Abstract
The Rho GTPase Rac is an important regulator of cancer cell migration and invasion; processes required for metastatic progression. We previously characterized the small molecule EHop-016 as a novel Rac inhibitor in metastatic breast cancer cells and recently found that EHop-016 was effective at reducing tumor growth in nude mice at 25 mg/kg bodyweight (BW). The purpose of this study was to compare the pharmacokinetics and bioavailability of EHop-016 at different dosages in a single dose input scheme (10, 20 and 40 mg/kg BW) following intraperitoneal (IP) and oral gavage (PO) administration to nude mice. We developed and validated a rapid and sensitive method for the quantitation of EHop-016 in mouse plasma by ultra high performance liquid chromatography coupled with electrospray ionization tandem mass spectrometry (UPLC/MS/MS). Separation was carried out on an Agilent Poroshell 120 EC-C18 column (3.0 × 50 mm) using organic and aqueous mobile phases. EHop-016 was identified from its accurate mass and retention time from the acquired full-scan chromatogram and quantified by its peak area. The validated method was linear (R2> 0.995) over the range of 5 – 1000 ng/mL (1/x2 weighting). Pharmacokinetic parameters were obtained by non-compartmental analysis using WinNonlin®. The area under the curve (AUC0-∞) ranged from 328 – 1869 ng·hr/mL and 133 – 487 ng·hr/mL for IP and PO dosing respectively. The elimination half-life (t1/2) ranged from 3.8 – 5.7 hours and 3.4 – 26.8 hours for IP and PO dosing respectively. For both IP and PO administration, the AUC0-∞values were proportional to the tested doses demonstrating linear PK profiles. The relative bioavailability of EHop-016 after oral gavage administration ranged from 26% - 40%. These results support further preclinical evaluation of EHop-016 as a new anti-cancer therapy.
Keywords: EHop-016, pharmacokinetics, UPLC/MS/MS, Rac, cancer
1. Introduction
The small molecule EHop-016 (Table 1) is an effective Rho GTPase Rac-specific inhibitor. We previously reported that EHop-016 inhibits Rac activity of metastatic cancer cells with an IC50 of 1 μM. EHop-016 was found to disrupt the interaction of Rac with the guanine nucleotide exchange factor Vav2 and to inhibit the activity of the Rac downstream effector p21-activated kinase 1 (PAK1), Rac-directed lamellipodia formation and directed cell migration. Additionally, EHop-016 decreased Akt and Jun kinase (JNK) activities and the expression of c-Myc and Cyclin D in breast and prostate cancer cell lines. At higher concentrations (>10 μM) EHop-016 also inhibited the activity of the Rho GTPase Cdc42, reduced cell viability, and activated apoptosis [1,2]. Other reports confirm our results and show that EHop-016 inhibits human T-lymphocyte and murine melanoma cell migration, as well as human and murine leukemic cell growth [3-5]. Since Rac and Cdc42 mediated cell signaling and migration/invasion are integral to cancer metastasis as well as immune and stromal cell function, in addition to being a potential anticancer compound, EHop-016 is a valuable tool for probing Rac/Cdc42/Vav activities in biological systems.
Table 1.
Name, structure, formula, and molecular weight of EHop-016.
| Compound ID |
Compound Name |
Structure | Formula | Molecular Weight |
|---|---|---|---|---|
| EHop-016 | (N4-(9-Ethyl-9H-carbazol-3-yl)-N2-(3- morpholin-4-yl-propyl)-pyrimidine-2,4- diamine) |
***** | C25H30N6O | 430.55 |
To validate an in vivo anticancer role for EHop-016, we recently characterized the pharmacological effects of EHop-016 in a mouse model of experimental metastasis. At 25 mg/kg body weight (BW), EHop-016 was effective at reducing mammary fat pad tumor growth, metastasis, and angiogenesis in athymic nude mice. A role for EHop-016 in angiogenesis inhibition was confirmed in vitro by demonstrating inhibition of Rac activity and capillary tube formation of endothelial cells [2]. There are currently no reports describing the pharmacokinetics (PK) of EHop-016 in an experimental mouse model. Such PK data are essential to explain the mechanisms of drug distribution and elimination to attain a better understanding of the pharmacology of EHop-016. To address this question, we first developed and fully validated a rapid and sensitive method for the quantification of Ehop-016 in mouse plasma by ultra high performance liquid chromatography coupled with electrospray ionization tandem mass spectrometry (UPLC/MS/MS). UPLC/MS/MS is the standard of choice for pharmacokinetic studies of new preclinical drug compounds because of its high sensitivity and specificity [6]. In particular, multiple reaction monitoring (MRM) using a triple quadrupole detector is a highly specific detection method with very low background interference. Therefore, this method was applied to evaluate the pharmacokinetics of EHop-016 in nude mice.
The aim of this study was to develop and validate an UPLC/MS/MS bioanalytical method to quantify the Rac inhibitor EHop-016 and evaluate its pharmacokinetic parameters in healthy mice. Therefore, we determined the time-course of plasma concentrations after intraperitoneal (IP) injection and oral gavage (per oral, PO) administration after a single dose input scheme at 10 mg/kg, 20 mg/kg, and 40 mg/kg BW EHop-016.
Materials and Methods
2.1. Materials
Organic solvents acetonitrile (ACN), methanol (MeOH), and dimethyl sulfoxide (DMSO) were purchased from Sigma. Formic acid was from Agilent. Ammonium fluoride and ultrapure water were from Sigma. Non-sterile mouse plasma containing sodium citrate was from Equitech-Bio, Inc. EHop-016 and EHop-0141 were used as analytical standards, and EHop-016 was synthesized as previously described by us [1]. The synthesis of EHop-0141 is described in Supplementary Data. A primary stock solution of EHop-016 analyte (2 mg/mL) was prepared by dissolving 10 mg of analyte in 4 mL of DMSO and 1 mL of MeOH. A stock solution of EHop-0141 internal standard (21.9 mg/mL) was prepared by dissolving 7.3 mg of analyte in 334 μL of DMSO. Analyte stock solutions were stored in the dark at −20°C. Standard solutions were prepared by diluting primary stock solutions in either mouse plasma or a solution of 65% deionized water (dH2O) and 35% organic (50% ACN:50%MeOH).
