Abstract
Dietary intake of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) and their respective enrichment in cell membranes have been negatively associated with atherosclerotic lesion development. This effect may be mediated, in part, by dampened inflammatory response of macrophages triggered by toll-like receptor 4 (TLR4) activation. This study investigated the influence of membrane fatty acid profile on TLR4-mediated inflammation in RAW 264.7 macrophages. Cells pretreated with myristic acid (MA), EPA, DHA or vehicle control for 24 h were stimulated with ultra-pure LPS, a specific TLR4 agonist, for 6 h or 24 h, corresponding to early and late stages of TNFα and IL-6 protein induction. Treatment significantly increased cell membrane MA, EPA, and DHA by 4.5-, 20.6-, and 8.9-fold, respectively. MA significantly increased IL-6 secretion 6 h post-exposure to the fatty acid, but did not change TNFα secretion in response to any other treatment condition. EPA and DHA significantly reduced TNFα secretion by 36% and 41%, respectively, in cells stimulated for 24 h but not 6 h. In contrast, EPA and DHA significantly reduced IL-6 secretion at both 6 h (67% and 72%, respectively) and 24 h (69% and 72%, respectively). MA or DHA treatment had no significant effect compared to vehicle on factors influencing cellular LPS recognition, including LPS-cell association, and cell surface expression of TLR4, TLR4-MD2 complex, and CD14. These data suggest that membrane fatty acid profiles influence the TLR4-mediated inflammatory response in macrophages, via mechanisms that occur downstream of TLR4 receptor activation.
Keywords: Eicosapentaenoic acid, Docosahexaenoic acid, Macrophages, Toll-like receptor 4, Tumor necrosis factor alpha, Interleukin 6
Introduction
Evidence has accumulated establishing a relationship between toll-like receptor 4 (TLR4) signaling and atherosclerotic lesion initiation and progression [1]. TLR4 is a type 1 transmembrane protein belonging to the TLR family of pattern recognition receptors and is expressed in plasma membranes of macrophages, endothelial and dendritic cells. The prototypical TLR4 ligand is lipopolysaccharide (LPS), a major constituent of the outer cell membrane of gram-negative bacteria [2]. Additionally, endogenous TLR4 agonists have been identified including saturated fatty acids [3]. Activation of TLR4 by LPS requires assembly with myeloid differentiation 2 (MD2) and cluster of differentiation 14 (CD14), as well as dimerization of two TLR4 molecules, all localized in lipid rafts [4]. TLR4 downstream signaling triggers NFκB and MAPK pathways which regulate the gene activation of various proinflammatory cytokines including TNFα and IL-6 [5].
A prominent role of TLR4 signaling in the development of atherosclerotic plaque is supported by both human and animal data. Macrophages and endothelial cells in atherosclerotic plaque from both humans [6, 7] and apoE-null mice [7] preferentially express TLR4, and have elevated levels compared to unaffected arterial tissue. The potential role of TLR4 signaling in lesion development is best evidenced by the studies that have used apoE-null mice deficient in TLR4 or myeloid differentiation primary response gene 88 (MyD88) who demonstrate significantly smaller aortic plaque size, lipid content, and macrophages infiltration [8, 9].
Observational evidence suggests that diets rich in omega-3 fatty acids, particularly the very long-chain omega-3 fatty acids, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), are atheroprotective [10]. Plasma phospholipid EPA and DHA concentrations are inversely associated with intima-media thickness [11], and prospectively with narrowing of coronary artery diameter [12] and intracranial atherosclerotic stenosis [13]. These associations may be partially due to the anti-inflammatory effects of EPA and DHA on macrophages, which play a significant role in augmenting the inflammatory response in the arterial intima [14]. Consumption of EPA and DHA supplements or fish-rich diets in humans [15–21] and animals [22, 23], and addition of exogenous EPA and DHA to macrophage cell lines [24–29], have been shown to lower circulating inflammatory marker concentrations and/or LPS-induced pro-inflammatory cytokine and prostaglandin secretion.
The influence of EPA and DHA on macrophage inflammatory activity due specifically to TLR4 activation is not well defined. Reduction in pro-inflammatory cytokine production in LPS-stimulated macrophage systems, as discussed previously, implies involvement of TLR4. However, much of the prior experimentation involved impure LPS, which activates both TLR4 and TLR2, unlike ultra-pure LPS, which is a specific TLR4 agonist [30, 31]. Likewise, few studies have investigated the influence of EPA and DHA cell membrane enrichment on the expression of TLR4 and its receptor complex components, and TLR4 activation in response to LPS. Incorporation of DHA into BV-2 cells, a murine microglial cell line, decreased cell surface expression of TLR4 and CD14 [32]. Similarly, pretreatment of human THP-1 monocytes with EPA or DHA reduced LPS-induced CD14 protein expression and LPS-cell binding [33]. Using both macrophage and non-macrophage cell lines it has been demonstrated that the anti-inflammatory effects of EPA and DHA and pro-inflammatory effects of saturated fatty acids were due to inhibition and activation, respectively, of TLR4 itself rather than downstream signaling components [34–37]. However, none of these effects were related to changes in cell membrane fatty acid profile.
Based on the available information, we hypothesized that enrichment of cell membranes with EPA or DHA will dampen the inflammatory response of macrophages to ultra-pure LPS through decreased cell surface expression of TLR4 and its associated molecules, MD2 and CD14, and that enrichment of cell membranes with a representative saturated fatty acid would have the opposite effect. To test our hypotheses, we enriched RAW 264.7 cell membranes with EPA, DHA, or a saturated fatty acid and measured pro-inflammatory cytokine secretion and cell surface expression of TLR4 and its receptor complex components in both TLR4-activated and non-activated cells. Although an increase in EPA or DHA membrane content reduced cytokine secretion levels, it did not coincide with a change in cell surface expression of TLR4 or its receptor complex components.
