Abstract
Synovial joint morphogenesis occurs through the condensation of mesenchymal cells into a non-cartilaginous region known as interzone, and the specification of progenitor cells that commit to the articular fate. Although several signaling molecules are expressed by the interzone, the mechanism is poorly understood. For treatments of cartilage injuries, it is critical to discover the presence of joint progenitor cells in adult tissues and their expression gene pattern. Potential stem cells niches have been found in different joint regions, such as the surface zone of articular cartilage, synovium and groove of Ranvier. Inherited joint malformation as well as joint degenerating conditions are often associated with other skeletal defects, and may be seen as the failure of morphogenic factors to establish the correct microenvironment in cartilage and bone. Therefore, exploring how joints form can help us understand how cartilage and bone are damaged and to develop drugs to reactivate this developing mechanism.
Keywords: synovial joint development, interzone, joint progenitor cells, articular cartilage, cytokines/chemokines, TGFβReceptor2, joint inherited diseases, joint acquired diseases, osteoarthritis
INTRODUCTION
Formation and positioning of synovial joints are critically important during evolution allowing successful adaptation of vertebrate limbs to a variety of ecological environments. Because of the variety of tissues comprising the synovial joint (articular cartilage, synovial fluid, ligaments, joint capsule) disease or trauma affecting one tissue has wide-ranging implications on the health and function of the joint as a whole organ (1). Inherited joint malformations, although relatively rare, present with a wide range of forms and severity and are often associated with skeletal defects, suggesting a reciprocal cause/effect relationship (2, 3). On the other hand, degenerating conditions that affect joint function, such as osteoarthritis (OA) and inflammatory rheumatoid arthritis (RA), are the single largest cause of disability in the adult population (4, 5), and while our understanding of the pathology of these diseases has greatly improved, the only successful treatment for restoring joint function in end-stage diseases is total joint replacement (6). To some extent, this lack of effective medical treatments is due to the fact that the articular cartilage is avascular, and this limits its reparative capacity (7). Although physiologically and clinically significant there is limited information on the mechanisms and signaling molecules that lead to joint morphogenesis. A greater understanding of joint formation can provide critical insight on the pathogenesis of joint degeneration and to develop novel reparative strategies to restore long-term joint function following trauma and disease.
JOINT MORPHOGENESIS
During skeletogenesis, long bone formation initiates as uninterrupted mesenchyme condensations in the early limb buds, which undergo differentiation to chondrocytes (1, 6). Joint formation becomes morphologically evident with the appearance of a region called the interzone at the sites of future joint location where the resident cells flatten and form a clear separation of the previous uninterrupted cartilaginous skeletal anlagen (8–9). In most species, the interzone is a tripartite structure consisting of two outer layers adjacent to the epiphyseal end of the future bones and an intermediate zone, that adopt a nonchondrogenic phenotype, as indicated by the loss of chondrogenic markers such as Sox-9 and Collagen 2, and the expression of new sets of genes including Gdf5, Wnt14 and Wnt4 (10–12). The pivotal role the interzone in joint formation is widely recognized, as its microsurgical removal results in joint ablation (8–9, 13). Ultrastructural analysis in developing rat embryos indicated that the outer interzone layers participate in initial lengthening of long bone anlagen, while articular chondrocytes largely derive from the intermediate layer (14). Despite wide recognition that the interzone region is essential for joint formation, there is limited information of the gene expression environment in which interzone cells emerge and distinguish themselves from adjacent growth plate chondrocytes (14–16). Numerous studies in recent years have reported several signaling molecules, growth factors, transcription factors, and other regulatory molecules expressed by the interzone: these include GDF-5, Wnt-14, Wnt-4, Wnt-16, Gli3, CD44; BMP antagonists Chordin and Noggin; fibroblast growth factor family member FGF-2, FGF-4 and FGF-13; transcription factors Cux1 and ERG (10, 12, 17–20). GDF-5 is expressed in the early interzone in mouse and chick embryo limb joints and lack of GDF-5 in the natural mouse mutant brachypodism, causes widespread joint defects and skeletal growth retardation (21). Subsequent studies established that Gdf-5-expressing cells give rise to most if not all joint tissues, including articular cartilage, ligaments and inner synovial lining (14). The current view is that GDF-5 has at least two roles in skeletogenesis. At early stages, it is expressed throughout the condensation and would stimulate recruitment and differentiation of chondrogenic cells. At later stages, when its expression becomes restricted to the interzone, GDF-5 would promote interzone cell function and joint development (22). Iwamoto et al. reported that there is a close spatiotemporal expression of GDF-5 with the transcriptional factor ERG (one of the ets gene family member) in developing mouse embryo joints which suggests that ERG acts downstream of GDF-5 leading chondrocytes to become joint forming cells (9).
Wnt14 and the BMP antagonist Noggin are anti-chondrogenic factors that are expressed early during interzone formation. Targeted misexpression of Wnt14 in developing chicken digit rays induced ectopic joints, with upregulation of Gdf5, Wnt4, CD44 and down-regulation of Col2, Sox9 (10) while ablation of Wnt-14 along with Wnt-4 impairs joint formation and causes some fusions (23). In vitro studies show that Wnt14 reversed chondrocyte differentiation in predifferentiated micromass culture, reflecting what happened in vivo(10, 24).
Experimental ablation of Noggin in mouse prevents limb joint formation and causes skeletal fusions (17). The role of Noggin is conserved between mouse and humans, and at least two human syndromes, which are characterized by multiple synostoses (absence of joints), are due to mutations in Noggin (25). The expression of another BMP antagonist, Chordin, is restricted to the joint interzone, emphasizing the role of BMP antagonism during joint development. The significant concept stemming from these studies is that joint formation involves the intervention of gene products with chondrogenic and anti-chondrogenic activities, the former critical for formation of fibro-cartilaginous joint structures and the latter for maintaining the initial mesenchymal character of cells present at joint sites (15). Thus, it is not surprising that the joint interzone appears to be also an essential regulator of skeletal development, and vice-versa (24). One example is shown by the conditional inactivation of Ext1 in developing joints in Gdf5-expressing cells. Ext1 encodes a subunit of the Ext1/Ext2 Golgi-associated protein complex responsible for heparan sulfate (HS) synthesis. Heparan sulfate (HS) is an essential component of cell surface and matrix-associated proteoglycans and mutations in HS-synthesizing and modifying enzymes cause several skeletal phenotypes, such as hereditary multiple exostoses (HME), described later in joint genetic disease chapter. Interestingly, mutation of Ext1 in Gdf5-expressing cells, led to lack of a distinct mesenchymal interzone with consequent joint fusion. They also found abnormal BMP and hedgehog activity and signaling leading to a delayed growth and lengthening of long bones indicating that defects in joint formation reverberate on, and delay, overall long bone growth (26).
Recent studies have identified an important role for HIF-1 and HIF-2 in the regulation of skeletal development as well as joint formation and homeostasis. In addition, overexpression of HIF-1 and HIF-2 has been clinically associated with osteoarthritis (27).
The critical role of Ihh and parathyroid Hormone-Related protein (PTHrP) during endochondral bone formation is well known, but also their importance in synovial joint formation (28–30) has been reported. Ihh null have mice failed joint development (28, 31, 32) and have abnormal distribution and function of Gdf5-expressing interzone-associated cells (17, 29, 31, 33). These studies suggest that in the developing diaphysis, secreted Ihh could diffuse and reach the epiphyseal ends of the long bone analgen, where it would regulate interzone formation (1). Using a PTHrP-Lac-Z knockin mouse containing a β-gal reporter under endogenous PTHrP gene regulatory sequences, Chen et al. have confirmed two distinct PTHrP β-gal positive subpopulations: in the articular cartilage and in the proliferative chondrocytes, that are maintained through adulthood (34) (35). A role for PTHrP in articular cartilage maintenance has been recently proposed by Gdf5-Cre-targeted knock-down of PTHrP in mouse articular chondrocytes that led to accelerated development of OA in a DMM model (36). In addition, systemic administration of recombinant human PTH in mice inhibited cartilage degeneration and induced cartilage regeneration following meniscal/ligamentous injury (37).