2.2. Instrumentation
The analysis was performed on an Agilent automated UPLC system coupled to a triple quadrupole MS/MS. The data was collected and analyzed by the Agilent MassHunter software package (Version B.05.01).The UPLC separations were performed on a Poroshell 120 EC-C18 column (3 × 50.0mm), 2.7μm particle size (Agilent, CA),maintained at 40°C, under gradient conditions. The mobile phases were 1mM ammonium fluoride in dH2O (Solution A) and 50% ACN/50% MeOH/0.1% formic acid (Solution B) and were equilibrated at an initial composition of 65% A:35% B. Subsequently, the percentage of B was increased by a linear gradient to 98% from 2.5 minutes to 3.0 minutes. The content of B was decreased by a linear gradient to 35% from 4.5 minutes to 5.0 minutes. The column was then stabilized for 1.5 minutes prior to the next injection. The total run time for analysis was 6.5 minutes and the injection volume was 1.0 μL. The 6460 triple quadrupole MS/MS with an electrospray ionization source was operated in the positive ionization mode using the following parameters: electrospray voltage +3848V, gas temperature 300°C, gas flow rate 8 L/min, sheath gas temperature 375°C, sheath gas flow rate 11 L/min, nebulizer pressure 30 psi, and capillary current 4000 nA. An instrument autotune was performed prior to each analysis or when necessary using a certified calibration solution. The mass spectra of [M+H]+ ions were recorded from 100 to 800 m/z. EHop-016 with precursor ion [M+H]+ = 431.6 and EHop-0141 with precursor ion [M+H]+ = 443.6 were used as analytical standards monitoring the MRM transitions [M+H]+ = 179.1 and [M+H]+ = 124.1 for EHop-016 and EHop-0141, respectively. Nitrogen (99.95%) was used as sheath gas.
2.3. Sample Extraction Procedure
Plasma samples were extracted by a standard protein precipitation method as follows: 100μL of plasma was transferred to a 1.5mL eppendorf tube. When appropriate, 50μL of internal standard (500 ng/mL prepared in 50% dH2O and 50% MeOH) was added to sample. Proteins in the matrix were precipitated by addition of 450 μL of ACN followed by vortexing for 10 minutes using a DVX-2500 at 2,500 rpm. Samples were then centrifuged for 10 minutes at 3,300 rpm at 4°C using a Sorvall Legend 17R. Supernatant was transferred to borosilicate glass tubes and dried using a Savant Speed Vac. Dried samples were reconstituted by adding 200 μL of 65% dH2O/35% organic (50%ACN:50%MeOH) solution and vortexed for ten minutes at 2,500 rpm. Samples were transferred to autosampler vials, sealed, and centrifuged at 3000 rpm on a Sorvall ST 16R centrifuge for 3 minutes prior to injection onto the UPLC system.
2.4. Method Validation
Calibration curves were prepared using working solutions of standards at 5, 20, 50, 60, 100, 250, 500, and 1000 ng/mL EHop-016 in mouse plasma. Quality control samples were prepared using working solutions of 10, 30, 75, and 750 ng/mL. The lower limit of quantification (LLOQ) was defined as the lowest concentration (5 ng/mL) that could be quantified with acceptable accuracy and precision. The upper limit of quantification (ULOQ) was defined as the highest concentration (1000 ng/mL) that could be quantified with acceptable accuracy and precision. Intra-day precision and accuracy were measured by comparing calculated concentrations of LLOQ, ULOQ, and four QC concentrations (n=6 replicates per concentration level) against their nominal concentrations in one batch. Inter-day precision and accuracy were measured by comparing the calculated concentrations of LLOQ, ULOQ, and four QC concentrations from six independent batches run on separate days (n=12, 2 replicates per concentration level). The accuracy and precision of the method were described by percent relative error (RE%) and relative standard deviation (RSD%), respectively. Recovery of EHop-016 was measured in spiked mouse plasma at three concentrations (5.0 ng/mL, 250 ng/mL, and 1000 ng/mL) based upon six replicates and compared to EHop-016 in a solution of 65% ammonium fluoride/35% organic solvent (50%MeOH:50%ACN) at the same concentrations. Recovery was calculated by comparing the peak areas of extracted samples to unextracted samples. Matrix effect was evaluated by comparing the peak areas of unextracted samples to neat samples. EHop-016 stability was measured under several conditions. Benchtop stability (5 and 1000 ng/mL) was evaluated for five hours. Post-preparation response of the four QC concentrations (10, 30, 75, and 750 ng/mL) was determined after 237 hours at ambient temperature. Freeze-thaw stability at −80°C (5 and 1000 ng/mL) was evaluated for twelve 12 - hour cycles and for six 24 - hour cycles. Long term stability at −20°C (5 and 1000 ng/mL) was evaluated after 26 and 111 days. EHop-016 was considered stable when the accuracy (% RE) was within ± 15 %.
2.5. Animals
All experimental procedures were conducted under approved protocol #A8180112 by The University of Puerto Rico School of Medicine Institutional Animal Care and Use Committee (IACUC), in accordance with the principles and procedures outlined in the NIH Guideline for the Care and Use of Laboratory Animals. Three to four-week-old female athymic nude (Nu/Nu) mice (Charles River Laboratories, Inc. Wilmington, MA) were housed under pathogen-free conditions in HEPA-filtered cages and kept on a 12 h light/dark cycle, and controlled temperature (22-24°C), and humidity (25%). Food and water were given ad libitum. After acclimation, animals were treated with EHop-016. EHop-016 was prepared as a stock solution of 32 mg/mL in cremophor:ethanol (50:50) dissolved by ultra-sonication for 5 minutes. The final concentrations of 2, 4, and 8 mg/mL EHop-016 were diluted in a cremophor:ethanol:PBS (12.5:12.5:75) solution. Each mouse (average weight of 20 g) was administered a single, 0.1 mL dose of 2 mg/mL, 4 mg/mL, or 8 mg/mL EHop-016, that corresponded to 10, 20, or 40 mg/kg BW Ehop-106 respectively, by IP or PO injection. Subsequently, four to five mice/group were sacrificed at 0.5, 1.0, 4.0, 8.0, and 12.0 hours by cervical dislocation and blood (~ 1.0 mL) was collected by cardiac puncture exsanguination. Blood was immediately collected in sodium citrate buffer on ice and centrifuged for 20 minutes at 4°C. Plasma was then recovered and stored at ±80°C until analysis.