Materials and Methods
Cell Culture
Murine macrophage-like cell line RAW 264.7 cells (ATCC, Manassas, VA) were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM; Invitrogen, Grand Island, NY) supplemented with 10% fetal bovine serum (FBS, endotoxin < 25 EU/mL; Sigma-Aldrich, St. Louis, MO), 100 U/mL of penicillin, and 100 μg/mL streptomycin (MP Biomedicals, LLC, Santa Anna, CA) at 37°C in a 5% CO2 humidified incubator. Fatty acids were prepared from fatty acid sodium salts obtained from Nu-Check Prep, Inc., Elysian, MN (> 99% purity) with the exception of DHA which was obtained from Sigma-Aldrich (≥ 95% purity). Fatty acid sodium salts were combined with fatty acid-free, low endotoxin bovine serum albumin (BSA; Sigma-Aldrich) at a 2:1 molar ratio. Cells were pretreated with 100 μM of the fatty acid or BSA for 24 h. After fatty acid pretreatment, cells were stimulated with 100 ng/mL of ultra-pure LPS (Invivogen, San Diego, CA) of the E. coli 0111:B4 strain for the indicated times in DMEM containing 10% FBS in the presence or absence of the fatty acid/BSA complex. Cellular protein concentration was measured using the bicinchoninic acid method (Pierce Inc., Rockford, IL).
Fatty acid profile of cell membranes
Cells were collected by scraping in phosphate buffered saline (PBS). A portion of the cell suspension was used for protein determination. The remaining cells were stored at −80°C prior to analysis. At the time of analysis, samples were quick-thawed and cells membranes were isolated by washing thrice with sodium chloride (0.9% buffered to pH 7.4) and pelleted by centrifugation at 1300 × g at 4 °C for 5 min. Membrane lipids were extracted, methylated, and quantified as described previously [38, 39].
TNFα and IL-6 secretion
Fatty acid-treated macrophages were stimulated with 100 ng/mL ultra-pure LPS for 0, 6, or 24 h in the absence or presence of the fatty acid/BSA complex. After centrifugation at 1500 rpm at 4 °C for 10 min, supernatants were collected and stored at −80 °C until analysis. TNFα and IL-6 in the culture supernatants were quantified by commercial DuoSet enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Minneapolis, MN) following manufacturer’s protocols.
Flow Cytometry
Flow cytometry was used to detect cell surface receptors and fluorescein isothiocyanate-conjugated LPS (FITC-LPS) associated with intact cells as previously described with minor modifications [40]. For the detection of cell surface receptors, cells were pretreated with fatty acid as indicated above with or without ultra-pure LPS for stimulation. One million cells were blocked with 1 μg anti-mouse CD16/CD32 (BD Biosciences, San Jose, CA) in 100 μL for 5 min at 4°C and then labeled with 0.25 μg anti-TLR4-APC (R&D Systems), 0.5 μg anti-TLR4/MD2-APC (eBioscience), 0.5 μg anti-CD14-PE (eBioscience), or their isotype controls in 100 μL blocking solution for 30 min at room temperature. To assess the effect of LPS-cell association, fatty acid-treated cells were harvested and suspended in the original culture media containing the treatment fatty acid, and incubated with LPS-FITC (1 μg/mL final concentration) for 1 h at 37°C. Fluorescent labeled cells were washed and resuspended in the staining buffer (R&D Systems), and analyzed on an Accuri Flow Cytometer (BD Biosciences).
Statistical Analysis
Differences among mean values were tested using one- or two-way analysis of variance (ANOVA) followed by Tukey’s test for multiple comparisons (GraphPad Prism 6, La Jolla, CA). P values < 0.05 were considered to be significantly different.
Results
Ultra-pure LPS-induced secretion of TNFα and IL-6
Under basal (unstimulated) conditions neither TNFα nor IL-6 was detectable in the culture supernatants. Exposure to ultra-pure LPS induced the secretion of both TNFα and IL-6, indicating TLR4 activation (Fig. 1). TNFα and IL-6 secretion differed in both induction time (TNFα secretion was induced earlier than IL-6 secretion) and magnitude (TNFα secretion was approximately twice that of IL-6 through the majority of the 24-h incubation period).
Fig.1.
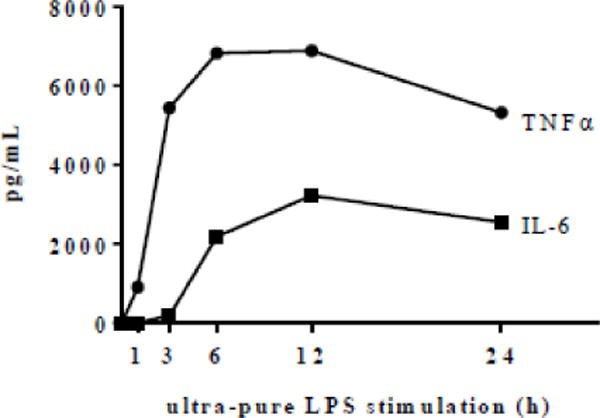
Time course of ultra-pure LPS-induced TNFα and IL-6 secretion. RAW 264.7 cells were stimulated with ultra-pure LPS (100 ng/mL) for a 24 h period. TNFα and IL-6 concentrations in culture supernatants were determined by ELISA
Effect of fatty acid pretreatment on cell membrane fatty acid profiles
At baseline (control), palmitic acid (PA) and stearic acid were the most abundant fatty acids in cell membranes, comprising 22.56% and 18.89% of total fatty acids, while the other major saturated fatty acid, MA, was found in much smaller proportions (3.69 %) (Table 1). Given that the proportion of MA was closer to the proportions of EPA (0.41%) and DHA (1.91%) (Table 1), and because the abundance of MA but not PA was shown to be increased several-fold in macrophages [29], we chose MA as the saturated fatty acid treatment.
Table 1.
