Abstract
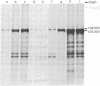
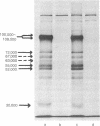
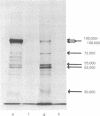
High-titer antiserum raised in rats against the tumor (T) antigen of polyoma virus was used to purify the T antigen by the Staphylococcus protein A antibody adsorbent technique. Sodium dodecyl sulfate/polyacrylamide gel electrophoresis allowed the identification of a protein with an apparent molecular weight of 100,000-108,000 as a major component induced in lytically infected mouse cells. In cells infected by ts A mutants this component was temperature sensitive. Several minor components were also observed. In pulse and chase experiments there was a slight decrease in electrophoretic mobility of T antigen during the chase period at the permissive temperature, suggesting that the T antigen is a modified protein. In two lines of transformed cells, the amount of T antigen seemed to be considerably less than in lytically infected cells, but the size of the antigen appeared to be equal.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmad-Zadeh C., Allet B., Greenblatt J., Weil R. Two forms of simian-virus-40-specific T-antigen in abortive and lytic infection. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1097–1101. doi: 10.1073/pnas.73.4.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLACK P. H., ROWE W. P., TURNER H. C., HUEBNER R. J. A SPECIFIC COMPLEMENT-FIXING ANTIGEN PRESENT IN SV40 TUMOR AND TRANSFORMED CELLS. Proc Natl Acad Sci U S A. 1963 Dec;50:1148–1156. doi: 10.1073/pnas.50.6.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin T. L. Host range mutants of polyoma virus. Proc Natl Acad Sci U S A. 1970 Sep;67(1):394–399. doi: 10.1073/pnas.67.1.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Carroll R. B., Smith A. E. Monomer molecular weight of T antigen from simian virus 40-infected and transformed cells. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2254–2258. doi: 10.1073/pnas.73.7.2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRIED M. ISOLATION OF TEMPERATURE-SENSITIVE MUTANTS OF POLYOMA VIRUS. Virology. 1965 Apr;25:669–671. doi: 10.1016/0042-6822(65)90098-x. [DOI] [PubMed] [Google Scholar]
- Fogel M., Gilden R., Defendi V. Polyoma virus-induced "complement-fixing antigen" in tumors and infected cells and detected by immunofluorescence. Proc Soc Exp Biol Med. 1967 Apr;124(4):1047–1052. doi: 10.3181/00379727-124-31920. [DOI] [PubMed] [Google Scholar]
- Francke B., Eckhart W. Polyoma gene function required for viral DNA synthesis. Virology. 1973 Sep;55(1):127–135. doi: 10.1016/s0042-6822(73)81014-1. [DOI] [PubMed] [Google Scholar]
- Fried M., Griffin B. E., Lund E., Robberson D. L. Polyoma virus--a study of wild-type, mutant and defective DNAs. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):45–52. doi: 10.1101/sqb.1974.039.01.009. [DOI] [PubMed] [Google Scholar]
- Fried M., Griffin B. E. Organization of the genomes of polyoma virus and SV40. Adv Cancer Res. 1977;24:67–113. doi: 10.1016/s0065-230x(08)61013-1. [DOI] [PubMed] [Google Scholar]
- HABEL K. SPECIFIC COMPLEMENT-FIXING ANTIGENS IN POLYOMA TUMORS AND TRANSFORMED CELLS. Virology. 1965 Jan;25:55–61. doi: 10.1016/0042-6822(65)90251-5. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Kimura G., Itagaki A., Summers J. Rat cell line 3y1 and its virogenic polyoma- and sv40- transformed derivatives. Int J Cancer. 1975 Apr 15;15(4):694–706. doi: 10.1002/ijc.2910150419. [DOI] [PubMed] [Google Scholar]
- Kuchino T., Yamaguchi N. Characterization of T antigen in cells infected with a temperature-sensitive mutant of simian virus 40. J Virol. 1975 Jun;15(6):1302–1307. doi: 10.1128/jvi.15.6.1302-1307.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lewis A. M., Jr, Rowe W. P. Studies of nondefective Adenovirus 2-Simian virus 40 hybrid viruses. 8. Association of Simian virus 40 transplantation antigen with a specific region of the early viral genome. J Virol. 1973 Oct;12(4):836–840. doi: 10.1128/jvi.12.4.836-840.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxman M. N., Takemoto K. K., Eckhart W. Polyoma T antigen synthesis by temperature-sensitive mutants of polyoma virus. Virology. 1972 Sep;49(3):675–682. doi: 10.1016/0042-6822(72)90524-7. [DOI] [PubMed] [Google Scholar]
- Paulin D., Cuzin F. Polyoma virus T antigen. I. Synthesis of modified heat-labile T angiten in cells transformed with the ts-a mutant. J Virol. 1975 Feb;15(2):393–397. doi: 10.1128/jvi.15.2.393-397.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulin D., Gaudray P., Cuzin F. Purification of polyoma T antigen from transformed cells. Biochem Biophys Res Commun. 1975 Aug 18;65(4):1418–1426. doi: 10.1016/s0006-291x(75)80387-1. [DOI] [PubMed] [Google Scholar]
- RAPP F., BUTEL J. S., FELDMAN L. A., KITAHARA T., MELNICK J. L. DIFFERENTIAL EFFECTS OF INHIBITORS ON THE STEPS LEADING TO THE FORMATION OF SV40 TUMOR AND VIRUS ANTIGENS. J Exp Med. 1965 Jun 1;121:935–944. doi: 10.1084/jem.121.6.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- TODARO G. J., GREEN H. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J Cell Biol. 1963 May;17:299–313. doi: 10.1083/jcb.17.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P. Altered patterns of protein synthesis in infection by SV40 mutants. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):9–15. doi: 10.1101/sqb.1974.039.01.004. [DOI] [PubMed] [Google Scholar]