2.6. Pharmacokinetic Data Analysis
The pharmacokinetics of EHop-016 after both IP and PO administration to nude mice was studied at different dosages in a single dose input scheme (10, 20 and 40 mg/kg body weight). All experimental data of plasma drug concentration are expressed as a mean from four to five animals per time-point. The pooled data analysis was performed by non-compartmental analysis (NCA) using a combined linear/log linear trapezoidal rule approach (WinNonlin professional software, Version 2.1, Pharsight Inc., 1997, NC, USA). A time zero value was considered for extrapolation purposes. The linear trapezoidal rule was used up to peak level, after which the logarithmic trapezoidal rule was applied. Lambda z is a first-order rate constant associated with the terminal (log linear) segment of the curve, as estimated by linear regression of the terminal data points. The largest adjusted regression was selected in order to estimate lambda z, with one caveat: if the adjustment did not improve, but was rather within 0.0001 of the largest value, the regression with larger number of points was used. For all data sets, model-independent metrics typically reported in pharmacokinetic studies were tabulated. Parameters extrapolated to infinity using the moments of the curve, such as mean residence time, (MRT), were computed based on the last predicted level, where the predicted value is based on the linear regression performed to estimate terminal lambda z rate constant. Computing these parameters based on the last observed level was discouraged in order to avoid larger estimation errors. Time-to-peak values were determined as the time of maximum observed level (i.e. maximum plasma concentration) considering the entire curve.
2.7. Statistical Analysis
Statistical analysis was performed using GraphPad Prism 6.0 (GraphPad software, Inc., San Diego, CA). Experimental values are expressed as mean ± standard error of the mean (SEM). Statistical analysis was performed using a Student’s t-test or one-way ANOVA. A p value of < 0.05 was considered significant.
3. Results
3.1. Performance and Optimization of the Analytical Methods
The UPLC/MS/MS and chromatography systems were first optimized for the analysis of EHop-016 from plasma using Mass Hunter Optimizer Sequence algorithm. Figure 1A shows the precursor ion of EHop-016 ([M + H]+1 = 431.6 m/z) and Figure 1B shows the four MRM ions 179.1, 286.1, 316.2, and 344.2 m/z, observed in the positive ionization mode. The ion 179.1 was used for quantitative analysis and the ion 286.1 was used for qualitative analysis. No fragments were detected in the negative ionization mode. Dwell time did not affect EHop-016 detection and therefore, a dwell time of 100 ms was selected for method validation. Cell accelerator voltage (CAV) reduced EHop-016 detection, and a CAV of 3 was selected for method validation. Finally, electron multiplier voltage (EMV) increased EHop-016 detection, and an EMV of 100 was selected for method validation (data not shown).
Figure 1.
(A) Full-scan precursor ion mass spectra of EHop-016 (m/z 100-800). (B) Positive ion mass spectra [M+H]+, m/z 179.1, 286.1, 316.2, and 344.2 amu (C,D) Typical total ion chromatograms of EHop-016 obtained after extraction of (C) blank mouse plasma and (D) mouse plasma spiked with EHop-016 (5 ng/mL). (E) Total ion chromatograms of EHop-016 (tR 1.17) and internal standard EHop-0141 (tR 1.63), with respective structures.
Several mobile phases were evaluated in order to achieve the optimum response by minimizing tailing, as well as achieving adequate separation. An organic mobile phase of 50% ACN and 50% MeOH with 0.1% formic acid and an aqueous mobile phase of water with 1 mM ammonium fluoride were selected. The use of ammonium fluoride resulted in good peak shape. Under these conditions, the retention time of EHop-016 was validated to be 1.17 (Fig 1 C, D). The specific analytical properties of EHop-016 are shown in Table 1.
Next, the sample extraction procedure was optimized. There were no significant differences in the recovery of EHop-016 between the anti-coagulant reagents sodium citrate and heparin (data not shown). In order to determine an appropriate extraction solvent for the detection of EHop-016 in mouse plasma matrix, several organic solvents were compared. While there were no significant differences in EHop-016 recovery after extraction using MeOH, ACN, and a mixture containing 50% MeOH and 50% ACN, there was a substantial decrease in recovery after extraction using MeOH + 0.1% formic acid (Supplementary Data). Therefore, all subsequent extractions were performed using ACN (100%) as the solvent.
During the development of the bioanalytical method, the LLOQ and the ULOQ were determined to be 5 ng/mL and 1000 ng/mL, respectively. Carryover was one of the principle factors controlling the dynamic range of the method. It was necessary to ensure that a blank sample injected after the injection of a high concentration sample did not demonstrate the presence of significant (>20% of LLOQ) residual analyte [7]. The observed carryover was identified to be autosampler, and not column-related, since it was reduced significantly by extensive washing of the autosampler injection syringe and needle 5X between injections.
3.2. Method Validation
Bioanalytical method validations were performed according to the guidelines specified by the U.S. Department of Health and Human Services Guidance for Industry [7].
3.2.1. Selectivity
Selectivity was evaluated by pooling six independent sources of blank mouse plasma and comparing the chromatograms with the same pooled plasma spiked with 5 ng/mL EHop-016 [8]. Figure 1C shows a representative chromatogram of a blank mouse plasma sample compared to the same plasma spiked with 5ng/mL EHop-016 (Fig. 1D). There was no significant interference observed from the blank mouse plasma samples.