Selected fatty acid composition (mol%) after 24-h fatty acid pretreatment and prior to ultra-pure LPS stimulation
| Fatty acid | Control
|
MA
|
EPA
|
DHA
|
||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| SFA | 48.63 | 8.18 | 53.96 | 1.68 | 47.46 | 5.42 | 45.93 | 2.10 |
| 14:0 | 3.81a | 2.47 | 16.53b | 4.39 | 2.46a | 0.14 | 2.69a | 0.45 |
| 16:0 | 23.05ab | 2.20 | 20.03a | 1.51 | 24.00b | 1.92 | 23.71b | 0.34 |
| 18:0 | 19.33 | 3.39 | 15.62 | 1.30 | 18.65 | 3.12 | 17.24 | 0.93 |
| MUFA | 35.85 | 6.10 | 31.29 | 2.39 | 25.05 | 2.72 | 26.32 | 6.02 |
| 16:1n-9 | 6.15 | 0.69 | 3.77 | 0.34 | 2.83 | 0.87 | 2.60 | 0.29 |
| 16:1n-7 | 3.35 | 1.15 | 4.15 | 1.11 | 2.32 | 0.54 | 2.39 | 0.71 |
| 18:1n-9 | 17.52 | 4.25 | 13.55 | 0.63 | 12.61 | 1.15 | 13.05 | 0.51 |
| 181n-7 | 9.51 | 1.34 | 9.63 | 2.10 | 6.69 | 3.43 | 7.66 | 4.46 |
| PUFA | 13.85 | 3.18 | 12.29 | 2.76 | 25.06 | 8.78 | 25.56 | 8.99 |
| n-6 PUFA | 9.27 | 1.27 | 7.86 | 1.62 | 4.64 | 0.29 | 6.54 | 1.05 |
| 18:2 | 2.44 | 0.26 | 1.98 | 0.40 | 1.42 | 0.61 | 2.12 | 0.57 |
| 20:4 | 5.69a | 0.90 | 4.79a | 1.14 | 2.51b | 0.35 | 3.61ab | 0.66 |
| 22:4 | 0.45 | 0.12 | 0.34 | 0.06 | 0.32 | 0.04 | 0.26 | 0.05 |
| n-3 PUFA | 4.58 | 2.36 | 4.43 | 1.23 | 20.42 | 8.57 | 19.02 | 9.62 |
| 20:5 | 0.41a | 0.21 | 0.46a | 0.15 | 8.39b | 3.70 | 0.45a | 0.16 |
| 22:5 | 1.74a | 0.95 | 1.52a | 0.52 | 10.39b | 5.70 | 1.08a | 0.05 |
| 22:6 | 1.92a | 1.27 | 1.86a | 0.65 | 0.69a | 0.26 | 16.92b | 9.88 |
MA, myristic acid; EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; SFA, saturated fatty acid; MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acid. Fatty acids that comprised less than 1 mol% of total fatty acids are not included, but are included in the calculations. Values are mean ± SD of 3 independent experiments. Mean values within a row without common letters statistically differ at P < 0.05 determined by one-way ANOVA adjusted with Tukey’s post-hoc test for multiple comparison. Mean values assigned a letter were included in statistical analysis.
RAW 264.7 cells pretreated with 100 μM MA, EPA, or DHA for 24 h resulted in 4.5-fold, 20.6-fold, and 8.9-fold, increase in MA, EPA and DHA, respectively (all P < 0.05), all primarily at the expense of oleic acid and to a lesser extent, arachidonic acid (AA) (Table 1). Pretreatment with EPA also resulted in a 6.0-fold increase (P < 0.05) in docosapentaenoic acid (DPA), an elongation product of EPA.
EPA and DHA but not MA attenuate TLR4-mediated TNFα and IL-6 secretion
The effect of MA, EPA and DHA enrichment on TLR4-mediated TNFα and IL-6 secretion was assessed 6 and 24 h post-exposure to ultra-pure LPS, which corresponded to the early and late stages of TNFα and IL-6 protein induction as determined by secretion time-course data (Fig. 1). There was no significant effect of MA compared to BSA on TNFα or IL-6 secretion at either time point. After 24 h of ultra-pure LPS stimulation, EPA and DHA pretreatment resulted in 36% and 41% lower TNFα secretion, respectively (both P < 0.05), compared to BSA (Fig. 2a). A similar pattern was observed in the samples collected after 6 h, however, the difference did not reach statistical significance. Interestingly, cells pretreated with EPA or DHA, secreted significantly less IL-6 after both 6 h (67% and 72%, respectively) and 24 h (69% and 76%, respectively) post-stimulation, compared to BSA (Fig. 2b). In response to EPA or DHA pretreatment the decrease in IL-6 secretion was significantly greater than TNFα secretion. Removing the fatty acids from the culture media prior to ultra-pure LPS stimulation produced a similar pattern, suggesting that the effects did not depend on the presence of treatment fatty acids in the culture media during ultra-LPS stimulation (Fig. 2c and 2d).
Fig. 2.
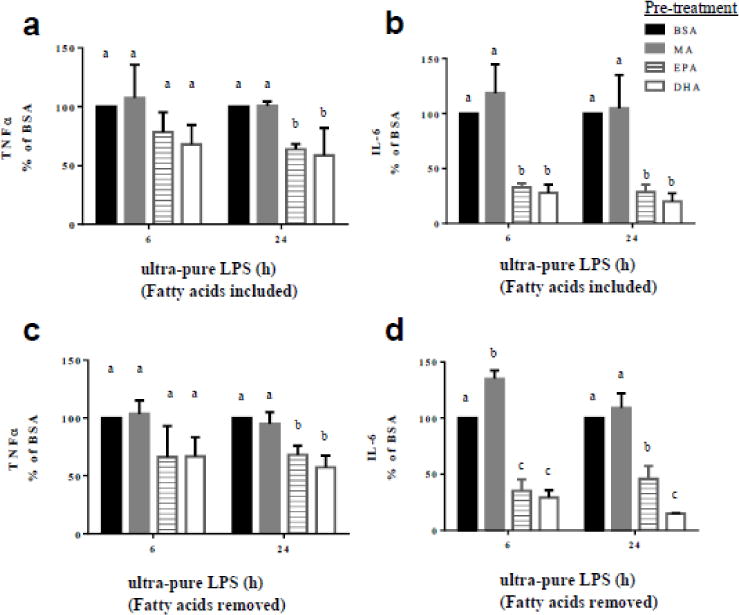
Ultra-pure LPS-induced secretion of TNFα and IL-6 from fatty acid-pretreated RAW 264.7 cells. RAW 264.7 cells were pretreated with the respective fatty acids (100 μM) for 24 h and then stimulated with ultra-pure LPS (100 ng/mL) for 6 or 24 h. Top panel. Production of TNFα (a) and IL-6 (b) from cells pretreated with respective fatty acid and continued to be exposed to respective fatty acids during stimulation. Bottom panel. Production of TNFα (d) and IL-6 (d) from cells pretreated with respective fatty acids, and for which respective fatty acids were removed before stimulation. Concentration of cytokines in the culture supernatants were determined by ELISA. Values are mean ± SD of 3 independent experiments normalized to BSA-treated cells. For each time point, bars without common letters statistically differ at P < 0.05 determined by one-way ANOVA adjusted with Tukey’s post-hoc test for multiple comparisons.