Proper spatial positioning of synovial joints is crucial in skeletogenesis. Sohaskey et al. reported that mice carrying an insertional mutation in the Jaws gene (abnormal joint with splitting) die perinatally with striking skeletal defects, including ectopic interphalangeal joints (38). These ectopic joints develop along the longitudinal axis and persist at birth, suggesting that JAWS is uniquely required for the orientation and consequent positioning of interphalangeal joints within the endochondral skeleton.
Transgenic mice overexpressing fibroblast growth factor receptors (FGFR) 1 and 3 exhibit joint defects similar to the symphalangism that occurs in Apert syndrome, a rare congenital disorder characterized by craniosynostosis, midfacial malformation and symmetrical syndactyly. In this mice some synovial joints failed to develop and the presumptive joint space was replaced by cartilage. (39)
A recent study by Gao et al. reported a critical role of Osr1 and Osr2, the mammalian homologs of the odd-skipped family of zinc finger transcription factors required for Drosophila leg joint formation, in mouse synovial joint formation. They report that Osr1 and Osr2 and are both strongly expressed in the developing synovial joint cells and are required for Gdf5, Wnt4 and Wnt14 expressions (40).
Genetic manipulation of the TGF-β system has revealed critical roles in both joint development and skeletogenesis (41–43). In transgenic mice, overexpression of a dominant negative Tgfbr2 (DNIIR) resulted in OA(40). In humans, mutations of the Tgfbr2 have been associated with Loeys-Dietz syndrome, a Marfan-like syndrome that leads early onset of OA (OMIM#609192) (44,45). In previous studies, by generating a TGF-β type II receptor (TβRII) reporter mouse (β-Gal and GFP), we showed that TβRII is highly and specifically expressed in the developing joint (43). By generating a mouse in which the TβRII signaling is conditionally inactivated in mesenchyme limb buds starting at E9.5 (Tgfbr2Prx1KO), we demonstrated that the lack of TβRII signaling led to complete absence of interphalangeal joints and was associated with down-regulation of key joint morphogenic factors (such as Noggin, GDF5 and the Notch ligand, Jagged 1). Interestingly, lack of joint was associated with up-regulation of many inflammatory cytokines/chemokines in the presumptive interzone, such as MCP-5 and Il 36 (43, 46, 47); blockade of MCP-5 receptor was able to rescue the TβRII-mediated joint deficiency (47), suggesting that cytokines/chemokines may have a regulatory role during development, mediating proper interzone formation.
There is compelling evidence that Notch signaling is critical for progenitor cell survival and for their maintenance in an undifferentiated state, which sets the boundaries that segregates two distinct cell populations during development (48–52). Notch1 is a regulator of great importance for cell fate commitment during both early growth and in adult tissues and has been shown to play a critical role in the cell fate determinant in different stem cell niche structures such as the skin, teeth and nervous system (53). Hayes et al. reported that Notch 1 is expressed in the developing and post-natal articular surface (54). The Notch family members are also expressed in adult articular cartilage (55) and over 70% of chondrocytes on the surface zone of articular cartilage express Notch 1 receptor (16), suggesting a significant role of the Notch signaling in regulating articular cartilage homeostasis during adult life (55,56)
Despite important advances on multiple fronts, there are still fundamental aspects of joint formation that remain unclear and in particular, how joints acquire their diverse morphology and organization from embryonic life to adulthood. A better understanding of genes involved in patterning processes will be critical to link embryonic development with joint disease in adult, in both inherited and acquired debilitating diseases.
PROGENITOR STEM CELL NICHE
The view of articular cartilage as a non-regenerative organ has been challenged in recent years. The superficial zone of articular cartilage demonstrates a distinct pattern regarding stem cell markers (Notch-1, Stro-1, and vascular cell adhesion-1) (57) and has been hypothesized to harbor stem cells (58). Studies regarding cartilage development have shed light on the question of whether any of the joint tissues harbors potential joint progenitor cells. Through the use of BrdU (bromo-deoxyuridine) injections to localize slow-cycling cells, a trait characteristic of progenitor cells, a few potential joint stem cell niches have been determined. A Notch-1 positive cell population has been isolated from the surface zone of articular cartilage (59); these cells possess high colony-forming efficiency that was abolished when Notch signaling was blocked (16).
Karystinou et al reported the isolation and characterization of multipotent MSCs from adult human synovium, that could be expanded for up to 30 population doubling, with limited senescence and maintenance of multilineage differentiation capacity toward chondrogenesis, osteogenesis, adipogenesis and skeletal myogenesis (60). In response to injury of various types, including trauma, the synovial membrane rapidly becomes hyperplastic, which seems to be sustained mainly by stromal synovial fibroblasts (61–63). The biologic function of synovial cell proliferation is believed to have a pivotal role in joint homeostasis and deregulation of this process is thought to contribute to the formation of pannus, which in RA causes destruction of cartilage and bone (63). Although very little is known about the identity of the synovial cells that proliferate following injury, this study provides the first evidence of the existence in vivo, within postnatal knee synovium, of non-hematopoietic, non-endothelial stromal cells with MSC phenotype (64–66) which proliferate following articular cartilage injury and, under specific experimental conditions, differentiated into chondrocytes in area of cartilage metaplasia within the synovium (67).
An area of potential interest with regard to joint progenitor cells is the perichondrial groove of Ranvier. This area is located at the periphery of the epiphyseal growth plate and has been demonstrated to contain proliferating cells (68). Studies by Karlsson et al. in the knee of sexually mature rabbits have proved the existence of different subpopulations of progenitor cells, in the articular cartilage, in the groove of Ranvier, as well as the epiphyseal plate near the groove of Ranvier; however, whereas the progenitor cells in the surface layer of the articular cartilage seem to lose their progenitor properties and become dispersed throughout the articular cartilage as they grow older, the progenitor cells in the groove of Ranvier were positive for several marker associated with stem cell niche (including Stro-1, Jagged-1 and BMPr1a) and maintain their progenitor properties and localization over time (58).
We have addressed in the previous section the critical role of the TβRII in joint-element development. Our finding that mice lacking of TβRII signaling in developing limbs failed interzone formation and lacked interphalangeal joints, led us to investigate the expression pattern of TβRII-expressing cells and their characterization as joint progenitors(69) By using a Tgfbr2-β-Gal-GFP-BAC mouse previously described, we have recently characterized the TβRII-expressing cells as joint progenitors(69), clustering in a contiguous niches that comprises the groove of Ranvier and the synovio-entheseal complex, including part of the perichondrium, the synovium, the articular cartilage superficial layer and the tendon’s enthuses(69) Developmental-stage studies showed that TβRII expression was in synchrony with expression of joint-morphogenic genes such as Noggin, Gdf-5, Notch1 and Jagged1. Pre-natal and post-natal BrdU-incorporation studies showed that within this synovio-entheseal articular cartilage niche most of the TβRII-expressing cells exhibit stem cell traits. In addition, TβRII-expressing cells isolated from embryonic limb mesenchyme expressed joint progenitor markers in a time- and TGF-β-dependent manner. TβRII-expressing cells were maintained in their specific niches from embryonic life throughout early adulthood, although characterized by a progressive age-dependent reduction in number.
The functional characterization of adult joint progenitors, together with the identification of embryonic markers that are preserved in postnatal joints, opens prospects for potential ways to reactivate the joint-forming ability of such cells, to implement their survival during aging and to evaluate their role in joint genetic disease as well as joint degenerative pathologies, such as arthritis.