3.2.2. Internal Standard
Several compounds were evaluated as potential internal standards for method validation. Of those tested, compound EHop-0141 was selected for the following reasons. First, as shown in Figure 1E, EHop-0141 is very similar in structure to EHop-016. Both share the same carbazole group, but where EHop-016 contains a pyrimidine core, EHop-0141 contains a triazole core. Secondly, EHop-016 elutes at a retention time of 1.17 during the initial 2.5 minute isocratic phase of the 6.5 minute runtime. It was important to select a standard, which also eluted in this isocratic phase to reduce potential problems associated with drifting in retention time during a gradient. EHop-0141 was found to elute during the initial 2.5 minute isocratic phase in 35% organic solvent (elution time was 1.63). And finally, EHop-0141 did not interfere with the elution of EHop-016.
3.2.3. Linearity
Linearity was determined using eight calibrant concentrations (5, 20, 50, 60, 100, 250, 500, and 1000 ng/mL EHop-016). The dynamic range established was 5 – 1000 ng/mL. Least-squares linear regression was calculated using MassHunter Quantitative software by analysis of concentrations and the relative response (RR) as determined by the ratio of the EHop-016 peak area to the EHop-0141 peak area. Calibration curves were evaluated using weighting of 1/x2, and for a linear fit with a determination coefficient (R2) greater than 0.995 in all validation runs.
3.2.3 Accuracy and precision
Accuracy and precision of the method were validated for both intra-day and inter-day analysis. The intra-day and inter-day accuracy results (RE%) for EHop-016 ranged from 0.2% to 7.1% and −2.9% to 9.5% respectively. The intra-day and inter-day precision results (RSD%) for EHop-016 ranged from 0.7% to 3.5% and 1.7% to 5.5%. (Table 2). Therefore, the validated method was both very accurate and precise.
Table 2.
Intra-day and inter-day accuracy and precision of EHop-016 in mouse plasma.
| Analyte | Spiked Level (ng/mL) |
Intra-Day (n=6) | Inter-Day (n=12) | ||
|---|---|---|---|---|---|
|
| |||||
| Accuracy (RE %) |
Precision (RSD %) |
Accuracy (RE %) |
Precision (RSD %) |
||
| 5 | 7.1 | 3.5 | 0.7 | 2.4 | |
| 10 | 1.4 | 2.0 | −2.9 | 5.5 | |
| EHop-016 | 30 | 4.0 | 1.8 | 3.1 | 1.7 |
| 75 | 0.2 | 0.7 | 2.3 | 3.4 | |
| 750 | 5.2 | 1.9 | 9.5 | 3.1 | |
| 1000 | 6.2 | 1.0 | −0.3 | 5.2 | |
3.2.4 Matrix and Recovery Effects
Serum protein including albumin, globulins, and fibrinogens are a major component of plasma. Plasma contains a mixture of dissolved proteins, amino acids, peptides, glucose, carbohydrates, vitamins, electrolytes, hormones and lipids, all of which may lead to matrix effects [9]. One cause of observed matrix effects can be due to non-specific binding of analytes to matrix components. Herein, the observed matrix effect was tolerable, i.e. ~ 75%. Importantly, this matrix effect was consistent across the entire dynamic range of the method. In addition, the observed recovery of EHop-016 after extraction was ~ 65% (Table 3).
Table 3.
Evaluation of matrix and recovery effects by comparing the areas of neat, unextracted, and extracted samples for solvent and matrix-matched calibrants. Matrix % = Unextracted peak area/Neat peak area x 100, Recovery % = Extracted peak area/Unextracted peak area x 100.
| Spiked Level (ng/mL) |
5 | 250 | 1000 | CV% |
|---|---|---|---|---|
| Neat | 1442 | 70109 | 251190 | -- |
|
| ||||
| Unextracted | 1129 | 53640 | 196246 | -- |
|
| ||||
| Extracted | 734 | 33490 | 131528 | -- |
|
| ||||
| Matrix (%) | 78 | 77 | 78 | 0.7 |
|
| ||||
| Recovery (%) | 65 | 62 | 67 | 3.9 |
3.2.5 Stability
The stability of EHop-016 in mouse plasma was investigated under several conditions in this study (Table 4). Results show that EHop-016 was stable after incubation at ambient temperature for both five hoursand 237 hours. EHop-016 was stable after 12 freeze-thaw cycles lasting 12 hours and after six freeze-thaw cycles lasting 24 hours. Long-term stability at –20°C showed that EHop-016 was also stable after 26 days and 111 days. These combined results support the robust stability of EHop-016.
Table 4.