DHA or MA membrane enrichment was not accompanied by a change in receptor abundance or LPS-cell association
Although EPA and DHA had similar effects, EPA effects could not be solely attributed to cell membrane EPA enrichment given the significant conversion of EPA to DPA in cell membranes. Therefore, we focused on DHA to further investigate potential mechanisms. We hypothesized that the proportion of DHA in the membrane could influence TLR4 signaling by altering cell LPS recognition. Therefore, we measured cell surface expression of TLR4, TLR4-MD2 complex and CD14. Cells enriched with MA or DHA compared to BSA treated cells showed no significant difference in cell surface expression of TLR4, TLR4-MD2 complex, or CD14, before or after ultra-pure LPS stimulation (10 min – 360 min; Fig. 3a – 3c). We also assessed the influence of MA and DHA membrane enrichment on LPS-cell surface association as an additional measure of LPS recognition but found no significant difference in either groups relative to the BSA control (Fig. 3d).
Fig. 3.
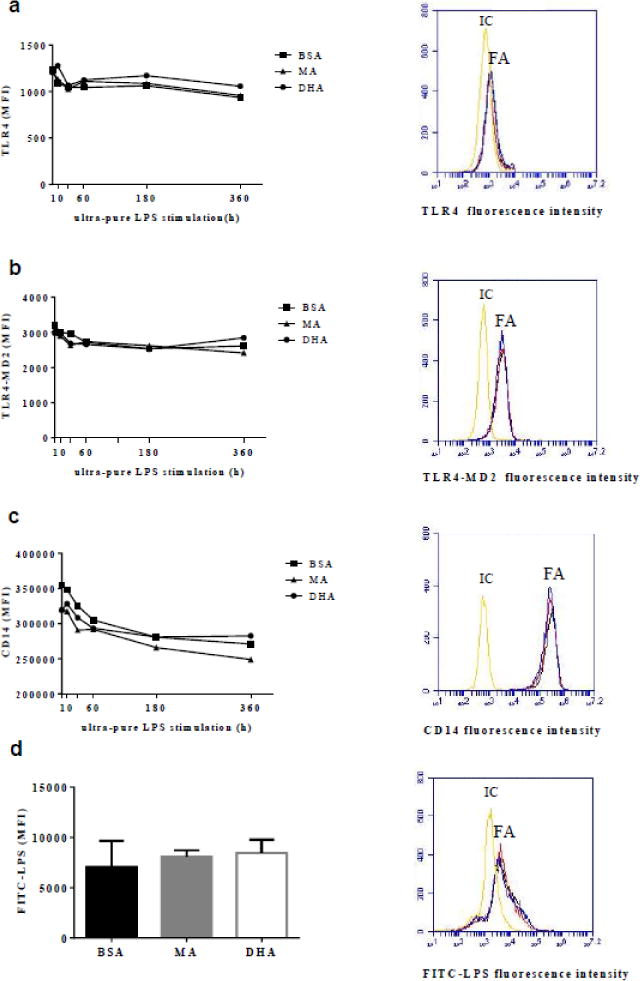
Cell surface expression of TLR4 (a), TLR4-MD2 (b), and CD14 (c), and LPS-cell surface association (d) were determined by flow cytometry. Left. Mean fluorescence intensities (MFI) associated with BSA-, MA-, or DHA-pre-treated cells stimulated with ultra-pure LPS for the times indicated (a–c), or with FITC-LPS for 1 h (d). Right. Representative histograms for indicated markers under different fatty acid pretreatments (BSA: black, MA: red, DHA: blue) compared with corresponding isotype control (IC) in unstimulated cells (a–c) or cells incubated with FITC-LPS for 1 h (d). Values are expressed as mean fluorescence intensity (MFI) ± SD, or just MFI for clarity (a–c) of 3 independent experiments.
Discussion
In the present study, we found that pretreating RAW 264.7 cells with MA, EPA or DHA for 24 h increased the proportion of these fatty acids in the cell membrane. Inflammatory response induced by specific TLR4 activation was suppressed in cells enriched with EPA and DHA, but was not in cells enriched with MA. Interestingly, EPA and DHA reduced IL-6 secretion to a greater extent than TNFα secretion, indicating that their anti-inflammatory effects are cytokinespecific rather than universal inhibition. Moreover, there was no significant effect of DHA or membrane enrichment on cell surface expression of TLR4, TLR4-MD2 complex, CD14, or LPS-cell surface association, further suggesting that DHA may inhibit signaling downstream of the receptor.
This study provides evidence that EPA and DHA inhibit TNFα and IL-6 production induced by TLR4 activation. By using ultra-pure LPS, which is specific for TLR4, we minimized the possibility of activating TLR2, which can be activated by impure LPS [31]. The mechanism of inhibition appears to be related to their incorporation into cell membranes rather than their interaction with ultra-pure LPS or TLR4 in the culture media. This is supported by the observation that the effect of EPA or DHA was similar between the two conditions tested: with and without the respective fatty acid in the culture media during stimulation. The fact that the two treatment conditions produced similar results also suggest that any effect due to the extracellular presence of EPA or DHA during stimulation is not dose-dependent, since washed cells would be expected to have a much smaller concentration of EPA or DHA in the culture media even when considering release of incorporated EPA or DHA back into the culture media due to membrane turnover [41].
