Abstract
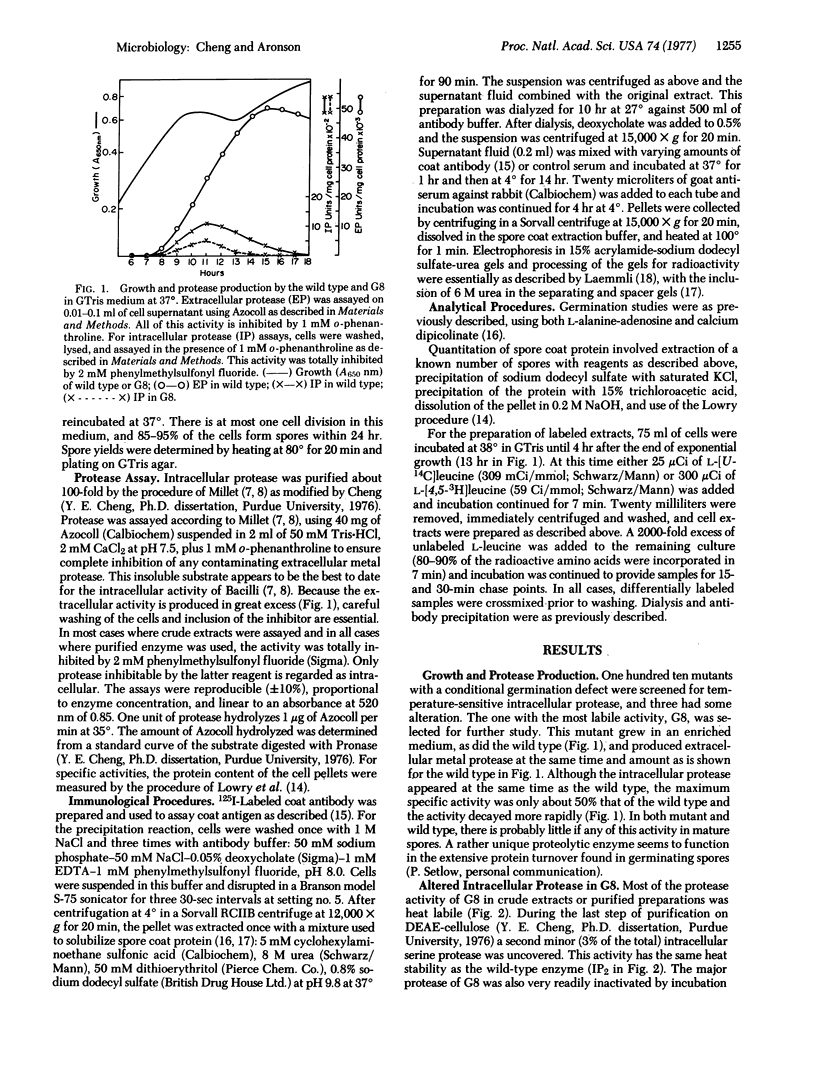
A mutant with an alteration in the major intracellular serine protease produced by postexponential Bacillus cereus was isolated by screening mutants defective in spore germination. The purified enzyme from the mutant is more labile to heat and alkaline pH than the protease from the wild type. Protease activity appears at the same time as in the wild type but only reaches 50% of the specific activity and decays more rapidly during sporulation. Coincident with the decay is a decrease in the rate of protein turnover. Generation of amino acids by turnover seems to be important for sporulation because the number of spores produced by the mutant is increased 4- to 10-fold by addition of casamino acids. As anticipated, the mutant produces spores that germinate poorly but, surprisingly, these spores are very deficient in coat protein. Coat antigen is present in cell extracts of mutant and wild type, however, both as large molecules not found on mature spores and as spore coat protein monomers. The large molecules rapidly disappear in a pulse chase experiment in the wild type with some increase in the coat monomers. In mutant extracts, however, this large coat antigen is slowly and improperly processed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronson A. I., Angelo N., Holt S. C. Regulation of extracellular protease production in Bacillus cereus T: characterization of mutants producing altered amounts of protease. J Bacteriol. 1971 Jun;106(3):1016–1025. doi: 10.1128/jb.106.3.1016-1025.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson A. I., Fitz-James P. C. Properties of Bacillus cereus spore coat mutants. J Bacteriol. 1975 Jul;123(1):354–365. doi: 10.1128/jb.123.1.354-365.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson A. I., Fitz-James P. Structure and morphogenesis of the bacterial spore coat. Bacteriol Rev. 1976 Jun;40(2):360–402. doi: 10.1128/br.40.2.360-402.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn D., Aronson A. I., Golub E. S. Development of a quantitative immunological assay for the study of spore coat synthesis and morphogenesis. J Bacteriol. 1973 Jan;113(1):313–321. doi: 10.1128/jb.113.1.313-321.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito J., Spizizen J. Increased rate of asporogenous mutations following treatment of Bacillus subtilis spores with ethyl methanesulfonate. Mutat Res. 1971 Sep;13(1):93–96. doi: 10.1016/0027-5107(71)90130-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leighton T. J., Dor R. H., Warren R. A., Kelln R. A. The relationship of serine protease activity to RNA polymerase modification and sporulation in Bacillus subtilis. J Mol Biol. 1973 May 5;76(1):103–122. doi: 10.1016/0022-2836(73)90083-1. [DOI] [PubMed] [Google Scholar]