Stability of EHop-016 in mouse plasma (n = 6)
| Conditions | Concentration (ng / mL) |
Accuracy (% RE) |
Precision (% RSD) |
|
|---|---|---|---|---|
| Nominal | Measured (Mean ± S.D.) |
|||
| Benchtop (5 Hr) | 5 | 4.67 ± 0.36 | −6.6 | 7.8 |
| 1000 | 999.39 ± 17.23 | −0.1 | 1.7 | |
|
| ||||
| Post Preparation (237 Hours) |
10 | 10.79 ± 0.25 | 7.88 | 2.30 |
| 30 | 32.20 ± 1.40 | 7.32 | 4.36 | |
| 75 | 83.79 ± 2.06 | 11.72 | 2.45 | |
| 750 | 838.83 ± 22.57 | 11.84 | 2.69 | |
|
| ||||
| Freeze-Thaw 12 Cycles (12 Hr) |
5 | 5.57 ± 0.05 | 11.3 | 0.9 |
|
1000 |
1127.70 ± 14.36 |
12.8 |
1.3 |
|
|
| ||||
| Freeze-Thaw 6 Cycles (24 Hr) |
5 | 5.71 ± 0.34 | 14.3 | 5.9 |
|
1000 |
1116.32 ± 27.38 |
11.6 |
2.5 |
|
|
| ||||
| Long Term Stability 26 Days |
5 | 5.24 ± 0.08 | 4.9 | 1.4 |
| 1000 | 1004.39 ± 25.98 | 0.4 | 2.6 | |
|
| ||||
| Long Term Stability 111 Days |
5 | 4.94 ± 0.29 | −1.3 | 5.8 |
| 1000 | 956.77 ± 79.26 | −4.3 | 8.3 | |
3.3. Pharmacokinetics and Oral Bioavailability in Nude Mice
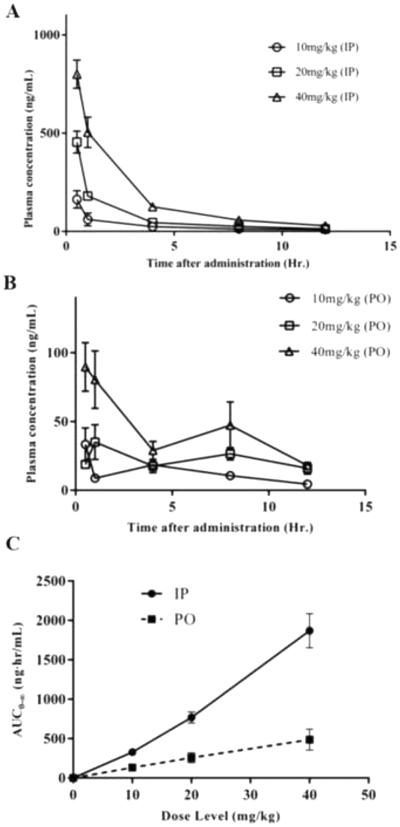
We applied this developed and fully validated bioanalytical method to investigate the pharmacokinetics of EHop-016 in nude mice following intraperitoneal (IP) and oral (PO) administration of EHop-016 (10 mg/kg, 20 mg/kg, and 40 mg/kg BW). As shown in Figure 2A, the Cmax plasma drug concentrations ranged from 163 – 835 ng/mL after IP administration of the three tested doses. As expected, the plasma drug concentrations were lower (~10 times) after PO administration as seen in Figure 2B compared to the corresponding dosages given by IP administrations. Here, the Cmax plasma drug concentrations ranged from 26 – 90 ng/mL after PO administration of the three tested doses. Pharmacokinetic parameters corresponding to each average disposition profile are presented in Table 5. Due to the large fluctuations seen in the measured plasma concentrations for the 20 mg/kg oral dose, modeling analysis for this dose was not performed. However, typical metrics directly computed from raw data in the plasma disposition profile of this dose (i.e., Cmax, tmax and AUC0-t) were determined without modeling. Systemic drug exposures, as measured by the AUC0-t values, ranged from 328 – 1870 ng·hr/mL for IP administrations and from 134 – 487 ng·hr/mL for PO administrations, respectively. As expected, the pharmacokinetics of EHop-016 for both IP and PO administration were linear as shown by the AUC0-t versus dose proportionality relationships (R2 > 0.95) seen in Figure 2C. The AUC0-t/dose normalizations as seen in Table 5 were not significantly different for either IP (p = 0.1261) or PO (p = 0.9545) further supporting the linear kinetics. The estimated elimination half-life parameters (t½) ranged from 5.73 – 4.15 hours after IP administration. The half-lives of the drug for PO administration were very similar to those for the IP doses and ranged from 3.44 - 5.87 hours. The volumes of distribution (Vz/F) of EHop-016 ranged from 2.2 – 4.1 L (IP) and 6.5 – 10.6 L (PO). The clearances (Cl/F) of EHop-016 ranged from 394 - 494 mL/hr after IP administration and from 1251 - 1315 mL/hr after PO administration. The clearance values were not significantly different for IP administration (p = 0.5870). The mean residence time (MRT) values ranged from 4.21 – 6.75 hours after IP administration. And, the MRT values ranged from 6.59 – 8.76 hours after oral administration. The relative bioavailabilities (Frel., PO to IP) were estimated to be about 40% (10 mg/kg BW), 33% (20 mg/kg BW) and 26% (40 mg/kg BW).
Figure 2.
Blood plasma concentration–time profile of EHop-016 in nude mice following (A) intraperitoneal (IP) injection or (B) per oral (PO) administration. (C) Pharmacokinetic behavior of EHop-016 after intraperitoneal (IP) and oral gavage (PO) administration at specified doses. All data are expressed as the mean ± S.E.M. where n = 5 (IP) and n = 4 (PO).
Table 5.
Pharmacokinetic parameters following intraperitoneal (IP) and oral gavage (PO) administration of EHop-016 (Mean ± S.E.M). For all ANOVA tests performed, p values < 0.05 were considered significant.
| IP (10 mg/kg), n=5 |
IP (20 mg/kg), n=5 |
IP (40 mg/kg), n=5 |
PO (10 mg/kg), n=4 |
PO (20 mg/kg), n=4 |
PO (40 mg/kg), n=4 |
|
|---|---|---|---|---|---|---|
| AUC0-t
(ng·hr/mL) |
328.04 ± 42.1 | 768.4 ± 70.6 | 1869.8 ± 216.9 | 133.7 ± 22.5 | 257.03 ± 62.3 | 486.8 ± 116.1 |
| AUC0-t/Dose | 32.80 ± 4.21a | 38.42 ± 3.53a | 46.75 ± 5.42a | 13.37 ± 2.25* | 12.85 ± 3.11* | 12.17 ± 2.9* |
| Tmax (hr) |
0.5 | 0.5 | 0.5 | 0.5 | 1.0 | 0.5 |
| Cmax (ng/mL) |
163.3 ± 17.7 | 454.8 ± 50.3 | 834.5 ± 88.9 | 26.1 ± 10.1 | 35.1 ± 11.0 | 89.7 ± 17.6 |
| Lambda-z (hr −1) | .12 ± 0.01 | .15 ± .01 | .18 ± .01 | .20 ± .01 | NC† | .12 ± .001 |
| t1/2 (hr) | 5.73 ± 0.43 | 4.66 ± .35 | 3.79 ± .12 | 3.44 ± .15 | NC† | 5.87 ± .06 |
| Vz/F (mL) | 4088.3 ± 297.2 | 3123.2 ± 92.7 | 2157.5 ± 337.1 | 6533.4 ± 1784.9 | NC† | 10595.0 ± 3386.7 |
| Cl/F (mL/hr) | 494.2 ± 79.4§ | 464.7 ± 51.5§ | 394.2 ± 46.6§ | 1315.3 ± 281.7 | NC† | 1251.4 ± 388.1 |
| MRT INF (hr) | 6.75 ± .27 | 4.73 ± .27 | 4.21 ± .14 | 6.59 ± .37 | NC† | 8.76 ± .43 |
| Frel(%) | 40.8 ± 1.1 | 33.4 ± 4.9 | 26.0 ± 3.1 |
Statistically insignificant (p = 0.1261).