Pro-inflammatory effects of saturated fatty acids have been demonstrated to involve activation of TLR4 [42, 43]. To further define the role of saturated fatty acids, we investigated whether exposure to saturated fatty acids and subsequent modification of the membrane fatty acid profile could enhance the inflammatory response of macrophages to the TLR4 ligand, LPS, by upregulating receptor expression and LPS-cell binding. For this purpose, we chose to study MA. MA appeared to have a slight effect on TLR4-mediated induction of pro-inflammatory cytokines, as a small enhancement in TNFα (non-significant) and IL-6 (significant) was observed in some stimulatory conditions. However, these effects were not accompanied by a significant change in cell surface expression of TLR4, TLR4-MD2 complex, CD14, or LPS-cell binding, suggesting that membrane MA content has no significant influence on TLR4 signaling in response to LPS. Studies using much shorter pretreatment time periods (2 or 3 h) have similarly reported no significant additive effect of MA on LPS-induced TNFα and IL-6 secretion in THP-1 macrophages [29, 44]. While the literature suggests that saturated fatty acids are heterogeneous in their ability to augment inflammation initiated by TLR4 activation, the present study further confirmed the findings reported in the previous studies [45, 46] by providing evidence that the saturated fatty acids do not effect TLR4 signaling by modifying the abundance of cell surface TLR4 and CD14, or LPS-cell binding.
It has been proposed that increasing the proportion of very long-chain omega-3 fatty acids in the cell membrane modulates immune cell function, for example, in T cells by influencing membrane receptor distribution and activity [47, 48]. However, evidence from the current work suggests that EPA and DHA may exert their anti-inflammatory effects in macrophages downstream of TLR4 activation. Inhibition of TNFα secretion by either EPA or DHA was much weaker than that of IL-6. A similar observation has been reported in peripheral blood mononuclear cells isolated from subjects who consumed DHA supplements [18], and after addition of EPA or DHA to the culture media of human THP-1 [29, 49] and murine J774 [24, 25] macrophage cell lines. If EPA or DHA treatment decreased TNFα and IL-6 secretion primarily through inhibition of TLR4 activation, a similar relative decline induced by the two fatty acids would be predicted because TLR4 initiates the signaling pathway. Since this was not the case, we hypothesize that inhibition might occur downstream of TLR4 activation, at a point in the signaling pathway that has differential regulatory influences on TNFα and IL-6 production.
Although significant incorporation of DHA into cell membranes was achieved through the pretreatment conditions employed in the current study (100 μM DHA for 24 h), these effects were not accompanied by a decrease in cell surface expression of TLR4 receptor, TLR4-MD2 complex, CD14 in the absence or presence of LPS, or LPS-cell association in RAW 264.7 cells. These results further support the hypothesis that disruption in TLR4 signaling occurs downstream of TLR4 activation. It is difficult to directly compare our findings with those of previous studies, which differ from the present study in cell type and/or treatment conditions. Chronic pretreatment with DHA (24–72 h) was observed to reduce the abundance of membrane TLR4 and CD14 in BV-2 microglial cells [32] and LPS binding to THP-1 cells [33]. It has been previously reported that acute stimulation with LPS (7 minutes) in the presence of DHA (without pretreatment) resulted in down-regulation of TLR4 expression in lipid raft membrane fractions in RAW 264.7 cells and inhibition of TLR4 and MD2 association in lipid rafts of Ba/F3 cells [35]. On the basis of these data it was postulated that this inhibition by DHA of TLR4 recruitment into lipid rafts was attributable to disruption in lipid raft formation due to changes in polar lipid-fatty acid composition. However, given the short exposure to DHA and lack of pretreatment with the fatty acid, it is unlikely that there was an appreciable modification to the lipid raft fatty acid composition. Nevertheless, this prior report suggests that the chronic exposure to DHA used in our study may have influenced TLR4 abundance and TLR4 and MD2 association only in membrane lipid rafts where activation of TLR4, engagement with associated molecules and signaling occur. However, the unequal inhibitory effect of DHA on TNFα and IL-6 secretion suggest that inhibition also occurs downstream of the receptor. For the most part, the results of this study largely concur with previous work that supports anti-inflammatory mechanisms independent of TLR4 signaling, such as activation of G-protein coupled receptor 120, which intercepts pro-inflammatory signaling pathways upstream of MAPK and NFκB activation or peroxisome proliferator-activated receptor gamma, which inhibits NFκB activation [8, 28].
In summary, our study confirms that enriching macrophages with EPA or DHA attenuates inflammatory activity initiated by TLR4 activation. However, the effect of EPA and DHA varies in potency depending on specific cytokines (TNFα and IL-6). This and the lack of influence of DHA on factors influencing LPS recognition and TLR4 activation suggest that DHA exerts its effects downstream of TLR4 activation.
Acknowledgments
This work was supported by the NIH grant: NHLBI-T32-HL069772 (NLS) and the USDA agreement No. 58-1950-0-0014.
Abbreviations
- AA
Arachidonic acid (20:4n-6)
- ANOVA
Analysis of variance
- APC
Allophycocyanin
- BSA
Bovine serum albumin
- CD14
Cluster of differentiation 14
- DHA
Docosahexaenoic acid (22:6n-3)
- DMEM
Dulbecco’s Modified Eagle’s Medium
- DPA
Docosapentaenoic acid (22:5n-3)
- ELISA
Enzyme-linked immunosorbent assay
- EPA
Eicosapentaenoic acid (20:5n-3)
- FBS
Fetal bovine serum
- FITC-LPS
Fluorescein isothiocyanate-conjugated lipopolysaccharide
- GPR120
G-protein coupled receptor
- IC
Isotype control
- IL-6
Interleukin 6
- LPS
Lipopolysaccharide
- MA
Myristic acid (14:0)
- MAPK
Mitogen activated protein kinase
- MD2
Myeloid differentiation 2
- MFI
Mean fluorescence intensity
- MyD88
Myeloid differentiation primary response gene 88
- NFκB
Nuclear factor kappa B
- PBS
Phosphate buffered saline
- PE
Phycoerythrin
- TLR2
Toll-like receptor 2
- TLR4
Toll-like receptor 4
- TNFα
Tumor necrosis factor alpha
Footnotes
Any opinions, findings, conclusion, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the U.S. Department of Agriculture.