JOINT GENETIC DISEASES
Genetic constitution is a factor in the development of most diseases, including those of bones and joints. Evidence for the influence of genes in most disease development is expressed in two ways. In polygenic disorders, the genetic influence is indirect, based only on the higher rate of recurrence in some families compared to the general population. The second group follows Mendelian inheritance, where a specific gene is directly responsible for the disease and the gene is passed on to subsequent generations. These diseases may also appear spontaneously as new mutations. There are over 5000 documented Mendelian disorders, and over 500 of these affect bones and joints (70). Some of these single gene disorders affect many tissues, and the skeletal system, including joints, is one of many organs involved (71), others are directly related to joint malformation. It is beyond the scope of this review to give a detailed list of all the joint genetic diseases; however, we will provide some examples in Table 1. There are very few genes that are known to be necessary and/or sufficient to initiate the joint formation process, among them Noggin, Gdf5, Tgfbr2 and Wnt14 (10,17,22,41). The factor(s) that induce the expression of these joint morphogenic molecules are undefined. Gong et al. (25) demonstrated that five dominant mutations in the Noggin gene in unrelated families segregate with proximal symphalangism (SYM1A, OMIM#185800), an autosomal dominant disorder characterized by a complete bony fusion between the proximal and middle phalanges of the digit. Another form of proximal symphalangism (SYM1B) is caused by a heterozygous missense mutation in the Gdf5 (OMIM #615298).
Table 1.
Joint Genetic Diseases
| Location | Gene locus | Inheritance | Phenotype | Clinical Synopsis (skeletal) |
|---|---|---|---|---|
| 17q22 | Noggin | Autosomal dominant | Proximal symphalangism 1A | Joint fusion Proximal interphalangeal joint synostoses Distal interphalangeal joint synostoses (occasional) Carpal and tarsal bone fusion |
| 20q11.22 | Gdf5, CDMP1, SYNS2, OS5,BDA1C, SYM1b | Autosomal dominant | Proximal symphalangism 1B | Joint fusion Distal interphalangeal joint fusions Flat feet Absence of the cuboid bone Bone fusion (proximal/middle phalanges) |
| 3p24.1 | Tgfbr2 | Loeys-Dietz syndrome type 2 (formerly, Marfan syndrome type 2) | Joint laxity Osteoporosis (some patients) Low-impact fractures Arachnodactyly Camptodactyly Brachydactyly Contractures |
|
| 1p13-p11 | Notch2 | Autosomal dominant | Hajdu-Cheney syndrome | Joint laxity Short stature Osteopenia Osteoporosis Pathologic fractures Acroosteolysis |
| 8q12.1 | Jaws/Impad1 | Autosomal recessive | Chondrodysplasia with joint dislocations, GRAPP type | Joint dislocations Shortening and deformity of the limbs Brachydactyly Longitudinal splitting of the proximal phalanx of forefinger Splitting of the first metacarpal in two parts Short stature |
| 8q24.11 | Ext1 | Autosomal dominant | Multiple cartilaginous exostoses, type 1 | Cartilaginous protuberances at ends of long bones Short metacarpal Exostoses in juxtaepiphyseal regions of long bones Genu valgum Madelung-like forearm deformities Bilateral overriding of single toes |
| 3q13.33 | Hgd | Autosomal recessive | Alkaptonuria (AKU) | Ochronotic pigmentation of connective tissue Ochronotic arthritis Ochronotic arthropathy Chronic joint pain Degeneration of intervertebral disks Fusion of vertebral bodies |
We reported that TβRII functions as a master regulator for the expression of key joint marker genes, such as Gdf5 and Noggin, and is necessary for proper joint development (43). The joint phenotype observed in mice lacking the Tgfbr2 gene in limbs is similar to that in patients with SYM1. In humans, heterozygous mutations of the Tgfbr2 gene have been associated with Loeys-Dietz syndrome (OMIM #610168), a Marfan-like syndrome characterized by several defects including joint hypermotility and/or contractures that lead to early onset of OA (44,45). It has been hypothesized that in Loeys-Dietz syndrome, an excess of TGF-β signaling accounts for the pathological manifestations (72).
Several lines of evidence have demonstrated the importance of Notch signaling in joint morphogenesis and genetic inactivation of the Notch signaling in Drosophila, resulting in loss of joints (73). A derangement of Notch signaling has been reported in articular synoviocytes from patients with RA, characterized by aberrant synoviocyte proliferation (74). Heterozygous mutation in the Notch2 gene causes the Hajdu-Cheney syndrome (HJCYS, OMIM #102500), a rare autosomal dominant disorder that, in addition to severe skeletal abnormalities including acroosteolysis, is characterized by joint laxity. Mutations and deletions in Jagged-1 (Notch ligand) have been found in patients with Alagille syndrome characterized by intrahepatic cholestasis, eye and cardiac abnormalities, as well as skeletal defects in particular in the fingers (75, 76).
Mouse studies have indicated that embryonic long bone development is altered by mutations in Ext genes members (Ext1, Ext2 and Ext3) of the exostosin protein family and responsible for the synthesis of heparan sulphate (HS)(77). Recent data have shown that loss of Ext-1 expression postnatally is not only affecting the growth and organization of long bones, but is incompatible with normal joint structure, resembling human OA (78, 79). In humans, HS deficiency due to mutation in the genes encoding exostosin-1, -2, -3, causes multiple hereditary exostoses (EXT, OMIM #133700), an autosomal dominant disorder characterized by multiple projections of bone capped by cartilage, most numerous in the metaphyses of long bones, but also occurring on the diaphyses of long bones. Deformity of the legs, forearms (resembling Madelung deformity), and hands is frequent (80).
Mutations in the Jaws gene (joints abnormal with splitting), also known as Impad1 (inositol monophosphatase domain-containing protein 1) cause the GPAPP type of chondrodysplasia with joint contractures, an autosomal recessive disorder characterized by short stature, chondrodysplasia with brachydactyly, congenital joint dislocations, micrognathia, cleft palate, and facial dysmorphism (OMIM #614078).
Alkaptonuria (AKU, OMIM #203500) is an autosomal recessive metabolic disorder caused by homozygous or compound heterozygous mutations in the homogentisate 1,2-dioxygenase gene (HGD) and characterized by accumulation of homogentisic acid, leading to darkened urine, ochronotic pigmentation of fibrous tissues including cartilage, tendons, ligaments, chronic joint pain, and destruction of the cardiac valves. Ultimately, patients develop severe secondary OA.
Current knowledge of joint biology and pathologies has not been translated, however, into effective therapies to treat joint conditions (103, 104). Knowing how joints form during fetal life, express certain genes that become established in early post-natal life and are maintained in adult life would shed light on the homeostasis of the whole joint and could lead to novel treatment for many joint disorders.
CAN WE UNDERSTAND BETTER OA FROM JOINT DEVELOPMENT?
OA is a complex disease caused by the interaction of multiple genetic and environmental factors and the major cause of disability in the adult population. Although articular cartilage degeneration is the primary concern in OA, it is now the prevailing paradigm that OA is a disease of the entire joint and homeostasis and integrity of articular cartilage depends also on the biochemical interplay with subchondral bone; thus, it not surprising that mutation in genes that are needed during skeletogenesis may lead to OA later in life.
We have addressed the critical role of the TGF-β system in both joint development and skeletogenesis (41–43). The scope of this section is to provide an example of how genes that are so critical in joint morphogenesis may also have key roles in joint degenerating disease, such as OA. TGF-βs bind to TGF-β type II receptor (TβRII) leading to TβRII-TβRI complex formation, which then activates the signaling cascade through R-Smad dependent (Smad-2, -3,-4) and Smad-independent pathways (81, 82).
In human and mouse embryonic cartilage, TGF-βs are expressed in the endochondral template (83–86). Using laser capturing microdissection (LCM) we have obtained RNA samples from E13.5 progenitor joint-forming interzone cells and from the adjacent growth plate chondrocytes. Each population-related mRNA was subjected to microarray analysis. We have found that Tgfbr2 expression was 12.3 times higher, while Tgfbr1 and Tgfbr3 were slightly lower in the interzone cells. In mice, targeted manipulation of the TGF-β system genes have revealed their critical, but still undefined, function in skeletogenesis (41, 42, 87, 88). R-Smad gene targeting in mice has led to variable phenotypes ranging from early lethality (Smad2 and Smad4 ablation) to a normal phenotype at birth with progressive OA in adulthood (Smad3 ablation) (89–92). In transgenic mice, overexpression of a dominant negative Tgfbr2 (DNIIR) can result in OA in adults (42); in chick embryos, however, implanted beads releasing TGF-β induce extra digit formation (93). In humans, mutations of the Tgfbr2 have been associated with Loeys-Dietz syndrome (44, 45, 72).
