-2010-05-286161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramanathan R., Burbelo P.D., Groot S. A luciferase immunoprecipitation systems assay enhances the sensitivity and specificity of diagnosis of Strongyloides stercoralis infection. J Infect Dis. 2008;198:444–451. doi: 10.1086/589718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bisoffi Z., Buonfrate D., Sequi M. Diagnostic accuracy of five serologic tests for Strongyloides stercoralis infection. PLoS Negl Trop Dis. 2014;8:e2640. doi: 10.1371/journal.pntd.0002640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burbelo P.D., Issa A.T., Ching K.H. Rapid, simple, quantitative, and highly sensitive antibody detection for lyme disease. Clin Vaccine Immunol. 2010;17:904–909. doi: 10.1128/CVI.00476-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burbelo P.D., Hoshino Y., Leahy H. Serological diagnosis of human herpes simplex virus type 1 and 2 infections by luciferase immunoprecipitation system assay. Clin Vaccine Immunol. 2009;16:366–371. doi: 10.1128/CVI.00350-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burbelo P.D., Swedo S.E., Thurm A. Lack of serum antibodies against Borrelia burgdorferi in children with autism. Clin Vaccine Immunol. 2013;20:1092–1093. doi: 10.1128/CVI.00643-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cohen J.I., Ali M.A., Bayat A. Detection of antibodies to varicella-zoster virus in recipients of the varicella vaccine using a luciferase immunoprecipitation system sssay. Clin Vaccine Immunol. 2014;21:1288–1291. doi: 10.1128/CVI.00250-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sashihara J., Burbelo P.D., Savoldo B., Pierson T.C., Cohen J.I. Human antibody titers to Epstein-Barr virus (EBV) gp350 correlate with neutralization of infectivity better than antibody titers to EBV gp42 using a rapid flow cytometry-based EBV neutralization assay. Virology. 2009;391:249–256. doi: 10.1016/j.virol.2009.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burbelo P.D., Ching K.H., Mattson T.L. Rapid antibody quantification and generation of whole proteome antibody response profiles using LIPS (luciferase immunoprecipitation systems) Biochem Biophys Res Commun. 2007;352:889–895. doi: 10.1016/j.bbrc.2006.11.140. [DOI] [PubMed] [Google Scholar]
- 43.Burbelo P.D., Kovacs J.A., Ching K.H. Proteome-wide anti-hepatitis C virus (HCV) and anti-HIV antibody profiling for predicting and monitoring the response to HCV therapy in HIV-coinfected patients. J Infect Dis. 2010;202:894–898. doi: 10.1086/655780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Makuria A.T., Raghuraman S., Burbelo P.D. The clinical relevance of persistent recombinant immunoblot assay-indeterminate reactions: insights into the natural history of hepatitis C virus infection and implications for donor counseling. Transfusion. 2012;52:1940–1948. doi: 10.1111/j.1537-2995.2011.03524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kubofcik J., Fink D.L., Nutman T.B. Identification of Wb123 as an early and specific marker of Wuchereria bancrofti infection. PLoS Negl Trop Dis. 2012;6:e1930. doi: 10.1371/journal.pntd.0001930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Katano H., Iwasaki T., Baba N. Identification of antigenic proteins encoded by human herpesvirus 8 and seroprevalence in the general population and among patients with and without Kaposi's sarcoma. J Virol. 2000;74:3478–3485. doi: 10.1128/jvi.74.8.3478-3485.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zheng D., Wan J., Cho Y.G. Comparison of humoral immune responses to Epstein-Barr virus and Kaposi's sarcoma-associated herpesvirus using a viral proteome microarray. J Infect Dis. 2011;204:1683–1691. doi: 10.1093/infdis/jir645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Burbelo P.D., Meoli E., Leahy H.P. Anti-HTLV antibody profiling reveals an antibody signature for HTLV-I-associated myelopathy/tropical spastic paraparesis (HAM/TSP) Retrovirology. 