Contributor Information
Kaori L. Honda, Email: kaori.honda@tufts.edu.
Stefania Lamon-Fava, Email: stefania.lamon-fava@tufts.edu.
Nirupa R. Matthan, Email: nirupa.matthan@tufts.edu.
Dayong Wu, Email: dayong.wu@tufts.edu.
References
- 1.Pasterkamp G, Van Keulen JK, De Kleijn DP. Role of Toll-like receptor 4 in the initiation and progression of atherosclerotic disease. Eur J Clin Invest. 2004;34(5):328–334. doi: 10.1111/j.1365-2362.2004.01338.x. [DOI] [PubMed] [Google Scholar]
- 2.Gay NJ, Gangloff M. Structure and function of Toll receptors and their ligands. Annu Rev Biochem. 2007:76141–165. doi: 10.1146/annurev.biochem.76.060305.151318. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz EA, Reaven PD. Lipolysis of triglyceride-rich lipoproteins, vascular inflammation, and atherosclerosis. Biochim Biophys Acta. 2012;1821(5):858–866. doi: 10.1016/j.bbalip.2011.09.021. [DOI] [PubMed] [Google Scholar]
- 4.Janssens S, Beyaert R. Role of Toll-like receptors in pathogen recognition. Clin Microbiol Rev. 2003;16(4):637–646. doi: 10.1128/CMR.16.4.637-646.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawai T, Akira S. Toll-like receptor downstream signaling. Arthritis Res Ther. 2005;7(1):12–19. doi: 10.1186/ar1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edfeldt K, Swedenborg J, Hansson GK, Yan ZQ. Expression of toll-like receptors in human atherosclerotic lesions: a possible pathway for plaque activation. Circulation. 2002;105(10):1158–1161. [PubMed] [Google Scholar]
- 7.Xu XH, Shah PK, Faure E, Equils O, Thomas L, Fishbein MC, Luthringer D, Xu XP, Rajavashisth TB, Yano J, Kaul S, Arditi M. Toll-like receptor-4 is expressed by macrophages in murine and human lipid-rich atherosclerotic plaques and upregulated by oxidized LDL. Circulation. 2001;104(25):3103–3108. doi: 10.1161/hc5001.100631. [DOI] [PubMed] [Google Scholar]
- 8.Michelsen KS, Wong MH, Shah PK, Zhang W, Yano J, Doherty TM, Akira S, Rajavashisth TB, Arditi M. Lack of Toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E. Proc Natl Acad Sci U S A. 2004;101(29):10679–10684. doi: 10.1073/pnas.0403249101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bjorkbacka H, Kunjathoor VV, Moore KJ, Koehn S, Ordija CM, Lee MA, Means T, Halmen K, Luster AD, Golenbock DT, Freeman MW. Reduced atherosclerosis in MyD88-null mice links elevated serum cholesterol levels to activation of innate immunity signaling pathways. Nat Med. 2004;10(4):416–421. doi: 10.1038/nm1008. [DOI] [PubMed] [Google Scholar]
- 10.Kelley DS, Adkins Y. Similarities and differences between the effects of EPA and DHA on markers of atherosclerosis in human subjects. Proc Nutr Soc. 2012;71(2):322–331. doi: 10.1017/S0029665112000080. [DOI] [PubMed] [Google Scholar]
- 11.Sekikawa A, Kadowaki T, El-Saed A, Okamura T, Sutton-Tyrrell K, Nakamura Y, Evans RW, Mitsunami K, Edmundowicz D, Nishio Y, Nakata K, Kadota A, Otake T, Miura K, Choo J, Abbott RD, Kuller LH, Curb JD, Ueshima H, group EJS Differential association of docosahexaenoic and eicosapentaenoic acids with carotid intima-media thickness. Stroke. 2011;42(9):2538–2543. doi: 10.1161/STROKEAHA.110.613042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erkkila AT, Matthan NR, Herrington DM, Lichtenstein AH. Higher plasma docosahexaenoic acid is associated with reduced progression of coronary atherosclerosis in women with CAD. J Lipid Res. 2006;47(12):2814–2819. doi: 10.1194/jlr.P600005-JLR200. [DOI] [PubMed] [Google Scholar]
- 13.Kim YJ, Kim OY, Cho Y, Chung JH, Jung YS, Hwang GS, Shin MJ. Plasma phospholipid fatty acid composition in ischemic stroke: importance of docosahexaenoic acid in the risk for intracranial atherosclerotic stenosis. Atherosclerosis. 2012;225(2):418–424. doi: 10.1016/j.atherosclerosis.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 14.Libby P. Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32(9):2045–2051. doi: 10.1161/ATVBAHA.108.179705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meydani SN, Endres S, Woods MM, Goldin BR, Soo C, Morrill-Labrode A, Dinarello CA, Gorbach SL. Oral (n-3) fatty acid supplementation suppresses cytokine production and lymphocyte proliferation: comparison between young and older women. J Nutr. 1991;121(4):547–555. doi: 10.1093/jn/121.4.547. [DOI] [PubMed] [Google Scholar]
- 16.Meydani SN, Lichtenstein AH, Cornwall S, Meydani M, Goldin BR, Rasmussen H, Dinarello CA, Schaefer EJ. Immunologic effects of national cholesterol education panel step-2 diets with and without fish-derived N-3 fatty acid enrichment. J Clin Invest. 1993;92(1):105–113. doi: 10.1172/JCI116537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trebble T, Arden NK, Stroud MA, Wootton SA, Burdge GC, Miles EA, Ballinger AB, Thompson RL, Calder PC. Inhibition of tumour necrosis factor-alpha and interleukin 6 production by mononuclear cells following dietary fish-oil supplementation in healthy men and response to antioxidant co-supplementation. Br J Nutr. 2003;90(2):405–412. doi: 10.1079/bjn2003892. [DOI] [PubMed] [Google Scholar]