In previous studies, we demonstrated that lack of TβRII signaling in developing limbs was associated with complete absence of interphalangeal joints as well as with impaired growth plate development (43).
By combining LCM with microarray analysis we were also able to show that interzone cells express a gene pattern that is distinct from adjacent growth plate chondrocytes and such gene regulation is impaired in mutant limbs lacking TβRII. We performed a pathway analysis using the PANTHER (protein analysis through evolutionary relationships) Classification System (Celera) to classify genes into canonical pathways. This enabled us to identify functional categories in the joint-forming interzone cells of wild type mice that were different from mutants. We determined that in the interzone cells the “inflammation mediated by chemokines and cytokines” was the most down-regulated pathway in the wild type interzone compared to mutant. Specifically, we found that monocyte chemoattractant protein-5 (MCP-5) and Interleukin 36-α (IL-36α) were among the most down-regulated genes in this pathway (46, 47). It has been reported that MCP5, MCP-1 and their sole common receptor CCR2, as well as IL-36α are increased in the inflamed joints of patients with different forms of arthritis and in rodent models for arthritis (94–97). While the role of inflammatory cytokines in the arthritic process appears indisputable, their role in joint development has never been evaluated. A finding of cytokine involvement in joint formation would determine a shift in our current view of joint cells as passive victims of the disruptive force of cytokines but as active participants in maintaining control of the cytokine effects and therefore joint integrity. It has been shown that in healthy cartilage, a balance between cytokines and anabolic growth factors is critical to maintain proper tissue homeostasis (98, 99). Supported by our previously published data and other current evidence, in Figure 1 we propose a model for the role of TβRII signaling in regulating cell commitment to joint-forming cells or chondrocytes within the joints (47). Specifically, TβRII signaling, by blocking critical inflammatory cytokines expression in the interzone, halts Collagen 2 expression and induces interzone specific markers (i.e. Gdf5, Noggin). In mutant mice, lack of the TβRII expression in developing limbs, leads to disregulated levels of cytokine/chemokines in the interzone (such as MCP-5 and IL-36α) which, in turn, block expression of interzone specific markers while increasing Collagen 2-expressing cells, with consequent accumulation of chondrocytes within the joint region and impaired of joint formation.
Figure 1. A model for the TβRII-mediated regulation of joint development.
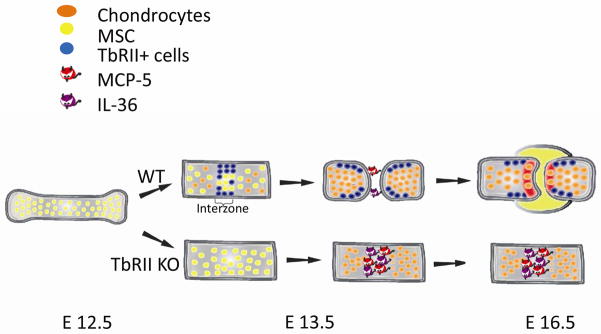
TβRII down-regulates MCP-5/IL-36α expressions in the interzone, allowing expression of key joint markers (such as Gdf5) while inhibiting Collagen2 expression. Lack of the TβRII signaling leads to up-regulation of interzone MCP-5/IL-36α with consequent lack of joint formation.
The activation of the inflammatory cytokine/chemokine cascade is associated with the more common forms of arthritis (100). The generally accepted hypothesis is that during the OA/RA process, the increased levels of cytokines and chemokines are the last step of a long lasting damage process that sees the joint as a passive target. To determine the role of TβRII signaling in joint homeostasis we generate a genetically modified mouse to tamoxifen induces, a Cre-conditional inactivation of floxed Tbfbr2 in osteochondro progenitors starting at postnatal day 3 of life (46). We found that postnatal TβRII signaling inactivation was associated with high expression of IL36α and MCP-5 (unpublished data) in the articular cartilage and synovium and progressive OA development(46). Blockage of IL36α signaling led to rescue of the OA phenotype(46). In a mouse model of post-traumatic OA, namely caused by a destabilization of the medial meniscus (DMM) surgically-induced after medial meniscotibial ligament transection, we found that an intense early cellular response of TβRII positive cells that gradually decreased with OA progression and was associated with an increase of MCP-5 (101). Blockage of the MCP-5 signaling as well as implant of TβRII positive cells improved OA lesions and decreased subchondral bone reaction (101, 102). These findings suggest that TβRII signaling and cellular response is critical in joint development and it is an active mechanism to preserve joint homeostasis in adulthood. In this respect we provide a novel perspective to evaluate the OA process that results from the failure of an active cell joint population, expressing TβRII, to maintain a controlled cytokine environment rather than from the damage induced by a systemic influx of inflammatory cells and cytokines against a passive target (the joint).
CONCLUSIONS
Joint biology has been the subject of extensive research activity, and much has been learned about the structure and composition of articular cartilage, ligaments, synovium, and joint capsule, and the specific roles each of these tissues plays in joint function in developing and adult organisms Much is also known about susceptibility of joint tissues to damage and malfunction during natural aging and congenital or acquired skeletal conditions (20). Diseases that target and disrupt structure and function in the articular cartilage, synovium, meniscus and subchondral bone result in painful, debilitating and costly conditions for patients and society as a whole. The articular cartilage is avascular, and this presents limitations on its reparative capacity and for drug delivery. Joint homeostasis is maintained preserving the articular cells to proceed down the differentiation pathway that leads to chondrocyte hypertrophy and bone replacement such as it occurs in the growth plate cartilage. Many joint degenerating conditions may be seen as the failure of morphogenic factors to establish the correct microenvironment in cartilage and bone. Therefore, exploring how joints form can lead us to understand how cartilage and bone are damaged and develop drugs that are able to reactivate this developing mechanism. This information would have major biomedical relevance, as it could lead to novel repair strategies to restore joint function following trauma, as well as providing insight on the pathogenesis of acquired and inherited joint diseases.
Acknowledgments
This work was supported by a NIH-NIAMS Grant 1R01AR057042-03 (to A.S.), NC-TraCS Institute (NIH-CTSA) (to L.L.), NIH-NIAMS Grant 1R03AR063232-01(to L.L.) and Arthritis National Research Foundation (to L.L.).
Footnotes
Conflict of Interest
L Longobardi, T Li, L Tagliafierro, JD Temple, HH Willcockson, P Ye, A Esposito, F Xu, and A Spagnoli all declare no conflicts of interest.
Human and Animal Rights and Informed Consent
All studies by the authors involving animal and/or human subjects were performed after approval by the appropriate institutional review boards. When required, written informed consent was obtained from all participants.