Statistically insignificant (p = 0.9545).
Statistically insignificant (p = 0.5870). Pharmacokinetic parameters were calculated by non-compartmental modeling using WinNonlin.
NC = Not calculated. These PK parameters could not be accurately calculated from the concentration-time profile which was measured.
4. Discussion
The importance of the Rho GTPase Rac mediated cell signaling in tumor progression and metastasis is well documented [10-15]. While several Rac inhibitors have been characterized, none have been developed for clinical applications [16-19]. We have reported that Ehop-016 inhibits Rac activity in metastatic cancer cells with a lower IC50 than other Rac inhibitors characterized so far [1]. Moreover, we recently tested the pharmacological effects of the Rac inhibitor EHop-016 in an experimental model of metastasis in nude mice and showed that a dose of 25 mg/kg BW was effective at reducing tumor growth, metastasis, and angiogenesis [2].
The present study was aimed to characterize the pharmacokinetic profiles of EHop-016 after IP and PO administration in healthy mice to establish future dosing regimens and to further understand the pharmacology of the compound. In order to pursue this objective, we developed and validated the first ever-reported method for the detection of EHop-016 in mouse plasma by UPLC coupled to tandem mass spectrometry. EHop-016 ionized in the positive ionization mode, and did not ionize in the negative ionization mode. This ionization pattern was likely due to protonation of its basic amino functional groups. We chose to use acetonitrile as the extracting agent because it resulted in the maximum ionization of EHop-016. Interestingly, we observed a significant decrease in ionization when EHop-016 was extracted in methanol and 0.1% formic acid. This decrease could be due to selective binding between a matrix protein(s) and a product formed from the interaction between formic acid and EHop-016. This method was developed to be highly sensitive, reaching a lower limit of detection of 5 ng/mL. In addition, the quantitative linear dynamic range of the developed method, 5 – 1000 ng/mL, was greater than two orders of magnitude. Furthermore, EHop-016 was found to be very stable at all of the conditions tested in our validation. In general, most small molecule drug compounds show greater stability compared to biological therapeutics [20-22]. The high sensitivity and the dynamic linear range of the method in combination with the stability of EHop-016 were adequate for using the method to study the pharmacokinetics of EHop-016 in mice.
This study presents the first analysis of the pharmacokinetics of the Rac inhibitor EHop-016 in mice. Both routes of administration analyzed, IP and PO, present very typical trajectories following rapid absorption inputs (Figure 2A and 2B). After IP administration, a bi-phasic decay pattern is shown, with the faster, initial portion of the curves accounting for most of the observed variability in the kinetic profiles (Figure 2A). Similar linear kinetic patterns are observed across the entire range of the three IP doses, as judged by the shape and slopes of the curves. These profiles show a sharp decline after one hour post-dose, without any plateau being observed, and the plasma samples collected 12 hours post-dose showed very low drug concentration values, suggesting that no detectable levels of EHop-016 will be measured beyond this time-point. The estimated elimination half-life values, as well as the mean residence times (MRT), confirm the rapid elimination of this drug from the plasma of the mouse.
Oral administration of EHop-016 resulted in larger fluctuations in the corresponding plasma concentrations (Figure 2B). This trend could be a consequence of variability in absorption from the gastrointestinal systems between animals. The increase and decrease in plasma concentrations was especially significant in the oral 20 mg/kg, and for this reason, non-compartmental analysis was only performed for the 10 mg/kg and 40 mg/kg oral doses. The absorption rates, lambda-z, were very similar for both IP and oral administrations. The time to peak, Tmax, values were the same for both IP and oral doses, except for the 20 mg/kg oral dose. All doses reached a maximum concentration (Cmax) after 0.5 hr whereas the 20 mg/kg dose demonstrated a Tmax of 1.0 hour. The larger AUC0-t values observed after the IP doses represent an increase of 3-4 times in the extent of absorption by IP administrations. Since AUC0-t is an approximate measure of the overall drug exposure in mouse, the greater AUC0-t values in the IP doses suggest a higher bioavailability by this ROA as confirmed by the Frel (%) values in Table 5.
The rapid absorption rate of EHop-016 by both IP and PO administration is comparable to reports for other anti-cancer small molecule drugs in mouse models. For example, a similar time-to-peak (Tmax) of 0.5 hr was reported after PO administration of 10 mg/kg of lapatinib in male mice. In addition, the extent of systemic bioavailability (F) after 10 mg/kg of oral input were very similar for lapatinib and EHop-016, although higher for lapatinib (F = 43 – 50% for lapatinib versus 40% for EHop-016) [23]. According to the schedule-dependence hypothesis, it has been speculated that anti-tumoral drug effects are more favorably influenced by the duration of drug exposure than by peak levels [24]. Consequently, the PK characteristics of this drug suggest a better drug exposure after IP administrations (AUC0-t) with a larger duration of systemic exposure.
The calculated systemic clearance values (Cl/F), 394.2 – 494.2 mL/hr, following IP doses, show rapid terminal disposition behaviors at the elimination phase of this drug indicating removal by mostly single-pass extraction or filtration. These estimated F-adjusted clearance values surpassed the expected glomerular filtration rate (GFR) in mice of 18 mL/hr, as well as the renal plasma flow (RPF), 75mL/hr [25,26]. Accordingly, we speculate more than one mechanism besides renal excretion is involved in the elimination of the drug from the plasma (e.g. a drug metabolic pathway). Future urine analysis will clarify if EHop-016 was eliminated rapidly by excretion only. The estimated Cl/F values of 1315.3 mL/hr and 1251.4 mL/hr, after oral administration of 10 mg/kg and 40 mg/kg respectively, were faster. Nonetheless, since the calculation of clearance in NCA is dependent upon the AUC, it is possible that the higher Cl/F estimations in the oral gavage inputs are in part a consequence of lower AUC due to poor bioavailability.