- 18.Vedin I, Cederholm T, Freund Levi Y, Basun H, Garlind A, Faxen Irving G, Jonhagen ME, Vessby B, Wahlund LO, Palmblad J. Effects of docosahexaenoic acidrich n-3 fatty acid supplementation on cytokine release from blood mononuclear leukocytes: the OmegAD study. Am J Clin Nutr. 2008;87(6):1616–1622. doi: 10.1093/ajcn/87.6.1616. [DOI] [PubMed] [Google Scholar]
- 19.Wallace FA, Miles EA, Calder PC. Comparison of the effects of linseed oil and different doses of fish oil on mononuclear cell function in healthy human subjects. Br J Nutr. 2003;89(5):679–689. doi: 10.1079/BJN1079/2002821. [DOI] [PubMed] [Google Scholar]
- 20.Zhang J, Wang C, Li L, Man Q, Meng L, Song P, Froyland L, Du ZY. Dietary inclusion of salmon, herring and pompano as oily fish reduces CVD risk markers in dyslipidaemic middle-aged and elderly Chinese women. Br J Nutr. 2012;108(8):1455–1465. doi: 10.1017/S0007114511006866. [DOI] [PubMed] [Google Scholar]
- 21.Zhang J, Wang C, Li L, Man Q, Song P, Meng L, Du ZY, Froyland L. Inclusion of Atlantic salmon in the Chinese diet reduces cardiovascular disease risk markers in dyslipidemic adult men. Nutr Res. 2010;30(7):447–454. doi: 10.1016/j.nutres.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 22.Wang S, Wu D, Matthan NR, Lamon-Fava S, Lecker JL, Lichtenstein AH. Reduction in dietary omega-6 polyunsaturated fatty acids: eicosapentaenoic acid plus docosahexaenoic acid ratio minimizes atherosclerotic lesion formation and inflammatory response in the LDL receptor null mouse. Atherosclerosis. 2009;204(1):147–155. doi: 10.1016/j.atherosclerosis.2008.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yaqoob P, Calder P. Effects of dietary lipid manipulation upon inflammatory mediator production by murine macrophages. Cell Immunol. 1995;163(1):120–128. doi: 10.1006/cimm.1995.1106. [DOI] [PubMed] [Google Scholar]
- 24.Martins de Lima-Salgado T, Coccuzzo Sampaio S, Cury-Boaventura MF, Curi R. Modulatory effect of fatty acids on fungicidal activity, respiratory burst and TNF-alpha and IL-6 production in J774 murine macrophages. Br J Nutr. 2011;105(8):1173–1179. doi: 10.1017/S0007114510004873. [DOI] [PubMed] [Google Scholar]
- 25.Oliver E, McGillicuddy FC, Harford KA, Reynolds CM, Phillips CM, Ferguson JF, Roche HM. Docosahexaenoic acid attenuates macrophage-induced inflammation and improves insulin sensitivity in adipocytes-specific differential effects between LC n-3 PUFA. J Nutr Biochem. 2012;23(9):1192–1200. doi: 10.1016/j.jnutbio.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 26.Weldon SM, Mullen AC, Loscher CE, Hurley LA, Roche HM. Docosahexaenoic acid induces an anti-inflammatory profile in lipopolysaccharide-stimulated human THP-1 macrophages more effectively than eicosapentaenoic acid. J Nutr Biochem. 2007;18(4):250–258. doi: 10.1016/j.jnutbio.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 27.Zhao Y, Joshi-Barve S, Barve S, Chen LH. Eicosapentaenoic acid prevents LPS-induced TNF-alpha expression by preventing NF-kappaB activation. J Am Coll Nutr. 2004;23(1):71–78. doi: 10.1080/07315724.2004.10719345. [DOI] [PubMed] [Google Scholar]
- 28.Zhao G, Etherton TD, Martin KR, Vanden Heuvel JP, Gillies PJ, West SG, Kris-Etherton PM. Anti-inflammatory effects of polyunsaturated fatty acids in THP-1 cells. Biochem Biophys Res Commun. 2005;336(3):909–917. doi: 10.1016/j.bbrc.2005.08.204. [DOI] [PubMed] [Google Scholar]
- 29.Wang S, Wu D, Lamon-Fava S, Matthan NR, Honda KL, Lichtenstein AH. In vitro fatty acid enrichment of macrophages alters inflammatory response and net cholesterol accumulation. Br J Nutr. 2009;102(4):497–501. doi: 10.1017/S0007114509231758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rutledge HR, Jiang W, Yang J, Warg LA, Schwartz DA, Pisetsky DS, Yang IV. Gene expression profiles of RAW264.7 macrophages stimulated with preparations of LPS differing in isolation and purity. Innate Immun. 2012;18(1):80–88. doi: 10.1177/1753425910393540. [DOI] [PubMed] [Google Scholar]
- 31.Hirschfeld M, Ma Y, Weis JH, Vogel SN, Weis JJ. Cutting edge: repurification of lipopolysaccharide eliminates signaling through both human and murine toll-like receptor 2. J Immunol. 2000;165(2):618–622. doi: 10.4049/jimmunol.165.2.618. [DOI] [PubMed] [Google Scholar]
- 32.De Smedt-Peyrusse V, Sargueil F, Moranis A, Harizi H, Mongrand S, Laye S. Docosahexaenoic acid prevents lipopolysaccharide-induced cytokine production in microglial cells by inhibiting lipopolysaccharide receptor presentation but not its membrane subdomain localization. J Neurochem. 2008;105(2):296–307. doi: 10.1111/j.1471-4159.2007.05129.x. [DOI] [PubMed] [Google Scholar]
- 33.Chu AJ, Walton MA, Prasad JK, Seto A. Blockade by polyunsaturated n-3 fatty acids of endotoxin-induced monocytic tissue factor activation is mediated by the depressed receptor expression in THP-1 cells. J Surg Res. 1999;87(2):217–224. doi: 10.1006/jsre.1999.5762. [DOI] [PubMed] [Google Scholar]