References
Papers of particular interest, published recently, have been highlighted as:
*Of importance
- 1.Pacifici M, Koyama E, Iwamoto M. Mechanisms of synovial joint and articular cartilage formation: recent advances, but many lingering mysteries. Birth Defects Res C Embryo Today. 2005 Sep;75(3):237–48. doi: 10.1002/bdrc.20050. [Research Support, N.I.H., Extramural Research Support, U.S. Gov’t, P.H.S. Review] [DOI] [PubMed] [Google Scholar]
- 2.Ikegawa S. Genetic analysis of skeletal dysplasia: recent advances and perspectives in the post-genome-sequence era. J Hum Genet. 2006;51(7):581–6. doi: 10.1007/s10038-006-0401-x. [DOI] [PubMed] [Google Scholar]
- 3.Pauli RM. The natural histories of bone dysplasias in adults--vignettes, fables and just-so stories. Am J Med Genet C Semin Med Genet. 2007 Aug 15;145C(3):309–21. doi: 10.1002/ajmg.c.30135. [DOI] [PubMed] [Google Scholar]
- 4.Goldring MB, Goldring SR. Osteoarthritis. J Cell Physiol. 2007 Dec;213(3):626–34. doi: 10.1002/jcp.21258. [DOI] [PubMed] [Google Scholar]
- 5.Hunter DJ. In the clinic Osteoarthritis. Ann Intern Med. 2007 Aug 7;147(3):ITC8-1–ITC8-16. doi: 10.7326/0003-4819-147-3-200708070-01008. [DOI] [PubMed] [Google Scholar]
- 6.Khan IM, Redman SN, Williams R, Dowthwaite GP, Oldfield SF, Archer CW. The development of synovial joints. Curr Top Dev Biol. 2007;79:1–36. doi: 10.1016/S0070-2153(06)79001-9. [Review] [DOI] [PubMed] [Google Scholar]
- 7.Redman SN, Oldfield SF, Archer CW. Current strategies for articular cartilage repair. Eur Cell Mater. 2005;9:23–32. doi: 10.22203/ecm.v009a04. discussion 23–32. [DOI] [PubMed] [Google Scholar]
- 8.Hyde G, Dover S, Aszodi A, Wallis GA, Boot-Handford RP. Lineage tracing using matrilin-1 gene expression reveals that articular chondrocytes exist as the joint interzone forms. Dev Biol. 2007 Apr 15;304(2):825–33. doi: 10.1016/j.ydbio.2007.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iwamoto M, Tamamura Y, Koyama E, Komori T, Takeshita N, Williams JA, et al. Transcription factor ERG and joint and articular cartilage formation during mouse limb and spine skeletogenesis. Dev Biol. 2007 May 1;305(1):40–51. doi: 10.1016/j.ydbio.2007.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hartmann C, Tabin CJ. Wnt-14 plays a pivotal role in inducing synovial joint formation in the developing appendicular skeleton. Cell. 2001 Feb 9;104(3):341–51. doi: 10.1016/s0092-8674(01)00222-7. [DOI] [PubMed] [Google Scholar]
- 11.Pacifici M, Koyama E, Shibukawa Y, Wu C, Tamamura Y, Enomoto-Iwamoto M, et al. Cellular and molecular mechanisms of synovial joint and articular cartilage formation. Ann N Y Acad Sci. 2006 Apr;1068:74–86. doi: 10.1196/annals.1346.010. [Research Support, N.I.H., Extramural Review] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Storm EE, Huynh TV, Copeland NG, Jenkins NA, Kingsley DM, Lee SJ. Limb alterations in brachypodism mice due to mutations in a new member of the TGF beta-superfamily. Nature. 1994 Apr 14;368(6472):639–43. doi: 10.1038/368639a0. [DOI] [PubMed] [Google Scholar]
- 13.Holder N. An experimental investigation into the early development of the chick elbow joint. J Embryol Exp Morphol. 1977 Jun;39:115–27. [PubMed] [Google Scholar]
- 14.Koyama E, Shibukawa Y, Nagayama M, Sugito H, Young B, Yuasa T, et al. A distinct cohort of progenitor cells participates in synovial joint and articular cartilage formation during mouse limb skeletogenesis. Dev Biol. 2008 Apr 1;316(1):62–73. doi: 10.1016/j.ydbio.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koyama E, Ochiai T, Rountree RB, Kingsley DM, Enomoto-Iwamoto M, Iwamoto M, et al. Synovial joint formation during mouse limb skeletogenesis: roles of Indian hedgehog signaling. Annals of the New York Academy of Sciences. 2007 Nov;1116:100–12. doi: 10.1196/annals.1402.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dowthwaite GP, Bishop JC, Redman SN, Khan IM, Rooney P, Evans DJ, et al. The surface of articular cartilage contains a progenitor cell population. J Cell Sci. 2004 Feb 29;117(Pt 6):889–97. doi: 10.1242/jcs.00912. [DOI] [PubMed] [Google Scholar]
- 17.Brunet LJ, McMahon JA, McMahon AP, Harland RM. Noggin, cartilage morphogenesis, and joint formation in the mammalian skeleton. Science. 1998 May 29;280(5368):1455–7. doi: 10.1126/science.280.5368.1455. [DOI] [PubMed] [Google Scholar]
- 18.Iwamoto M, Higuchi Y, Koyama E, Enomoto-Iwamoto M, Kurisu K, Yeh H, et al. Transcription factor ERG variants and functional diversification of chondrocytes during limb long bone development. J Cell Biol. 2000 Jul 10;150(1):27–40. doi: 10.1083/jcb.150.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lizarraga G, Lichtler A, Upholt WB, Kosher RA. Studies on the role of Cux1 in regulation of the onset of joint formation in the developing limb. Dev Biol. 2002 Mar 1;243(1):44–54. doi: 10.1006/dbio.2001.0559. [DOI] [PubMed] [Google Scholar]
- 20.Archer CW, Dowthwaite GP, Francis-West P. Development of synovial joints. Birth Defects Res C Embryo Today. 2003 May;69(2):144–55. doi: 10.1002/bdrc.10015. [DOI] [PubMed] [Google Scholar]
- 21.Settle SH, Jr, Rountree RB, Sinha A, Thacker A, Higgins K, Kingsley DM. Multiple joint and skeletal patterning defects caused by single and double mutations in the mouse Gdf6 and Gdf5 genes. Dev Biol. 2003 Feb 1;254(1):116–30. doi: 10.1016/s0012-1606(02)00022-2. [DOI] [PubMed] [Google Scholar]
- 22.Storm EE, Kingsley DM. GDF5 coordinates bone and joint formation during digit development. Dev Biol. 1999 May 1;209(1):11–27. doi: 10.1006/dbio.1999.9241. [DOI] [PubMed] [Google Scholar]
- 23.Spater D, Hill TP, O’Sullivan RJ, Gruber M, Conner DA, Hartmann C. Wnt9a signaling is required for joint integrity and regulation of Ihh during chondrogenesis. Development. 2006 Aug;133(15):3039–49. doi: 10.1242/dev.02471. [DOI] [PubMed] [Google Scholar]
- 24.Guo X, Day TF, Jiang X, Garrett-Beal L, Topol L, Yang T. Wnt/beta-catenin signaling is sufficient and necessary for synovial joint formation. Genes Dev. 2004 Oct;18(19):2404–17. doi: 10.1101/gad.1230704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gong Y, Krakow D, Marcelino J, Wilkin D, Chitayat D, Babul-Hirji R, Hudgins L, Cremers CW, Cremers FP, Brunner HG, Reinker K, Rimoin DL, Cohn DH, Goodman FR, Reardon W, Patton M, Francomano CA, Warman ML. Heterozygous mutations in the gene encoding noggin affect human joint morphogenesis. Nat Genet. 1999 Mar;21(3):302–4. doi: 10.1038/6821. [DOI] [PubMed] [Google Scholar]