The estimated volumes of distribution (Vz/F) ranged from 2.2 – 4.1 L for IP doses, and 6.5 – 10.6 L for PO doses, respectively. In comparison with the blood volume of approximately 1.5 – 2.0 mL in a mouse (BW, 20-25 g), these remarkably large “apparent” volumes of distribution suggest either a significantly high extent of drug distribution from plasma into the innermost peripheral tissues or a considerable binding to tissue proteins. The rapid decrease in detection after 30 minutes may reflect significant uptake of EHop-016 into peripheral tissues (i.e., blood-tissue partitioning). EHop-016 is a highly lipid-soluble drug, with a pKa of 5.0, supporting its rapid diffusion through passive mechanisms. However, poor bioavailability and rapid clearance values (Vz is strongly influenced by Cl) could be in part responsible for the excessively large Vz estimates. As a matter of fact, the estimated Vz/F values are larger than actual volumes due to the bioavailability, F<1, after any extravascular administration (IP or PO). The Vss of this drug after IP doses was only 1.3 L (±0.25 SEM; 1.94% CV) to 1.8 L (± 0.30 SEM; 16% CV). These Vss values of approximately 65 to 72 L/kg BW suggest that less than 1% of the drug is in the bloodstream.
5. Conclusion
In conclusion, the Rac inhibitor EHop-016 shows promising pharmacological activity in a mouse model of experimental metastasis. Understanding the pharmacokinetics of Ehop-016 in mice provides valuable information for further developing EHop-016 as an anti-cancer therapeutic. Based on results from this study describing a PK analysis using non-compartmental modeling with the necessary dose proportionality (linearity) assessments, we can conclude that no saturable or capacity-limited (dose-dependent) processes account for the observed systemic disposition behavior of EHop-016, in the mouse model, for the selected dosage range (10 - 40 mg/kg BW). In summary, this study supports the continued development of the Rac inhibitor EHop-016 as a potential anti-cancer therapeutic.
Supplementary Material
Highlights.
A rapid and sensitive method was validated for detection of EHop-016 in mouse plasma by UPLC/MS/MS.
EHop-016 pharmacokinetics in mice was quantified using a dosing scheme of 10, 20, and 40 mg/kg BW.
EHop-016 is bioavailable after intraperitoneal and oral administration.
Acknowledgements
We would like to thank Dr. Valance Washington for providing us with mouse plasma and Dr. Joseph Bloom for providing valuable technical feedback in the bioanalytical method development. We also acknowledge the statistical advice from the Research Centers in Minority Institutions (RCMI) Translational Research Network (RTRN) Data Coordinating Center (DCC).
Grant numbers: This study was supported by National Institute on Minority Health and Health Disparities of the National Institutes of Health (NIMHHD/NIH) U54MD008149 to SD; Title V PPOHA P031M10505 and Title V Cooperative P031S130068 from U.S. Department of Education to UCC; and UPR RCM NIH/NIMHHD grants 5U54CA096297 and R25GM061838 to THB.
List of Abbreviations
- IP
Intraperitoneal
- ROA
Route of administration
- AUC
Area under the curve
- BW
Bodyweight
- Vz
Volume of distribution
- ACN
Acetonitrile
- MeOH
Methanol
- UPLC/MS/MS
Ultra Performance Tandem Mass Spectrometry
- DMSO
Dimethyl Sulfoxide
- CAV
Cell accelerator voltage
- EMV
Electron multiplier voltage
- PO
Per oral
- MRT
Mean residence time
- Frel
Relative bioavailability
- t1/2
Half-life
- Cmax
Maximum concentration
- PK
Pharmacokinetics
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Montalvo-Ortiz BL, Castillo-Pichardo L, Hernandez E, Humphries-Bickley T, De LM-P, Cubano LA, et al. Characterization of EHop-016, a novel small molecule inhibitor of Rac GTPase. J.Biol.Chem. 2012 doi: 10.1074/jbc.M111.334524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Castillo-Pichardo L, Humphries-Bickley T, De La Parra C, Forestier I, Martinez-Ferrer M, Hernandez E, Vlaar C, Ferrer Y, Washington AV, Cubano L, Rodriguez-Orengo J, Dharmwardhane S. The Rac Inhibitor EHop-016 Inhibits Mammary Tumor Growth and Metastasis in a Nude Mouse Model. Translational Oncology. 2014;7:5. doi: 10.1016/j.tranon.2014.07.004. Num. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Maes H, Van ES, Krysko DV, Vandenabeele P, Nys K, Rillaerts K, Garg AD, Verfaillie T, Agostinis P. BNIP3 supports melanoma cell migration and vasculogenic mimicry by orchestrating the actin cytoskeleton. Cell Death. Dis. 2014;5:e1127. doi: 10.1038/cddis.2014.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Martin H, Mali RS, Ma P, Chatterjee A, Ramdas B, Sims E, Munugalavadla V, Ghosh J, Mattingly RR, Visconte V, Tiu RV, Vlaar CP, Dharmawardhane S, Kapur R. Pak and RacGTPases promote oncogenic KIT-induced neoplasms. J Clin.Invest. 2013;123:4449–4463. doi: 10.1172/JCI67509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Manes TD, Pober JS. TCR-driven transendothelial migration of human effector memory CD4 T cells involves Vav, Rac, and myosin IIA. J Immunol. 2013;190:3079–3088. doi: 10.4049/jimmunol.1201817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Want E, Compton BJ, Hollenbeck T, Siuzdak G. The Applications of Mass Spectrometry in Pharmacokinetic Studies. Spectroscopy. 2003;17:681–691. IOS Press. [Google Scholar]
- [7].Guidance for Industry Bioanalytical Method Validation. http://www.fda.gov/downloads/ Drugs/Guidances/ucm070107.pdf.