- Linn T. G., Greenleaf A. L., Shorenstein R. G., Losick R. Loss of the sigma activity of RNA polymerase of Bacillus subtilis during sporulation. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1865–1869. doi: 10.1073/pnas.70.6.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel J. F., Millet J. Physiological studies on early-blocked sporulation mutants of Bacillus subtilis. J Appl Bacteriol. 1970 Mar;33(1):220–227. doi: 10.1111/j.1365-2672.1970.tb05246.x. [DOI] [PubMed] [Google Scholar]
- Millet J., Aubert J. P. Etude de la mégatériopeptidase, protéase exocellulaire de Bacillus megaterium. 3. Biosynthèse et rôle physiologique. Ann Inst Pasteur (Paris) 1969 Oct;117(4):461–473. [PubMed] [Google Scholar]
- Millet J. Caractérisation d'une endopeptidase cytoplasmique chez Bacillus megaterium en voie de sporulation. C R Acad Sci Hebd Seances Acad Sci D. 1971 Mar 29;272(13):1806–1809. [PubMed] [Google Scholar]
- Millet J., Larribe M., Aubert J. P. Mutant thermosensible de B. subtilis affecté dans la sporulation et la sérylprotéase extracellulaire. Biochimie. 1976;58(1-2):109–117. doi: 10.1016/s0300-9084(76)80361-6. [DOI] [PubMed] [Google Scholar]
- Reysset G., Millet J. Characterization of an intracellular protease in B. subtillus during sporulation. Biochem Biophys Res Commun. 1972 Oct 17;49(2):328–334. doi: 10.1016/0006-291x(72)90414-7. [DOI] [PubMed] [Google Scholar]
- STRANGE R. E., DARK F. A. Cell-wall lytic enzymes at sporulation and spore germination in Bacillus species. J Gen Microbiol. 1957 Oct;17(2):525–537. doi: 10.1099/00221287-17-2-525. [DOI] [PubMed] [Google Scholar]
- Sadoff H. L., Celikkol E., Engelbrecht H. L. Conversion of bacterial aldolase from vegetative to spore form by a sporulation-specific protease. Proc Natl Acad Sci U S A. 1970 Jul;66(3):844–849. doi: 10.1073/pnas.66.3.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadoff H. L., Hitchins A. D., Celikkol E. Properties of fructose 1,6-diphosphate aldolases from spores and vegetative cells of Bacillus cereus. J Bacteriol. 1969 Jun;98(3):1208–1218. doi: 10.1128/jb.98.3.1208-1218.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow P., Primus G. Protein metabolism during germination of Bacillus megaterium spores. I. Protein synthesis and amino acid metabolism. J Biol Chem. 1975 Jan 25;250(2):623–630. [PubMed] [Google Scholar]