- 26.Mundy C, Yasuda T, Kinumatsu T, Yamaguchi Y, Iwamoto M, Enomoto-Iwamoto M, Koyama E, Pacifici M. Synovial joint formation requires local Ext1 expression and heparan sulfate production in developing mouse embryo limbs and spine. Dev Biol. 2011 Mar 1;351(1):70–81. doi: 10.1016/j.ydbio.2010.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Husa M, Liu-Bryan R, Terkeltaub R. Shifting HIFs in osteoarthritis. Nat Med. 2010 Jun;16(6):641–4. doi: 10.1038/nm0610-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rankin EB, Giaccia AJ, Schipani E. A central role for hypoxic signaling in cartilage, bone, and hematopoiesis. Curr Osteoporos Rep. Jun;9(2):46–52. doi: 10.1007/s11914-011-0047-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lanske B, Karaplis AC, Lee K, Luz A, Vortkamp A, Pirro A, et al. PTH/PTHrP receptor in early development and Indian hedgehog-regulated bone growth. Science. 1996 Aug 2;273(5275):663–6. doi: 10.1126/science.273.5275.663. [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 30.Vortkamp A, Lee K, Lanske B, Segre GV, Kronenberg HM, Tabin CJ. Regulation of rate of cartilage differentiation by Indian hedgehog and PTH-related protein. Science. 1996 Aug 2;273(5275):613–22. doi: 10.1126/science.273.5275.613. [DOI] [PubMed] [Google Scholar]
- 31.Wallis GA. Bone growth: coordinating chondrocyte differentiation. Curr Biol. 1996 Dec 1;6(12):1577–80. doi: 10.1016/s0960-9822(02)70776-8. [Review] [DOI] [PubMed] [Google Scholar]
- 32.Koyama E, Leatherman JL, Noji S, Pacifici M. Early chick limb cartilaginous elements possess polarizing activity and express hedgehog-related morphogenetic factors. Dev Dyn. 1996 Nov;207(3):344–54. doi: 10.1002/(SICI)1097-0177(199611)207:3<344::AID-AJA11>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 33.Kohno H, Shigeno C, Kasai R, Akiyama H, Iida H, Tsuboyama T, et al. Synovial fluids from patients with osteoarthritis and rheumatoid arthritis contain high levels of parathyroid hormone-related peptide. J Bone Miner Res. 1997 May;12(5):847–54. doi: 10.1359/jbmr.1997.12.5.847. [Clinical Trial Controlled Clinical Trial Research Support, Non-U.S. Gov’t] [DOI] [PubMed] [Google Scholar]
- 34.Nakamura T, Aikawa T, Iwamoto-Enomoto M, Iwamoto M, Higuchi Y, Pacifici M, et al. Induction of osteogenic differentiation by hedgehog proteins. Biochem Biophys Res Commun. 1997 Aug 18;237(2):465–9. doi: 10.1006/bbrc.1997.7156. [DOI] [PubMed] [Google Scholar]
- 35.Mountford P, Zevnik B, Duwel A, Nichols J, Li M, Dani C, et al. Dicistronic targeting constructs: reporters and modifiers of mammalian gene expression. Proc Natl Acad Sci U S A. 1994 May 10;91(10):4303–7. doi: 10.1073/pnas.91.10.4303. [Research Support, Non-U.S. Gov’t] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen X, Macica CM, Dreyer BE, Hammond VE, Hens JR, Philbrick WM, et al. Initial characterization of PTH-related protein gene-driven lacZ expression in the mouse. J Bone Miner Res. 2006 Jan;21(1):113–23. doi: 10.1359/JBMR.051005. [Research Support, N.I.H., Extramural] [DOI] [PubMed] [Google Scholar]
- 37.Sampson ER, Hilton MJ, Tian Y, Chen D, Schwarz EM, Mooney RA, et al. Teriparatide as a chondroregenerative therapy for injury-induced osteoarthritis. Sci Transl Med. 2011 Sep 21;3(101):101ra93. doi: 10.1126/scitranslmed.3002214. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sohaskey ML, Yu J, Diaz MA, Plaas AH, Harland RM. JAWS coordinates chondrogenesis and synovial joint positioning. Development. 2008 Jul;135(13):2215–20. doi: 10.1242/dev.019950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Q, Green RP, Zhao G, Ornitz DM. Differential regulation of endochondral bone growth and joint development by FGFR1 and FGFR3 tyrosine kinase domains. Development. 2001 Oct;128(19):3867–76. doi: 10.1242/dev.128.19.3867. [DOI] [PubMed] [Google Scholar]
- 40.Gao Y, Lan Y, Liu H, Jiang R. The zinc finger transcription factors Osr1 and Osr2 control synovial joint formation. Dev Biol. 2011 Apr 1;352(1):83–91. doi: 10.1016/j.ydbio.2011.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ito Y, Yeo JY, Chytil A, Han J, Bringas P, Jr, Nakajima A, et al. Conditional inactivation of Tgfbr2 in cranial neural crest causes cleft palate and calvaria defects. Development. 2003 Nov;130(21):5269–80. doi: 10.1242/dev.00708. [DOI] [PubMed] [Google Scholar]
- 42.Serra R, Johnson M, Filvaroff EH, LaBorde J, Sheehan DM, Derynck R, et al. Expression of a truncated, kinase-defective TGF-beta type II receptor in mouse skeletal tissue promotes terminal chondrocyte differentiation and osteoarthritis. J Cell Biol. 1997 Oct 20;139(2):541–52. doi: 10.1083/jcb.139.2.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spagnoli A, O’Rear L, Chandler RL, Granero-Molto F, Mortlock DP, Gorska AE, et al. TGF-beta signaling is essential for joint morphogenesis. J Cell Biol. 2007 Jun 18;177(6):1105–17. doi: 10.1083/jcb.200611031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Loeys BL, Chen J, Neptune ER, Judge DP, Podowski M, Holm T, et al. A syndrome of altered cardiovascular, craniofacial, neurocognitive and skeletal development caused by mutations in TGFBR1 or TGFBR2. Nat Genet. 2005 Mar;37(3):275–81. doi: 10.1038/ng1511. [DOI] [PubMed] [Google Scholar]
- 45.Jones KB, Sponseller PD, Erkula G, Sakai L, Ramirez F, Dietz HC, 3rd, et al. Symposium on the musculoskeletal aspects of Marfan syndrome: meeting report and state of the science. J Orthop Res. 2007 Mar;25(3):413–22. doi: 10.1002/jor.20314. [DOI] [PubMed] [Google Scholar]
- 46.Li T, Longobardi L, Myers TJ, Temple JD, Esposito A, Spagnoli A. Tgfbeta Signaling Regulates Interlekin-36 alpha in Joint Development and Osteoarthritis. Abstract Presented to ASBMR Meeting; 2013; 2013. [Google Scholar]
- 47.Longobardi LLT, Myers TJ, O’Rear L, Ozkan H, Li Y, Contaldo C, Spagnoli A. TGF-β Type II Receptor/MCP-5 Axis: at the Crossroad between Joint and Growth Plate Development. Developmental Cell. 2012 doi: 10.1016/j.devcel.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hansson EM, Lendahl U, Chapman G. Notch signaling in development and disease. Seminars in cancer biology. 2004 Oct;14(5):320–8. doi: 10.1016/j.semcancer.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 49.Lai EC. Notch signaling: control of cell communication and cell fate. Development. 2004 Mar;131(5):965–73. doi: 10.1242/dev.01074. [DOI] [PubMed] [Google Scholar]
- 50.Bray SJ. Notch signalling: a simple pathway becomes complex. Nature reviews. 2006 Sep;7(9):678–89. doi: 10.1038/nrm2009. [DOI] [PubMed] [Google Scholar]
- 51.Milner LA, Bigas A. Notch as a mediator of cell fate determination in hematopoiesis: evidence and speculation. Blood. 1999 Apr 15;93(8):2431–48. [PubMed] [Google Scholar]
- 52.Chiba S. Notch signaling in stem cell systems. Stem cells (Dayton, Ohio) 2006 Nov;24(11):2437–47. doi: 10.1634/stemcells.2005-0661. [DOI] [PubMed] [Google Scholar]
- 53.Lovschall H, Mitsiadis TA, Poulsen K, Jensen KH, Kjeldsen AL. Coexpression of Notch3 and Rgs5 in the pericyte-vascular smooth muscle cell axis in response to pulp injury. Int J Dev Biol. 2007;51(8):715–21. doi: 10.1387/ijdb.072393hl. [DOI] [PubMed] [Google Scholar]