- [8].Whitmire M, Ammerman J, de Lisio P, Killmer J, Kyle D, Mainstone E, Porter L, Zhang T. LC-MS/MS Bioanalysis Method Development, Validation, and Sample Analysis: Points to Consider When Conducting Nonclinical and Clinical Studies in Accordance with Current Regulatory Guidances. J. Anal Bioanal Tech. 2011 S4:001.doi:10.4172/2155-9872.S4-001. [Google Scholar]
- [9].Hall Terence G., IneseSmukste. Bresciano Karen R., Wang Yunxia, McKearn David, Savage Ronald E. Identifying and Overcoming Matrix Effects in Drug Discovery and Development, Tandem Mass Spectrometry - Applications and Principles, DrJeevanPrasain. 2012 ISBN: 978-953-51-0141-3, InTech, DOI: 10.5772/32108. Available from: http://www.intechopen.com/books/tandem-mass-spectrometry-applications-and- principles/identifying-and-overcoming-matrix-effects-in-drug-discovery-and-development.
- [10].Azios NG, Krishnamoorthy L, Harris M, Cubano LA, Cammer M, Dharmawardhane SF. Estrogen and resveratrol regulate Rac and Cdc42 signaling to the actin cytoskeleton of metastatic breast cancer cells. Neoplasia. 2007;9:147–158. doi: 10.1593/neo.06778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Baugher PJ, Krishnamoorthy L, Price JE, Dharmawardhane SF. Rac1 and Rac3 isoform activation is involved in the invasive and metastatic phenotype of human breast cancer cells. Breast Cancer Res. 2005;7:R965–R974. doi: 10.1186/bcr1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Chan AY, Coniglio SJ, Chuang YY, Michaelson D, Knaus UG, Philips MR, Symons M. Roles of the Rac1 and Rac3 GTPases in human tumor cell invasion. Oncogene. 2005;24:7821–7829. doi: 10.1038/sj.onc.1208909. [DOI] [PubMed] [Google Scholar]
- [13].Mira JP, Benard V, Groffen J, Sanders LC, Knaus UG. Endogenous, hyperactive Rac3 controls proliferation of breast cancer cells by a p21-activated kinase-dependent pathway. ProcNatlAcadSci U S A. 2000;97:185–189. doi: 10.1073/pnas.97.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Yoshida T, Zhang Y, Rivera Rosado LA, Chen J, Khan T, Moon SY, Zhang B. Blockade of Rac1 activity induces G1 cell cycle arrest or apoptosis in breast cancer cells through downregulation of cyclin D1, survivin, and X-linked inhibitor of apoptosis protein. Mol.CancerTher. 2010;9:1657–1668. doi: 10.1158/1535-7163.MCT-09-0906. [DOI] [PubMed] [Google Scholar]
- [15].Mack NA, Whalley HJ, Castillo-Lluva S, Malliri A. The diverse roles of Rac signaling in tumorigenesis. Cell Cycle. 2011 May 15;10(10):1571–81. doi: 10.4161/cc.10.10.15612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Gao Y, Dickerson JB, Guo F, Zheng J, Zheng Y. Rational design and characterization of a Rac GTPase-specific small molecule inhibitor. Proc.Natl.Acad.Sci.U.S.A. 2004;101:7618–7623. doi: 10.1073/pnas.0307512101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ferri N, Corsini A, Bottino P, Clerici F, Contini A. Virtual screening approach for the identification of new Rac1 inhibitors. J.Med.Chem. 2009;52:4087–4090. doi: 10.1021/jm8015987. [DOI] [PubMed] [Google Scholar]
- [18].Shutes A, Onesto C, Picard V, Leblond B, Schweighoffer F, Der CJ. Specificity and mechanism of action of EHT 1864, a novel small molecule inhibitor of Rac family small GTPases. J Biol.Chem. 2007;282:35666–35678. doi: 10.1074/jbc.M703571200. [DOI] [PubMed] [Google Scholar]
- [19].Zins K, Lucas T, Reichl P, Abraham D, Aharinejad S. A Rac1/Cdc42 GTPase-specific small molecule inhibitor suppresses growth of primary human prostate cancer xenografts and prolongs survival in mice. PLoS.One. 2013;8:e74924. doi: 10.1371/journal.pone.0074924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Zhang J. Mammalian cell culture for biopharmaceutical production. In: Baltz RH, Davies JE, Demain AL, editors. Manual of Industrial Microbiology and Biotechnology. Third American Society of Microbiology; Washington, DC: 2010. pp. 157–178. [Google Scholar]
- [21].Sekhon BS, Saluja V. Biosimilars: an overview. Biosimilars. 2011;1(1):1–11. [Google Scholar]
- [22].Wurm FM. Production of recombinant protein therapeutics in cultivated mammalian cells. Nat Biotechnology. 2004;22(11):1393–1398. doi: 10.1038/nbt1026. [DOI] [PubMed] [Google Scholar]
- [23].Center for Drug Evaluation and Research Pharmacology Review(s) Application Number: 22-059. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2007/022059s000_ PharmR_P1.pdf.
- [24].Weiss AJ, Manthei R. A hypothesis concerning the relationship of cellular pharmacokinetics to optimal scheduling of anti-cancer agents. Oncology. 1988;45(6):448–52. doi: 10.1159/000226664. [DOI] [PubMed] [Google Scholar]
- [25].Qi Z, Whitt I, Mehta A, Jin J, Zhao M, Harris RC, Fogo AB, Breyer MD. Serial determination of glomerular filtration rate in conscious mice using FITC-inulin clearance. Am J Physiol Renal Physiol. 2004;286:F590–F596. doi: 10.1152/ajprenal.00324.2003. [DOI] [PubMed] [Google Scholar]
- [26].Sällström J, Fridén M. Simultaneous determination of renal plasma flow and glomerular filtration rate in conscious mice using dual bolus injection. Journal of Pharmacological and Toxicological Methods. 2013 May-Jun;67(3):187–93. doi: 10.1016/j.vascn.2013.01.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.