- 34.Lee JY, Plakidas A, Lee WH, Heikkinen A, Chanmugam P, Bray G, Hwang DH. Differential modulation of Toll-like receptors by fatty acids: preferential inhibition by n-3 polyunsaturated fatty acids. J Lipid Res. 2003;44(3):479–486. doi: 10.1194/jlr.M200361-JLR200. [DOI] [PubMed] [Google Scholar]
- 35.Wong SW, Kwon MJ, Choi AM, Kim HP, Nakahira K, Hwang DH. Fatty acids modulate Toll-like receptor 4 activation through regulation of receptor dimerization and recruitment into lipid rafts in a reactive oxygen species-dependent manner. J Biol Chem. 2009;284(40):27384–27392. doi: 10.1074/jbc.M109.044065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang S, Rutkowsky JM, Snodgrass RG, Ono-Moore KD, Schneider DA, Newman JW, Adams SH, Hwang DH. Saturated fatty acids activate TLR-mediated proinflammatory signaling pathways. J Lipid Res. 53(9):2002–2013. doi: 10.1194/jlr.D029546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee JY, Ye J, Gao Z, Youn HS, Lee WH, Zhao L, Sizemore N, Hwang DH. Reciprocal modulation of Toll-like receptor-4 signaling pathways involving MyD88 and phosphatidylinositol 3-kinase/AKT by saturated and polyunsaturated fatty acids. J Biol Chem. 2003;278(39):37041–37051. doi: 10.1074/jbc.M305213200. [DOI] [PubMed] [Google Scholar]
- 38.Lecker JL, Matthan NR, Billheimer JT, Rader DJ, Lichtenstein AH. Impact of dietary fat type within the context of altered cholesterol homeostasis on cholesterol and lipoprotein metabolism in the F1B hamster. Metabolism. 2010;59(10):1491–1501. doi: 10.1016/j.metabol.2010.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matthan NR, Ip B, Resteghini N, Ausman LM, Lichtenstein AH. Long-term fatty acid stability in human serum cholesteryl ester, triglyceride, and phospholipid fractions. J Lipid Res. 2010;51(9):2826–2832. doi: 10.1194/jlr.D007534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang J, Pae M, Meydani SN, Wu D. Epigallocatechin-3-gallate inhibits expression of receptors for T cell regulatory cytokines and their downstream signaling in mouse CD4+ T cells. J Nutr. 2012;142(3):566–571. doi: 10.3945/jn.111.154419. [DOI] [PubMed] [Google Scholar]
- 41.Norris PC, Dennis EA. Omega-3 fatty acids cause dramatic changes in TLR4 and purinergic eicosanoid signaling. Proc Natl Acad Sci U S A. 2012;109(22):8517–8522. doi: 10.1073/pnas.1200189109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee JY, Sohn KH, Rhee SH, Hwang D. Saturated fatty acids, but not unsaturated fatty acids, induce the expression of cyclooxygenase-2 mediated through Toll-like receptor 4. J Biol Chem. 2001;276(20):16683–16689. doi: 10.1074/jbc.M011695200. [DOI] [PubMed] [Google Scholar]
- 43.Huang S, Rutkowsky JM, Snodgrass RG, Ono-Moore KD, Schneider DA, Newman JW, Adams SH, Hwang DH. Saturated fatty acids activate TLR-mediated proinflammatory signaling pathways. J Lipid Res. 2012;53(9):2002–2013. doi: 10.1194/jlr.D029546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haversen L, Danielsson KN, Fogelstrand L, Wiklund O. Induction of proinflammatory cytokines by long-chain saturated fatty acids in human macrophages. Atherosclerosis. 2009;202(2):382–393. doi: 10.1016/j.atherosclerosis.2008.05.033. [DOI] [PubMed] [Google Scholar]
- 45.Chang CF, Chau YP, Kung HN, Lu KS. The lipopolysaccharide-induced proinflammatory response in RAW264.7 cells is attenuated by an unsaturated fatty acid-bovine serum albumin complex and enhanced by a saturated fatty acid-bovine serum albumin complex. Inflamm Res. 2012;61(2):151–160. doi: 10.1007/s00011-011-0399-1. [DOI] [PubMed] [Google Scholar]
- 46.Schwartz EA, Zhang WY, Karnik SK, Borwege S, Anand VR, Laine PS, Su Y, Reaven PD. Nutrient modification of the innate immune response: a novel mechanism by which saturated fatty acids greatly amplify monocyte inflammation. Arterioscler Thromb Vasc Biol. 2010;30(4):802–808. doi: 10.1161/ATVBAHA.109.201681. [DOI] [PubMed] [Google Scholar]
- 47.Fan YY, Ly LH, Barhoumi R, McMurray DN, Chapkin RS. Dietary docosahexaenoic acid suppresses T cell protein kinase C theta lipid raft recruitment and IL-2 production. J Immunol. 2004;173(10):6151–6160. doi: 10.4049/jimmunol.173.10.6151. [DOI] [PubMed] [Google Scholar]
- 48.Stulnig TM, Huber J, Leitinger N, Imre EM, Angelisova P, Nowotny P, Waldhausl W. Polyunsaturated eicosapentaenoic acid displaces proteins from membrane rafts by altering raft lipid composition. J Biol Chem. 2001;276(40):37335–37340. doi: 10.1074/jbc.M106193200. [DOI] [PubMed] [Google Scholar]
- 49.Mullen A, Loscher CE, Roche HM. Anti-inflammatory effects of EPA and DHA are dependent upon time and dose-response elements associated with LPS stimulation in THP-1-derived macrophages. J Nutr Biochem. 2010;21(5):444–450. doi: 10.1016/j.jnutbio.2009.02.008. [DOI] [PubMed] [Google Scholar]