- 54.Hayes AJ, Dowthwaite GP, Webster SV, Archer CW. The distribution of Notch receptors and their ligands during articular cartilage development. J Anat. 2003 Jun;202(6):495–502. doi: 10.1046/j.1469-7580.2003.00185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Karlsson C, Brantsing C, Egell S, Lindahl A. Notch1, Jagged1, and HES5 are abundantly expressed in osteoarthritis. Cells Tissues Organs. 2008;188(3):287–98. doi: 10.1159/000121610. [DOI] [PubMed] [Google Scholar]
- 56.Sassi N, et al. The role of the Notch pathway in healthy and osteoarthritic articular cartilage: From experimental models to ex vivo studies. Arthritis Res Ther. 2011;13(2):208. doi: 10.1186/ar3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grogan SP, Miyaki S, Asahara H, D’Lima DD, Lotz MK. Mesenchymal progenitor cell markers in human articular cartilage: normal distribution and changes in osteoarthritis. Arthritis Res Ther. 2009;11(3):R85. doi: 10.1186/ar2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Henriksson H, Thornemo M, Karlsson C, Hagg O, Junevik K, Lindahl A, et al. Identification of cell proliferation zones, progenitor cells and a potential stem cell niche in the intervertebral disc region: a study in four species. Spine (Phila Pa 1976) 2009 Oct 1;34(21):2278–87. doi: 10.1097/BRS.0b013e3181a95ad2. [DOI] [PubMed] [Google Scholar]
- 59.Hayes AJ, Benjamin M, Ralphs JR. Extracellular matrix in development of the intervertebral disc. Matrix Biol. 2001 Apr;20(2):107–21. doi: 10.1016/s0945-053x(01)00125-1. [DOI] [PubMed] [Google Scholar]
- 60.Karystinou A, Dell’Accio F, Kurth TB, Wackerhage H, Khan IM, Archer CW, et al. Distinct mesenchymal progenitor cell subsets in the adult human synovium. Rheumatology (Oxford) 2009 Sep;48(9):1057–64. doi: 10.1093/rheumatology/kep192. [DOI] [PubMed] [Google Scholar]
- 61.Amizuka N, Warshawsky H, Henderson JE, Goltzman D, Karaplis AC. Parathyroid hormone-related peptide-depleted mice show abnormal epiphyseal cartilage development and altered endochondral bone formation. J Cell Biol. 1994 Sep;126(6):1611–23. doi: 10.1083/jcb.126.6.1611. [Research Support, Non-U.S. Gov’t] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pessler F, Dai L, Diaz-Torne C, Gomez-Vaquero C, Paessler ME, Zheng DH, et al. The synovitis of “non-inflammatory” orthopaedic arthropathies: a quantitative histological and immunohistochemical analysis. Ann Rheum Dis. 2008 Aug;67(8):1184–7. doi: 10.1136/ard.2008.087775. [DOI] [PubMed] [Google Scholar]
- 63.Buckley CD, Filer A, Haworth O, Parsonage G, Salmon M. Defining a role for fibroblasts in the persistence of chronic inflammatory joint disease. Ann Rheum Dis. 2004 Nov;63(Suppl 2):ii92–ii5. doi: 10.1136/ard.2004.028332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.De Bari C, Dell’Accio F, Luyten FP. Human periosteum-derived cells maintain phenotypic stability and chondrogenic potential throughout expansion regardless of donor age. Arthritis Rheum. 2001 Jan;44(1):85–95. doi: 10.1002/1529-0131(200101)44:1<85::AID-ANR12>3.0.CO;2-6. [Research Support, Non-U.S. Gov’t] [DOI] [PubMed] [Google Scholar]
- 65.De Bari C, Dell’Accio F, Vandenabeele F, Vermeesch JR, Raymackers JM, Luyten FP. Skeletal muscle repair by adult human mesenchymal stem cells from synovial membrane. J Cell Biol. 2003 Mar 17;160(6):909–18. doi: 10.1083/jcb.200212064. [Research Support, Non-U.S. Gov’t] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.De Bari C, Dell’Accio F, Luyten FP. Failure of in vitro-differentiated mesenchymal stem cells from the synovial membrane to form ectopic stable cartilage in vivo. Arthritis Rheum. 2004 Jan;50(1):142–50. doi: 10.1002/art.11450. [In Vitro Research Support, Non-U.S. Gov’t] [DOI] [PubMed] [Google Scholar]
- 67.De Bari C, Kurth TB, Augello A. Mesenchymal stem cells from development to postnatal joint homeostasis, aging, and disease. Birth Defects Res C Embryo Today. 2010 Dec;90(4):257–71. doi: 10.1002/bdrc.20189. [Research Support, Non-U.S. Gov’t Review] [DOI] [PubMed] [Google Scholar]
- 68.Shapiro F, Holtrop ME, Glimcher MJ. Organization and cellular biology of the perichondrial ossification groove of ranvier: a morphological study in rabbits. J Bone Joint Surg Am. 1977 Sep;59(6):703–23. [PubMed] [Google Scholar]
- 69.Li T, Longobardi L, Myers TJ, Temple JD, Chandler RL, Ozkan H, et al. Joint TGF-beta type II receptor-expressing cells: ontogeny and characterization as joint progenitors. Stem Cells Dev. May 1;22(9):1342–59. doi: 10.1089/scd.2012.0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McKusick VA. First South-North Human Genome Conference. Genomics. 1992 Dec;14(4):1121–3. doi: 10.1016/s0888-7543(05)80146-6. [DOI] [PubMed] [Google Scholar]
- 71.McCarthy EF. Genetic diseases of bones and joints. Semin Diagn Pathol. Feb;28(1):26–36. doi: 10.1053/j.semdp.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 72.Ramirez F, Dietz HC. Marfan syndrome: from molecular pathogenesis to clinical treatment. Current opinion in genetics & development. 2007 Jun;17(3):252–8. doi: 10.1016/j.gde.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 73.Shirai T, Yorimitsu T, Kiritooshi N, Matsuzaki F, Nakagoshi H. Notch signaling relieves the joint-suppressive activity of Defective proventriculus in the Drosophila leg. Dev Biol. 2007 Dec 1;312(1):147–56. doi: 10.1016/j.ydbio.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 74.Ando K, Kanazawa S, Tetsuka T, Ohta S, Jiang X, Tada T, et al. Induction of Notch signaling by tumor necrosis factor in rheumatoid synovial fibroblasts. Oncogene. 2003 Oct 30;22(49):7796–803. doi: 10.1038/sj.onc.1206965. [DOI] [PubMed] [Google Scholar]
- 75.Oda T, Elkahloun AG, Pike BL, Okajima K, Krantz ID, Genin A, et al. Mutations in the human Jagged1 gene are responsible for Alagille syndrome. Nat Genet. 1997 Jul;16(3):235–42. doi: 10.1038/ng0797-235. [DOI] [PubMed] [Google Scholar]
- 76.Boyer J, Crosnier C, Driancourt C, Raynaud N, Gonzales M, Hadchouel M, et al. Expression of mutant JAGGED1 alleles in patients with Alagille syndrome. Human genetics. 2005 May;116(6):445–53. doi: 10.1007/s00439-005-1262-7. [DOI] [PubMed] [Google Scholar]
- 77.Busse M, Feta A, Presto J, Wilen M, Gronning M, Kjellen L, et al. Contribution of EXT1, EXT2, and EXTL3 to heparan sulfate chain elongation. J Biol Chem. 2007 Nov 9;282(45):32802–10. doi: 10.1074/jbc.M703560200. [DOI] [PubMed] [Google Scholar]
- 78.Mundy C, Yasuda T, Kinumatsu T, Yamaguchi Y, Iwamoto M, Enomoto-Iwamoto M, et al. Synovial joint formation requires local Ext1 expression and heparan sulfate production in developing mouse embryo limbs and spine. Dev Biol. Mar 1;351(1):70–81. doi: 10.1016/j.ydbio.2010.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sgariglia F, Candela ME, Huegel J, Jacenko O, Koyama E, Yamaguchi Y, et al. Epiphyseal abnormalities, trabecular bone loss and articular chondrocyte hypertrophy develop in the long bones of postnatal Ext1-deficient mice. Bone. Nov;57(1):220–31. doi: 10.1016/j.bone.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Peterson HA. Multiple hereditary osteochondromata. Clin Orthop. 1989;239:222–230. [PubMed] [Google Scholar]