2008;5:96. doi: 10.1186/1742-4690-5-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cohen J.I., Jaffe E.S., Dale J.K. Characterization and treatment of chronic active Epstein-Barr virus disease: a 28-year experience in the United States. Blood. 2011;117:5835–5849. doi: 10.1182/blood-2010-11-316745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Burbelo P.D., Issa A.T., Ching K.H. Distinct profiles of antibodies to Kaposi's sarcoma-associated herpesvirus antigens in patients with Kaposi sarcoma, multicentric Castlemen's disease, and primary effusion lymphoma. J Infect Dis. 2010;198:444–451. doi: 10.1086/652869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mendoza D., Johnson S.A., Peterson B.A. Comprehensive analysis of unique cases with extraordinary control over HIV replication. Blood. 2012;119:4645–4655. doi: 10.1182/blood-2011-10-381996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Enose-Akahata Y., Abrams A., Johnson K.R., Maloney E.M., Jacobson S. Quantitative differences in HTLV-I antibody responses: classification and relative risk assessment for asymptomatic carriers and ATL and HAM/TSP patients from Jamaica. Blood. 2012;119:2829–2836. doi: 10.1182/blood-2011-11-390807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Burbelo P.D., Bayat A., Rhodes C.S. HIV antibody characterization as a method to quantify reservoir size during curative interventions. J Infect Dis. 2014;209:1613–1617. doi: 10.1093/infdis/jit667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lipkin W.I. Microbe hunting. Microbiol Mol Biol Rev. 2010;74:363–377. doi: 10.1128/MMBR.00007-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Finkbeiner S.R., Holtz L.R., Jiang Y. Human stool contains a previously unrecognized diversity of novel astroviruses. Virol J. 2009;6:161. doi: 10.1186/1743-422X-6-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kapoor A., Li L., Victoria J. Multiple novel astrovirus species in human stool. J Gen Virol. 2009;90:2965–2972. doi: 10.1099/vir.0.014449-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Burbelo P.D., Ching K.H., Esper F. Serological studies confirm the novel astrovirus HMOAstV-C as a highly prevalent human infectious agent. PLoS One. 2011;6:e22576. doi: 10.1371/journal.pone.0022576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Burbelo P.D., Dubovi E.J., Simmonds P. Serology-enabled discovery of genetically diverse hepaciviruses in a new host. J Virol. 2012;86:6171–6178. doi: 10.1128/JVI.00250-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kapoor A., Simmonds P., Gerold G. Characterization of a canine homolog of hepatitis C virus. Proc Natl Acad Sci U S A. 2011;108:11608–11613. doi: 10.1073/pnas.1101794108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alagaili A.N., Briese T., Mishra N. Middle East respiratory syndrome coronavirus infection in dromedary camels in Saudi Arabia. MBio. 2014;5:e00884–14. doi: 10.1128/mBio.00884-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Burbelo P.D., Ching K.H., Morse C.G. Altered antibody profiles against common infectious agents in chronic disease. PLoS One. 2013;8:e81635. doi: 10.1371/journal.pone.0081635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sosenko J.M., Skyler J.S., Palmer J.P. The prediction of type 1 diabetes by multiple autoantibody levels and their incorporation into an autoantibody risk score in relatives of type 1 diabetic patients. Diabetes Care. 2013;36:2615–2620. doi: 10.2337/dc13-0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jonsson R., Theander E., Sjostrom B., Brokstad K., Henriksson G. Autoantibodies present before symptom onset in primary Sjögren syndrome. JAMA. 2013;310:1854–1855. doi: 10.1001/jama.2013.278448. [DOI] [PubMed] [Google Scholar]
- 64.Hamlin K.L., Moss D.M., Priest J.W. Longitudinal monitoring of the development of antifilarial antibodies and acquisition of Wuchereria bancrofti in a highly endemic area of Haiti. PLoS Negl Trop Dis. 2012;6:e1941. doi: 10.1371/journal.pntd.0001941. [DOI] [PMC free article] [PubMed] [Google Scholar]