- 81.Massague J, Gomis RR. The logic of TGFbeta signaling. FEBS letters. 2006 May 22;580(12):2811–20. doi: 10.1016/j.febslet.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 82.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003 Oct 9;425(6958):577–84. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 83.Lawler S, Candia AF, Ebner R, Shum L, Lopez AR, Moses HL, et al. The murine type II TGF-beta receptor has a coincident embryonic expression and binding preference for TGF-beta 1. Development. 1994 Jan;120(1):165–75. doi: 10.1242/dev.120.1.165. [DOI] [PubMed] [Google Scholar]
- 84.Pelton RW, Saxena B, Jones M, Moses HL, Gold LI. Immunohistochemical localization of TGF beta 1, TGF beta 2, and TGF beta 3 in the mouse embryo: expression patterns suggest multiple roles during embryonic development. J Cell Biol. 1991 Nov;115(4):1091–105. doi: 10.1083/jcb.115.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Millan FA, Denhez F, Kondaiah P, Akhurst RJ. Embryonic gene expression patterns of TGF beta 1, beta 2 and beta 3 suggest different developmental functions in vivo. Development. 1991 Jan;111(1):131–43. doi: 10.1242/dev.111.1.131. [DOI] [PubMed] [Google Scholar]
- 86.Serra R, Chang C. TGF-beta signaling in human skeletal and patterning disorders. Birth Defects Res C Embryo Today. 2003 Nov;69(4):333–51. doi: 10.1002/bdrc.10023. [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S. Review] [DOI] [PubMed] [Google Scholar]
- 87.Dunker N, Krieglstein K. Targeted mutations of transforming growth factor-beta genes reveal important roles in mouse development and adult homeostasis. Eur J Biochem. 2000 Dec;267(24):6982–8. doi: 10.1046/j.1432-1327.2000.01825.x. [DOI] [PubMed] [Google Scholar]
- 88.Baffi MO, Slattery E, Sohn P, Moses HL, Chytil A, Serra R. Conditional deletion of the TGF-beta type II receptor in Col2a expressing cells results in defects in the axial skeleton without alterations in chondrocyte differentiation or embryonic development of long bones. Dev Biol. 2004 Dec 1;276(1):124–42. doi: 10.1016/j.ydbio.2004.08.027. [DOI] [PubMed] [Google Scholar]
- 89.Weinstein M, Yang X, Li C, Xu X, Gotay J, Deng CX. Failure of egg cylinder elongation and mesoderm induction in mouse embryos lacking the tumor suppressor smad2. Proc Natl Acad Sci U S A. 1998 Aug 4;95(16):9378–83. doi: 10.1073/pnas.95.16.9378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yang X, Chen L, Xu X, Li C, Huang C, Deng CX. TGF-beta/Smad3 signals repress chondrocyte hypertrophic differentiation and are required for maintaining articular cartilage. J Cell Biol. 2001 Apr 2;153(1):35–46. doi: 10.1083/jcb.153.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhu Y, Richardson JA, Parada LF, Graff JM. Smad3 mutant mice develop metastatic colorectal cancer. Cell. 1998 Sep 18;94(6):703–14. doi: 10.1016/s0092-8674(00)81730-4. [DOI] [PubMed] [Google Scholar]
- 92.Sirard C, de la Pompa JL, Elia A, Itie A, Mirtsos C, Cheung A, et al. The tumor suppressor gene Smad4/Dpc4 is required for gastrulation and later for anterior development of the mouse embryo. Genes Dev. 1998 Jan 1;12(1):107–19. doi: 10.1101/gad.12.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ganan Y, Macias D, Duterque-Coquillaud M, Ros MA, Hurle JM. Role of TGF beta s and BMPs as signals controlling the position of the digits and the areas of interdigital cell death in the developing chick limb autopod. Development. 1996 Aug;122(8):2349–57. doi: 10.1242/dev.122.8.2349. [DOI] [PubMed] [Google Scholar]
- 94.Robinson E, Keystone EC, Schall TJ, Gillett N, Fish EN. Chemokine expression in rheumatoid arthritis (RA): evidence of RANTES and macrophage inflammatory protein (MIP)-1 beta production by synovial T cells. Clin Exp Immunol. 1995 Sep;101(3):398–407. doi: 10.1111/j.1365-2249.1995.tb03126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hayashida K, Nanki T, Girschick H, Yavuz S, Ochi T, Lipsky PE. Synovial stromal cells from rheumatoid arthritis patients attract monocytes by producing MCP-1 and IL-8. Arthritis Res. 2001;3(2):118–26. doi: 10.1186/ar149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Koch AE, Kunkel SL, Harlow LA, Johnson B, Evanoff HL, Haines GK, et al. Enhanced production of monocyte chemoattractant protein-1 in rheumatoid arthritis. J Clin Invest. 1992 Sep;90(3):772–9. doi: 10.1172/JCI115950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.De Benedetti F, Pignatti P, Bernasconi S, Gerloni V, Matsushima K, Caporali R, et al. Interleukin 8 and monocyte chemoattractant protein-1 in patients with juvenile rheumatoid arthritis. Relation to onset types, disease activity, and synovial fluid leukocytes. J Rheumatol. 1999 Feb;26(2):425–31. [PubMed] [Google Scholar]
- 98.Pujol JP, Chadjichristos C, Legendre F, Bauge C, Beauchef G, Andriamanalijaona R, et al. Interleukin-1 and transforming growth factor-beta 1 as crucial factors in osteoarthritic cartilage metabolism. Connect Tissue Res. 2008;49(3):293–7. doi: 10.1080/03008200802148355. [DOI] [PubMed] [Google Scholar]
- 99.Murray LA, Argentieri RL, Farrell FX, Bracht M, Sheng H, Whitaker B, et al. Hyper-responsiveness of IPF/UIP fibroblasts: interplay between TGFbeta1, IL-13 and CCL2. Int J Biochem Cell Biol. 2008;40(10):2174–82. doi: 10.1016/j.biocel.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 100.Szekanecz Z, Halloran MM, Volin MV, Woods JM, Strieter RM, Kenneth Haines G, 3rd, et al. Temporal expression of inflammatory cytokines and chemokines in rat adjuvant-induced arthritis. Arthritis Rheum. 2000 Jun;43(6):1266–77. doi: 10.1002/1529-0131(200006)43:6<1266::AID-ANR9>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 101.Lara Longobardi JDT, D’Onofrio nunzia, Ozkan Huseyin, Esposito Alessandra, Willcockson Helen H, Li Tieshi, Myers Timothy J, Ye Ping, Moats-Staats Billie M, Balestrieri Marialuisa, Spagnoli Anna. A Role for Tgf-betaRII/MCP5 Axis during Post-traumatic Osteoarthritis and Potential Role od PTHrP in Mediating MCP5 Effect. Abstract presented at the OARSI Meeting; Paris. 2014. [Google Scholar]
- 102.Lara Longobardi HO, Temple Joseph D, Li Tieshi, Esposito Alessandra, Myers Timothy J, Spagnoli Anna. Inhibition of MCP5 Signaling Decreases osteoarthritisc Lesions in a Murine Model of Post-traumatic Osteoarthritis. Abstract presented at the ASBMR Meeting; Baltimore, MD, USA. 2013. [Google Scholar]
- 103.Bird HA. Controversies in the treatment of osteoarthritis. Clin Rheumatol. 2003 Sep;22(3):165–7. doi: 10.1007/s10067-003-0705-6. [DOI] [PubMed] [Google Scholar]
- 104.Brandt KD. Non-surgical treatment of osteoarthritis: a half century of “advances”. Ann Rheum Dis. 2004 Feb;63(2):117–22. doi: 10.1136/ard.2002.004606. [DOI] [PMC free article] [PubMed] [Google Scholar]


